Abstract
Large-conductance Ca2+-activated K+ channels (BK, KCa1.1, MaxiK) are important regulators of urinary bladder function and may be an attractive therapeutic target in bladder disorders. In this study, we established a high-throughput fluorometric imaging plate reader–based screening assay for BK channel activators and identified a small-molecule positive modulator, NS19504 (5-[(4-bromophenyl)methyl]-1,3-thiazol-2-amine), which activated the BK channel with an EC50 value of 11.0 ± 1.4 µM. Hit validation was performed using high-throughput electrophysiology (QPatch), and further characterization was achieved in manual whole-cell and inside-out patch-clamp studies in human embryonic kidney 293 cells expressing hBK channels: NS19504 caused distinct activation from a concentration of 0.3 and 10 µM NS19504 left-shifted the voltage activation curve by 60 mV. Furthermore, whole-cell recording showed that NS19504 activated BK channels in native smooth muscle cells from guinea pig urinary bladder. In guinea pig urinary bladder strips, NS19504 (1 µM) reduced spontaneous phasic contractions, an effect that was significantly inhibited by the specific BK channel blocker iberiotoxin. In contrast, NS19504 (1 µM) only modestly inhibited nerve-evoked contractions and had no effect on contractions induced by a high K+ concentration consistent with a K+ channel–mediated action. Collectively, these results show that NS19504 is a positive modulator of BK channels and provide support for the role of BK channels in urinary bladder function. The pharmacologic profile of NS19504 indicates that this compound may have the potential to reduce nonvoiding contractions associated with spontaneous bladder overactivity while having a minimal effect on normal voiding.
Introduction
Ca2+-activated large conductance K+ channels (BK, KCa1.1, MaxiK, Slo1), encoded by KCNMA, are unique in the family of K+-selective ion channels by showing dual activation by both Ca2+ and membrane depolarization. The ability to respond strongly to increases in intracellular Ca2+ resulting from action potential activity makes this channel an important negative-feedback mechanism for Ca2+ entry in many excitable cell types. In addition to direct regulation by intracellular Ca2+ and voltage, BK channel activity is also modulated by other factors, such as phosphorylation state, pH, and the presence of regulatory β-subunits. Alternative splice variants of the KCNMA1 α-subunit with distinct basic physiologic and pharmacological properties have been described (Salkoff et al., 2006). BK channels are expressed in both excitable and nonexcitable cells and are involved in many cellular functions, including regulation of the tone of vascular, uterine, gastrointestinal, airway and bladder smooth muscle, neuronal excitability, as well as neurotransmitter and hormone release (Ghatta et al., 2006; Lu et al., 2006). As a consequence of their physiologic roles, BK channels have long been regarded as attractive therapeutic targets for a number of diseases, including urinary bladder dysfunction disorders.
In urinary bladder smooth muscle (UBSM), BK channels have been shown to be key regulators of excitability and contractility. Bladder emptying is mediated by the activation of parasympathetic nerves, which release ATP and acetylcholine to increase UBSM excitability (action potentials) and thereby contractility (Heppner et al., 2005; Petkov et al., 2005; Nausch et al., 2010). BK pore-forming α and accessory β1- and β4-subunits are expressed in UBSM cells (Ohya et al., 2000; Ohi et al., 2001a,b; Petkov et al., 2001; Werner et al., 2007; Chen and Petkov, 2009; Hristov et al., 2011), and the BK channel is believed to play an important modulatory role in urinary bladder function through action potential shortening and membrane potential hyperpolarization (Heppner et al., 1997). Consistent with this role, inhibiting BK channel activity with the specific BK blocker iberiotoxin (IbTx) has been shown to increase spontaneous phasic, agonist-induced, and nerve-evoked contractions of detrusor strips from humans (Darblade et al., 2006), pigs (Buckner et al., 2002), guinea pigs (Heppner et al., 1997; Kobayashi et al., 2000; Mora and Suarez-Kurtz, 2005), rats (Uchida et al., 2005), and mice (Herrera et al., 2005).
In bladder strips from mice in which the BK pore-forming α-subunit has been deleted (slo−/− mice), contractility is markedly increased in response to both cholinergic and purinergic pathway stimulation (Thorneloe et al., 2005; Werner et al., 2007). In vivo, slo−/− mice show a pronounced increase in voiding frequency and display nonvoiding bladder contractions (Meredith et al., 2004; Thorneloe et al., 2005). Studies on BK β1-KO mice also support a role for the BK channel, and specifically the β1-subunit, in modulation of smooth muscle contractility in the cardiovascular system and bladder (Petkov et al., 2001). Taken together, studies using specific toxins and/or BK-deficient mice to block or reduce BK channel function suggest that positive modulators of BK may have beneficial effects in unstable or overactive bladders.
A number of small-molecule, positive BK modulators have been identified, including the synthetic benzimidazolin-2-one derivative NS1619 (1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-1,3-diaza-1,3-dihydroinden-2-one) and the bisarylurea NS1608 (4-chloro-2-{3-[m-(trifluoromethyl)phenyl]ureido}phenol). More recent studies have identified the bisarylthiourea NS11021 [5-(2-{3-[3,5-bis(trifluoromethyl)phenyl]thioureido}-5-bromophenyl)-1H-1,2,3,4-tetrazole], the oxindole BMS-204352 [(3S)-3-(5-chloro-2-methoxyphenyl)-3-fluoro-6-(trifluoromethyl)-2-indolinone], the natural modulator dihydrosoyasaponin-1 (for review, see Ghatta et al., 2006; Nardi and Olesen, 2008), the GoSlo-SR family of anthraquinone analogs (Roy et al., 2012, 2014), as well as the tetrahydroquinoline racemate “X” and its two enantiomers “Y” and “Z” (Ponte et al., 2012). Several of these modulators have shown activity in pharmacological studies in ex vivo organ bath studies using bladder strips (Imai et al., 2001; Tanaka et al., 2003; Malysz et al., 2004; Mora and Suarez-Kurtz, 2005; Ponte et al., 2012). However, despite the long-standing interest in the BK channel as a promising therapeutic target, the known activators of BK channels suffer to a varying degree from limited selectivity (Holland et al., 1996; Bentzen et al., 2009) as well as undesirable physiochemical and pharmacodynamic properties that compromise their usefulness in validating the BK channel as a viable target or their value as therapeutic agents in their own right. Accordingly, there is a continued interest in identifying new positive BK channel modulators with improved properties.
In the present study, we introduce NS19504 (5-[(4-bromophenyl)methyl]-1,3-thiazol-2-amine) identified in a high-throughput fluorescence imaging plate reader (FLIPR) screening campaign as a new BK channel activator. The compound was validated and characterized further in automated and manual patch-clamp experiments, in which it was shown to activate heterologously expressed and native BK channels in a concentration-dependent manner. A favorable selectivity profile was revealed in a screen of 68 receptors and by functional tests on Nav, Cav, SK, and IK channels. NS19504 potently inhibits urinary bladder spontaneous phasic contractions (SPCs) while having only a modest effect on contractions evoked by electrical field stimulation (EFS) and no effect on high K+-induced contractions. These findings suggest that NS19504 may have the potential to alleviate bladder overactivity without compromising the capacity for voluntary bladder emptying.
Materials and Methods
NS19504 was synthesized from readily available starting materials, as described by Obushak et al. (2004). The compound is also commercially available and can be purchased from Enamine (Kiev, Ukraine). IbTx was purchased from Tocris Bioscience (Bristol, UK) and dissolved in water.
Cells.
Establishment of human embryonic kidney (HEK) 293 cells stably expressing hBK channels (α-subunit) has previously been described (Ahring et al., 1997). The cells were grown in Dulbecco's minimum essential medium supplemented with 10% fetal calf serum and selection antibiotics (G418; Invitrogen, Taastrup, Denmark) and maintained at 37°C in a 5% CO2 atmosphere.
Smooth muscle cells from guinea pig urinary bladder were isolated, as described before (Layne et al., 2010). Briefly, pieces (2 × 2 mm) of guinea pig detrusor were incubated for 20 minutes at 37°C in dissection solution (80 mM monosodium glutamate, 55 mM NaCl, 6 mM KCl, 10 mM glucose, 10 mM HEPES, and 2 mM MgCl2, adjusted to pH 7.3) containing 1 mg/ml papain (Worthington Biochemical, Freehold, NJ) and 1 mg/ml dithioerythritol. Tissue pieces were then transferred to dissection solution containing 1 mg/ml collagenase (type II; Sigma-Aldrich, Brøndby, Denmark) and 100 µM CaCl2 and incubated for 6 minutes at 37°C. After rinses in ice-cold dissection solution, tissue pieces were gently triturated using a fire-polished Pasteur pipette to release smooth muscle cells. Smooth muscle cells were used the same day.
For culturing of dorsal root ganglia (DRG) cells, see Supplemental Methods.
High-Throughput Screening.
