Abstract
We examined α7β2-nicotinic acetylcholine receptor (α7β2-nAChR) expression in mammalian brain and compared pharmacological profiles of homomeric α7-nAChRs and α7β2-nAChRs. α-Bungarotoxin affinity purification or immunoprecipitation with anti-α7 subunit antibodies (Abs) was used to isolate nAChRs containing α7 subunits from mouse or human brain samples. α7β2-nAChRs were detected in forebrain, but not other tested regions, from both species, based on Western blot analysis of isolates using β2 subunit–specific Abs. Ab specificity was confirmed in control studies using subunit-null mutant mice or cell lines heterologously expressing specific human nAChR subtypes and subunits. Functional expression in Xenopus oocytes of concatenated pentameric (α7)5-, (α7)4(β2)1-, and (α7)3(β2)2-nAChRs was confirmed using two-electrode voltage clamp recording of responses to nicotinic ligands. Importantly, pharmacological profiles were indistinguishable for concatenated (α7)5-nAChRs or for homomeric α7-nAChRs constituted from unlinked α7 subunits. Pharmacological profiles were similar for (α7)5-, (α7)4(β2)1-, and (α7)3(β2)2-nAChRs except for diminished efficacy of nicotine (normalized to acetylcholine efficacy) at α7β2- versus α7-nAChRs. This study represents the first direct confirmation of α7β2-nAChR expression in human and mouse forebrain, supporting previous mouse studies that suggested relevance of α7β2-nAChRs in Alzheimer disease etiopathogenesis. These data also indicate that α7β2-nAChR subunit isoforms with different α7/β2 subunit ratios have similar pharmacological profiles to each other and to α7 homopentameric nAChRs. This supports the hypothesis that α7β2-nAChR agonist activation predominantly or entirely reflects binding to α7/α7 subunit interface sites.
Introduction
Several nicotinic acetylcholine receptor (nAChR) subtypes are expressed widely along the entire neuraxis and are involved in many of the physiologic functions of the central and peripheral nervous systems (Albuquerque et al., 2009; Hurst et al., 2013). nAChR activity controls important aspects of synaptic function and brain development, including the proliferation and differentiation of neural progenitors, neural migration, and neuronal maturation (Griguoli and Cherubini, 2012; Picciotto et al., 2012; Yakel, 2013). Furthermore, nAChR dysfunction may play an important role in a variety of neurologic diseases, including neurodegenerative and psychiatric diseases (Gotti and Clementi, 2004; Lewis and Picciotto, 2013).
α4β2- and homomeric α7-nAChRs are the most widely expressed subtypes in mammalian brain. The latter are thought to contain five identical agonist binding sites located at subunit interfaces in extracellular domains (Gotti and Clementi, 2004; Whiteaker et al., 2007). Pharmacological hallmarks of α7-nAChRs are their high sensitivity to antagonism by snake venom–derived polypeptide toxins such as α-bungarotoxin (α-Bgtx) and α-cobratoxin (α-Cbtx), and their sensitivity to choline [a product of acetylcholine (ACh) hydrolysis] as an agonist (Albuquerque et al., 1997, 2009). α7-nAChRs are highly expressed in the cortex, hippocampus, and subcortical limbic regions, and (at lower levels) in the thalamus and basal ganglia. α7-nAChRs that are located on or near nerve terminals are involved in control of neurotransmitter release, whereas α7-nAChRs on dendrites or soma apposed to cholinergic synaptic endings play roles in classic neurotransmission. In both cases, the high calcium permeability of α7-nAChRs may also result in altered intracellular signaling and gene transcription (Dajas-Bailador and Wonnacott, 2004; Albuquerque et al., 2009). α7-nAChRs also may be associated with extrasynaptic volume transmission (Lendvai and Vizi, 2008).
