Abstract
Objective
To evaluate the correlation of preretrieval quantitative serum hCG level with oocyte maturity.
Design
Retrospective cohort study.
Setting
Military assisted reproductive technology (ART) program.
Patient(s)
Fresh autologous ART cycles.
Intervention(s)
Serum hCG level the day before oocyte retrieval.
Main Outcome Measure(s)
Linear regression was used to correlate serum hCG levels and oocyte maturity rates. Normal oocyte maturity was defined as ≥ 75% and the Wilcoxon rank sum test was used to compare serum hCG levels in patients with normal and low oocyte maturity. Threshold analysis was performed to determine hCG levels that could predict oocyte maturity.
Result(s)
A total of 468 ART cycles were analyzed. Serum hCG level was not correlated with hCG dose; however, it was negatively correlated with body mass index (BMI). Serum hCG levels did not differ between patients with oocyte maturity of <75% and ≥ 75%. Serum hCG levels did not correlate with oocyte maturity rates. Receiver operator characteristic and less than efficiency curves failed to demonstrate thresholds at which hCG could predict oocyte maturity.
Conclusion(s)
Serum hCG levels were not correlated with oocyte maturity. Although a positive hCG was reassuring that mature oocytes would be retrieved for most patients, the specific value was not helpful.
Keywords: Assisted reproductive technologies, infertility, in vitro fertilization
Human chorionic gonadotropin has been used for oocyte maturation induction during assisted reproduction cycles for more than 30 years (1). Because of the biochemical similarity between LH and hCG their cellular effects are moderated by a single polypeptide known as the LH/hCG receptor. This allows hCG to be used as a surrogate for the LH surge during the controlled ovarian hyper-stimulation (COH) portion of assisted reproductive technology (ART). After administration of hCG, oocytes resume meiosis and oocyte retrieval is timed 34–36 hours later (2, 3).
Retrieving a higher percentage of mature oocytes correlates with pregnancy and live birth in ART (4). Consequently, ensuring that oocytes are mature upon retrieval is an important component of the simulation process in ART (5, 6). Oocyte maturity has been correlated with patient age, differences in COH dosing protocols, follicular steroid hormone levels, and duration of ovarian stimulation (7–9).
The endogenous LH surge triggers completion of the first meiotic division, follicular rupture, oocyte maturation, and ovulation. Because the hCG trigger is used as a surrogate for the LH surge in ART, serum levels of hCG after administration may be evaluated to assess whether they have an influence on clinical outcomes during ART. Previous studies have demonstrated that serum values of hCG on the day after injection are similar using different routes of injection (10) and correlated to the patient's body mass index (BMI) (11). However, there are minimal data evaluating the impact of the serum hCG level before oocyte retrieval on ART outcomes. The objective of this study was to evaluate the impact of serum hCG level on oocyte maturity during ART cycles.
Materials and Methods
Study Design
This study was a retrospective analysis of 468 ART cycles performed at Walter Reed National Military Medical Center-Bethesda from December 2010 through April 2012. Institutional Review Board approval was obtained. All fresh autologous ART cycles proceeding to oocyte retrieval during the study period were included in the analysis. Exclusion criteria were frozen ET cycles and cycles cancelled before oocyte retrieval due to poor response. Measurement of serum hCG level on the day before oocyte retrieval was initiated in our program in December 2010 as a mechanism to insure proper hCG administration before retrieval. The study period represented the first year and a half of the new protocol.
Stimulation Protocol
All patients received a stimulation protocol of microdose flare or luteal dose lupron pituitary down-regulation with recombinant FSH and urinary hMG ovarian stimulation, as previously described (11). An evening injection of IM hCG (APP pharmaceuticals/USA) at a dose of 5,000 or 10,000 IU was administered when three or more follicles were more than 18 mm in mean diameter (12). All patients undergoing stimulation in our program received this formulation from the facility pharmacy. Patients were given the 5,000-unit dose of hCG if they had an E2 >5,000 pg/mL or were given antagonist rescue for ovarian hyperstimulation syndrome (OHSS) risk (12). Serum hCG levels were measured by commercially available ELISA kit on the morning after hCG administration. The detection limit is 0.7 mIU/mL and the intra-assay and interassay coefficients of variation (CV) are 3.2% and 4.9%, respectively. Ultrasound-guided transvaginal oocyte retrieval was performed 36 hours after hCG injection and ET was performed either 3 or 5 days after oocyte retrieval depending on patient history and embryo quality. Luteal phase was supported with IM P in oil or vaginal P tablets depending on patient history and preference (12).