To identify new BK channel modulators, we established a Tl+ influx assay, exploiting the fact that Tl+ can constitute a surrogate for K+ influx. The screening campaign was performed using HEK293 cells stably expressing hBK channels and loaded with the Tl+-sensitive fluorescent dye benzothiazole coumarin acetoxymethyl ester (BTC-AM). A high-throughput screening (HTS) campaign using the FLIPR methodology was launched to identify potential modulators of hBK channels in a compound collection of 174,879 compounds, of which 11,261 were proprietary, and the remainder was comprised of commercially available screening libraries. HEK293 cells stably expressing hBK channels were seeded on 384-well, clear-bottom, black-walled Optiplates (Corning, Corning, NY) coated with poly-D-lysine (10 µg/ml) at a density of ∼3 × 106 cells/ml in 20 µl Dulbecco's minimum essential medium containing 10% fetal calf serum. After incubation overnight at 37°C in a humidified 5% CO2 environment, cells were washed once in Cl−-free assay buffer (in mM: 140 Na+ gluconate, 2.5 K+-gluconate, 6 Ca2+ gluconate, 1 Mg2+ gluconate, 5 glucose, 10 HEPES, pH 7.3) and then loaded with the fluorescent dye BTC-AM (Invitrogen) for 1 hour in loading buffer [assay buffer containing an additional 2 µM BTC-AM, 2 mM amaranth (Sigma-Aldrich), and 1 mM tartrazine (Sigma-Aldrich)]. Assay plates were tested in a FLIPR (Molecular Devices, Sunnyvale, CA) using the 488-nm line of an argon laser for excitation and a 540 ± 30-nm bandpass filter for emission in a two-addition protocol. Following collection of a baseline signal, test compounds from the compound library were added in Cl−-free assay buffer to give a final test concentration of 30 µM [final dimethylsulfoxide (DMSO) concentration <0.1%]. Thereafter, stimulus buffer (Cl−-free assay buffer supplemented with 6 µM A23187, 18.9 mM Tl2SO4, 12.5 mM K2SO4, 2 mM amaranth, and 1 mM tartrazine) was added to the assay plates to yield final concentrations of Tl+ and A23187 of 9.45 mM and 1.5 µM, respectively. Test compound responses were normalized to the stimulus buffer response. Screening hits were retested in quadruplicate for confirmation of activity. Compounds for which the activity was confirmed were subsequently subjected to concentration-response testing (0.1 nM to 100 µM) in quadruplicate. In each test plate, 16 wells served as negative controls and 16 wells served as positive controls (addition of either Cl−-free assay or stimulus buffer alone).
For data analysis, values from individual wells in the HTS campaign were background-corrected by subtraction of the averaged value of the negative control wells and normalized to the fluorescence peak value of the positive control response. The screening window coefficient Z′ describing the assay quality was estimated as described by Zhang et al. (1999). A Z′ ≥ 0.25 was required for quality control of the screening plates. The hit threshold was defined as the mean of the test wells on a given plate plus 3 × S.D.
Confirmed FLIPR hits were subsequently validated in an automated electrophysiology assay (QPatch) (see Supplemental Material; Supplemental Fig. 1). Finally, the hits underwent a clustering procedure based on chemoinformatic descriptor-based and manual methods.
Patch-Clamp Electrophysiology.
HEK293 cells expressing hBK channels (approximately 75% confluent) were washed with phosphate-buffered saline, harvested by TrypLE Express (Gibco/Life Technologies, Nærum, Denmark) treatment, and transferred to petri dishes containing coverslips. Membrane currents were recorded at room temperature using the whole-cell or inside-out configuration of the patch-clamp technique. Coverslips were transferred to the 15 µl recording chamber, and the cells were continuously superfused at 1 ml/min. An integrated Ag/AgCl pellet electrode served as reference. Patch pipettes (∼2 MΩ) were pulled from borosilicate tubes with an outside diameter of 1.32 mm using a horizontal electrode puller, and an electronically controlled micromanipulator was used for positioning pipettes. Recordings, experimental control, and data acquisition were performed using an EPC-9 amplifier and Pulse software (HEKA, Lambrecht, Germany).
In patch-clamp experiments, the saline on the extracellular side contained (in mM): 144 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, and 10 HEPES (pH 7.4 with NaOH). The intracellular saline contained (in mM): 154 KCl, 10 HEPES, 10 EGTA, as well as MgCl2 and CaCl2 to yield free concentrations of 1 mM Mg2+ and 0.03 µM (whole-cell) or 0.3 µM (inside-out) Ca2+ (whole-cell: 1.7 MgCl2 and 2.4 CaCl2; inside-out: 1.2 and 7.6). The intracellular saline was adjusted to pH 7.2 with 1 M KOH.
Whole-cell currents were recorded during 150-millisecond voltage ramps ranging from −120 to +30 mV elicited every 5 seconds from a holding potential of −90 mV. In inside-out experiments, currents were elicited by applying either a voltage ramp (200-millisecond linear voltage ramp from −120 to +80 mV every 5 seconds from a holding potential of −90 mV) or a voltage step protocol (from a holding potential of −90 mV, 20 mV steps, 50-millisecond duration, were applied from −120 to +120 mV, followed by a 60-millisecond step to −120 mV). Sample rate was 10 kHz to allow for proper resolution of peak tail currents. Serial resistance (Rs) compensation (80%) was performed in the whole-cell experiments. Data are reported in the absence of leak subtraction.
For the calculation of steady-state activation curves, peak tail currents were measured at −120 mV. Steady-state activation curves were fitted to Boltzmann equations of the following form:
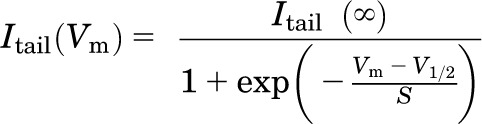 |
where V1/2 is the potential at which half-maximal activation is obtained and S is the slope factor. When results from several experiments were pooled, data were normalized with respect to fitted Itail (∞) from the individual experiments.
To test for selectivity toward other classes of ion channels, recordings of whole-cell voltage-clamp experiments were conducted using rat dorsal root ganglion neurons and HEK293 cell lines expressing hIK, hSK3, hSK2, and rNav1.2 channels, respectively. hIK, hSK2, and hSK3 currents were activated by using a pipette solution with free [Ca2+]i buffered at 0.4 µM, and currents were measured upon application of voltage ramps from −120 mV to +30 mV every 5 seconds. rNav1.2 channels in HEK293 as well as Nav and Cav channels endogenously expressed in DRG neurons were activated by 15-millisecond voltage steps to 0 mV (see Supplemental Material).
Patch-Clamp Electrophysiology of Single Freshly Isolated Guinea Pig USBM Cells.
Whole-cell BK channel currents in response to 200-millisecond voltage ramps (−100 to +50 mV; holding potential, −50 mV) were recorded using the conventional configuration of the patch-clamp technique (Axopatch 200B amplifier and Clampex software; Molecular Devices) at room temperature. Currents were filtered at 2 KHz and digitized at 20 KHz. The bathing solution contained (in mM): 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH 7.4. The patch pipettes had tip resistance of ∼3 MOhm. The pipette solution contained (in mM) KCl 128.6, KOH 11.4, NaOH 10, CaCl2 3.2, EGTA 5.0, Mg 1.091, pH 7.2. The free Ca2+ concentration was calculated to be 300 nM (WEBMAXC Standard, http://www.stanford.edu/~cpatton/webmaxcS.htm; Chris Patton, Stanford University).
Guinea Pig Bladder Myography.
Juvenile guinea pigs of either sex were euthanized by isoflurane anesthesia, followed by exsanguination, according to a protocol approved by the Institutional Animal Care and Use Committee of the University of Vermont. The urinary bladder was removed and kept in ice-cold Ca2+-free HEPES-buffered solution (Ca-free HEPES, in mM: 55 NaCl, 5.6 KCl, 2 MgCl2, 80 sodium glutamate, 10 HEPES, 10 glucose, pH adjusted to 7.3 with NaOH). The caudal part of the urinary bladder (proximal urethra, trigone, distal ureters) was removed, and the remaining tissue was cut open with a longitudinal incision. Residual urine was rinsed off the tissue with ice-cold Ca-free HEPES. After removal of the urothelium, the detrusor was cut into eight strips (∼6 × 1.5 mm), and loops made from silk suture were attached to either end of the tissue strips.
For myography, the strips were transferred to the organ bath of a MyoMED myograph system (Med Associates, Georgia, VT) and, by means of the loops, attached to a force transducer. The strips were bathed in physiologic saline solution (PSS; in mM: 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, 0.023 EDTA, 11 glucose). PSS was warmed to 37°C and bubbled with 95% O2 and 5% CO2 to provide oxygenation and maintain physiologic pH. Initially, a tension of 10 mN was applied and the tissue was allowed to equilibrate for 1 hour. During equilibration, the PSS was changed every 20 minutes. Most strips developed SPCs during equilibration. The effect of 1 µM NS19504 was tested on SPCs. After equilibration, IbTx (100 nM) or apamin (Apa; 300 nM) was added to the bath, as applicable. After 30 minutes of incubation, 1 µM NS19504 was added to the bath. Contractile force was analyzed before and 20 minutes after adding NS19504. Vehicle (DMSO) controls were run in parallel.
For concentration-response curves, stock solution of NS19504 in DMSO was added in an additive fashion directly to the bath to yield 0.1, 0.3, 1, 3, 10, and 30 µM NS19504. Contractile force was analyzed before adding NS19504 and then 20 minutes after each concentration increase.