Affinity purification of nAChRs using snake venom α-toxins has been performed from brain tissue of various species. Extracts from whole rat brain appear to be predominantly composed of homomeric α7-nAChRs (Drisdel and Green, 2000). However, homomeric α7- and α8-nAChRs (and heteromeric α7α8-nAChRs) have been identified in chick central nervous system extracts (Keyser et al., 1993; Gotti et al., 1994). Furthermore, studies using heterologous systems have shown that α7 subunits can form functional channels when combined with α5 (Girod et al., 1999), β2 (Khiroug et al., 2002), β3 (Palma et al., 1999), or β4 subunits (Criado et al., 2012). Fluorescently tagged nAChR α7 and β2 subunits were recently used to characterize the formation of α7β2-nAChRs, and functional differences between α7- and α7β2-nAChRs have been suggested (Murray et al., 2012). Coexpression of β2 and α7 subunits caused a significant decrease in agonist-evoked whole cell current amplitudes, but this decrease occurs without affecting the concentration-response characteristics of a range of common agonists and antagonists (Murray et al., 2012). Other studies have shown that α7 and β2 subunits are coexpressed in rat basal forebrain cholinergic neurons and appear to form heteromeric α7β2-nAChRs with subtly different biophysical and pharmacological properties from those of homomeric α7-nAChRs (Liu et al., 2009). In addition, interaction of these putative α7β2-nAChRs with oligomeric forms of amyloid-β (Aβ1-42) may be relevant in the etiology of Alzheimer disease (Liu et al., 2013).
These previous studies suggest that the function and pharmacology of α7*-nAChRs (where the asterisk denotes the known or possible presence of other nAChR subunits than α7; Lukas et al., 1999) may be more complex than previously thought, and that α7β2-nAChR expression may be restricted to forebrain areas. However, heteromeric α7*-nAChRs have not yet been directly detected biochemically, nor have they been definitively identified in human brain. We used the α7-nAChR–selective ligand, α-Bgtx, to affinity purify α7*-nAChRs from selected brain areas of humans or of wild-type (WT) or β2 subunit-null mutant (KO) mice. The subunit compositions of these isolated α7*-nAChRs were analyzed by Western blot analysis using subunit-specific anti-α7 or anti-β2 antibodies. The results show expression of α7β2-nAChRs in both WT mouse and human forebrain samples, but not in brains from β2 KO mice. Moreover, concatemeric (linked subunit) constructs, the Xenopus oocyte system, and two-electrode voltage clamp (TEVC) recording were used to confirm functional expression of α7β2-nAChRs. This work defined α7 and β2 subunit stoichiometries that enable α7β2-nAChR function and showed similar pharmacological characteristics across α7- and α7β2-nAChR subtypes. The results confirm commonalities in expression of α7β2-nAChRs in humans and mice, and support hypotheses linking α7β2-nAChRs, cholinergic signaling loss, and roles for Aβ1-42 in etiopathogenesis of at least a subset of human dementias.
Materials and Methods
Animals and Materials
This study used 4- to 6-month-old male, pathogen-free, C57BL/6 WT, α7 KO, or β2 KO mice (Picciotto et al., 1995; Orr-Urtreger et al., 1997) obtained from Dr. U. Maskos (Pasteur Institute, Paris, France). All animal experiments were conducted in accordance with the European Community Council Directive (86/609/EEC) of November 24, 1986.