Outcomes
The primary outcome of the study was oocyte maturity. Oocyte maturity rates were calculated as the number meiosis II zygotes per patient divided by the number of oocyte retrieved, determined by the presence of a polar body to indicate that the zygote had completed meiosis II. Normal oocyte maturity was defined as ≥75% and poor oocyte maturity was defined as <75% (7, 12, 13).
Statistics
Univariate linear regression was used to correlate serum hCG levels with oocyte maturity rates. Multivariate linear regression was used to control for potential confounders to include BMI, age, and dose of hCG. The Wilcoxon rank sum test was used to compare nonparametric continuous data and the Student's t test to compare parametric continuous data. In addition, patients with serum hCG level below the 5th percentile (hCG ≤ 50 mIU/mL) were compared to patients with hCG level above the 95th percentile (≥300 mIU/mL). To determine whether there was an hCG threshold below which abnormal oocyte maturity occurred, less than efficiency curves were generated. The curves calculated the oocyte maturity below each hCG threshold beginning at <40 mIU/mL and continuing in increments of 10 mIU/mL. Thresholds values were set such that any value <75% would be considered abnormal maturity rate. Data were analyzed with SPSS software (IBM).
Results
Four hundred sixty-eight ART cycles met inclusion criteria and were included in the analysis. When comparing differences between patients with normal and low oocyte maturity, there was no statistically significant difference between the two groups with respect to age, ovarian reserve, pituitary down-regulation, days of stimulation, BMI, and E2 levels on the day of serum hCG measurement (Table 1). Mean serum hCG levels for the entire cohort were 372 ± 191 mIU/mL (range, 27–700 mIU/mL). Fifty-seven patients received a dose of 5,000 IU of hCG and 411 patients received a 10,000-IU dose. Oocyte maturity was similar between patients receiving 5,000 and 10,000 units of hCG, respectively (75.6% ± 19% vs. 76.7% ± 18%; P=not significant [NS]).
Table 1.
Comparison of demographics in patients with oocyte maturity <75% and ≥75%.
| Oocyte maturity <75% (n = 180) | Oocyte maturity ≥75% (n = 288) | |
|---|---|---|
| Age (y) | 34 ± 5 | 35 ± 5 |
| Days of stimulation | 11 ± 2 | 11 ±3 |
| BMI (kg/m2) | 26.62 ± 4.77 | 25.91 ± 4.47 |
| Day 3 FSH (IU/L) | 6.9 ± 2.49 | 6.78 ± 2.2 |
| Total AFC | 16 ± 11 | 16 ± 8 |
| E2 on day of hCG administration +1 (pg/mL) | 7,421 ± 4,648 | 3,953 ± 2,016 |
| hCG peak (mIU/mL) | 132 ± 86 | 145 ± 95 |
Note: There were no differences in age, days of stimulation, body mass index (BMI), ovarian reserve, E2, and hCG levels on day of hCG administration +1. Data presented as mean ± SD. AFC = antral follicle count.
Levy. Preretrieval serum hCG. Fertil Steril 2013.
There were 180 patients with low oocyte maturity <75% with a mean serum hCG level of 132 ± 86 mIU/mL. There were 288 patients with normal oocyte maturity ≥ 75% with a mean serum hCG level of 145 ± 95 mIU/mL (Fig. 1). The mean serum hCG level in patients with normal and low oocyte maturity was statistically similar (P=NS). The lower 5th percentile of serum hCG levels were all below 50 mIU/mL (range, 27–50 mIU/mL). Patients above the 95th percentile of serum hCG all had levels of more than 300 mIU/mL (range, 300–700 mIU/mL). Patients with serum hCG levels in the 5th percentile had similar oocyte maturity when compared with patients with an hCG level in the 95th percentile (82% vs. 80%; P=NS).
Figure 1.
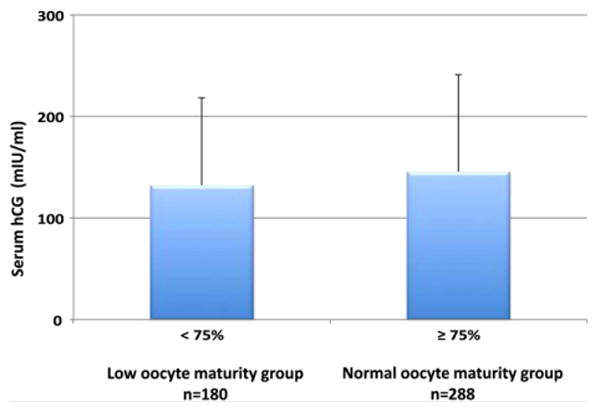
Comparison of serum hCG levels on the day before oocyte retrieval in patients with normal oocyte maturity (≥75%) and low oocyte maturity (<75%). Data are expressed as mean ± SE and the hCG levels were similar between the two groups.
