Abstract
The study of muscle physiology has undergone many changes over the past 25 years and has moved from purely physiological studies to those intimately intertwined with molecular and cell biological questions. To ask these questions, it is necessary to be able to transfer genetic reagents to cells both in culture, and ultimately, in living animals. Over the past 10 years, a number of different chemical and physical approaches have been developed to transfect skeletal, smooth, and cardiac muscle living systems with varying success and efficiency. This review will provide a survey of these methods and describe some more recent developments in the field of in vivo gene transfer to these various muscle types. Both gene delivery for overexpression of desired gene products and delivery of nucleic acids for downregulation of specific genes and their products will be discussed to aid the physiologist, cell biologist, and molecular biologist in their studies on whole animal biology.
The study of muscle physiology has undergone tremendous changes in the last 25 years. In the 1970s and 1980s, much of the research on skeletal, smooth, and cardiac muscle focused on defining the biophysical factors governing muscle properties as they related to vascular resistance, cardiac pacing, and force generation. In the 1990s the emphasis shifted towards cell biology as the focus moved from the organ to the cellular level. Now, many studies must deal with the molecular biology of these systems to understand the mechanisms at the molecular level. At the other extreme, molecular biology has seen a similar evolution over same time, going from studies on DNA sequence and promoter analysis of muscle-specific genes in in vitro systems to studying the roles of these genes and their impact on cell biology in cultured muscle cells. With the advent of the genomic and proteomic revolution, many researchers have begun to turn their attention to studying the roles of various genes and their regulation in living systems in the context of the living organisms themselves. With these transitions in mind, it is clear that we must have ways to study gene function in living tissue.
Perhaps the most powerful way to do this is to utilize gene transfer strategies to deliver genes and other DNAs or RNAs to cells within tissues to study their roles in the context of the animal itself. Such approaches can be used to upregulate gene expression, study transcriptional and post-transcriptional regulation of various genes and gene products, and to down-regulate expression of desired targets. However, to succeed in all of these studies, efficient ways to deliver nucleic acids to muscle cells in vivo must be used. This review will focus on the techniques used to deliver genes to skeletal, smooth, and cardiac muscle in vivo and will discuss various approaches to upregulate and downregulate expression in living systems using gene transfer.
Transfection Strategies
A number of methods have been developed to introduce exogenous nucleic acids into cells. However, while many of these approaches work to varying degrees on cells in culture, they are almost always much less effective in vivo. There are two main types of transfection strategies: chemical and physical. Chemical methods include the use of cationic liposomes (“lipoplex”), polymers (“polyplex”), combinations of the two (“lipopolyplex”), calcium phosphate, and DEAE dextran. In almost all of these chemical methods, the reagents promote transfection by complexing with the DNA to neutralize the charge, condensing the DNA, mediating interaction and attachment to the cell membrane, and promoting entry into the cell, typically via endocytosis and subsequent endosomal escape. In addition to the chemical methods, a number of physical methods exist that promote the direct entry of uncomplexed DNA into the cell. These methods can include microinjection of individual cells, hydroporation, electroporation, ultrasound, and biolistic delivery (i.e., the gene gun). In the case of hydroporation, recent data from Dexi Liu and colleagues suggest that hydrodynamic delivery of DNA, at least in the liver, casues transient pores to open in the cell membrane (hence the term, “hydroporation”), which close within 10 minutes of high volume injection, and allow entry of DNA into the cytoplasm (135). Similarly, electroporation, at the appropriate field strength, causes limited and focal membrane destabilization giving rise to pores that exist for the lifetime of the field (111). Membrane pores are also induced by high frequency sound waves (“acoustic cavitation”) and can be amplified by microbubbles using ultrasound (63). In contrast to these techniques, the gene gun “shoots” DNA-coated particles into cells, breaching the plasma membrane by physical force. While it is clear that some of these approaches are unsuited for in vivo delivery (microinjection of individual cells within a tissue could be seen by some as inefficient), others have had varying success in animals. Out of all of the methods, the most widely used and effective methods use lipids or polymers, direct injection into bulk tissue, or electroporation.
Skeletal Muscle
In 1990, Jon Wolff and colleagues performed a simple experiment that resulted in an elegant and highly effective method for gene delivery to skeletal muscle (127). Purified, protein-free plasmids expressing β-galactosidase were suspended in saline and directly injected into the quadricep muscle group of mice. Several days later, the muscles were removed and assayed for gene expression. Surprisingly, many fibers within the injected muscle expressed high levels of the gene product. The majority of expressing cells appeared to be adjacent to the needle injection track, but many cells throughout the muscle expressed the injected gene. This approach was termed “naked DNA injection” and has been shown by numerous groups to give extremely high levels of gene expression in vivo (72). In most cases, either strong viral promoters such as the CMV immediate early promoter (CMViep), the SV40 early promoter, or the RSV long terminal repeat promoter, or skeletal muscle promoters such as the β-actin promoter are used to drive expression of the transgene (47). Relatively low levels of plasmid are required to achieve robust gene expression (typically 10 to 50 μg of plasmid is injected per muscle in mice, resulting in nanogram to microgram levels of gene product). Reasonable expression tends to develop within 6 hours and peaks within 2 days, although expression has been seen as early as 2 minutes following injection (cells around the needle track express first) (28). Most importantly, the levels of gene expression persist for extended periods of time. Experiments in mice have detected gene expression out to 19 months following a single injection of plasmid (126). Long term gene expression has also been detected in larger animals and non-human primates (71). However, although expression persists for a very long time, the levels drop from their day 2 peak to about 10 to 20% of this maximum by 7 to 14 days and remain at this level for extended periods (126). This drop in expression could be due to combinations of a variety of factors, including promoter choice, promoter inactivation or downregulation (49), DNA methylation (50), immune responses generated against the produced transgene (49, 61), or inflammatory responses against CpG motifs in the plasmids (130, 136). It should also be stressed that the levels and duration of expression are also dependent on the transgene product being produced. This approach has been used for many purposes, including the production of secreted proteins using muscles as bioreactors (e.g., factor VIII for clotting disorders or growth factors to aid growth) and for DNA vaccines.
An approach to increase the efficiency of this method was developed by applying external electric fields to the injected muscle. Electroporation has been used extensively to transfer DNA to bacteria, yeast, and mammalian cells in culture for the past 20 years (6, 99). More recently, it has been applied to intact tissues in living animals, including skeletal, smooth, and cardiac muscle. Electroporation uses electrical fields to create transient pores in the cell membrane that exist for the lifetime of the electric field and allow the entry of normally impermeable macromolecules into the cytoplasm (111). The electric field also results in the electrophoretic movement of the added DNA, aiding in gene delivery (104). Surprisingly, at the appropriate field strengths, the application of these fields to tissues results in relatively little damage or trauma although some low level of necrosis and changes in gene expression may be detected (8, 39, 81, 88). When a square wave electric field at the appropriate strength was applied to mouse quadricep muscles following direct injection of the plasmid, the levels of gene expression jumped between 100 and 1000-fold (2, 81, 86). Both the distribution of cells taking up and expressing the DNA increases upon electroporation as does the absolute amount of gene product per cell (this is likely due to increased delivery of plasmids into each cell)(Figure 1). Indeed, many reports demonstrate that between 50 and 80% of fibers within a given muscle group can be transfected using this approach (29, 81, 86). As with direct injection, gene expression is long lasting. Further, it has been used successfully in a number of different species, up to non-human primates (93).
Figure 1. Electroporation mediated gene transfer to skeletal muscle.
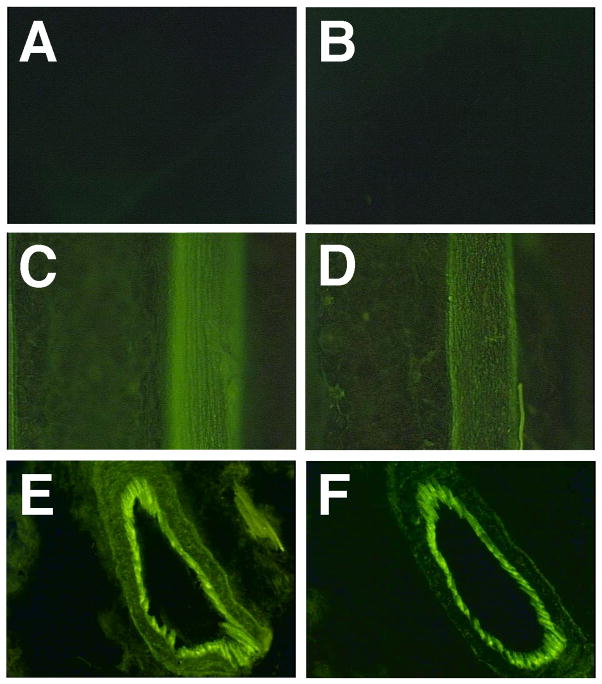
Balb/c mice were anesthetized and 50 μg of pEGFP-N1 (BD Clontech, Palo Alto CA) in 100 μl of saline was injected into the right and left quadriceps. Immediately following injection, a series of eight 10 msec pulses at 200 V/cm were delivered to the muscles of right leg (B); the left leg received DNA but was not electroporated. Twenty-four hours later, both muscles were exposed and photographed using a fluorescence dissecting microscope. As can be seen, robust gene expression in skeletal muscle is detected using electroporation.
The application of the electric field can be accomplished using either plate electrodes placed on the surface of the leg or using needle electrodes that are inserted into the DNA-injected muscle (Figure 2). For needle arrays, there are several types, including two- and six-needle arrays, both of which work very well. For plate arrays, any number of commercially available or homemade electrodes will work, from two rods placed on either side of the leg, to “caliper” electrodes which have metal plates attached to calipers so that the gap between the electrodes can be accurately measured to set the field parameters. A number of different pulsing parameters have been used, with both high voltage-short pulse length and low voltage-long pulse length being effective. Two such combinations that work extremely well are to apply a set of 6–8 pulses of 20 msec duration each at a field strength of between 100 to 400 V/cm (low voltage, long pulse) or a similar train of 100 μsec pulses at 900 to 1000 V/cm (high voltage, short pulse)(87, 120). Briefly, plasmids purified by standard laboratory techniques (e.g., Qiagen kits, Promega kits, etc) are suspended in physiological saline (140 mM NaCl), typically in a buffer that contains 10 mM Tris, pH8, and 1 mM EDTA., although any buffer should suffice. Between 10 and 100 μg of plasmid in 50 to 100 μl are injected into the desired muscle group of the anesthetized mouse so that the tissue bulges slightly with the injected fluid. For larger animals, increased volumes and amounts of plasmid can be administered. It has been demonstrated that when small volumes are used, the distribution of gene expression is more focal around the site of injection and not dispersed evenly throughout the tissue. Immediately following DNA injection, electrodes are placed on the skin on either side of the injected muscle and a series of pulses are delivered. Because an electric field is being applied, the muscles will contract, although no lasting effects have been observed. However, at higher field strengths with long pulse times, tissue damage can occur (8, 81, 88).