Nerve-evoked contractions were induced by EFS. A PHM-152V stimulator was used to generate square pulses of 20 V amplitude and 0.2-millisecond duration. Bursts of pulses were delivered via platinum electrodes parallel to the tissue strip for 2 seconds at 20 Hz every 2 minutes. After 30 minutes of stimulation, 1 µM NS19504 or 0.1% DMSO (vehicle) was added to the bath. Contractile force was analyzed before application of NS19504 or DMSO and 20 minutes thereafter.
For K+-induced depolarization of strips, the PSS in the organ bath was replaced with PSS containing 60 mM K+ (K+ PSS, in mM: 64.9 NaCl, 58.8 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, 0.023 EDTA, 11 glucose). After 15 minutes, 1 µM NS19504 or DMSO (0.1%) was added and contractile force was measured before and 15 minutes after application of NS19504 or DMSO.
For myography data analysis, we analyzed amplitude, force integral (area under the curve) and frequency of SPCs, amplitude and force integral of EFS-evoked contractions, and amplitude of contractions evoked by 60 mM K+ PSS. Data were collected before and after application of NS19504 (1 µM) and compared using Student’s t tests. To compare different treatments, we expressed the value after treatment as percentage of the value before treatment (% of control) and performed Student’s t tests; one- or two-way analyses of variance (ANOVAs) were used to compare two or more groups. Half-maximal effective concentrations (EC50) were obtained from fits of concentration-response curve data to a sigmoidal concentration-response function (GraphPad Prism; GraphPad Software, La Jolla, CA). The number of experiments (n) refers to the number of tissue strips (from at least four guinea pigs).
All statistical comparisons were performed using GraphPad Prism software; P values <0.05 were considered statistically significant. All data are presented as mean ± S.E.M.
Results
Fluorescence-Based Tl+ Influx Assay for the Identification of hBK Modulators.
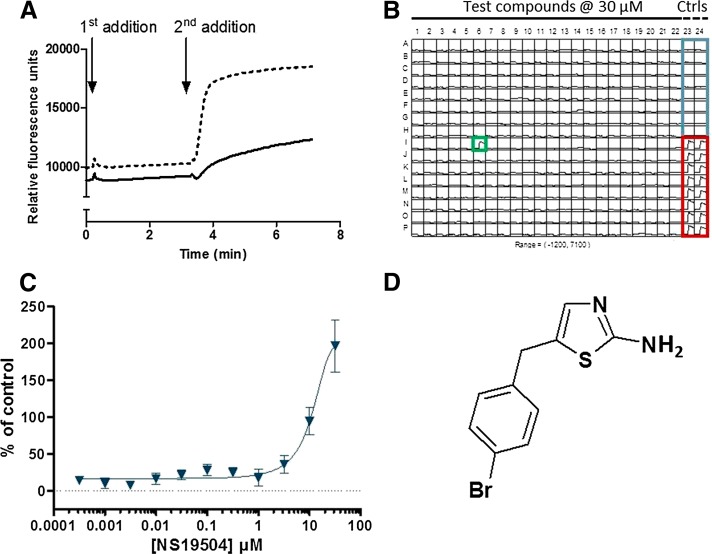
A HTS campaign was launched to test for potential modulators of hBK channels in a compound collection of 174,879 compounds. As described in Materials and Methods, the screening campaign was performed as a FLIPR-based Tl+ influx assay. Figure 1A demonstrates that an increased fluorescence signal was readily detected upon addition of the Ca2+ ionophore A23187 (second addition) in the presence of a BK-positive modulator, in this two-addition Tl+ influx assay. The screening assay was performed in 384-well format, as demonstrated in Fig. 1B, in which columns 23 and 24 served as controls. As part of the assay validation procedure, the ability of known BK channel openers to potentiate the basic stimulus response was confirmed. These BK channel openers included, among others, NS1619 and NS11021 (data not shown). The screening campaign delivered 345 confirmed hits, which correspond to a hit rate of 0.2%. The confirmed FLIPR hits were subsequently validated in an automated patch-clamp assay using QPatch. Supplemental Figure 1 shows an example of a test of the compound NS19504 in the QPatch system. The resulting 231 validated hits were clustered in 30 putative chemical series, including a particularly interesting group of benzothiazoleamine compounds. The prototype compound of this group, NS19504, activated BK channels concentration-dependently in the Tl+ assay (Fig. 1C) with an estimated EC50 value of 11.0 ± 1.4 µM (n = 4). To rule out any potential nonspecific fluorescent effects of NS19504, the compound was also tested in the presence of the BK channel antagonist paxilline. In this study, 5 µM paxilline completely abolished the modulatory response of NS19504 (data not shown). NS19504 has a structure markedly different from previously reported BK activators (Fig. 1D) as well as a low molecular mass of 269.2 Da and good calculated physicochemical properties (ACDlabs Software version 12), showing a cLogD of 2.5 at pH 7.4, a calculated intrinsic solubility of 0.097 mg/ml [measured solubilities of 0.02 mg/ml (water), 9.0 mg/ml (15% methyl-β-cyclodextrin), 0.43 mg/ml (15% hydroxypropyl-β-cyclodextrin), and 3.6 mg/ml (10% Cremophor), respectively], and a polar surface area of 67.2 Å2. In addition, NS19504 is devoid of an acidic group in contrast to most known small-molecule BK channel activators. NS19504 was therefore selected for further characterization as a promising chemical lead.
Fig. 1.
FLIPR-based hBK assay. (A) Fluorescence emission from HEK293 cells stably overexpressing BK and loaded with BTC-AM followed over time on a fluorescence-imaging plate reader (FLIPR). In the first addition, cells are stimulated with either Cl−-free buffer (full line) or Cl−-free buffer containing a BK activator to be tested (dashed line). In the second addition, a Tl+-based stimulus buffer containing the Ca2+ ionophore A23187 and K2SO4 (for slight depolarization, because hBK channels are voltage- as well as Ca2+-sensitive) at final concentrations of 1.5 µM and 12.5 mM, respectively, is added (all traces). Note that, under control conditions, there is a minor increase in the fluorescence signal upon addition of stimulus buffer (full line), whereas in the presence of a hBK-positive modulator, there is a marked increase in the response after the second addition (dashed line). (B) A representative example of a screening plate showing the fluorescence traces over time. Wells A1-P22 were exposed to test compound at a concentration of 30 µM. One positive modulator hit (green square) was identified on the illustrated screening plate. Columns 23 and 24 contain control wells: buffer control (blue square, wells A23:H24) and a proprietary BK channel modulator used as positive control (red square, wells I23:P24). (C) Typical example of a concentration-response curve for NS19504 tested in 1/2-log dilutions ranging from 0.316 nM to 31.6 µM. Each compound concentration was tested in four individual wells, and responses were normalized toward the proprietary BK channel modulator. Symbols and error bars depict average responses and S.D. (D) Structure of NS19504. Ctrls, controls.
NS19504 Activation of BK Channel Currents.