Levy. Preretrieval serum hCG. Fertil Steril 2013.
In univariate linear regression, serum hCG level was negatively correlated with BMI (r = −0.48, P<.001). Serum hCG level did not correlate with the dose of hCG administered. Oocyte maturity rates were not correlated with the level of serum hCG (P=NS). In univariate regression analysis, oocyte maturity was not correlated with age, serum hCG level, dose of hCG, BMI, total oocytes retrieved, days of stimulation, gonadotropin dose, or peak serum E2 levels. Oocyte maturity was positively correlated with the total number of mature oocytes (P< .001), meaning that patients with more total mature oocytes also has a higher percentage of their total oocyte yield that was mature. Similarly, patients with a diagnosis of diminished ovarian reserve had a lower maturity rate when compared with all other patient groups (69% ± 24%vs.77%± 17%; P= .01). In regression analysis there was a significant negative correlation with diminished ovarian reserve and oocyte maturity.
Less than efficiency curves demonstrated that maturity rates remained above 75% at all thresholds of hCG measurements (Fig. 2A). The lowest hCG thresholds had some of the highest oocyte maturity rates. At no point in the threshold analysis did oocyte maturity rates drop below the 75% threshold. Receiver operator characteristic curves also did not demonstrate an ability of serum hCG level to predict normal oocyte maturity with an area under the curve of 0.546 (Fig. 2B).
Figure 2.
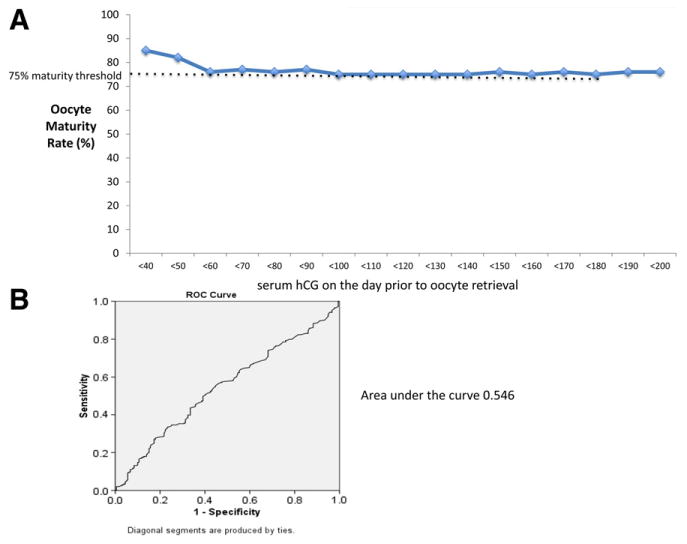
(A) Less than efficiency curve of oocyte maturity based on serum hCG thresholds. The curves calculated the oocyte maturity below each hCG threshold beginning at <40 mIU/mL and continuing in increments of 10 mIU/mL. Thresholds values were set such that any value <75% (black dashed line) would be considered abnormal maturity rate. (B) Receiver operator characteristic (ROC) curve for the ability of serum hCG level to predict oocyte maturity. Area under the curve = 0.546.
Levy. Preretrieval serum hCG. Fertil Steril 2013.
Discussion
The objective of this study was to evaluate the impact of the preretrieval serum hCG levels on oocyte maturity and to determine whether there was an hCG threshold level below which abnormal oocyte maturity was more likely to occur. The data demonstrated that serum hCG values did not correlate with oocyte maturity and there was no hCG threshold below which abnormal oocyte maturity occurred. The presence of any detectable level of serum hCG, the lowest of which was 27 mIU/mL in this cohort, resulted in good oocyte maturity.
Poor oocyte maturity has been directly correlated with decreased rates of fertilization and clinical pregnancy (13). If a particular threshold level of hCG corresponded with increased oocyte maturity potential and increased likelihood of clinical pregnancy, then hCG trigger injection concentrations could be tailored to target this threshold. During the past 30 years, hCG has been used to induce oocyte maturation, given the biochemical similarity between LH and hCG (1). Although there have been prior studies evaluating oocyte maturity in relation to patient age, differences in COH dosing protocols, FSH hormone levels, and number of ovarian stimulation cycles, there are limited data evaluating the relationship between hCG levels on the day after injection and subsequent oocyte maturity (6–8).