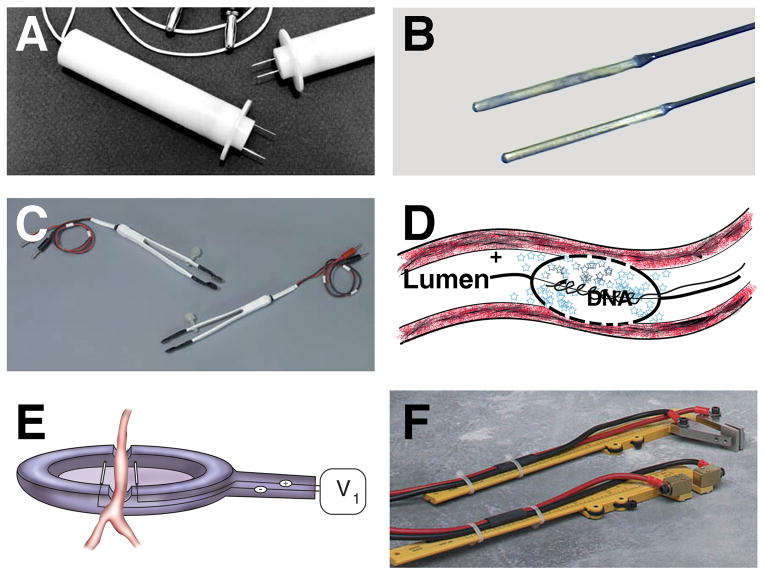
Figure 2. Electroporator probes and electrodes.
A number of different electrodes for in vivo electroporation-mediated gene transfer have been developed. Some electrodes can be used for multiple tissue applications (A, B, C, and F), whereas others are limited to certain tissues, such as the vasculature (D and E). Two-needle electrodes (A), “Genetrode” rod electrodes (B), “Tweezertrode” tweezer electrodes (C), porous balloon-catheter electrode (25, 26)(D), spoon electrode (79)(E), and caliper electrodes (F) are shown. Electrodes shown in A, B, C, and F are from BTX (Harvard Instruments). The small blue stars in panel D represent plasmids.
Electroporation of skeletal muscle has been used extensively to express a number of therapeutic and physiological genes in multiple animal models (for an excellent review, see (3). Because direct DNA delivery coupled with electroporation results in such high level expression, this technique has been used to exploit skeletal muscle as a bioreactor to produce and secrete a number of proteins that act in an exocrine manner, such as Factor IX for hemophilia (37), Erythropoetin for thalassemia (96, 101), growth hormone-releasing hormone to increase body weight (30, 31), and various interleukins. Genes that act on skeletal muscles themselves have also been delivered using this approach, including dystrophin (36, 90) and IGF-1 (106). Finally, electroporation of skeletal muscle has also been used to express antigens for development of DNA vaccines, in multiple animal models (7, 93).
Although electroporation greatly increases gene transfer and expression in skeletal muscle, a number of studies have evaluated the use of adjunct reagents to increase gene transfer even further, in both electroporated and non-electroporated muscles. Early studies from Wolff’s lab, who developed the naked DNA injection technique, demonstrated that injection of the muscles with bupivacaine at doses that induced limited muscle degeneration, prior to DNA injection resulted in increased gene transfer and expression (20). More recently, several groups have shown that injection of hyaluronidase into muscle, prior to DNA delivery and electroporation, also increased gene transfer and expression (36, 42, 84, 85, 88). In this case, the enzyme partially degrades the extracellular matrix surrounding the myotubes, and this is thought to aid in uptake of the DNA into the cells, resulting in up to four-fold higher gene transfer versus electroporation alone.
Apart from direct DNA injection with or without electroporation, a number of chemical methods and techniques have been tested for DNA delivery to skeletal muscle in vivo, with varying degrees of success. Cationic liposomes, such as Lipofectin, DOTAP:DOPE, etc., have revolutionized transfections of cultured cells in the lab due to their ease of use and typically high transfection efficiency. However, while many of these reagents can be used to productively transfect myoblasts and myoblast cell lines in culture, they show almost no transfection activity in differentiated myotubes in culture or in skeletal muscle in vivo (48, 72). Similarly, cationic polymers including polyethyleneimine (PEI) which are highly efficient for in vitro delivery and delivery to other tissues such the lung, have also not performed well in differentiated myotubes, in vitro or in vivo (14, 68).
Several other physical methods are worth mentioning. First, particle bombardment has been used in several studies to transfer genes to various skeletal muscles with success (62, 133). This technique coats gold particles with plasmids and the particles are delivered into the tissue using a “gene gun” that uses pressure to shoot the particles into the tissue. In one study where this method was compared to direct DNA injection, the gene gun performed best in the muscle of young rats, and yielded between 10- and 100-fold more expression than direct DNA injection (62). The only drawback to this technique is that it traditionally requires the muscle to be exposed (although a new device can penetrate multiple layers of the skin and transfect sub-epithelial tissues (27)) and induces some degree of trauma. Microbubble ultrasound has also been used to deliver DNA to skeletal muscle. DNA is delivered directly or with a microbubble agent such as Optison and ultrasound waves are concentrated on the area of the tissue where gene transfer is desired (73). Wide distribution is achieved as is high level gene expression with the benefit of being able to direct the waves to specific regions of interest. Finally, hydrostatic pressure, or hydroporation, has been used successfully to transfer genes to skeletal muscle in a number of organisms. The original versions of this technique delivered a large volume of DNA via the tail vein over a short period of time and resulted in tremendous expression in the liver (70). Wolff and others have applied this method to isolated limbs to target skeletal muscle by injecting large volumes of DNA into the circulation over a short period of time and achieved relatively uniform, high-level expression in most muscle groups fed by the vasculature in the limb (11, 19, 69, 134). This has now been applied to animals from mice to non-human primates with success. However, the only drawback to this approach is that some tissue damage does occur, but the ability to target the majority of muscles with a given limb is attractive.
Smooth Muscle
Smooth muscle, especially in the vasculature and airways, is an extremely important target for gene transfer and expression studies. However, perhaps the major challenge to transfecting smooth muscle cells (SMCs) in vivo is that they are surrounded by other cell types (endothelial and adventitial cells in the vasculature and epithelial cells in the airways). Thus, unless the tissue is damaged, they are largely inaccessible to DNA transfer reagents. Indeed, it has been well documented for viral vector-mediated delivery as well as lipoplex and polyplex-mediated delivery to vascular SMCs, that unless the endothelial layer is denuded, typically by angioplasty, essentially no gene transfer to the smooth muscle layer is detected when the reagents are delivered from the lumen (55, 83). Similarly, when the transfection complexes (viral or non-viral) are delivered from the adventitial surface, very little expression is found in the medial SMC layer, and almost none in the intima (118), although one recent study has had success using adventitial delivery of plasmids using the reagent Effectene (a proprietary non-liposomal transfection reagent from Qiagen) to transfer DNA to the SMCs (10). In airways, the situation is the same with agents delivered from the airways being primarily targeted to the airway epithelial cells and those delivered systemically via the vasculature being restricted to the endothelial cells. It should be stressed, however, that a variety of liposomal reagents, including DOTAP/DOPE, Lipofectin, Lipofectamine, etc, and cationic polymers such as PEI, Effectene, and dendrimers, are effective for transfecting smooth muscle cells both in vitro and in vivo, if the reagents have direct contact with the cells (after injury) in vivo (5, 44, 74, 89, 114, 118). Polymer-coated stents have also been used to transfer DNA directly to smooth muscle cells of the vasculature in vivo, but again, as for all other chemical transfection reagents, damage to the endothelium is a prerequisite for SMC gene transfer (59, 97, 113). Thus, if the desired experiments can be performed using endothelial or epithelial denuded tissues, these techniques can be used.
Another limitation for smooth muscle transfection in vivo is that direct DNA injection cannot effectively be used since in most instances the smooth muscle layers are too thin to inject reliably. This is especially true in the vasculature. With the exception of a few large vessels, the vessel wall is too thin to be injected with DNA. Moreover, even if DNA could be delivered to the walls by injection, the architecture of the vessel wall would prevent the even distribution of the DNA throughout it. Similar limitations apply in airways, bladder, digestive tract, and uterus. However, despite these limitations, several approaches have been developed to transfect smooth muscle cells in vivo.
One way around the inaccessibility of the smooth muscle cells within a tissue is to transfect cultured SMCs and then engraft them back into the host in hopes that they will home to the appropriate smooth muscle layer. The advantage of such an approach is that it is much easier to transfect cells in culture than it is in vivo. Several attempts at this have been made with little success, because although the cells can be transfected and express after delivery in vivo, they do not necessarily engraft into the smooth muscle layer to any great extent. In one study, transfected SMCs were injected intravenously, intraperitoneally, or intramuscularly (34). Although cells injected by all routes expressed for 4 to 7 days, there were no data to show where these cells actually localized since the gene expressed encoded for a secreted protein. In another case, a stent was embedded with transfected SMCs and placed (with accompanying endothelial cell injury) into the coronary arteries of pigs (94). These cells continued to express their gene product for up to one month after stent placement, but in all animals, expressing cells were detected only within the mesh of the stent; no engraftment of transfected cells into the vascular smooth muscle layer occurred.
As seen in skeletal muscle, pressure can be used to deliver genes and other nucleic acids to the vascular wall. Surprisingly, hydrostatic delivery of DNA via the circulation does not result in significant delivery and expression of genes within the vessel wall (11, 69, 134). Thus, to target the vessel wall, a different method that transfects cells in explanted vessels which are then transplanted into the host has been developed (121). Vessel segments are removed and placed in a container along with the plasmid or other nucleic acid, and the container is briefly pressurized (typically vessels are placed in a dialysis bag that is tied off at one end and attached to a syringe at the other). After gene transfer, the vessel is then either cultured ex vivo for study or transplanted into a recipient. The nice feature to this approach is that gene transfer to all cell types within the vessel is achieved without injuring the endothelial layer. This approach has been used to transfer plasmids for vascular SMC gene expression, anti-sense RNA to inhibit gene expression, and decoy oligonucleotides to downregulate transcription factor activity (32, 77, 78, 121).
Ultrasound has also been used in the vasculature to transfer genes to the smooth muscle cells. As seen in other tissues, the use of a microbubble contrast agent such as Optison, greatly increased gene transfer using ultrasound over DNA alone or ultrasound using DNA without microbubbles (52, 115). Microbubble ultrasound was able to give high level gene expression of either of two reporter genes in the vessel wall. Further, the levels of expression were roughly the same for balloon angioplasty-injured and uninjured vessels. Unfortunately, in both studies, cellular localization of gene expression following transfer in uninjured vessels was not determined. Since the levels of expression were roughly equivalent in denuded and intact vessels in these studies, it is likely that the SMCs are targeted to some degree without injury. However, as with chemical transfection methods, in the injured vessels, significant gene delivery and expression was found in the smooth muscle layer (115).
Electroporation to target smooth muscle
As in skeletal muscle, electroporation works well in vivo for gene delivery to smooth muscle. To date, vascular, airway, bladder, and intestinal smooth muscle have been successfully transfected in vivo using electroporation. Because the tissues cannot be directly injected, as skeletal muscle can be, alternative approaches have been used to get the DNA to the tissue in the first place. To do this, DNA can be delivered from the inside (lumen, airway, or intravesical space) or the outside (adventitia, via the circulation for the airways, or subserosal space for the bladder) of the tissue, prior to delivery of the electric field.
Vascular smooth muscle
The first use of electroporation for these tissues was described by Martin et al., where genes were transferred to the mesenteric vasculature of rats using adventitial DNA delivery (79). Vessels of the rat mesenteric vascular tree were exteriorized in anesthetized animals, placed in an electrode resembling a spoon with two wires flanking a notch for the vessel (Figure 3), covered with a solution of plasmid, and electroporated with a series of 8 square wave electric pulses of 10 msec duration at an optimal field strength of 200 V/cm. Following electroporation, the vessel was removed from the electrode, the vessels and the intestine were returned to the abdomen and the animals recovered without incident Gene expression was detected as early as 6 hours following electroporation. With the CMV immediate early promoter used to drive the reporter gene, expression peaked between days 1 and 3 and then dropped to baseline by day 7, after which gene expression cannot be detected (79, 131). Such short duration of gene expression with the CMV promoter has been seen in other tissues and is likely the result of promoter inactivation (see below). The use of other promoters, such as the Ubiquitin C promoter, can drive much longer gene expression (on the order of weeks not days, see below). Gene transfer using this approach is dose-dependent, time-dependent, and highly dependent on field strength (79). No gene transfer and expression above background is seen without an applied electric field or at 50 V/cm. At 100 V/cm, reasonable, but variable, gene expression was detected, and at 200 V/cm, consistent, high-level gene transfer and expression is seen. When the field strength was increased to 400 V/cm, tissue damage was detected and the levels of gene expression declined.