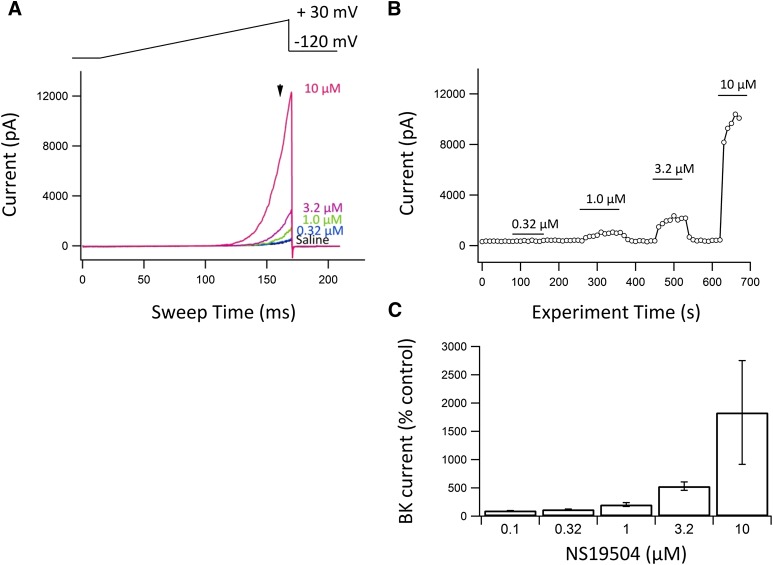
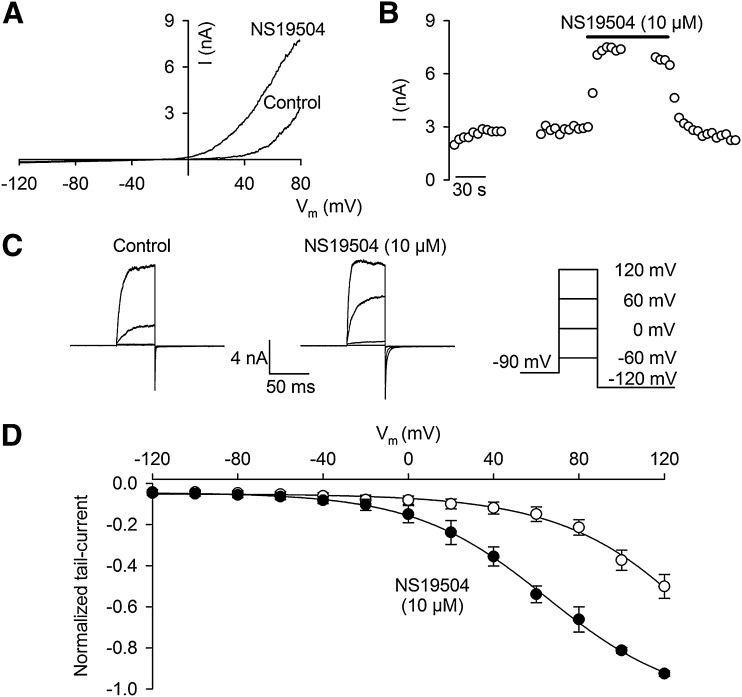
NS19504 was further characterized in whole-cell patch-clamp experiments on the same hBK/HEK293 cell line as used in the FLIPR and QPatch assays. Figure 2A shows individual current responses to an applied voltage ramp in the absence and presence of NS19504 in concentrations from 0.32 to 10 µM. Figure 2B shows the time course of the entire experiment as the current measured at a ramp potential of 25 mV (indicated by the arrow in Fig. 2A). Figure 2C is a summary bar graph showing the percentage increase in BK current at the various concentrations tested. Clearly, the BK channel is reversibly modulated in a concentration-dependent manner starting at submicromolar concentrations of NS19504. Because the BK channel is expressed at high levels in the cell line used, we were not able to apply higher concentrations of NS19504 (voltage control issue). To record under a controlled Ca2+ concentration ([Ca2+]i), we chose to proceed the characterization of NS19504 with recordings from inside-out patches (Fig. 3). Under control conditions and at a [Ca2+]i of 0.3 µM, applying voltage ramps from −120 to +80 mV activated an outwardly rectifying BK current with a threshold for activation approximately +40 mV (Fig. 3A). Application of NS19504 (10 µM) resulted in an increase in current at potentials positive to 0 mV, indicating that less membrane depolarization is needed for opening of BK channels. Figure 3B shows the time course of an experiment in which current was measured at +80 mV. Application of NS19504 (10 µM) induced a fast increase in current that reached a new steady level within seconds and completely reversed to control levels upon compound washout. To determine the effect of NS19504 on the steady-state voltage activation curve, we measured tail currents at −120 mV after 50-millisecond steps to potentials from −120 to +120 mV. Tail currents were analyzed once a steady current level was obtained both in the absence and presence of NS19504 (indicated by breaks in the time course record in Fig. 3B). The peak tail current reflects the relative fraction of open channels at the previous step potential. Figure 3C shows the current in response to steps to −60, 0, +60, and +120 mV from a holding potential of −90 mV and the inward tail current in response to the subsequent step to −120 mV. Plotting tail currents as a function of the step potential yielded the activation curves depicted in Fig. 3D. Under control conditions, the threshold for activation was approximately +40 mV, and the fitted curve yielded a V1/2 of approximately +120 mV. In the presence of 10 µM NS19504, the voltage activation curve was left-shifted with a threshold for activation of 0 mV and V1/2 of +60 mV, indicating more current at a given potential.
Fig. 2.
Activation of hBK channels measured in whole-cell patch-clamp experiments. (A) hBK-mediated currents recorded from voltage ramps (top) before (Saline) and in the presence of NS19504 in the concentrations indicated to the right of the traces. Currents were measured at a ramp potential of 25 mV as indicated by arrow. (B) The currents at +25 mV were measured and plotted as a function of time. At the 0.03 µM free Ca2+ used in this experiment, activation was prominent from a concentration of 1 µM NS19504. Due to the high numbers of channels in the HEK293 cell line, the currents were too large to test higher concentrations of the compound. (C) Summary bar graph showing percentage BK current (control = 100%) at various concentrations of NS19504. The average values ± S.E.M. are 0.1 µM: 100% (n = 1); 0.32 µM: 121 ± 5.7% (n = 6); 1 µM: 205 ± 34% (n = 6); 3.2 µM: 530 ± 74% (n = 6); and 10 µM: 1834 ± 917 (n = 4).
Fig. 3.
Electrophysiological characterization and activation of BK channels by NS19504. Current was measured in inside-out patches obtained from HEK293 cells stably expressing BK channels. Experiments were conducted at a physiologic K+ gradient (4/154 mM K+) and at a free intracellular Ca2+ concentration of 0.3 µM. (A) Current-voltage (I-V) relationships measured in the absence (Control) or presence of 10 µM NS19504 in the intracellular/bath solution. Currents were elicited by applying linear voltage ramps from −120 to +80 mV from a holding potential of −90 mV. (B) BK current measured at +80 mV depicted as a function of time. NS19504 (10 µM) was applied to the inside of the patch, indicated by the bar. Breaks in the recording indicate periods where voltage step protocols were applied. Data are from a single experiment representative of four independent experiments. (C) BK current activated by membrane potential steps, as illustrated in the drawing to the right. (D) Normalized tail currents depicted as a function of step potential. Data are mean ± S.E.M. of four independent experiments. Peak tail currents were measured by stepping to −120 mV after obtaining steady current activation at depolarized potentials either in the absence or presence of 10 µM NS19504.
Selectivity Testing.
The functional effect of NS19504 on the IK and SK Ca2+-activated K+ channels was tested in whole-cell experiments (see Supplemental Material). The pipette saline with a free Ca2+ of 0.4 µM was used in these experiments, and using this saline in experiments with hBK 0.3 µM NS19504 increased the current relatively to the baseline current to 164 ± 26% (n = 4), whereas 1 µM augmented it to 569 ± 96% (n = 3). On hIK a modest increase in current was observed at 1 µM NS19504 (119 ± 5% S.E., n = 5), whereas clear activation was observed at 10 µM (234 ± 29%, n = 5). At hSK3 and hSK2 channels, the effects of 10 µM NS19504 were 113 ± 5%, n = 7 and 290 ± 80%, n = 3, respectively.
Furthermore, NS19504 (10 µM) inhibited negligibly 3 ± 3% of the peak current mediated by Nav1.2 channels expressed in HEK293 cells (n = 6) (see Supplemental Material; Supplemental Fig. 2) and 18% (n = 2) of the Nav current in DRG neurons. Finally, NS19504 at 10 µM had no effect on high-threshold Cav channels in DRG neurons (n = 3) (for experimental details, see Supplemental Material).
To further address receptor selectivity, we tested binding of NS19504 (10 μM) at 68 receptors in radioligand receptor-binding assays (Ricerca, LeadProfilingScreen, Taipei, Taiwan). Effects (defined as >50% inhibition) were observed against the following: norepinephrine transporter (SLC6A2; 74%), dopamine transporter (SLC6A3; 75%), and sigma nonopioid intracellular receptor 1 (σ1R; 59%). No effect was observed against the other 65 channels and receptors tested, including L- and N-type Ca2+ channels; Nav, KATP, and hERG channels; muscarinic M1, M2, and M3 receptors; neuropeptide Y1 and Y2 receptors; NK1 receptor; and purinergic P2X and P2Y receptors (Supplemental Table 1). These observations provide experimental support for the selectivity profile of NS19504 for BK channels.
NS19504 Activation of BK Channels in Bladder Smooth Muscle Cells.
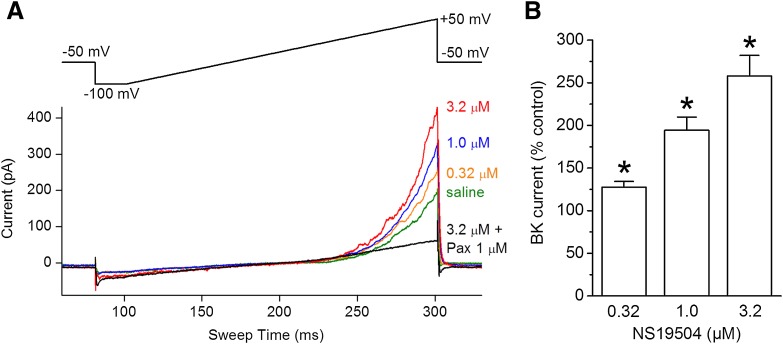
To test the effect of NS19504 on native BK channel currents in urinary bladder, smooth muscle cells were isolated from guinea pig bladder and whole-cell responses to voltage ramps were investigated (Fig. 4). NS19504 increased whole-cell currents induced by voltage ramps of 200 milliseconds (−100 to +50 mV, holding potential −50 mV, 300 nM free Ca2+) in a concentration-dependent manner. At a concentration of 0.32 µM, NS19504 increased the response to 127 ± 7% of control (n = 7). When 1.0 µM NS19504 was applied, a response to 194 ± 16% (n = 8) was observed; at a concentration of 3.2 µM, NS19504 increased the response to 258 ± 24% (n = 8); and at a concentration of 10 µM, NS19504 increased the response to 561 ± 114% (n = 7; data not shown). The augmented response was abolished by the BK channel inhibitor paxilline (1 µM), supporting the BK selectivity of NS19504 in guinea pig urinary bladder cells.