(±)-[3H]epibatidine (Epi; specific activity, 66Ci/mmol) and [125I]-α-Bgtx (specific activity of 200–216 Ci/mmol) were purchased from Perkin Elmer (Waltham, MA). Nonradioactive α-Bgtx, Epi, and nicotine were purchased from Tocris Bioscience (Bristol, UK, or Minneapolis, MN), as were dihydro-β-erythroidine (DHβE), and methyllycaconitine (MLA). Sazetidine-A (also known as AMOP-H-OH) was kindly supplied by Dr. Alan Kozikowski (University of Illinois, Chicago, IL, USA). 1,2-bis-N-cytisinylethane (CC4) also was used (Riganti et al., 2005). α-Cbtx and all other reagents were sourced from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Human Tissues
Human cerebellum was provided by the Newcastle Brain Tissue Resource on the basis of a collaboration with Dr. Jennifer Court (Newcastle upon Tyne General Hospital, Newcastle upon Tyne, UK). Samples were all collected by the Newcastle Brain Tissue Resource with informed consent and appropriate ethical approval. Case details are shown in Table 1. The approvals and method for categorizing the subjects’ smoking status are outlined in the methods section of Court et al. (2005). Human basal forebrain tissue was provided by Dr. Emanuele Sher (Lilly Research Center, Windlesham, Surrey, UK), and was also collected with appropriate informed consent in accordance with all applicable laws and regulations.
TABLE 1.
Details of cases sampled for receptor analysis
Values are the mean ± S.E.M.
| Brain Region | Cases | Age | Postmortem Delay | Male/Female |
|---|---|---|---|---|
| n | yr | h | ||
| Basal forebrain | 4 | 65.7 ± 9.4 | 2 > 8 | 3/1 |
| 2 (2–6) | ||||
| Cerebellum smokers | 4 | 73.0 ± 3.9 | >8 | 2/2 |
| Cerebellum nonsmokers | 4 | 68.7 ± 6.6 | >8 | 2/2 |
There were no significant differences between groups for age.
Transfected Cells
Human α2-, α3-, β2-, and β4-nAChR subunit clones in the mammalian expression vector pcDNΑ3 were gifts from Dr. Sergio Fucile (University of Rome, Rome, Italy). The human α7-nAChR subunit clone in pcDNΑ3 was a gift of Dr. Roberta Benfante (CNR Institute of Neuroscience, Milan, Italy). HEK293 and SH-SY5Y cells were transiently transfected using the Ca3(PO4)2 method or the Jet-PEI reagent (Polyplus, Euroclone, Italy) transfection. For the α7 plasmid, 1.5 × 106 cells were transfected with 6 μg plasmid using the Jet-PEI. For each of the α2, α3, α4, and β2 or β4 subunits, 20 μg plasmids for 1.5 × 106 cells was used, with the Ca3(PO4)2 method. nAChR expression by cells was analyzed 24 hours after transfection.
Antibody Production and Characterization
We used affinity-purified, subunit-specific polyclonal antibodies (Abs), produced in rabbit against peptides derived from the C-terminal (COOH) or intracytoplasmic loop (CYT) of human or mouse nAChR subunit sequences, as previously described (Gotti et al., 2006; Grady et al., 2009). The Ab against the COOH peptide (SAPNFVEAVSKDFA) was used for α7 subunits in mouse and human tissues. Abs directed against the α7 mouse CYT peptide (PSGDPDLAKILEEVRYIANRFRC) or the human CYT peptide (QMQEADISGYIPNGQMQEADISGYIPNG) were used for mouse and human tissues, respectively. For the β2 subunit, we used antibodies directed against two different cytoplasmic human β2 peptides: RQREREGAGALFFREAPGADSCTY [β2(1)] and cgIADHMRSEDDDQSVREDWKYV [β2(2)].
The specificity of the affinity-purified Abs was tested by immunoprecipitation studies using α7 WT or α7 KO hippocampus and β2 WT or β2 KO mouse cortex (the results are shown in Supplemental Fig. 1). The same Abs also were tested by means of Western blotting (Supplemental Fig. 1). To exclude any cross-reactivity between nAChR subunits, anti-β2(1)- or anti-α7 human subunit Abs were also tested by means of immunoprecipitation studies and Western blotting in HEK293 cells transfected to express human α2β4-, α4β2-, α4β4, or α3β4-nAChR subtypes or in SH-SY5Y cells transfected to express human α7-nAChRs (see above) (results are shown in Supplemental Fig. 2).