Figure 3. Electroporation mediated gene transfer to vascular smooth muscle.
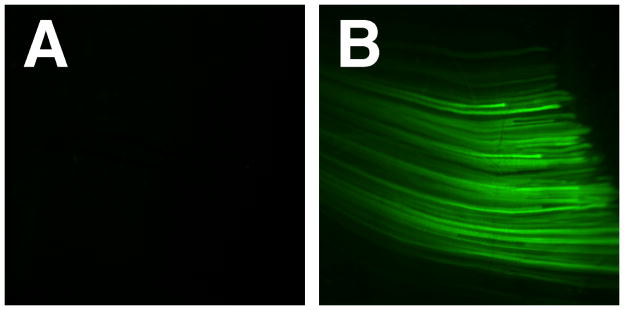
GFP expression can be seen in multiple cell layers in the mesenteric vasculature of rats following electroporation. Neurovascular bundles were untreated (A) or bathed in 0.5 mg/ml pEGFP-N1 without electroporation (B). Vessels in panels C through F were bathed in plasmid and were electroporated at 200 V/cm using eight pulses of 10 msec duration each, as described (79, 131). Gene transfer and expression of GFP are seen in both the artery (C) and vein (D) of an individual neurovascular bundle. Cross-sections of electroporated vessels show that gene expression can be detected throughout most cells within the vessel, including adventitial, smooth muscle, and endothelial cells (E and F).
Gene expression of reporter genes was restricted to areas that were bathed in the DNA and received the electric field and thus, localized to the adventitial cells, smooth muscle cells, and endothelial cells within the vessels (79, 131). In fact, the majority of the gene expression was detected within the endothelial cells, suggesting that a significant amount of DNA can move through the tissue, cells, and elastic lamina of these vessels. Similar patterns of gene expression have also been detected in larger vessels, including the rat carotid (J. L. Young and D. A. Dean, unpublished). When quantified, up to nanogram levels of gene product per cm of vessel (100 μm in diameter) were produced (79, 131). Assuming that approximately 2% of the volume of the neurovascular bundle are actually cells (based on histological and morphometric analysis), 1 ng of a 100,000 dalton gene product would correspond to an intracellular concentration of approximately 0.1 μM. This concentration is more than sufficient to elicit physiological effects, as seen in multiple studies (51, 110). For example, Benoit and colleagues have used this approach to transfer dominant-negative mutants of PKC epsilon and demonstrated that this isoform of PKC plays a role in regulating β agonist-induced vascular smooth muscle contraction (110). In their study, expression levels of the dominant negative protein were high enough to abrogate PKCε-signaling in response to phenylephrine to the same degree as did the general PKC inhibitor chelerythrine. However, it should be noted that all effects, especially those using dominant negative inhibitors, are highly dependent on the protein, enzyme, activity, or response being measured. Thus, while the levels of expression obtained with electroporation may be high enough for one response, they may not be for all.
Although this technique may seem traumatic, electroporation at the optimal field strength parameters does not induce any histological changes, inflammatory response, or trauma (79). Further, when vasoreactive responses of the vessels were measured at the peak of gene expression (2 days) or long after gene expression had subsided (40 days) following electroporation, the vessels responded in a manner indistinguishable from control vessels in terms of constriction to phenylephrine and relaxation to adenosine and isoproterenol (79). Finally, our lab has also performed DNA microarray analyses on vessel segments pre- and post-electroporation and found that the act of electroporation does not affect the global patterns of gene expression to any degree (131).
Several other electroporation approaches for the vascular wall have also been developed by other groups using DNA delivered via the lumen. The advantage to this method is that with the appropriate device (e.g., single or double balloon catheter), vectors can be delivered to defined regions of the vasculature with relatively simple methods that are clinically routine. The disadvantages are that in order for vectors to be delivered to the vessel lumen using double balloon catheters, blood flow must be restricted, which may cause ischemia of the downstream vessels and tissues. Several studies from one group have used a porous balloon catheter that also contains two electrodes to deliver heparin or DNA through the porous balloon and electroporate at the same time (25, 26). The electrode system uses the guide wire as one electrode and an internal wrapped wire contained entirely within the balloon as the second electrode. When a voltage is applied, an electric field develops between the two electrodes, causing electroporation of the vessel wall and delivery of heparin or DNA to cells within the vessel wall. As with adventitial delivery and electroporation, transfer is detected within the endothelial cells and both the intimal and medial smooth muscle layers. Another group delivered DNA using a double balloon catheter and applied the electric field from the adventitial surface of the rabbit carotid using two “T” shaped electrodes (2.5 cm long × 0.5 cm wide) placed on either side of the vessel segment (82). In both of these cases, gene transfer was dependent on the electric field. No expression was seen at 0 V/cm, but gene transfer and expression increased as the field strength was raised to 100 and 200 V/cm and then decreased by 300 and 400 V/cm. Again, as in the studies by Dev and colleagues, gene transfer was detected in the endothelial and smooth muscle layers. More recently, a similar study was performed in the abdominal aorta of rats in which DNA was administered to the lumen and the vessel was electroporated from the adventitial surface, resulting in gene transfer and expression to all cell types in the aorta wall (51). With all of these methods, the levels of gene product expressed has been sufficient to elicit physiologic or therapeutic responses (25, 26, 51, 110).
Airway smooth muscle
Airway smooth muscle also suffers from the fact that it is buried beneath other cells and is hidden from standard gene transfer reagents. Our lab has recently shown that electroporation can also be used to target these cells without inducing any injury to the airways to make the cells accessible (21, 23, 76). Purified plasmid suspended in a physiologically compatible buffer containing 140 mM NaCl is administered to the airways of anesthetized mice or rats and the animals are electroporated using flat electrodes placed on either side of the chest. As for the vasculature, a series of 8 square wave pulses are delivered to the animals at an optimal field strength of 200 V/cm and pulse lengths of 10 msec and the animals recover without incident.
Gene transfer is dependent on field strength, with very little gene transfer and expression seen at values less than 100 V/cm and an optimum at 200 V/cm. However, it should be stated that other field strength and pulse length combinations may work very well, as has been seen with skeletal muscle (i.e., higher field with shorter pulses). Indeed, in one study, the pulse length was varied from 10 μsec to 10 msec using a constant field strength of 200 V/cm in the rat (76). Although gene transfer was seen with the 10 μsec pulses, expression increased by over 100-fold when the pulse length was raised to 10 msec. However, by increasing the dose of DNA, equivalent levels of expression can be achieved using the short pulse length.
Gene transfer using transthoracic electroporation was DNA dose-dependent, with 100 μg of DNA giving 10 ng of gene product per gram wet weight of lung in mice (23). In rats, the levels of expression are lower, with 100 μg giving 150 pg gene product per g wet weight at the longer pulse length (76). The level of gene expression is more than enough to elicit physiological effects as demonstrated by increases in alveolar fluid clearance following electroporation-mediated transfer of plasmids encoding a subunit of the Na+, K+-ATPase (75). Indeed, the physiological response was equivalent to that seen with recombinant Adenoviruses expressing the same gene product (35, 75). Most importantly, gene expression was detected throughout all cell types in the lung (21, 23, 76). Cell types that received and expressed DNA included alveolar type I and type II epithelial cells, endothelial cells, airway epithelial cells, and vascular and airway smooth muscle cells. By immunohistochemistry for the expressed gene product, there appeared to be little to no difference between the levels of gene expression in the various cell types. As for uniformity of gene transfer, there appears to be transfer and expression to all regions of the lung that is relatively homogeneous over a 2–3 fold range (21, 76).
Using smooth muscle specific promoters, we have also been able to limit gene expression following electroporation to the airway smooth muscle cells (T. Kuzniar and D. A. Dean, manuscript in preparation). This has been done in the rat and the mouse, both in vivo using transthoracic electroporation following tracheal delivery of DNA and in lung explants. Expression from plasmids utilizing smooth muscle-specific promoters was detected only in the smooth muscle layer, while expression from plasmids containing the CMV immediate early promoter or SV40 early promoter was seen in all cell types. When quantified, expression in the smooth muscle accounted for about 5% of total lung expression, which is slightly more than the relative ratio of smooth muscle cells to total cells. Thus, not only can the airway smooth muscle cells be transfected along with all other cell types in the lung, they can also be specifically targeted using the appropriate promoters and electroporation.
Bladder smooth muscle
Electroporation-mediated transfer of genes to bladder smooth muscle in rats has also been reported (53). Genes for luciferase, GFP, and nNOS were transferred to the bladder by injecting 50 μl of the DNA into the subserosal region of the exposed bladder wall following removal of urine from the bladder. Flat tweezer-like electrodes were placed on either side of the bladder and square wave pulses were delivered to the tissue. Following this, the abdomen was closed and gene expression was evaluated at later times. Gene transfer and expression was dependent on the field strength, with optimal expression seen at 225 V/cm using 8 pulses of 50 msec duration each. As seen in the lung (76), expression was also pulse length dependent, although the dependency was not as pronounced as in the lung. Also, the optimal pause between pulses was found to be 1 second; shorter pauses gave lower levels of expression. Further, although there was no trauma or tissue damage under the optimal conditions, at higher field strengths, tissue damage was detected.
Transfer of GFP resulted in significant levels of gene expression in the smooth muscle layer of the bladder. Not only was the reporter gene expressed, but transfer of nNOS resulted in enhanced staining for the transferred nNOS in the smooth muscle of the tissue sections. Further, NO production was increased by over 50% in bladder strips isolated from electroporated animals that had received the nNOS plasmid but not in control strips that received plasmid without electroporation or in strips that were electroporated without added DNA. Taken together, these results suggest that electroporation can be used to target smooth muscle cells within a number of different tissues.
Cardiac Muscle
As with smooth muscle, one of the limitations to transfecting cardiac muscle is that the cardiomyocytes are surrounded by an endothelial layer on the side to which is most easy to deliver DNA, namely the blood. Several different methods have been used to deliver genes to the myocardium using intravascular delivery, typically via the coronary or carotid arteries. However, because the muscle in the heart is relatively large, direct injection has proven a very effective technique for gene delivery, either alone, or in combination with polymers, lipids, ultrasound, or electroporation.
For delivery via the circulation, in most cases the plasmid is complexed with either liposomes or polymers for two reasons. First, the complexed cationic lipids or polymers condense the DNA to a smaller size and mediate interactions between the DNA and the cell membrane. Without such reagents, very little DNA would interact with the plasma membranes of cells and almost no DNA uptake would be observed. These complexes also serve to provide stability to the DNA and protection from degradation. In the absence of liposomes, the half-life of free DNA in the serum is on the order of several minutes at best (54). Thus, without the protective function of these chemical transfection reagents, the DNA would be degraded long before it ever got to the target cells. DNA has been complexed with a number of different lipids for gene transfer to the heart via the circulation. A number of studies have used a mixture of phosphatidylserine, cholesterol, and phosphatidylcholine mixed with inactivated
Hemagglutinating Virus of Japan (HVJ) particles (33, 105, 112). These HVJ-liposomes are fusogenic and promote fusion of the liposome with the endosomal membrane and uptake of the DNA into the cell. In several studies, mixtures of plasmid and HVJ liposomes were infused over the course of 10 minutes into either the aorta or coronary arteries of excised hearts maintained at 4° and then the hearts were transplanted into recipient mice or rats (105, 112). In one study, this method resulted in up to 50% of cardiomyocytes receiving and expressing the transgene for up to 14 days (the beta actin promoter was used to drive gene expression in these studies)(105). However, infusion through the coronary artery or aorta in vivo (i.e., an in situ heart) appeared to result in much less efficient uptake of DNA (less than 1.6% of cardiomyocytes)(33, 109). Unlike the Sawa study using HVJ liposomes in excised, transplanted hearts, the use of other lipids (DLRIE/DOPE) or dendrimers to complex with the DNA resulted in very low levels of gene transfer to either endothelial cells or cardiomyocytes (less than 1% of cells) when a similar infusion/transplant method was used (24, 108, 125).