Fig. 4.
NS19504 increases BK currents in freshly isolated single USBM cells. (A) Representative whole-cell currents in response to 200-millisecond voltage ramps from −100 to +50 mV (insert in top) before (saline) and in the presence of NS19504 in the concentrations indicated to the right of the traces. BK channel inhibitor paxilline (1 µM, Pax) added at the end of experiment eliminates the effect of NS19504 and decreases the currents below the control (saline) amplitude. (B) Summary of relative to control percent increase of whole-cell BK currents by 0.32, 1.0, and 3.2 µM NS19504 measured at +50 mV. BK currents were obtained by subtraction of the currents in the presence of paxilline (1 µM) from control currents and those in the presence of NS19504. Data are means ± S.E. (n = 7– 8; *P < 0.05, one-way ANOVA).
Effect of NS19504 on SPCs in Guinea Pig Bladder Strips.
We studied the effect of NS19504 in urothelium-denuded bladder strips from guinea pigs, as this preparation is highly suitable for analyzing SPCs of myogenic origin (Fig. 5). SPCs developed in most strips during equilibration. We measured amplitude, force integral, and frequency of SPCs. In controls, the amplitude was 0.89 ± 0.14 mN, the force integral was 2.28 ± 0.44 mN × s, and the frequency was 3.72 ± 0.32 minute−1. A representative recording is depicted in Fig. 5A.
Fig. 5.
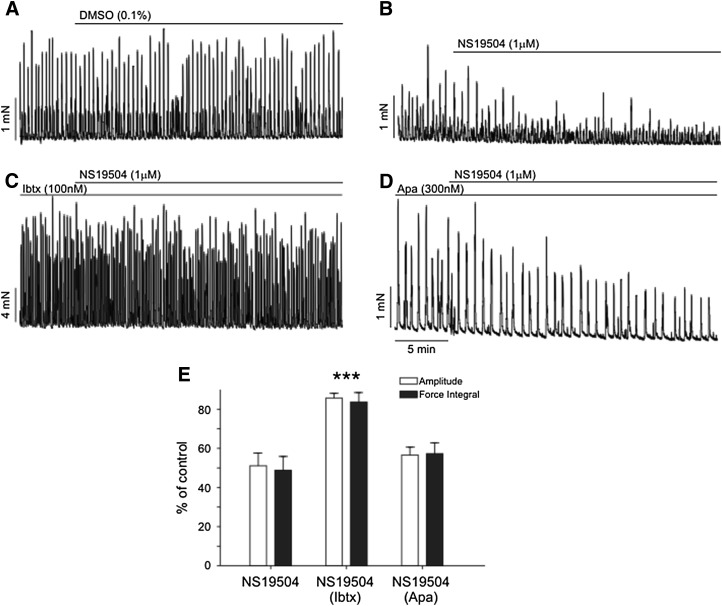
Effect of 1 µM NS19504 on spontaneous contractions of myogenic origin (SPCs, A–D). Representative recordings of SPCs in guinea pig detrusor strips under control conditions (0.1% DMSO) (A), with application of 1 µM NS19504 only (B), with application of 1 µM NS19504 to strips pretreated with 100 nM IbTx (C), and with application of 1 µM NS19504 to strips pretreated with 300 nM Apa (D). Application of 1 µM NS19504 reduced SPCs to 56% ± 4% of control, an effect that was blocked by pretreatment with IbTx, but not by pretreatment with Apa. (E) Summary of data. DMSO, vehicle control; NS19504, 1 µM NS19504; NS19504 (IbTx), 1 µM NS19504 in strips pretreated with IbTx; NS19504 (Apa), 1 µM NS19504 in strips pretreated with Apa (***P < 0.01 compared with NS19504).
Application of 1 µM NS19504 significantly reduced the amplitude of SPCs to 56.3% ± 4.4% of controls (from 1.14 ± 0.26 to 0.62 ± 0.13 mN, P = 0.0035, n = 10) and force integral to 53.7% ± 5.6% of control (from 3.02 ± 0.72 to 1.56 ± 0.39 mN × s, P = 0.0028, n = 10). The frequency of SPCs was also reduced by NS19504 to 76.5% ± 5.9% of control (from 4.6 ± 0.5 to 3.4 ± 0.3 minute−1, P = 0.0064, n = 10). A representative recording is shown in Fig. 5B.
The response to NS19504 was further investigated using two different toxin blockers of Ca2+-activated K+ channels, as follows: IbTx, which selectively blocks BK channels, and Apa, which selectively blocks SK channels. After pretreating with (or without) BK or SK channel blocker for 30 minutes to allow strips to equilibrate, we applied NS19504 (1 µM) or vehicle (0.1% DMSO) and then measured amplitude, force integral, and frequency of SPCs. The specific BK channel blocker IbTx (100 nM) alone (prior to application of NS19504 or vehicle) increased amplitude, force integral, and frequency of SPCs to 4.8 ± 0.8 mN, 9.0 ± 1.6 mN × s, and 8.5 ± 0.7 minutes−1, respectively. NS19504 (1 µM) had no effect on SPCs in IbTx-pretreated strips compared with DMSO time controls (P > 0.05, one-way ANOVA; representative recording in Fig. 5C), suggesting that NS19504 acts through BK channels. To confirm this, we tested the effect of NS19504 on strips pretreated with the SK channel blocker Apa (300 nM). As was the case with inhibition of BK channels, block of SK channels increased the amplitude and force integral of SPCs to 2.0 ± 0.3 mN and 6.1 ± 0.9 mN × s, respectively, but did not increase SPC frequency (2.6 ± 0.4 minutes−1 after Apa). In contrast to pretreatment with IbTx, Apa pretreatment did not inhibit the effects of subsequently added NS19504 (1 µM). In the presence of Apa, NS19504 reduced amplitude to 56.6% ± 4.0% of control, force integral to 57.3% ± 5.5% of control, and frequency to 77.1% ± 7.7% of control, effects that were not significantly different from those in strips treated with NS19504 alone (P > 0.05, one-way ANOVA; representative recording in Fig. 5D). Collectively, these data indicate that NS19504 (1 µM) reduces the amplitude, force integral, and frequency of the SPC by activating BK channels (Fig. 5E).
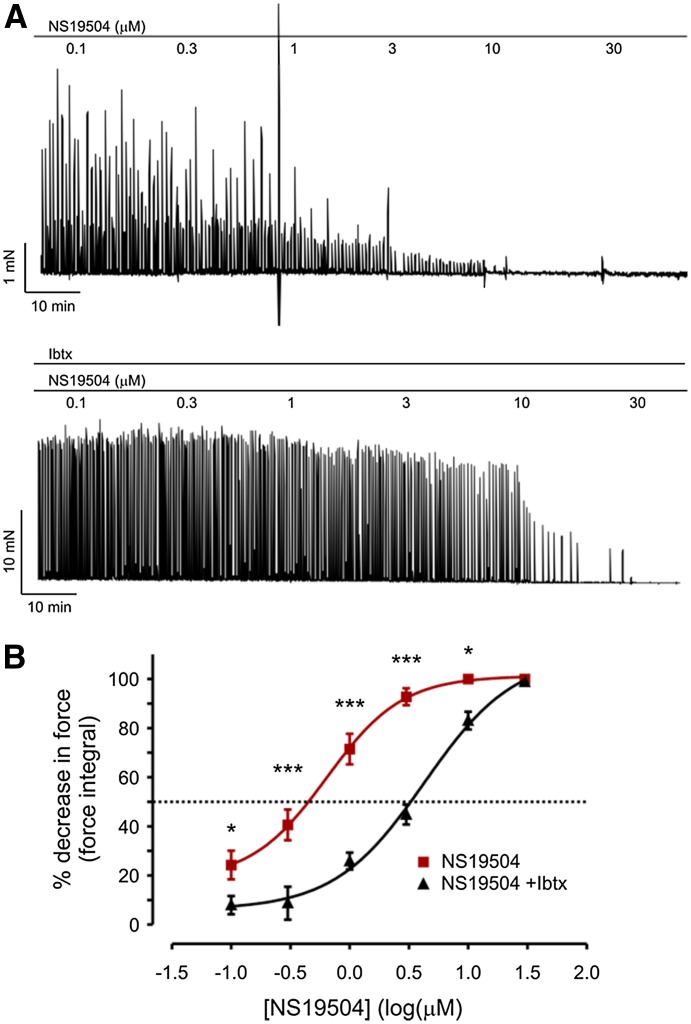
To investigate the ability of NS19504 to affect bladder strips in greater detail, we determined the relationship between NS19504 concentration and phasic contractions (Fig. 6). Stock solutions of NS19504 in DMSO were added directly to the bath. NS19504 decreased the force integral of SPCs in a concentration-dependent manner, producing a 24% decrease at 100 nM and completely eliminating SPCs at 10 µM (Fig. 6A). A fit of the data to a sigmoidal dose-response function yielded an EC50 for NS19504 of 640 nM (log EC50 = −0.19 ± 0.11 where EC50 is in µM units). To confirm that the effects of NS19504 were due to activation of BK channels, we also performed concentration-response experiments with detrusor strips pretreated with IbTx (100 nM). Inhibition of BK channels shifted the concentration-response curve to the right (Fig. 6B). In the presence of IbTx, the EC50 for NS19504 was approximately 4.3 µM, whereas the effect of NS19504 at concentrations ≤1 µM was effectively eliminated by IbTx.