Purification of α-Bgtx–Binding nAChRs
For studies using mice, approximately 100 mg of basal forebrain or hippocampus tissue microdissected from either WT or subunit-null mice were pooled in every experiment. The tissue was homogenized in 10 ml of 50 mM Na phosphate, pH 7.4, 1 M NaCl, 2 mM EDTA, 2 mM EGTA, and 2 mM phenylmethylsufonylfluoride (to covalently inactivate serine protease activity), and the homogenates were diluted and centrifuged for 1.5 hours at 60,000g. The entire membrane homogenization, dilution, and centrifugation procedure were then repeated, and the resulting pellets were collected, rapidly rinsed with 50 mM Tris HCl, pH 7, 120 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, and 2 mM phenylmethylsufonylfluoride. The washed pellets were then resuspended in 2 ml of the same buffer, further supplemented with 20 µg/ml of each of the following protease inhibitors: leupeptin, bestatin, pepstatin A, and aprotinin. Triton X-100 at a final concentration of 2% was added to the washed membranes, which were extracted for 2 hours at 4°C. The extracts were centrifuged for 1.5 hours at 60,000g, recovered, and an aliquot of the supernatants was collected for protein measurement using the BCA protein assay (Pierce Biotechnology, Rockford, IL), with bovine serum albumin (BSA) as the standard. Extracts (2 ml) were incubated with 200 µl Sepharose–α-Bgtx (concentration of coupled toxin 1 mg/ml of gel) and shaken overnight at 4°C. The following day, the beads were centrifuged, the supernatant was recovered, and the resins were washed 4–6 times by resuspension followed by centrifugation. After washing, the Sepharose–α-Bgtx beads with bound nAChRs (purified α-Bgtx–binding receptors) were incubated with one to two volumes of Laemmli sample buffer (125 mM Tris phosphate, 4% SDS, 20% glycerol, 0,02% bromophenol blue, and 10% 2-mercaptoethanol, pH 6.8) and boiled for 2 minutes. The supernatant was then recovered by centrifugation.
In the case of human tissue, α-Bgtx–binding sites were purified using the same procedure as that used for mouse tissue, starting from 600 mg of tissue (see Table 1 for subject details).
Binding Studies
[125I]-α-Bgtx.
The binding of [125I]-α-Bgtx to 2% Triton X-100 extracts of mouse tissues was determined by collection onto DEAE-Sepharose Fast Flow (GE Healthcare, Uppsala, Sweden). Triton extracts (250 µl) from each experimental group were incubated overnight with a saturating concentration (5 nM) of [125I]-α-Bgtx at 20°C in the presence of 2 mg/ml BSA. Specific radioligand binding was defined as total binding minus the nonspecific binding determined in the presence of 1 µM unlabeled α-Bgtx. Nonspecific binding averaged 30–40% of total binding. Binding to α7*-nAChRs could also be measured in an immunoprecipitation assay format. Receptor extracts were labeled with [125I]-α-Bgtx (5 nM in the presence or absence of 1 µM unlabeled α-Bgtx to define total and nonspecific binding). The labeled extract could then be bound to protein A beads via anti-α7 subunit Abs (described later in the Materials and Methods). Similar amounts of specific binding were recorded in either assay format, and nonspecific binding was between 10 and 15% of total binding.
[3H]Epi.
Binding of [3H]Epi to nAChRs in 2% Triton X-100 brain tissue extracts obtained was also assessed. [3H]Epi binds to multiple heteromeric nAChR subtypes with picomolar affinity and to α7-nAChR with nanomolar affinity. To ensure that the α7 nAChR did not contribute to [3H]Epi binding, in solubilized extracts, binding was performed in the presence of 1 μM α-Bgtx, which specifically binds to α7-nAChRs (and thus prevents [3H]Epi binding to these sites).