Direct injection of DNA, either as naked DNA or complexed with lipids or polymers, into the wall of the heart has been shown to give much higher levels of gene expression in cardiomyocytes, but usually with a more limited distribution of gene expression. Most studies have used naked DNA and have injected the DNA into multiple sites within the wall of the heart. In some cases, injections were performed following thoracotomy (58, 122) or using a subdiaphragmatic approach following a midline abdominal incision (1), but in most cases, less invasive procedures were used that employed echocardiography to guide percutaneous injections into the ventricular wall (95, 103, 116, 117). In the majority of studies, gene expression was limited to a relatively small number of cells around the injection sites (95, 116, 117). However, several studies have shown that the heart can express very high levels of gene product. In one study following gene transfer in rat hearts after direct injection of DNA, up to 800 ng of luciferase or 2 ng of VEGF per heart was expressed (103). This study used the CMV promoter to drive expression, and as found in other studies, expression peaked within the first three days and then dropped off dramatically. Further, this robust gene expression was detected using a dose of only 30 μg of DNA (103). Similarly, Kitis and colleagues found that the heart was even 10 to 100-times more efficient at gene expression than skeletal muscle, when the same dose of DNA was delivered using direct injection (58). The limitation to this approach is not that inadequate levels of gene product are produced, but rather that the distribution of expression is not uniform throughout the myocardium. One way that may be useful for increasing the distribution of gene expression following direct injection is to inject DNA complexed with PE6400 block copolymer or HVJ liposomes (4, 98). Using the block copolymer, much better distribution of gene expression compared to naked DNA was seen (98), although the absolute levels of expression (40 ng luciferase/heart in rats) was not as high as some other reports, this is still extremely good expression.
Several reports have also begun to use microbubble enhanced ultrasound to mediate gene delivery to the heart in vivo (18, 60). In this approach, DNA mixed with Optison resulted in gene transfer to the heart following infusion into the carotid artery or jugular vein. However, in both studies, no absolute levels of gene expression are reported (only relative light units for luciferase transfer) and no histological analysis was performed to localize the transferred genes. Thus, it is unclear whether this method yields high level gene transfer or whether it targets the cardiomyocytes or only the endothelial cells.
Finally, two studies have been reported using electroporation to transfer DNA to the heart (46, 124). In this study, hearts were removed from stage 18 embryonic chickens, placed in a bath of DNA, and electroporated at 200 V/cm using 10 msec square wave pulses delivered with a Grass stimulator. The hearts were then cultured ex vivo on a collagen gel in the presence of cell culture medium and analyzed for gene expression one to three days later. When no electric field was applied, no gene expression was detected in any of the hearts. However, when either 6 or 8 pulses were delivered to the hearts, significant levels of GFP or luciferase expression could be detected. Indeed, up to 30% of the cells in the heart showed GFP expression, most all of which was in cardiomyocytes. Another group performed very similar studies on excised and transplanted mouse hearts (124). They found good expression using naked DNA coupled with electroporation, but found that complexation of the DNA with dendrimer complexes increased the levels of gene transfer between 10- and 45-fold, using very similar electroporation parameters.
Regulating Gene Transfer and Expression
Once genes are delivered to the desired tissue, issues regarding levels and duration of expression, inducibility, and restriction of gene expression to the desired cell type become central to any experiment. Perhaps the easiest way to regulate most aspects of gene expression is at the level of the promoter. For most transient transfection studies, either in cultured cells or in animals, strong viral promoters, such as the CMV immediate early promoter, the SV40 early promoter, or the Rous Sarcoma Virus LTR promoter, are used. These promoters drive expression at high levels in most tissues and express rather ubiquitously in terms of species and cell types. Indeed, all have been shown to be highly active in all forms of muscle. More importantly, all of these promoters are easily accessible in a variety of commercially available expression plasmids. However, they do have certain drawbacks. In all cases, these promoters are sensitive to inflammatory responses and are regulated by NF-kB (38, 45). Thus, if an inflammatory response is mounted during the course of gene expression in a desired system, levels of expression may increase in response to increased NF-kB activation. While this may not be detrimental to some experiments, it could greatly confound experiments in which an assumption is made that the transferred transgene is uniformly and constitutively expressed. Another drawback to these universal promoters is that they do express in all cell types. Thus, if the transgene-expressing plasmid is transferred to endothelial, smooth muscle, and adventitial cells in the vasculature, the gene will be expressed in all layers of the tissue.
As an alternative to these strong viral promoters, ubiquitously active cellular promoters are often used to drive gene expression. The β actin promoter has been used extensively in a variety of tissues, such as the heart, and gives levels of expression that are at the same level as that seen with the CMV promoter (105). The elongation factor 1α (EF-1α) promoter has also been used effectively to drive gene expression in heart and other tissues (103). Other ubiquitously active endogenous promoters include the GAPDH, HSP70, and Ubiquitin C promoters. The major advantage to these promoters is that they are usually not subject to rapid silencing as are the viral promoters (see below), and can express genes for a much longer time.
One of the problems with many of these “strong” viral promoters is that they often express for only a limited duration of time in many tissues. For example, the CMV immediate early promoter gives a rapid burst of gene expression that usually lasts for less than a week in vivo, despite the fact that the plasmids can still be detected in the tissues. This has been seen in both smooth muscle in the vasculature and airways as well as in cardiomyocytes and other tissues (23, 98, 131). Similar findings have been reported for the SV40 early and EF-1α promoters in heart (103). However, it should be stressed that this is not the case in skeletal muscle, where gene expression from the CMV promoter (and many other promoters) persists for quite a long time. Indeed, it has even been shown that gene expression can be detected out to 19 months following intramuscular injection in mice (126). There are a number of potential explanations for the limited duration of expression in these and other tissues, including promoter inactivation (49), DNA methylation (50), altered plasmid chromatin structure, inflammation caused by CpG motifs in the DNA (130, 136), and immune responses to the transgene product (49, 61).
Several approaches have been taken to increase the duration of gene expression in vivo (41, 128, 129). The most straight-forward approach has been to evaluate alternative promoters for long-term gene expression. One of the most durable promoters identified to date has been that of the Ubiquitin C gene (41, 129). This promoter can drive gene expression at levels roughly equal to that seen with the CMV promoter, but expression persists for up to 6 months in a number of tissues, including vascular and airway smooth muscle (41, 129)(Dean, unpublished). Thus, using the appropriate promoter, either short or long term gene expression can be achieved in vivo.
Apart from wanting high level and either short or long-term gene expression, many times it is crucial to have gene expression restricted to a certain cell type within a tissue. One way to restrict gene expression to a desired cell type is to use a promoter that is active only in that cell type. Such cell-specific promoters abound. For example, in smooth muscle, the promoters for smooth muscle alpha actin, SM22α, and smooth muscle myosin heavy chain have all been used to drive high level expression that is cell-restricted. Other promoters may be generally restricted to all types of muscle in general, such as that from the gene for desmin (65). Synthetic promoters have also been developed that work in specific cell types, such as the synthetic C5-12 promoter that was constructed by randomly combining skeletal muscle-specific transcription factor consensus binding sites to form a promoter that acts uniquely in skeletal muscle (67). Similar promoters have been constructed for smooth muscle-specific expression (56, 100).
A second method for restricting gene expression to specific cell types relies on the fact that the nuclear import of plasmids in non-dividing cells is sequence specific (22). Our lab and others have demonstrated that plasmids containing certain sequences can enter the nucleus in a non-dividing cell in the absence of mitosis, whereas plasmids lacking such sequences remain in the cytoplasm, fail to express their genes, and are degraded (64, 131). Vacik and colleagues identified such a DNA sequence that mediates DNA nuclear import uniquely in non-dividing smooth muscle cells (119). When carried on a plasmid that is transfected into the cell, the smooth muscle gamma actin (SMGA) promoter binds to several smooth muscle cell-specific transcription factors in the cytoplasm (which is where all transcription factors and other proteins are synthesized) to form a DNA-protein complex. Since these proteins are destined for the nucleus, they contain specific nuclear localization signals for their nuclear import. As a result of the proteins binding to the SMGA promoter on the plasmid, the plasmid becomes covered with nuclear localization signals for nuclear entry. Because the transcription factors are expressed only in smooth muscle cells, the import complexes only form in these cells. Universally active DNA nuclear import sequences have been shown to act in vivo to increase gene transfer in all cell types, including both skeletal muscle and smooth muscle (9, 66, 131), whereas the SMGA sequences act specifically to restrict DNA nuclear import (and subsequent expression) to smooth muscle cells in the vasculature and airways (J. L. Young, W. E. Zimmer, and D. A. Dean, manuscript in preparation; T. Kuzniar and D. A. Dean, unpublished).
Another aspect of regulation is the ability to turn gene expression on and off at will. A number of systems have been developed to do just this, but the three most developed are the “tet on/off”, Geneswitch, and ecdysone-regulated systems. In all cases, a combination of ligand-binding, synthetic inducer/repressor proteins and promoter control regions are used to regulate transgene expression in a controllable fashion. The most used system is based on variations of the tetracycline resistance operon from bacteria and is called the “Tet on/off” system (43). The gene of interest whose level is to be controlled is placed behind a basal promoter that also contains binding sites for the tetracycline repressor or inducer (the Tet response element, or TRE). One of two fusion proteins, both of which bind doxycycline as an inducer, is then introduced into the cell, typically encoded for either in the genome (i.e., in a transgenic mouse) or on a second plasmid. The first fusion protein, the tetracycline transactivator, binds to the TRE and activates transcription of the gene of interest in the absence of doxycycline. Thus, addition of doxycycline renders the Tet activator unable to bind to the TRE and expression is turned off (Tet-OFF). The second fusion protein that can be used is the reverse tetracycline transactivator, which only binds to the TRE in the presence of doxycycline. In this case, in the absence of drug, expression is off, but addition of drug causes gene expression to be induced (Tet-ON). One advantage of this system is that expression can be controlled in a graded manner; the more doxycycline that is administered, the greater the level of induction or suppression. However, doxycycline is also a potent antibiotic and can result in side effects in animals.
The second system is very similar but is based on a protein/DNA sequence from drosophila. The ecdysone inducible system again consists of an fusion protein, in this case the transactivation domain of the glucocorticoid receptor is fused to the ecdysone-binding nuclear receptor protein, and an ecdysone response element that is placed upstream of a minimal promoter driving expression of the desired gene product. Upon addition of ecdysone, an insect hormone with no mammalian homologues, the fusion protein dimerizes, binds to the response element, and induces expression up to 1000-fold (91). One advantage to this system is that the synthetic receptor protein and response element bind to no other mammalian hormones or transcription factors, respectively, resulting in very low background expression in the absence of drug. Thus, expression can be very tightly controlled.
Finally, the Geneswitch uses a mifepristone-binding, progesterone receptor fused to the DNA binding domain of the yeast GAL4 protein, and the transactivation domain from the NF-kB p65 subunit (12). This fusion protein in turn binds to a GAL4 upstream activating sequence placed upstream of a minimal promoter to regulate gene expression. When the hormone is added, gene expression is turned on leading to up to a 50,000-fold induction of transgene (12, 123). When the drug is removed, expression returns to baseline within 5 days in vivo. This system has been used effectively to turn expression on and off repeatedly in the same animals, with no loss of induction with subsequent drug administrations.