Fig. 6.
NS19504 decreases SPC activity in a concentration-dependent manner. (A) Addition of increasing concentrations of NS19504 decreased SPC activity under control conditions (top); pretreatment with 100 nM IbTx inhibited the relaxing effect of NS19504 at concentrations lower than 1 µM (bottom). (B) Concentration-response curve. IbTx shifted the concentration-response curve to the right, with an EC50 for NS19504 of 640 nM in the absence and 4.27 µM in presence of IbTx (*P < 0.05; ***P < 0.01; two-way ANOVA).
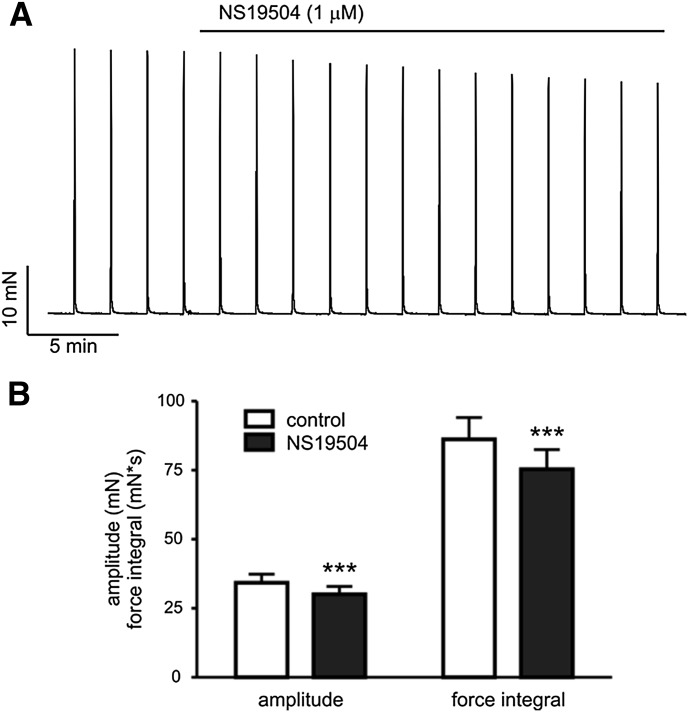
In addition to spontaneous contractions, nerve-evoked contractions elicited by EFS, which causes release of the neurotransmitters ATP and acetylcholine from parasympathetic nerves, were also measured (Fig. 7). The tissue was stimulated every 2 minutes with a 2-second burst of 40 pulses of 20 V amplitude and 0.2-millisecond duration. Amplitude and force integral of EFS-induced contractions were measured before and 20 minutes after the application of vehicle (0.1% DMSO) or NS19504 (1 µM). Application of vehicle did not significantly affect amplitude (31.8 ± 4.2 mN before and 30.6 ± 3.9 mN after treatment; P = 0.246, n = 6) or force integral (85.9 ± 15.4 mN × s before and 84.8 ± 13.8 mN × s after treatment; P = 0.745, n = 6). NS19504 (1 µM) modestly decreased force amplitude (34.3 ± 3.1 mN before and 30.1 ± 2.9 mN after treatment; P < 0.001, n = 8) and force integral (86.2 ± 7.9 mN × s before and 75.4 ± 7.1 mN × s after treatment; P < 0.001, n = 8; Fig. 7). Expressed as a percentage, NS19504 (1 µM) reduced amplitude to 87.6 ± 2.6% (P = 0.003) and force integral to 87.3 ± 0.9% (P = 0.004) of DMSO controls. Thus, NS19504 (1 µM) reduces nerve-evoked contraction, but the effect is small (13% reduction).
Fig. 7.
NS19504 modestly decreases nerve-evoked contractions. (A) Original recording of contractile response to EFS before and after application of 1 µM NS19504. (B) Summary data showing a statistically significant, but small, relaxing effect of NS19504 on EFS-induced contractions (***P < 0.01 compared with control; paired t test).
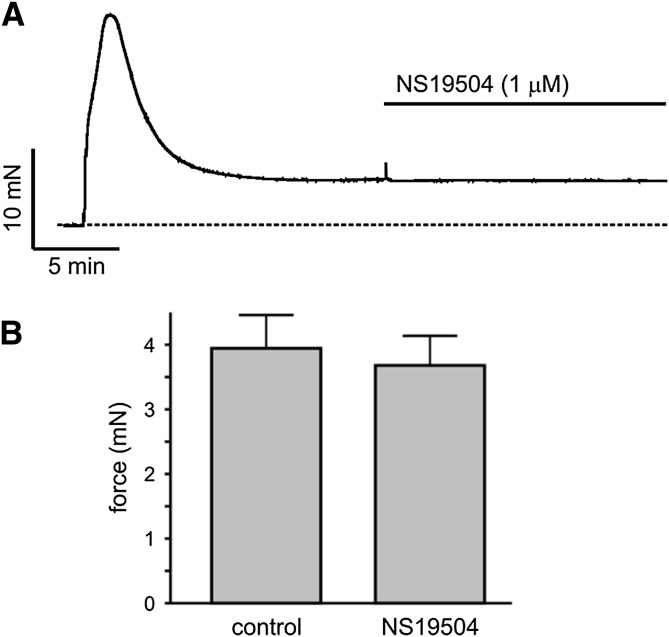
Finally, contractions were evoked by inducing depolarization with a high concentration of extracellular K+ (60 mM; K+ PSS) to activate voltage-gated Ca2+ channels (Fig. 8), and contractile force was measured before and 15 minutes after application of NS19504 (1 µM) or vehicle (0.1% DMSO). Depolarization of urinary bladder smooth muscles by application of K+ PSS caused a biphasic contraction, with an initial peak followed by a plateau phase (Fig. 8A). Force stabilized at a value of 4.7 ± 0.4 mN (n = 12) about 15 minutes after adding K+ PSS. Vehicle had no effect on steady-state contractions induced by K+ PSS (5.5 ± 0.6 mN before and 5.4 ± 0.7 mN after DMSO; P = 0.256, n = 5). Treatment with NS19504 (1 µM, 15 minutes) also failed to reduce contractile force (4.0 ± 0.5 mN in control and 3.7 ± 0.5 mN with NS19504; P = 0.200, n = 6; Fig. 8B). Collectively, these results demonstrate that NS19504 (1 µM) does not exert its function by inhibiting voltage-gated Ca2+ channels.
Fig. 8.
NS19504 does not inhibit depolarization-induced contraction. (A) Original recording of contractile force induced by 60 mM K+ PSS. After about 15 minutes, force reached a stable plateau and addition of 1 µM NS19504 had no effect. (B) Summary data (P = 0.200; paired t test, n = 6).
Discussion
The BK channel has long been pursued as a therapeutic target, and several studies using BK subtype-deficient mice and selective channel blockers have suggested that activation of BK channels may be beneficial in overactive bladder (OAB) disorders. Moreover, genetic transfer of DNA encoding the pore-forming BK subunit has been reported to be effective in animal models of OAB (Christ et al., 2001), strengthening support for the BK channel as a valid target in the treatment of OAB. Different classes of BK channel activators have been reported and shown to be effective in studies in vitro and in animal disease models. Two promising small-molecule BK channel activators, NS-8 and TA-1702, entered clinical trials for OAB, but none of them appear to be in active development. Therefore, there is a continued search for new small-molecule BK channel activators with more favorable properties (Nardi and Olesen, 2008).
In this study, we introduce NS19504, which represents a novel chemotype among BK activators. The structure of NS19504 is markedly different from that of well known BK activators, such as the benzimidazolidin-2-one NS1619, the quinolinone BMS-223131 [4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl)-6-(trifluoromethyl)-3,4-dihydro-1H-quinolin-2-one], the oxindole BMS-204352, the anthraquinone analog BK activators of the GoSlo-SR family (Roy et al., 2012, 2014), and the bisaryl thiourea NS11021 (Bentzen et al., 2007). NS19504 is characterized by a low mol. wt. and generally very good calculated physicochemical properties, including a low cLogD value and low polar surface area as well as the absence of an acidic function, which otherwise characterize most of the known small-molecule BK activators. Such properties may be considered promising with respect to the prospects of achieving good absorption, distribution, metabolism, and excretion and safety properties. In addition, the structure and low mol. wt. appear to provide a good starting point for generation of analogs. We have adopted the multiple parameter optimization (MPO) value introduced by Wager et al. (2010), who, based on investigations of approved central nervous system active drugs, developed the MPO approach to predict drug-like properties of small molecules and expressed this as the MPO score on a scale from 0 to 6, a score of ≥4 being desirable. The MPO value for NS19504 is 5.24 and close to the ideal of 6. Thus, NS19504 stands out from earlier reported compounds because of its low mol. wt. and absence of an acidic function, and therefore represents an interesting lead in the search for new BK channel modulators.