As for [125I]-α-Bgtx binding assays, binding sites were captured using DEAE-Sepharose Fast Flow, after overnight incubation of 250-µl aliquots of the extracts with 1 nM [3H]Epi at 4°C. Nonspecific binding (averaging 5–10% of total binding) was determined in parallel samples containing 100 nM unlabeled Epi.
Immunoprecipitation
For immunoprecipitation studies of heteromeric receptors present in human tissues, we used Abs specific for α2, α3, α4, α5, β2, or β4 subunits directed against human subunit peptides as previously described (Gotti et al., 2006). For α6 and β3 subunits, we used Abs directed against peptides of mouse subunit sequences, also as previously characterized and described (Grady et al., 2009). The immunoprecipitation capacities of the anti-human subunit Abs ranged from 90 to 100% of the [3H]Epi–labeled receptors (mean of three independent experiments). For immunoprecipitation experiments, affinity-purified Abs were covalently immobilized on agarose-Protein A beads at a concentration of 4 mg/ml wet resin. Immunoprecipitation was then performed by adding 20 µl agarose-Protein A beads with bound, affinity-purified Abs to 200 µl of 1 nM [3H]Epi–labeled extracts. After overnight incubation, immunoprecipitates were recovered by centrifugation and washed three times with phosphate-buffered saline containing 0.1% Triton X-100.
Immunoblotting and Densitometric Quantification of Western Blot Bands
nAChR subunit contents of tissue extracts or of α-Bgtx–binding complexes were analyzed by Western blotting. For the extracts loaded before and after the purification, 10 μg of proteins were loaded, whereas for the α-Bgtx–purified receptors a constant volume (40 µl), that depending on the tissue, may represent 1/10 or 1/20 of the total recovered Laemmli sample buffer-eluted receptors was loaded onto a 9% acrylamide (Bio-Rad, Hercules, CA) gel and subjected to SDS-PAGE. After SDS-PAGE, proteins were electrophoretically transferred to nitrocellulose membranes with 0.45-mm diameter pores (Schleicher and Schuell, Dassel, Germany). The blots were blocked overnight in 5% nonfat milk in Tris-buffered saline, washed in a buffer containing 5% nonfat milk and 0.3% Tween 20 in Tris-buffered saline, incubated for 2 hours with the primary antibody (1–2.5 mg/ml), and then incubated with the appropriate peroxidase-conjugated secondary Abs (Sigma-Aldrich). After 10 washes, peroxidase was detected using a chemiluminescent substrate (Pierce Biotechnology). The signal intensity of the Western blot bands was measured using an Epson 4500 gel scanner. The developed films were scanned as a TIFF image in eight-bit grayscale format at a resolution setting of 300 dpi. All of the films obtained from the separate experiments were acquired in the same way and scanned in parallel with a calibrated optical density step tablet from Stouffer (Stouffer Graphics Arts, Mishawaka, IN).
The images were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD) (Schneider et al., 2012). The pixel values of the images were transformed to optical density values by the program using the calibration curve obtained by acquiring the calibrated tablet with the same parameters as those used for the images. The immunoreactive bands were quantified in four separate experiments for the mouse hippocampus and basal forebrain as previously described (Grady et al., 2009)
Concatemeric α7*-nAChR Constructs
Fully pentameric nAChR concatemers were constructed from human nAChR subunit sequences. cDNAs encoding concatemers were created using the same subunit layout we have previously employed to encode high- and low-agonist-sensitivity α4β2*-nAChR isoforms and α3β4(α5[D/N])-nAChRs (George et al., 2012; Eaton et al., 2014). Subunits were arranged in the order α7-α7-α7-α7-α7 (α7 homopentamer), α7-α7-β2-α7-α7, or α7-β2-α7-β2-α7. Kozac and signal peptide sequences were removed from all subunit sequences with the exception of subunits expressed in the first position of the concatamer. Subunits were linked by alanine-glycine-serine repeats designed to provide a complete linker length (including the C-terminal tail of the preceding subunit) of 40 ± 2 amino acids. At the nucleotide level, linker sequences were designed to contain unique restriction sites that allow easy removal and replacement of individual α7 and β2 subunits. The protein sequences for the human nAChR subunits were encoded by synthetic nucleotide sequences optimized for expression systems (GeneArt; Life Technologies, Grand Island, NY). Optimization included minimization of high GC content sequence segments, improved codon usage, reduction of predicted RNA secondary structure formation, and removal of sequence repeats and possible alternative start and splice sites. Sequences of all subunits, together with their associated partial linkers, were confirmed by DNA sequencing (GeneArt). Each concatamer was subcloned into the pSGEM oocyte high-expression vector (from Dr. Michael Hollmann, Ruhr-Universitaet, Bochum, Germany). For comparison, homomeric α7-nAChRs were also expressed from unlinked individual subunits (cDNA clone also synthesized and optimized by GeneArt). The unlinked human α7 subunit cDNA was also subcloned into the pSGEM vector.