Approaches to down-regulate gene expression
Almost all of the previous discussion has focused on in vivo gene delivery to muscle cells for the purpose of overexpressing a gene product. However, this is only half the story for the utility of gene transfer to study cell physiology on the whole animal level. Many techniques have been developed to downregulate gene expression in cells instead of increase it. These include anti-sense RNA, DNAzymes, ribozymes, siRNA, decoy oligonucleotides, and dominant negative mutants, as well as transgenic and knock-out mice. Several excellent recent reviews have been published on these approaches (15, 107), so I will focus on only two that have been used in smooth muscle: DNAzymes and dominant negative mutants.
DNAzymes are catalytic oligonucleotides that selectively bind to an RNA substrate by Watson-Crick base pairing and cleave phosphodiester bonds, resulting in decreased target mRNA levels and subsequent protein production (57, 132). These reagents have been used effectively to downregulate a number of genes in cultured cells, and more recently in animal models. Several studies have targeted early growth response factor 1 (EGR-1) in the vasculature to reduce intimal hyperplasia in injured vessels using liposomes (16, 17, 102). Although the DNAzymes effectively reduced EGR-1 mRNA and protein levels in the intima and elicited a therapeutic effect, again, as seen for liposomal delivery of DNA, transfer of the nucleic acid to the smooth muscle required endothelial cell injury.
Our lab has used electroporation to deliver DNAzymes to vascular smooth muscle in uninjured, intact vessels in the rat (92). In this study, we targeted the PKCε gene, due to its implicated role in regulating agonist-induced vascular smooth muscle contraction (13, 80). Using a spoon-like electrode designed for plasmid transfer to the vasculature, 31-mer single stranded DNAzymes were transferred to the mesenteric vessels of rats in a field strength and dose-dependent manner. As for plasmids, optimal DNAzyme delivery (as determined by DNAzyme-mediated reduction of PKCε mRNA and protein levels) was achieved using eight 10 msec pulses at 200 V/cm and a dose of 100 μM oligonucleotide. Under these conditions, a 60% reduction in PKCε mRNA and protein were detected in smooth muscle cells in vivo. As for plasmid delivery, the advantage to this approach is that electroporation mediated delivery to smooth muscle without a requirement for endothelial cell damage. Thus, by choosing the desired gene product to be targeted, DNAzymes can be designed, synthesized, and delivered to tissues relatively easily to target various muscle types in vivo and effectively down-regulate endogenous gene expression.
A second approach for reducing gene activity in vivo is through the use of dominant negative mutants. Dominant negative mutants are proteins that are not only catalytically inactive themselves, but also have the ability to inactivate wild type versions of themselves in the cell. The easiest way to think about this is in terms of a protein that normally dimerizes in order to function. If a large amount in inactive monomer is added to the system, the active monomers are titrated out so that any dimers that form are either between two inactive mutants or an active monomer and an inactive mutant; in either case, the resulting dimers would be inactive. In one study, a dominant-negative PKCε mutant gene was used in the vasculature to study vasoconstriction in a rat model (110), based on studies suggesting that PKCε may play a role in adrenoreceptor mediated contraction of mesenteric arteries (13, 80). To examine the role of PKCε in vasoconstriction, a dominant-negative PKCε (PKCε-KN)(40) was transferred to vessels by electroporation from the adventitial surface using a spoon-like electrode and two days later, vessels were excised and mounted on a myograph for functional studies of phenylephrine-induced vasoconstriction (110). Not only did transfer of the dominant negative PKCε mutant attenuate phenylephrine responses compared to control, non-electroporated vessels, but the level of attenuation was indistinguishable from that achieved using the pharmacologic isoform-nonspecific PKC inhibitor chelerythrine.
Conclusions
The ability to transfer genes to the various cell types within whole animals has already altered the way we think about the prevention and treatment of many diseases. It is our hope that researchers will grasp the immense power of such techniques not just to treat disease, but also to study the physiology of the healthy body. The ability to manipulate distinct gene products within a signaling or biosynthetic pathway, or to alter structural interactions within and between cells is extremely useful and is technologically possible today. Despite the challenges and limitations surrounding their transfection, skeletal, smooth, and cardiac muscle can be effectively transfected in vivo, as the experimental approaches touched upon illustrate.
Acknowledgments
This work was supported in part by grants from the Sandler Program for Asthma Research and NIH grants HL59956 and HL71643. I thank members of my laboratory for helpful discussions and critical reading of this manuscript and Dr. Rui Zhou for providing unpublished images of GFP-expressing murine skeletal muscles.
References
- 1.Acsadi G, Jiao SS, Jani A, Duke D, Williams P, Chong W, Wolff JA. Direct gene transfer and expression into rat heart in vivo. New Biol. 1991;3:71–81. [PubMed] [Google Scholar]
- 2.Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol. 1998;16:867–870. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- 3.Andre F, Mir LM. DNA electrotransfer: its principles and an updated review of its therapeutic applications. Gene Ther. 2004;11 (Suppl 1):S33–42. doi: 10.1038/sj.gt.3302367. [DOI] [PubMed] [Google Scholar]
- 4.Aoki M, Morishita R, Higaki J, Moriguchi A, Kida I, Hayashi S, Matsushita H, Kaneda Y, Ogihara T. In vivo transfer efficiency of antisense oligonucleotides into the myocardium using HVJ-liposome method. Biochem Biophys Res Commun. 1997;231:540–545. doi: 10.1006/bbrc.1996.5762. [DOI] [PubMed] [Google Scholar]
- 5.Armeanu S, Pelisek J, Krausz E, Fuchs A, Groth D, Curth R, Keil O, Quilici J, Rolland PH, Reszka R, Nikol S. Optimization of nonviral gene transfer of vascular smooth muscle cells in vitro and in vivo. Mol Ther. 2000;1:366–375. doi: 10.1006/mthe.2000.0053. [DOI] [PubMed] [Google Scholar]
- 6.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Short protocols in molecular biology. 4. New York: John Wiley & Sons; 1999. [Google Scholar]
- 7.Babiuk S, Baca-Estrada ME, Foldvari M, Middleton DM, Rabussay D, Widera G, Babiuk LA. Increased gene expression and inflammatory cell infiltration caused by electroporation are both important for improving the efficacy of DNA vaccines. J Biotechnol. 2004;110:1–10. doi: 10.1016/j.jbiotec.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Bertrand A, Ngo-Muller V, Hentzen D, Concordet JP, Daegelen D, Tuil D. Muscle electrotransfer as a tool for studying muscle fiber-specific and nerve-dependent activity of promoters. Am J Physiol Cell Physiol. 2003;285:C1071–1081. doi: 10.1152/ajpcell.00104.2003. [DOI] [PubMed] [Google Scholar]
- 9.Blomberg P, Eskandarpour M, Xia S, Sylven C, Islam KB. Electroporation in combination with a plasmid vector containing SV40 enhancer elements results in increased and persistent gene expression in mouse muscle. Biochem Biophys Res Commun. 2002;298:505–510. doi: 10.1016/s0006-291x(02)02486-5. [DOI] [PubMed] [Google Scholar]
- 10.Bolz SS, Pohl U. Highly effective non-viral gene transfer into vascular smooth muscle cells of cultured resistance arteries demonstrated by genetic inhibition of sphingosine-1-phosphate-induced vasoconstriction. J Vasc Res. 2003;40:399–405. doi: 10.1159/000072830. [DOI] [PubMed] [Google Scholar]
- 11.Budker V, Zhang G, Danko I, Williams P, Wolff J. The efficient expression of intravascularly delivered DNA in rat muscle. Gene Ther. 1998;5:272–276. doi: 10.1038/sj.gt.3300572. [DOI] [PubMed] [Google Scholar]
- 12.Burcin MM, Schiedner G, Kochanek S, Tsai SY, O’Malley BW. Adenovirus-mediated regulable target gene expression in vivo. Proc Natl Acad Sci U S A. 1999;96:355–360. doi: 10.1073/pnas.96.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buus CL, Aalkjaer C, Nilsson H, Juul B, Moller JV, Mulvany MJ. Mechanisms of Ca2+ sensitization of force production by noradrenaline in rat mesenteric small arteries. J Physiol. 1998;510:577–590. doi: 10.1111/j.1469-7793.1998.577bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campeau P, Chapdelaine P, Seigneurin-Venin S, Massie B, Tremblay JP. Transfection of large plasmids in primary human myoblasts. Gene Ther. 2001;8:1387–1394. doi: 10.1038/sj.gt.3301532. [DOI] [PubMed] [Google Scholar]
- 15.Caplen NJ. Gene therapy progress and prospects. Downregulating gene expression: the impact of RNA interference. Gene Ther. 2004;11:1241–1248. doi: 10.1038/sj.gt.3302324. [DOI] [PubMed] [Google Scholar]
- 16.Cassidy KJ, Bull JL, Glucksberg MR, Dawson CA, Haworth ST, Hirschl R, Gavriely N, Grotberg JB. A rat lung model of instilled liquid transport in the pulmonary airways. J Appl Physiol. 2001;90:1955–1967. doi: 10.1152/jappl.2001.90.5.1955. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol. 2002;34:1345. doi: 10.1006/jmcc.2002.2086. [DOI] [PubMed] [Google Scholar]
- 18.Chen S, Shohet RV, Bekeredjian R, Frenkel P, Grayburn PA. Optimization of ultrasound parameters for cardiac gene delivery of adenoviral or plasmid deoxyribonucleic acid by ultrasound-targeted microbubble destruction. J Am Coll Cardiol. 2003;42:301–308. doi: 10.1016/s0735-1097(03)00627-2. [DOI] [PubMed] [Google Scholar]
- 19.Danialou G, Comtois AS, Matecki S, Nalbantoglu J, Karpati G, Gilbert R, Geoffroy P, Gilligan S, Tanguay JF, Petrof BJ. Optimization of regional intraarterial naked DNA-mediated transgene delivery to skeletal muscles in a large animal model. Mol Ther. 2005;11:257–266. doi: 10.1016/j.ymthe.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Danko I, Fritz JD, Jiao S, Hogan K, Lattendresse JS, Wolff JA. Pharmacological enhancement of in vivo gene expression in muscle. Gene Ther. 1994;1:114–121. [PubMed] [Google Scholar]