NS19504 was identified using a HTS approach employing a new FLIPR-based Tl+-flux assay for BK channel activators as a primary screen and an automated QPatch assay as a secondary screen. Our goal was to establish an assay for the detection of positive modulators of the hBK channel of sufficient quality to permit us to conduct a HTS campaign. It has previously been reported that Tl+ influx can be monitored in FLIPR assays to detect activation of K+ channels such as Kv7.2 and KCa2.3 (Weaver et al., 2004). In addition, our earlier studies demonstrated the capacity to detect activators of another Ca2+-activated K+ channel (hKCa3.1) in a similar assay (Hougaard et al., 2009; Jørgensen et al., 2013). The BK Tl+ influx assay described in the current study appears to be highly suitable for detecting new small-molecule BK activators. The average Z′ factor for the entire HTS campaign was estimated at 0.3. In the current study, more than 200 hits were validated by electrophysiological analysis.
NS19504 activated heterologously expressed recombinant BK channels and native BK channels in bladder myocyte cells and appeared to have selectivity for BK channels, as tested by receptor binding and electrophysiological analyses of activity toward other related channels as well as by evaluating integrated myographic responses in bladder tissue. Manual whole-cell patch-clamp experiments showed that 1 µM NS19504, a concentration that was highly efficacious at recombinant and native BK channels, had very little effect on IK or SK3 channels in an exogenous expression system; effects on IK channels were greater at 10 µM. Even at this higher concentration, NS19504 had a relatively small effect (∼10–20%) on exogenously expressed Nav1.2 currents and endogenous Nav currents in DRGs and no effect on Cav channels. Notably, radioligand-binding assays showed that NS19504 did not inhibit 65 of 68 receptors/channels tested (based on a >50% inhibitory threshold). The only exceptions were the norepinephrine transporter, dopamine transporter, and Sigma σ1. Moreover, bladder contractions evoked by 60 mM K+ PSS were not inhibited by 1 µM NS19504, suggesting that NS19504 does not affect voltage-dependent Ca2+ channels. On the basis of these findings, we suggest that NS19504 will be a highly useful pharmacological tool for selectively activating BK channels in native cells and in complex tissue preparations such as organ bath preparations of bladder and other tissues in which BK channel function is important.
In urothelium-denuded urinary bladder strips, NS19504 at 1 µM significantly reduced the amplitude, force integral, and frequency of SPCs. Phasic contractions, which are caused by Ca2+ entry during an action potential, are dependent on BK channel function, as evidenced by the fact that block of BK channels with IbTx increases the peak and duration of an action potential (Heppner et al., 1997). Consistent with this, the amplitudes of phasic contractions are increased in bladder preparations from BK-deficient Slo−/− mice as well as in bladders from wild-type mice treated with paxilline or IbTx. Given this, activation of BK channels would be predicted to decrease contraction amplitude and duration through effects on the action potential, as observed in this work. It is likely that only modest levels of BK channel activation by NS19504 are required to enhance the ability of BK channel to shorten the action potential.
The inhibitory effect of NS19504 on urinary bladder SPCs observed in the current study was prevented by the BK channel blocker IbTx (100 nM) but was unaffected by pretreatment with the selective SK channel blocker, Apa, supporting that NS19504 (1 µM) acts on urinary bladder smooth muscle via BK channels and not SK channels. It should be noted that both SK and BK channel blockers greatly increase the amplitude, force integral, and frequency of SPCs. Therefore, the lack of effect of NS19504 in the presence of IbTx was not due to an increase in force. In contrast to its inhibitory effect on SPCs, NS19504 (1 µM) had only a modest effect on nerve-evoked contractions (13% decrease in force integral) in guinea pig bladder strip preparations, although loss of BK channel function has been shown to result in a substantial increase of nerve-evoked contractions. Nerve-evoked contractions, which are dependent on both activation of UBSM purinergic and muscarinic receptors, trigger a burst of action potentials and concurrent calcium influx (Nausch et al., 2010) to contract urinary bladder smooth muscle. This presumably leads to a strong activation of BK channels, and therefore it is conceivable that the addition of an exogenous BK channel opener may have only a small additional effect.
Spontaneous nonvoiding contractions are not only a hallmark sign of overactive bladder, but are also believed to contribute to its pathogenesis (Brading, 1997; Andersson, 2010). Therefore, a compound capable of inhibiting nonvoiding contractions without affecting normal voiding elicited by nerve-evoked contractions may be considered to have a desired profile as a candidate to treat bladder overactivity. The current observations that NS19504 potently inhibits SPCs in guinea pig bladder strips while only modestly affecting EFS-induced contractions suggest a promising profile for treating overactive detrusor. A similar profile was previously reported for the BK activator NS11021 (Layne et al., 2010). However, NS19504 inhibits SPCs more potently than NS11021 by acting on BK channels without affecting SK or voltage-dependent Ca2+ channels. Thus, NS19504 appears to be more potent and selective and also has more favorable physicochemical properties compared with NS11021 and most other known BK activators.
In summary, in this study, we identified and characterized NS19504, which represents a new class of small molecules exhibiting fast BK channel activation with favorable physicochemical properties. We found that NS19504 is a good pharmacological tool and a promising lead for the future development of therapeutics targeting OAB disorders.
Supplementary Material
Acknowledgments
The authors thank Susanne K. Hansen, Gitte G. Sørensen, Charlotte Holtoft, and Charlotte L. Petersen for excellent technical assistance; David Brown for expertise and help on grouping HTS hits; and Elsebet Ø. Nielsen for excellent handling and interpretation of selectivity testing.
Abbreviations
- ANOVA
analysis of variance
- Apa
apamin
- BMS-204352
(3S)-3-(5-chloro-2-methoxyphenyl)-3-fluoro-6-(trifluoromethyl)-2-indolinone
- BMS-223131
4-(5-chloro-2-hydroxyphenyl)-3-(2-hydroxyethyl)-6-(trifluoromethyl)-3,4-dihydro-1H-quinolin-2-one
- BTC-AM
benzothiazole coumarin acetoxymethyl ester
- DMSO
dimethylsulfoxide
- DRG
dorsal root ganglia
- EFS
electrical field stimulation
- FLIPR
fluorometric imaging plate reader
- HEK
human embryonic kidney
- HTS
high-throughput screening
- IbTx
iberiotoxin
- MPO
multiple parameter optimization
- NS11021
5-(2-{3-[3,5-bis(trifluoromethyl)phenyl]thioureido}-5-bromophenyl)-1H-1,2,3,4-tetrazole
- NS1608
4-chloro-2-{3-[m-(trifluoromethyl)phenyl]ureido}phenol
- NS1619
1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-1,3-diaza-1,3-dihydroinden-2-one
- NS19504
5-[(4-bromophenyl)methyl]-1,3-thiazol-2-amine
- OAB
overactive bladder
- PSS
physiologic saline solution
- SPC
spontaneous phasic contraction
- UBSM
urinary bladder smooth muscle
Authorship Contributions
Participated in research design: Nardi, Jørgensen, Korsgaard, Rode, Olesen, Christophersen, Grunnet, Dyhring, Hougaard, Strøbæk, Rønn, Nausch, Nelson.
Conducted experiments: Nausch, Korsgaard, Bonev, Jørgensen, Rode, Nardi, Hougaard, Strøbæk.
Contributed new reagents or analytic tools: Nardi, Brown.
Performed data analysis: Nausch, Rode, Jørgensen, Bonev, Korsgaard, Hougaard, Dyhring, Grunnet, Nelson, Strøbæk, Rønn, Brown, Nardi.
Wrote or contributed to the writing of the manuscript: Nausch, Jørgensen, Korsgaard, Rode, Nardi, Hougaard, Grunnet, Brown, Christophersen, Olesen, Dyhring, Nelson, Rønn.
Footnotes
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R37-DK053832]; the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL044455 and 1P01-HL095488]; and the Totman Medical Research Trust (to M.T.N.).