In the present study, there was no significant difference between the mean hCG levels when comparing patients with oocyte maturity <75% and oocyte maturity ≥75%. In addition, when stratifying patients by hCG levels, there was no significant difference in oocyte maturity between patients with hCG levels at either extremes of the spectrum (5th vs. 95th percentile). These data do not support a dose-related relationship between serum hCG levels and percentage of oocyte maturity. Furthermore, the data did not support an hCG threshold below which abnormal oocyte maturity was more likely to occur.
In a case report evaluating oocyte maturation arrest, Lev-ran et al. proposed several mechanisms to account for failure of meiosis, including insufficient or absent LH effect, abnormalities in cumulus cell signaling mechanisms, and intrinsic oocyte factors (14). Levran et al. further proposed mechanisms to account for an insufficient LH effect that included incorrect timing of the hCG injection, lack of LH activity secondary to poor hCG quality or utilization of an inactive isomer, and abnormalities in the hormone delivery or dysfunctional LH receptors (14).
Serum hCG levels were found to have a strong negative correlation with BMI in the present study, a finding consistent with prior studies (15). However, neither BMI nor serum hCG level was found to correlate with oocyte maturity, suggesting that there is not a need to increase the dose of hCG in obese patients for the purpose of oocyte maturation. Similarly, the dose of hCG was not found to correlate with oocyte maturity and patients at the 5,000-unit dose had similar maturity rates compared with patients at the 10,000-unit dose.
There are limitations related to the retrospective design of this study. One limitation was the varied hCG dosages used for oocyte maturation induction. Although the majority of patients received a 10,000-IU hCG injection, a small percentage of patients received a 5,000-IU hCG injection. This variable was controlled for in the multiple linear regression analysis and in subgroup analysis based on the dose of hCG. The serum hCG concentration may also vary based on how long after injection it is measured. Patients typically administered the hCG injection between 8:00 pm and 1:00 am with a blood draw and serum hCG assay performed the following morning. Therefore, the injection was administered 6–11 hours before the time of serum measurement. One pharmacokinetics study demonstrated that serum hCG levels peak at 12 hours after injection and decrease during the course of 120 hours (15). However, that study did not draw blood levels between 0 and 12 hours, therefore it is unclear how early may hCG levels peak. We were unable to control for the time between injection and measurement of hCG in this study. Finally, the present study did not address several areas of research that remain. This includes the possibility to use urine hCG measurements to confirm proper drug administration, the cost effectiveness of measuring hCG on the day before oocyte retrieval, and whether patients with failed hCG medication delivery have better ART outcomes when identified by hCG measurement compared with patients identified by failure to retrieve oocytes on the first ovary during oocyte retrieval.
Measurement of serum hCG levels on the day before oocyte retrieval was implemented in our ART program for the purpose of detecting medication or administration errors that might lead to failure to obtain oocytes at retrieval. Reich-man et al. (16) recently analyzed more than 17,289 ART cycles in which hCG was measured at the same time point and identified 41 patients who had undetectable serum hCG levels (0.25% incidence). These 41 patients underwent a salvage dose of hCG and proceeded to oocyte retrieval 36 hours later. The 41 patients had similar oocyte maturity, implantation, and live birth compared with matched controls. Their data suggest that identifying patients with undetectable hCG levels can result in salvage dose administration and good ART cycle outcomes.
In conclusion, hCG levels were not correlated with oocyte maturity and there was no specific hCG level associated with an increase in oocyte maturity. However, the presence of detectable serum hCG levels was overall reassuring that mature oocytes would be obtained at oocyte retrieval. Further studies are warranted to determine modifiable factors that may predict and facilitate oocyte maturation to improve ART outcomes.