- 21.Dean DA. Electroporation of the vasculature and the lung. DNA Cell Biol. 2003;22:797–806. doi: 10.1089/104454903322625000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dean DA. Intracellular trafficking of nucleic acids. Expert Opin Drug Deliv. 2004;1:127–140. doi: 10.1517/17425247.1.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL. Electroporation as a method for high-level non-viral gene transfer to the lung. Gene Ther. 2003;10:1608–1615. doi: 10.1038/sj.gt.3302053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeBruyne LA, Li K, Chan SY, Qin L, Bishop DK, Bromberg JS. Lipid-mediated gene transfer of viral IL-10 prolongs vascularized cardiac allograft survival by inhibiting donor-specific cellular and humoral immune responses. Gene Ther. 1998;5:1079–1087. doi: 10.1038/sj.gt.3300694. [DOI] [PubMed] [Google Scholar]
- 25.Dev NB, Hofmann GA, Dev SB, Rabussay DP. Intravascular electroporation markedly attenuates neointima formation after balloon injury of the carotid artery in the rat. J Interventional Cardiol. 2000;13:331–338. [Google Scholar]
- 26.Dev NB, Preminger TJ, Hofmann GA, Dev SB. Sustained local delivery of heparin to the rabbit arterial wall with an electroporation catheter. Cathet Cardiovasc Diagn. 1998;45:337–345. doi: 10.1002/(sici)1097-0304(199811)45:3<337::aid-ccd28>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 27.Dileo J, Miller TE, Jr, Chesnoy S, Huang L. Gene transfer to subdermal tissues via a new gene gun design. Hum Gene Ther. 2003;14:79–87. doi: 10.1089/10430340360464732. [DOI] [PubMed] [Google Scholar]
- 28.Doh SG, Vahlsing HL, Hartikka J, Liang X, Manthorpe M. Spatial-temporal patterns of gene expression in mouse skeletal muscle after injection of lacZ plasmid DNA. Gene Ther. 1997;4:648–663. doi: 10.1038/sj.gt.3300460. [DOI] [PubMed] [Google Scholar]
- 29.Dona M, Sandri M, Rossini K, Dell’Aica I, Podhorska-Okolow M, Carraro U. Functional in vivo gene transfer into the myofibers of adult skeletal muscle. Biochem Biophys Res Commun. 2003;312:1132–1138. doi: 10.1016/j.bbrc.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 30.Draghia-Akli R, Ellis KM, Hill LA, Malone PB, Fiorotto ML. High-efficiency growth hormone-releasing hormone plasmid vector administration into skeletal muscle mediated by electroporation in pigs. Faseb J. 2003;17:526–528. doi: 10.1096/fj.02-0671fje. [DOI] [PubMed] [Google Scholar]
- 31.Draghia-Akli R, Fiorotto ML, Hill LA, Malone PB, Deaver DR, Schwartz RJ. Myogenic expression of an injectable protease-resistant growth hormone-releasing hormone augments long-term growth in pigs. Nat Biotechnol. 1999;17:1179–1183. doi: 10.1038/70718. [DOI] [PubMed] [Google Scholar]
- 32.Ehsan A, Mann MJ, Dell’Acqua G, Dzau VJ. Long-term stabilization of vein graft wall architecture and prolonged resistance to experimental atherosclerosis after E2F decoy oligonucleotide gene therapy. J Thorac Cardiovasc Surg. 2001;121:714–722. doi: 10.1067/mtc.2001.111204. [DOI] [PubMed] [Google Scholar]
- 33.Ellison KE, Bishopric NH, Webster KA, Morishita R, Gibbons GH, Kaneda Y, Sato B, Dzau VJ. Fusigenic liposome-mediated DNA transfer into cardiac myocytes. J Mol Cell Cardiol. 1996;28:1385–1399. doi: 10.1006/jmcc.1996.0130. [DOI] [PubMed] [Google Scholar]
- 34.Elmadbouh I, Rossignol P, Meilhac O, Vranckx R, Pichon C, Pouzet B, Midoux P, Michel JB. Optimization of in vitro vascular cell transfection with non-viral vectors for in vivo applications. J Gene Med. 2004;6:1112–1124. doi: 10.1002/jgm.604. [DOI] [PubMed] [Google Scholar]
- 35.Factor P, Dumasius V, Saldias F, Sznajder JI. Adenoviral-mediated overexpression of the NA,K-ATPase beta1 subunit gene increases lung edema clearance and improves survival during acute hyperoxic lung injury in rats. Chest. 1999;116:24S–25S. doi: 10.1378/chest.116.suppl_1.24s. [DOI] [PubMed] [Google Scholar]
- 36.Ferrer A, Foster H, Wells KE, Dickson G, Wells DJ. Long-term expression of full-length human dystrophin in transgenic mdx mice expressing internally deleted human dystrophins. Gene Ther. 2004;11:884–893. doi: 10.1038/sj.gt.3302242. [DOI] [PubMed] [Google Scholar]
- 37.Fewell JG, MacLaughlin F, Mehta V, Gondo M, Nicol F, Wilson E, Smith LC. Gene therapy for the treatment of hemophilia B using PINC-formulated plasmid delivered to muscle with electroporation. Mol Ther. 2001;3:574–583. doi: 10.1006/mthe.2001.0295. [DOI] [PubMed] [Google Scholar]
- 38.Fields BN, Knipe DM, Howley PM, Chanock RM, Melnick JL, Monath TP, Roizman B, Staus SE. Virology. New York: Raven Press; 1996. [Google Scholar]
- 39.Gehl J, Skovsgaard T, Mir LM. Vascular reactions to in vivo electroporation: characterization and consequences for drug and gene delivery. Biochim Biophys Acta. 2002;1569:51–58. doi: 10.1016/s0304-4165(01)00233-1. [DOI] [PubMed] [Google Scholar]
- 40.Genot EM, Parker PJ, Cantrell DA. Analysis of the role of protein kinase C-alpha, -epsilon, and -zeta in T cell activation. J Biol Chem. 1995;270:9833–9839. doi: 10.1074/jbc.270.17.9833. [DOI] [PubMed] [Google Scholar]
- 41.Gill DR, Smyth SE, Goddard CA, Pringle IA, Higgins CF, Colledge WH, Hyde SC. Increased persistence of lung gene expression using plasmids containing the ubiquitin C or elongation factor 1alpha promoter. Gene Ther. 2001;8:1539–1546. doi: 10.1038/sj.gt.3301561. [DOI] [PubMed] [Google Scholar]
- 42.Gollins H, McMahon J, Wells KE, Wells DJ. High-efficiency plasmid gene transfer into dystrophic muscle. Gene Ther. 2003;10:504–512. doi: 10.1038/sj.gt.3301927. [DOI] [PubMed] [Google Scholar]
- 43.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harada M, Toki Y, Numaguchi Y, Osanai H, Ito T, Okumura K, Hayakawa T. Prostacyclin synthase gene transfer inhibits neointimal formation in rat balloon-injured arteries without bleeding complications. Cardiovasc Res. 1999;43:481–491. doi: 10.1016/s0008-6363(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 45.Harms JS, Splitter GA. Interferon-gamma inhibits transgene expression driven by SV40 or CMV promoters but augments expression driven by the mammalian MHC I promoter. Hum Gene Ther. 1995;6:1291–1297. doi: 10.1089/hum.1995.6.10-1291. [DOI] [PubMed] [Google Scholar]
- 46.Harrison RL, Byrne BJ, Tung L. Electroporation-mediated gene transfer in cardiac tissue. FEBS Lett. 1998;435:1–5. doi: 10.1016/s0014-5793(98)00987-9. [DOI] [PubMed] [Google Scholar]
- 47.Hartikka J, Sawdey M, Cornefert-Jensen F, Margalith M, Barnhart K, Nolasco M, Vahlsing HL, Meek J, Marquet M, Hobart P, Norman J, Manthorpe M. An improved plasmid DNA expression vector for direct injection into skeletal muscle. Hum Gene Ther. 1996;7:1205–1217. doi: 10.1089/hum.1996.7.10-1205. [DOI] [PubMed] [Google Scholar]
- 48.Helbling-Leclerc A, Scherman D, Wils P. Cellular uptake of cationic lipid/DNA complexes by cultured myoblasts and myotubes. Biochim Biophys Acta. 1999;1418:165–175. doi: 10.1016/s0005-2736(99)00012-7. [DOI] [PubMed] [Google Scholar]
- 49.Herweijer H, Zhang G, Subbotin VM, Budker V, Williams P, Wolff JA. Time course of gene expression after plasmid DNA gene transfer to the liver. J Gene Med. 2001;3:280–291. doi: 10.1002/jgm.178. [DOI] [PubMed] [Google Scholar]
- 50.Hong K, Sherley J, Lauffenburger DA. Methylation of episomal plasmids as a barrier to transient gene expression via a synthetic delivery vector. Biomol Eng. 2001;18:185–192. doi: 10.1016/s1389-0344(01)00100-9. [DOI] [PubMed] [Google Scholar]
- 51.Hoshina K, Koyama H, Miyata T, Shigematsu H, Takato T, Dalman RL, Nagawa H. Aortic wall cell proliferation via basic fibroblast growth factor gene transfer limits progression of experimental abdominal aortic aneurysm. J Vasc Surg. 2004;40:512–518. doi: 10.1016/j.jvs.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 52.Huber PE, Mann MJ, Melo LG, Ehsan A, Kong D, Zhang L, Rezvani M, Peschke P, Jolesz F, Dzau VJ, Hynynen K. Focused ultrasound (HIFU) induces localized enhancement of reporter gene expression in rabbit carotid artery. Gene Ther. 2003;10:1600–1607. doi: 10.1038/sj.gt.3302045. [DOI] [PubMed] [Google Scholar]
- 53.Iwashita H, Yoshida M, Nishi T, Otani M, Ueda S. In vivo transfer of a neuronal nitric oxide synthase expression vector into the rat bladder by electroporation. BJU Int. 2004;93:1098–1103. doi: 10.1111/j.1464-410X.2003.04788.x. [DOI] [PubMed] [Google Scholar]
- 54.Kawabata K, Takakura Y, Hashida M. The fate of plasmid DNA after intravenous injection in mice: involvement of scavenger receptors in its hepatic uptake. Pharm Res. 1995;12:825–830. doi: 10.1023/a:1016248701505. [DOI] [PubMed] [Google Scholar]
- 55.Keogh MC, Chen D, Lupu F, Shaper N, Schmitt JF, Kakkar VV, Lemoine NR. High efficiency reporter gene transfection of vascular tissue in vitro and in vivo using a cationic lipid-DNA complex. Gene Ther. 1997;4:162–171. doi: 10.1038/sj.gt.3300374. [DOI] [PubMed] [Google Scholar]
- 56.Keogh MC, Chen D, Schmitt JF, Dennehy U, Kakkar VV, Lemoine NR. Design of a muscle cell-specific expression vector utilising human vascular smooth muscle alpha-actin regulatory elements [In Process Citation] Gene Ther. 1999;6:616–628. doi: 10.1038/sj.gt.3300866. [DOI] [PubMed] [Google Scholar]
- 57.Khachigian LM. Catalytic DNAs as potential therapeutic agents and sequence-specific molecular tools to dissect biological function. J Clin Invest. 2000;106:1189–1195. doi: 10.1172/JCI11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitsis RN, Buttrick PM, McNally EM, Kaplan ML, Leinwand LA. Hormonal modulation of a gene injected into rat heart in vivo. Proc Natl Acad Sci U S A. 1991;88:4138–4142. doi: 10.1073/pnas.88.10.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klugherz BD, Jones PL, Cui X, Chen W, Meneveau NF, DeFelice S, Connolly J, Wilensky RL, Levy RJ. Gene delivery from a DNA controlled-release stent in porcine coronary arteries. Nat Biotechnol. 2000;18:1181–1184. doi: 10.1038/81176. [DOI] [PubMed] [Google Scholar]