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Ahring PK, Strøbaek D, Christophersen P, Olesen SP, Johansen TE. (1997) Stable expression of the human large-conductance Ca2+-activated K+ channel alpha- and beta-subunits in HEK293 cells. FEBS Lett 415:67–70 [DOI] [PubMed] [Google Scholar]
- Andersson KE. (2010) Detrusor myocyte activity and afferent signaling. Neurourol Urodyn 29:97–106 [DOI] [PubMed] [Google Scholar]
- Bentzen BH, Nardi A, Calloe K, Madsen LS, Olesen SP, Grunnet M. (2007) The small molecule NS11021 is a potent and specific activator of Ca2+-activated big-conductance K+ channels. Mol Pharmacol 72:1033–1044 [DOI] [PubMed] [Google Scholar]
- Bentzen BH, Osadchii O, Jespersen T, Hansen RS, Olesen SP, Grunnet M. (2009) Activation of big conductance Ca(2+)-activated K (+) channels (BK) protects the heart against ischemia-reperfusion injury. Pflugers Arch 457:979–988 [DOI] [PubMed] [Google Scholar]
- Brading AF. (1997) A myogenic basis for the overactive bladder. Urology 50:57–67 [DOI] [PubMed] [Google Scholar]
- Buckner SA, Milicic I, Daza AV, Coghlan MJ, Gopalakrishnan M. (2002) Spontaneous phasic activity of the pig urinary bladder smooth muscle: characteristics and sensitivity to potassium channel modulators. Br J Pharmacol 135:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Petkov GV. (2009) Identification of large conductance calcium activated potassium channel accessory beta4 subunit in rat and mouse bladder smooth muscle. J Urol 182:374–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ GJ, Day NS, Day M, Santizo C, Zhao W, Sclafani T, Zinman J, Hsieh K, Venkateswarlu K, Valcic M, et al. (2001) Bladder injection of “naked” hSlo/pcDNA3 ameliorates detrusor hyperactivity in obstructed rats in vivo. Am J Physiol Regul Integr Comp Physiol 281:R1699–R1709 [DOI] [PubMed] [Google Scholar]
- Darblade B, Behr-Roussel D, Oger S, Hieble JP, Lebret T, Gorny D, Benoit G, Alexandre L, Giuliano F. (2006) Effects of potassium channel modulators on human detrusor smooth muscle myogenic phasic contractile activity: potential therapeutic targets for overactive bladder. Urology 68:442–448 [DOI] [PubMed] [Google Scholar]
- Ghatta S, Nimmagadda D, Xu X, O’Rourke ST. (2006) Large-conductance, calcium-activated potassium channels: structural and functional implications. Pharmacol Ther 110:103–116 [DOI] [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Nelson MT. (1997) Ca(2+)-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol 273:C110–C117 [DOI] [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Nelson MT. (2005) Elementary purinergic Ca2+ transients evoked by nerve stimulation in rat urinary bladder smooth muscle. J Physiol 564:201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera GM, Etherton B, Nausch B, Nelson MT. (2005) Negative feedback regulation of nerve-mediated contractions by KCa channels in mouse urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 289:R402–R409 [DOI] [PubMed] [Google Scholar]
- Holland M, Langton PD, Standen NB, Boyle JP. (1996) Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br J Pharmacol 117:119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard C, Fraser MO, Chien C, Bookout A, Katofiasc M, Jensen BS, Rode F, Bitsch-Nørhave J, Teuber L, Thor KB, et al. (2009) A positive modulator of K Ca 2 and K Ca 3 channels, 4,5-dichloro-1,3-diethyl-1,3-dihydro-benzoimidazol-2-one (NS4591), inhibits bladder afferent firing in vitro and bladder overactivity in vivo. J Pharmacol Exp Ther 328:28–39 [DOI] [PubMed] [Google Scholar]
- Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. (2011) Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol 301:C903–C912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Okamoto T, Yamamoto Y, Tanaka H, Koike K, Shigenobu K, Tanaka Y. (2001) Effects of different types of K+ channel modulators on the spontaneous myogenic contraction of guinea-pig urinary bladder smooth muscle. Acta Physiol Scand 173:323–333 [DOI] [PubMed] [Google Scholar]
- Jørgensen S, Dyhring T, Brown DT, Strøbæk D, Christophersen P, Demnitz J. (2013) A high-throughput screening campaign for detection of Ca(2+)-activated k(+) channel activators and inhibitors using a fluorometric imaging plate reader-based tl(+)-influx assay. Assay Drug Dev Technol 11:163–172 [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Adachi-Akahane S, Nagao T. (2000) Involvement of BK(Ca) channels in the relaxation of detrusor muscle via beta-adrenoceptors. Eur J Pharmacol 404:231–238 [DOI] [PubMed] [Google Scholar]
- Layne JJ, Nausch B, Olesen SP, Nelson MT. (2010) The BK channel activation by NS11021 decreases excitability and contractility of bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 298:R378–R384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, He T, Katusic ZS, Lee HC. (2006) Molecular mechanisms mediating inhibition of human large conductance Ca2+-activated K+ channels by high glucose. Circ Res 99:607–616 [DOI] [PubMed] [Google Scholar]
- Malysz J, Buckner SA, Daza AV, Milicic I, Perez-Medrano A, Gopalakrishnan M. (2004) Functional characterization of large conductance calcium-activated K+ channel openers in bladder and vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol 369:481–489 [DOI] [PubMed] [Google Scholar]
- Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. (2004) Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem 279:36746–36752 [DOI] [PubMed] [Google Scholar]
- Mora TC, Suarez-Kurtz G. (2005) Effects of NS1608, a BK(Ca) channel agonist, on the contractility of guinea-pig urinary bladder in vitro. Br J Pharmacol 144:636–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi A, Olesen SP. (2008) BK channel modulators: a comprehensive overview. Curr Med Chem 15:1126–1146 [DOI] [PubMed] [Google Scholar]
- Nausch B, Heppner TJ, Nelson MT. (2010) Nerve-released acetylcholine contracts urinary bladder smooth muscle by inducing action potentials independently of IP3-mediated calcium release. Am J Physiol Regul Integr Comp Physiol 299:R878–R888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obushak ND, Matiichuk VS, Vasylyshin RY, Ostapyuk YV. (2004) Heterocyclic syntheses on the basis of arylation products of unsaturated compounds. X. 3-Aryl-2-chloropropanals as reactants for the synthesis of 2-aminothiazole derivatives. Russian Journal of Organic Chemistry (Translation of Zhurnal Organicheskoi Khimii) 40:383–389 [Google Scholar]
- Ohi Y, Atsuki K, Tori Y, Ohizumi Y, Watanabe M, Imaizumi Y. (2001a) Imaging of Ca2+ release by caffeine and 9-methyl-7-bromoeudistomin D and the associated activation of large conductance Ca2+-dependent K+ channels in urinary bladder smooth muscle cells of the guinea pig. Jpn J Pharmacol 85:382–390 [DOI] [PubMed] [Google Scholar]
- Ohi Y, Yamamura H, Nagano N, Ohya S, Muraki K, Watanabe M, Imaizumi Y. (2001b) Local Ca(2+) transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder. J Physiol 534:313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya S, Kimura S, Kitsukawa M, Muraki K, Watanabe M, Imaizumi Y. (2000) SK4 encodes intermediate conductance Ca2+-activated K+ channels in mouse urinary bladder smooth muscle cells. Jpn J Pharmacol 84:97–100 [DOI] [PubMed] [Google Scholar]
- Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. (2001) Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537:443–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov GV, Nelson MT. (2005) Differential regulation of Ca2+-activated K+ channels by beta-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol 288:C1255–C1263 [DOI] [PubMed] [Google Scholar]
- Ponte CG, McManus OB, Schmalhofer WA, Shen DM, Dai G, Stevenson A, Sur S, Shah T, Kiss L, Shu M, et al. (2012) Selective, direct activation of high-conductance, calcium-activated potassium channels causes smooth muscle relaxation. Mol Pharmacol 81:567–577 [DOI] [PubMed] [Google Scholar]
- Roy S, Morayo Akande A, Large RJ, Webb TI, Camarasu C, Sergeant GP, McHale NG, Thornbury KD, Hollywood MA. (2012) Structure-activity relationships of a novel group of large-conductance Ca(2+)-activated K(+) (BK) channel modulators: the GoSlo-SR family. ChemMedChem 7:1763–1769 [DOI] [PubMed] [Google Scholar]
- Roy S, Large RJ, Akande AM, Kshatri A, Webb TI, Domene C, Sergeant GP, McHale NG, Thornbury KD, Hollywood MA. (2014) Development of GoSlo-SR-5-69, a potent activator of large conductance Ca2+-activated K+ (BK) channels. Eur J Med Chem 75:426–437 [DOI] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. (2006) High-conductance potassium channels of the SLO family. Nat Rev Neurosci 7:921–931 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Sasaki Y, Kimura Y, Fukui T, Hamada K, Ukai Y. (2003) A novel pyrrole derivative, NS-8, suppresses the rat micturition reflex by inhibiting afferent pelvic nerve activity. BJU Int 92:1031–1036 [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. (2005) Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol 289:F604–F610 [DOI] [PubMed] [Google Scholar]
- Uchida H, Shishido K, Nomiya M, Yamaguchi O. (2005) Involvement of cyclic AMP-dependent and -independent mechanisms in the relaxation of rat detrusor muscle via beta-adrenoceptors. Eur J Pharmacol 518:195–202 [DOI] [PubMed] [Google Scholar]
- Wager TT, Hou X, Verhoest PR, Villalobos A. (2010) Moving beyond rules: the development of a central nervous system multiparameter optimization (CNS MPO) approach to enable alignment of druglike properties. ACS Chem Neurosci 1:435–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CD, Harden D, Dworetzky SI, Robertson B, Knox RJ. (2004) A thallium-sensitive, fluorescence-based assay for detecting and characterizing potassium channel modulators in mammalian cells. J Biomol Screen 9:671–677 [DOI] [PubMed] [Google Scholar]
- Werner ME, Knorn AM, Meredith AL, Aldrich RW, Nelson MT. (2007) Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol 292:R616–R624 [DOI] [PubMed] [Google Scholar]
- Zhang JH, Chung TD, Oldenburg KR. (1999) A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4:67–73 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.