Acknowledgments
Supported, in part, by intramural research program of the Program in Reproductive and Adult Endocrinology, National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
G.L. has nothing to disclose. M.J.H. has nothing to disclose. C.R. has nothing to disclose. T.P. has nothing to disclose. J.P. has nothing to disclose. R.S.H. has nothing to disclose. J.H.S. has nothing to disclose. J.C. has nothing to disclose.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
References
- 1.Jones GS, Garcia JE, Rosenwaks Z. The role of pituitary gonadotropins in follicular stimulation and oocyte maturation in the human. J Clin Endocrinol Metab. 1984;59:178–80. doi: 10.1210/jcem-59-1-178. [DOI] [PubMed] [Google Scholar]
- 2.Fischer RA, Nakajima ST, Gibson M, Brumsted JR. Ovulation after intravenous and intramuscular human chorionic gonadotropin. Fertil Steril. 1993;60:418–22. [PubMed] [Google Scholar]
- 3.Saal W, Glowania HJ, Hengst W, Happ J. Pharmacodynamics and pharmacokinetics after subcutaneous and intramuscular injection of human chorionic gonadotropin. Fertil Steril. 1991;56:225–9. [PubMed] [Google Scholar]
- 4.Verberg MF, Eijkemans MJ, Macklon NS, Heijnen EM, Baart EB, Hohmann FP, et al. The clinical significance of the retrieval of a low number of oocytes following mild ovarian stimulation for IVF: a meta-analysis. Hum Reprod Update. 2009;15:5–12. doi: 10.1093/humupd/dmn053. [DOI] [PubMed] [Google Scholar]
- 5.Bosch E, Ezcurra D. Individualised controlled ovarian stimulation (iCOS): maximising success rates for assisted reproductive technology patients. Reprod Biol Endocrinol. 2011;9:82. doi: 10.1186/1477-7827-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halvaei I, Ali Khalili M, Razi MH, Nottola SA. The effect of immature oocytes quantity on the rates of oocytes maturity and morphology, fertilization, and embryo development in ICSI cycles. J Assist Reprod Genet. 2012;29:803–10. doi: 10.1007/s10815-012-9799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JE, Kim SD, Jee BC, Suh CS, Kim SH. Oocyte maturity in repeated ovarian stimulation. Clin Exper Reprod Med. 2011;38:234–7. doi: 10.5653/cerm.2011.38.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HJ, Jee BC, Suh CS, Kim SH, Moon SY. Oocyte maturity in relation to woman's age in in vitro fertilization cycles stimulated by single regimen. Yonsei Medical J. 2012;53:181–5. doi: 10.3349/ymj.2012.53.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee MS, Ben-Rafael Z, Meloni F, Mastroianni L, Jr, Flickinger GL. Relationship of human oocyte maturity, fertilization, and cleavage to follicular fluid prolactin and steroids. J IVF ET. 1987;4:168–72. doi: 10.1007/BF01555465. [DOI] [PubMed] [Google Scholar]
- 10.Sills ES, Drews CD, Perloe M, Kaplan CR, Tucker MJ. Periovulatory serum human chorionic gonadotropin (hCG) concentrations following subcutaneous and intramuscular nonrecombinant hCG use during ovulation induction: a prospective, randomized trial. Fertil Steril. 2001;76:397–9. doi: 10.1016/s0015-0282(01)01903-3. [DOI] [PubMed] [Google Scholar]
- 11.Elkind-Hirsch KE, Bello S, Esparcia L, Phillips K, Sheiko A, McNichol M. Serum human chorionic gonadotropin levels are correlated with body mass index rather than route of administration in women undergoing in vitro fertilization—embryo transfer using human menopausal gonad-otropin and intracytoplasmic sperm injection. Fertil Steril. 2001;75:700–4. doi: 10.1016/s0015-0282(00)01790-8. [DOI] [PubMed] [Google Scholar]
- 12.Levens ED, Whitcomb BW, Kort JD, Materia-Hoover D, Larsen FW. Micro-dose follicular flare: a viable alternative for normal-responding patients undergoing in vitro fertilization? Fertil Steril. 2009;91:110–4. doi: 10.1016/j.fertnstert.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill MJ, Chason RJ, Payson MD, Segars JH, Csokmay JM. GnRH antagonist rescue in high responders at risk for OHSS results in excellent assisted reproduction outcomes. Reprod Biomed Online. 2012;25:284–91. doi: 10.1016/j.rbmo.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levran D, Farhi J, Nahum H, Glezerman M, Weissman A. Maturationarrest of human oocytes as a cause of infertility: case report. Hum Reprod. 2002;17:1604–9. doi: 10.1093/humrep/17.6.1604. [DOI] [PubMed] [Google Scholar]
- 15.Chan CC, Ng EH, Chan MM, Tang OS, Lau EY, Yeung WS, et al. Bioavailability of hCG after intramuscular or subcutaneous injection in obese and non-obese women. Hum Reprod. 2003;18:2294–7. doi: 10.1093/humrep/deg446. [DOI] [PubMed] [Google Scholar]
- 16.Reichman DE, Greenwood E, Meyer L, Kligman I, Rosenwaks Z. Can in vitro fertilization cycles be salvaged by repeat administration of intramuscular human chorionic gonadotropin the day after failed injection? Fertil Steril. 2012;98:671–4. doi: 10.1016/j.fertnstert.2012.05.050. [DOI] [PubMed] [Google Scholar]