- 60.Kondo I, Ohmori K, Oshita A, Takeuchi H, Fuke S, Shinomiya K, Noma T, Namba T, Kohno M. Treatment of acute myocardial infarction by hepatocyte growth factor gene transfer: the first demonstration of myocardial transfer of a “functional” gene using ultrasonic microbubble destruction. J Am Coll Cardiol. 2004;44:644–653. doi: 10.1016/j.jacc.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 61.Latta-Mahieu M, Rolland M, Caillet C, Wang M, Kennel P, Mahfouz I, Loquet I, Dedieu JF, Mahfoudi A, Trannoy E, Thuillier V. Gene transfer of a chimeric trans-activator is immunogenic and results in short-lived transgene expression. Hum Gene Ther. 2002;13:1611–1620. doi: 10.1089/10430340260201707. [DOI] [PubMed] [Google Scholar]
- 62.Lauritzen HP, Reynet C, Schjerling P, Ralston E, Thomas S, Galbo H, Ploug T. Gene gun bombardment-mediated expression and translocation of EGFP-tagged GLUT4 in skeletal muscle fibres in vivo. Pflugers Arch. 2002;444:710–721. doi: 10.1007/s00424-002-0862-5. [DOI] [PubMed] [Google Scholar]
- 63.Lawrie A, Brisken AF, Francis SE, Cumberland DC, Crossman DC, Newman CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene Ther. 2000;7:2023–2027. doi: 10.1038/sj.gt.3301339. [DOI] [PubMed] [Google Scholar]
- 64.Lechardeur D, Sohn K-J, Haardt M, Joshi PB, Monck M, Graham RW, Beatty B, Squire J, O’Brodovich H, Lukacs GL. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene Ther. 1999;6:482–497. doi: 10.1038/sj.gt.3300867. [DOI] [PubMed] [Google Scholar]
- 65.Li H, Capetanaki Y. An E box in the desmin promoter cooperates with the E box and MEF-2 sites of a distal enhancer to direct muscle-specific transcription. Embo J. 1994;13:3580–3589. doi: 10.1002/j.1460-2075.1994.tb06665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li S, MacLaughlin FC, Fewell JG, Gondo M, Wang J, Nicol F, Dean DA, Smith LC. Muscle-specific enhancement of gene expression by incorporation of the SV40 enhancer in the expression plasmid. Gene Therapy. 2001;8:494–497. doi: 10.1038/sj.gt.3301419. [DOI] [PubMed] [Google Scholar]
- 67.Li X, Eastman EM, Schwartz RJ, Draghia-Akli R. Synthetic muscle promoters: activities exceeding naturally occurring regulatory sequences. Nat Biotechnol. 1999;17:241–245. doi: 10.1038/6981. [DOI] [PubMed] [Google Scholar]
- 68.Liang KW, Hoffman EP, Huang L. Targeted delivery of plasmid DNA to myogenic cells via transferrin-conjugated peptide nucleic acid. Mol Ther. 2000;1:236–243. doi: 10.1006/mthe.2000.0043. [DOI] [PubMed] [Google Scholar]
- 69.Liu F, Nishikawa M, Clemens PR, Huang L. Transfer of full-length Dmd to the diaphragm muscle of Dmd(mdx/mdx) mice through systemic administration of plasmid DNA. Mol Ther. 2001;4:45–51. doi: 10.1006/mthe.2001.0419. [DOI] [PubMed] [Google Scholar]
- 70.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 71.Liu MA, McClements W, Ulmer JB, Shiver J, Donnelly J. Immunization of non-human primates with DNA vaccines. Vaccine. 1997;15:909–912. doi: 10.1016/s0264-410x(96)00280-0. [DOI] [PubMed] [Google Scholar]
- 72.Lu QL, Bou-Gharios G, Partridge TA. Non-viral gene delivery in skeletal muscle: a protein factory. Gene Ther. 2003;10:131–142. doi: 10.1038/sj.gt.3301874. [DOI] [PubMed] [Google Scholar]
- 73.Lu QL, Liang HD, Partridge T, Blomley MJ. Microbubble ultrasound improves the efficiency of gene transduction in skeletal muscle in vivo with reduced tissue damage. Gene Ther. 2003;10:396–405. doi: 10.1038/sj.gt.3301913. [DOI] [PubMed] [Google Scholar]
- 74.Ma X, Glover C, Miller H, Goldstein J, O’Brien E. Focal arterial transgene expression after local gene delivery. Can J Cardiol. 2001;17:873–883. [PubMed] [Google Scholar]
- 75.Machado-Aranda D, Adir Y, Young JL, Briva A, Budinger GRS, Yeldandi A, Sznajder JI, Dean DA. Gene transfer of the Na+,K+-ATPase β1 subunit using electroporation increases lung liquid clearance in rats. Am J Respir Crit Care Med. 2005;171:204–211. doi: 10.1164/rccm.200403-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Machado-Aranda D, Adir Y, Young JL, Budinger GRS, Yeldandi A, Sznajder JI, Dean DA. Gene transfer of the Na+,K+-ATPase β1 subunit using electroporation increases lung liquid clearance in rats. Am J Respir Crit Care Med. 2004 doi: 10.1164/rccm.200403-313OC. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mann MJ, Gibbons GH, Hutchinson H, Poston RS, Hoyt EG, Robbins RC, Dzau VJ. Pressure-mediated oligonucleotide transfection of rat and human cardiovascular tissues. Proc Natl Acad Sci U S A. 1999;96:6411–6416. doi: 10.1073/pnas.96.11.6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mann MJ, Whittemore AD, Donaldson MC, Belkin M, Conte MS, Polak JF, Orav EJ, Ehsan A, Dell’Acqua G, Dzau VJ. Ex vivo gene therapy of human vascular bypass grafts with E2F decoy: the PREVENT single-centre, randomised, controlled trial. Lancet. 1999;354:1493–1498. doi: 10.1016/S0140-6736(99)09405-2. [DOI] [PubMed] [Google Scholar]
- 79.Martin JB, Young JL, Benoit JN, Dean DA. Gene transfer to intact mesenteric arteries by electroporation. J Vasc Res. 2000;37:372–380. doi: 10.1159/000025753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Masuo M, Reardon S, Ikebe M, Kitazawa T. A novel mechanism for the Ca(2+)-sensitizing effect of protein kinase C on vascular smooth muscle: inhibition of myosin light chain phosphatase. J Gen Physiol. 1994;104:265–286. doi: 10.1085/jgp.104.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mathiesen I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Therapy. 1999;6:508–514. doi: 10.1038/sj.gt.3300847. [DOI] [PubMed] [Google Scholar]
- 82.Matsumoto T, Komori K, Shoji T, Kuma S, Kume M, Yamaoka T, Mori E, Furuyama T, Yonemitsu Y, Sugimachi K. Successful and optimized in vivo gene transfer to rabbit carotid artery mediated by electronic pulse. Gene Ther. 2001;8:1174–1179. doi: 10.1038/sj.gt.3301502. [DOI] [PubMed] [Google Scholar]
- 83.McLean J, Thurston G, McDonald D. Sites of Uptake and Expression of Cationic Liposome DNA Complexes Injected Intravenously. In: Huang L, Hung M-C, Wagner E, editors. Nonviral vectors for Gene Therapy. San Diego: Academic Press; 1999. pp. 120–139. [Google Scholar]
- 84.McMahon JM, Signori E, Wells KE, Fazio VM, Wells DJ. Optimisation of electrotransfer of plasmid into skeletal muscle by pretreatment with hyaluronidase -- increased expression with reduced muscle damage. Gene Ther. 2001;8:1264–1270. doi: 10.1038/sj.gt.3301522. [DOI] [PubMed] [Google Scholar]
- 85.Mennuni C, Calvaruso F, Zampaglione I, Rizzuto G, Rinaudo D, Dammassa E, Ciliberto G, Fattori E, La Monica N. Hyaluronidase increases electrogene transfer efficiency in skeletal muscle. Hum Gene Ther. 2002;13:355–365. doi: 10.1089/10430340252792495. [DOI] [PubMed] [Google Scholar]
- 86.Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud J-M, Delaere P, Branellec D, Schwartz B, Scherman D. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci USA. 1999;96:4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mir LM, Bureau MF, Rangara R, Schwartz B, Scherman D. Long-term, high level in vivo gene expression after electric pulse-mediated gene transfer into skeletal muscle. C R Acad Sci III. 1998;321:893–899. doi: 10.1016/s0764-4469(99)80003-1. [DOI] [PubMed] [Google Scholar]
- 88.Molnar MJ, Gilbert R, Lu Y, Liu AB, Guo A, Larochelle N, Orlopp K, Lochmuller H, Petrof BJ, Nalbantoglu J, Karpati G. Factors influencing the efficacy, longevity, and safety of electroporation-assisted plasmid-based gene transfer into mouse muscles. Mol Ther. 2004;10:447–455. doi: 10.1016/j.ymthe.2004.06.642. [DOI] [PubMed] [Google Scholar]
- 89.Morishita R, Gibbons GH, Ellison KE, Nakajima M, Zhang L, Kaneda Y, Ogihara T, Dzau VJ. Single intraluminal delivery of antisense cdc2 kinase and proliferating-cell nuclear antigen oligonucleotides result in chronic inhibition of neointimal hyperplasia. Proc Natl Acad Sci USA. 1993;90:8474–8478. doi: 10.1073/pnas.90.18.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murakami T, Nishi T, Kimura E, Goto T, Maeda Y, Ushio Y, Uchino M, Sunada Y. Full-length dystrophin cDNA transfer into skeletal muscle of adult mdx mice by electroporation. Muscle Nerve. 2003;27:237–241. doi: 10.1002/mus.10283. [DOI] [PubMed] [Google Scholar]
- 91.No D, Yao TP, Evans RM. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc Natl Acad Sci U S A. 1996;93:3346–3351. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nunamaker EA, Zhang HY, Shirasawa Y, Benoit JN, Dean DA. Electroporation mediated delivery of catalytic oligodeoxynucleotides for manipulation of vascular gene expression. Am J Physiol Heart Circ Physiol. 2003;285:H2240–2247. doi: 10.1152/ajpheart.00350.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Otten G, Schaefer M, Doe B, Liu H, Srivastava I, zur Megede J, O’Hagan D, Donnelly J, Widera G, Rabussay D, Lewis MG, Barnett S, Ulmer JB. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine. 2004;22:2489–2493. doi: 10.1016/j.vaccine.2003.11.073. [DOI] [PubMed] [Google Scholar]
- 94.Panetta CJ, Miyauchi K, Berry D, Simari RD, Holmes DR, Schwartz RS, Caplice NM. A tissue-engineered stent for cell-based vascular gene transfer. Hum Gene Ther. 2002;13:433–441. doi: 10.1089/10430340252792567. [DOI] [PubMed] [Google Scholar]
- 95.Park SW, Gwon HC, Jeong JO, Byun J, Kang HS, You JR, Cho SS, Lee MJ, Lee Y, Kim S, Kim DK. Intracardiac echocardiographic guidance and monitoring during percutaneous endomyocardial gene injection in porcine heart. Hum Gene Ther. 2001;12:893–903. doi: 10.1089/104303401750195863. [DOI] [PubMed] [Google Scholar]
- 96.Payen E, Bettan M, Rouyer-Fessard P, Beuzard Y, Scherman D. Improvement of mouse beta-thalassemia by electrotransfer of erythropoietin cDNA. Exp Hematol. 2001;29:295–300. doi: 10.1016/s0301-472x(00)00679-2. [DOI] [PubMed] [Google Scholar]
- 97.Perlstein I, Connolly JM, Cui X, Song C, Li Q, Jones PL, Lu Z, DeFelice S, Klugherz B, Wilensky R, Levy RJ. DNA delivery from an intravascular stent with a denatured collagen-polylactic-polyglycolic acid-controlled release coating: mechanisms of enhanced transfection. Gene Ther. 2003;10:1420–1428. doi: 10.1038/sj.gt.3302043. [DOI] [PubMed] [Google Scholar]
- 98.Pitard B, Pollard H, Agbulut O, Lambert O, Vilquin JT, Cherel Y, Abadie J, Samuel JL, Rigaud JL, Menoret S, Anegon I, Escande D. A nonionic amphiphile agent promotes gene delivery in vivo to skeletal and cardiac muscles. Hum Gene Ther. 2002;13:1767–1775. doi: 10.1089/104303402760293592. [DOI] [PubMed] [Google Scholar]
- 99.Potter H, Weir L, Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984;81:7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ribault S, Neuville P, Mechine-Neuville A, Auge F, Parlakian A, Gabbiani G, Paulin D, Calenda V. Chimeric smooth muscle-specific enhancer/promoters: valuable tools for adenovirus-mediated cardiovascular gene therapy. Circ Res. 2001;88:468–475. doi: 10.1161/01.res.88.5.468. [DOI] [PubMed] [Google Scholar]
- 101.Samakoglu S, Fattori E, Lamartina S, Toniatti C, Stockholm D, Heard JM, Bohl D. betaMinor-globin messenger RNA accumulation in reticulocytes governs improved erythropoiesis in beta thalassemic mice after erythropoietin complementary DNA electrotransfer in muscles. Blood. 2001;97:2213–2220. doi: 10.1182/blood.v97.8.2213. [DOI] [PubMed] [Google Scholar]
- 102.Santiago FS, Lowe HC, Kavurma MM, Chesterman CN, Baker A, Atkins DG, Khachigian LM. New DNA enzyme targeting Egr-1 mRNA inhibits vascular smooth muscle proliferation and regrowth after injury. Nat Med. 1999;5:1438. doi: 10.1038/71020. [DOI] [PubMed] [Google Scholar]
- 103.Sarkar N, Blomberg P, Wardell E, Eskandarpour M, Sylven C, Drvota V, Islam KB. Nonsurgical direct delivery of plasmid DNA into rat heart: time course, dose response, and the influence of different promoters on gene expression. J Cardiovasc Pharmacol. 2002;39:215–224. doi: 10.1097/00005344-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 104.Satkauskas S, Bureau MF, Puc M, Mahfoudi A, Scherman D, Miklavcic D, Mir LM. Mechanisms of in vivo DNA electrotransfer: respective contributions of cell electropermeabilization and DNA electrophoresis. Mol Ther. 2002;5:133–140. doi: 10.1006/mthe.2002.0526. [DOI] [PubMed] [Google Scholar]
- 105.Sawa Y, Kaneda Y, Bai HZ, Suzuki K, Fujimoto J, Morishita R, Matsuda H. Efficient transfer of oligonucleotides and plasmid DNA into the whole heart through the coronary artery. Gene Ther. 1998;5:1472–1480. doi: 10.1038/sj.gt.3300750. [DOI] [PubMed] [Google Scholar]
- 106.Schakman O, Gilson H, de Coninck V, Lause P, Verniers J, Havaux X, Ketelslegers JM, Thissen JP. Insulin-Like Growth Factor-I Gene Transfer by Electroporation Prevents Skeletal Muscle Atrophy in Glucocorticoid-Treated Rats. Endocrinology. 2005;146:1789–1797. doi: 10.1210/en.2004-1594. [DOI] [PubMed] [Google Scholar]
- 107.Scherer LJ, Rossi JJ. Approaches for the sequence-specific knockdown of mRNA. Nat Biotechnol. 2003;21:1457–1465. doi: 10.1038/nbt915. [DOI] [PubMed] [Google Scholar]
- 108.Sen L, Hong YS, Luo H, Cui G, Laks H. Efficiency, efficacy, and adverse effects of adenovirus vs. liposome-mediated gene therapy in cardiac allografts. Am J Physiol Heart Circ Physiol. 2001;281:H1433–1441. doi: 10.1152/ajpheart.2001.281.3.H1433. [DOI] [PubMed] [Google Scholar]
- 109.Shinmura K, Morishita R, Aoki M, Higaki J, Ogihara T, Kaneda Y, Tani M. Catheter-delivered in vivo gene transfer into rat myocardium using the fusigenic liposomal mediated method. Jpn Heart J. 2000;41:633–647. doi: 10.1536/jhj.41.633. [DOI] [PubMed] [Google Scholar]
- 110.Shirasawa Y, Rutland TJ, Young JL, Dean DA, Joseph BN. Modulation of protein kinase C (PKC)-mediated contraction and the possible role of PKcepsilon in rat mesenteric arteries. Front Biosci. 2003;8:a133–138. doi: 10.2741/1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Somiari S, Glasspool-Malone J, Drabick JJ, Gilbert RA, Heller R, Jaroszeski MJ, Malone RW. Theory and in vivo application of electroporative gene delivery. Mol Ther. 2000;2:178–187. doi: 10.1006/mthe.2000.0124. [DOI] [PubMed] [Google Scholar]
- 112.Suzuki J, Morishita R, Amano J, Kaneda Y, Isobe M. Decoy against nuclear factor-kappa B attenuates myocardial cell infiltration and arterial neointimal formation in murine cardiac allografts. Gene Ther. 2000;7:1847–1852. doi: 10.1038/sj.gt.3301316. [DOI] [PubMed] [Google Scholar]
- 113.Takahashi A, Palmer-Opolski M, Smith RC, Walsh K. Transgene delivery of plasmid DNA to smooth muscle cells and macrophages from a biostable polymer-coated stent. Gene Ther. 2003;10:1471–1478. doi: 10.1038/sj.gt.3302010. [DOI] [PubMed] [Google Scholar]
- 114.Takeshita S, Gal D, Leclerc G, Pickering JG, Riessen R, Weir L, Isner JM. Increased gene expression after liposome-mediated arterial gene transfer associated with intimal smooth muscle cell proliferation. In vitro and in vivo findings in a rabbit model of vascular injury. J Clin Invest. 1994;93:652–661. doi: 10.1172/JCI117017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, Aoki M, Ogihara T, Yasufumi K, Morishita R. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 2002;105:1233–1239. doi: 10.1161/hc1002.105228. [DOI] [PubMed] [Google Scholar]
- 116.Tomiyasu K, Oda Y, Nomura M, Satoh E, Fushiki S, Imanishi J, Kondo M, Mazda O. Direct intra-cardiomuscular transfer of beta2-adrenergic receptor gene augments cardiac output in cardiomyopathic hamsters. Gene Ther. 2000;7:2087–2093. doi: 10.1038/sj.gt.3301329. [DOI] [PubMed] [Google Scholar]
- 117.Tomiyasu K, Satoh E, Oda Y, Nishizaki K, Kondo M, Imanishi J, Mazda O. Gene transfer in vitro and in vivo with Epstein-Barr virus-based episomal vector results in markedly high transient expression in rodent cells. Biochem Biophys Res Commun. 1998;253:733–738. doi: 10.1006/bbrc.1998.9835. [DOI] [PubMed] [Google Scholar]
- 118.Turunen MP, Hiltunen MO, Ruponen M, Virkamäki L, Szoka J, FC, Urtti A, Ylä-Herttuala S. Efficient adventitial gene delivery to rabbit carotid artery with cationic polymer–plasmid complexes. Gene Therapy. 1999;6:6–11. doi: 10.1038/sj.gt.3300800. [DOI] [PubMed] [Google Scholar]
- 119.Vacik J, Dean BS, Zimmer WE, Dean DA. Cell-specific nuclear import of plasmid DNA. Gene Therapy. 1999;6:1006–1014. doi: 10.1038/sj.gt.3300924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vicat JM, Boisseau S, Jourdes P, Laine M, Wion D, Bouali-Benazzouz R, Benabid AL, Berger F. Muscle transfection by electroporation with high-voltage and short- pulse currents provides high-level and long-lasting gene expression. Hum Gene Ther. 2000;11:909–916. doi: 10.1089/10430340050015518. [DOI] [PubMed] [Google Scholar]
- 121.von der Leyen HE, Braun-Dullaeus R, Mann MJ, Zhang L, Niebauer J, Dzau VJ. A pressure-mediated nonviral method for efficient arterial gene and oligonucleotide transfer. Hum Gene Ther. 1999;10:2355–2364. doi: 10.1089/10430349950017004. [DOI] [PubMed] [Google Scholar]
- 122.Wang J, Jiao S, Wolff JA, Knechtle SJ. Gene transfer and expression in rat cardiac transplants. Transplantation. 1992;53:703–705. [PubMed] [Google Scholar]
- 123.Wang XJ, Liefer KM, Tsai S, O’Malley BW, Roop DR. Development of gene-switch transgenic mice that inducibly express transforming growth factor beta1 in the epidermis. Proc Natl Acad Sci U S A. 1999;96:8483–8488. doi: 10.1073/pnas.96.15.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Y, Bai Y, Price C, Boros P, Qin L, Bielinska AU, Kukowska-Latallo JF, Baker JR, Jr, Bromberg JS. Combination of electroporation and DNA/dendrimer complexes enhances gene transfer into murine cardiac transplants. Am J Transplant. 2001;1:334–338. doi: 10.1034/j.1600-6143.2001.10408.x. [DOI] [PubMed] [Google Scholar]
- 125.Wang Y, Boros P, Liu J, Qin L, Bai Y, Bielinska AU, Kukowska-Latallo JF, Baker JR, Jr, Bromberg JS. DNA/dendrimer complexes mediate gene transfer into murine cardiac transplants ex vivo. Mol Ther. 2000;2:602–608. doi: 10.1006/mthe.2000.0201. [DOI] [PubMed] [Google Scholar]
- 126.Wolff JA, Ludtke JJ, Acsadi G, Williams P, Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum Mol Genetics. 1992;1:363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 127.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 128.Yew NS, Marshall J, Przybylska M, Wysokenski DM, Ziegler RJ, Rafter PW, Li C, Armentano D, Cheng SH. Increased duration of transgene expression in the lung with plasmid DNA vectors harboring adenovirus E4 open reading frame 3. Hum Gene Ther. 1999;10:1833–1843. doi: 10.1089/10430349950017518. [DOI] [PubMed] [Google Scholar]
- 129.Yew NS, Przybylska M, Ziegler RJ, Liu D, Cheng SH. High and sustained transgene expression in vivo from plasmid vectors containing a hybrid ubiquitin promoter. Mol Ther. 2001;4:75–82. doi: 10.1006/mthe.2001.0415. [DOI] [PubMed] [Google Scholar]
- 130.Yew NS, Zhao H, Przybylska M, Wu IH, Tousignant JD, Scheule RK, Cheng SH. CpG-Depleted Plasmid DNA Vectors with Enhanced Safety and Long-Term Gene Expression in Vivo. Mol Ther. 2002;5:731–738. doi: 10.1006/mthe.2002.0598. [DOI] [PubMed] [Google Scholar]
- 131.Young JL, Benoit JN, Dean DA. Effect of a DNA nuclear targeting sequence on gene transfer and expression of plasmids in the intact vasculature. Gene Ther. 2003;10:1465–1470. doi: 10.1038/sj.gt.3302021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Young JL, Dean DA. Non-Viral Gene Transfer Strategies for the Vasculature. Microcirculation Res. 2002;9:35–50. doi: 10.1038/sj/mn/7800120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zelenin AV, Kolesnikov VA, Tarasenko OA, Shafei RA, Zelenina IA, Mikhailov VV, Semenova ML, Kovalenko DV, Artemyeva OV, Ivaschenko TE, Evgrafov OV, Dickson G, Baranovand VS. Bacterial beta-galactosidase and human dystrophin genes are expressed in mouse skeletal muscle fibers after ballistic transfection. FEBS Lett. 1997;414:319–322. doi: 10.1016/s0014-5793(97)01019-3. [DOI] [PubMed] [Google Scholar]
- 134.Zhang G, Budker V, Williams P, Subbotin V, Wolff JA. Efficient expression of naked DNA delivered intraarterially to limb muscles of nonhuman primates. Hum Gene Ther. 2001;12:427–438. doi: 10.1089/10430340150504046. [DOI] [PubMed] [Google Scholar]
- 135.Zhang G, Gao X, Song YK, Vollmer R, Stolz DB, Gasiorowski JZ, Dean DA, Liu D. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 2004;11:675–682. doi: 10.1038/sj.gt.3302210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhao H, Hemmi H, Akira S, Cheng SH, Scheule RK, Yew NS. Contribution of Toll-like receptor 9 signaling to the acute inflammatory response to nonviral vectors. Mol Ther. 2004;9:241–248. doi: 10.1016/j.ymthe.2003.11.012. [DOI] [PubMed] [Google Scholar]