RNA Synthesis
Plasmids containing concatemeric α7-homopentameric or α7β2-nAChR constructs, or individual α7-nAChR subunits, were linearized with NheI (2 hours at 37°C), and the reaction mix was treated with proteinase K (30 minutes at 50°C). cRNAs were transcribed using the mMessage mMachine T7 kit (Applied Biosystems/Ambion, Austin, TX). Reactions were treated with TURBO DNase (1 U for 15 minutes at 37°C) and cRNAs were purified using the Qiagen RNeasy Clean-Up kit (Qiagen, Valencia, CA). cRNA purity was confirmed on a 1% agarose gel and preparations were stored at −80°C.
Xenopus Oocytes and RNA injection
Xenopus oocytes were purchased from Ecocyte Bioscience US (Austin, TX) and incubated upon arrival at 13°C. The tips of pulled glass micropipettes were broken to achieve an outer diameter of approximately 40 µm (resistance of 2–6 MΩ), and pipettes were used to inject 20–60 nl containing 10 ng of cRNA/oocyte. To improve functional expression of α7*-nAChRs, Ric-3 mRNA was also coinjected (Halevi et al., 2002). A ratio of 1:50 Ric-3/α7 subunit mRNA by mass was determined to be optimally effective in pilot experiments (data not shown).
TEVC Recording of α7- and α7β2-nAChR Function
TEVC recordings were made at room temperature (20°C) in oocyte saline solution (containing 82.5 mM NaCl, 2.5 mM KCl, 5 mM HEPES, 1.8 mM CaCl2·2H2O, and 1 mM MgCl2·6H2O, pH 7.4). Seven to 14 days after injection, Xenopus oocytes expressing concatenated α7*-nAChRs were voltage clamped at −70 mV with an Axoclamp 900A amplifier (Molecular Devices, Sunnyvale, CA). Recordings were sampled at 10 kHz (low-pass Bessel filter, 40 Hz; high-pass filter, DC), and the resulting traces were saved to disk (Clampex v10.2; Molecular Devices). Data from oocytes with leak currents (Ileak) > 50 nA were excluded from recordings.
Nicotinic Receptor Pharmacology
Fresh stock drug solutions (agonists: ACh, choline, nicotine, sazetidine, and CC4; antagonists: DHβE, MLA, mecamylamine, and α-Cbtx) were made daily and diluted as required. Agonists and antagonists were applied using a 16-channel, gravity-fed perfusion system with automated valve control (AutoMate Scientific, Inc.; Berkeley, CA). All solutions were supplemented with atropine sulfate (1.5 µM) to ensure that muscarinic ACh receptor responses were blocked and thus not recorded. Oocytes expressing loose subunits and/or concatemeric α7- or α7β2-nAChRs were perfused with nAChR agonists for 5 seconds with 60-second washout times between each subsequent application. Oocytes were preincubated with nAChR antagonists for 2 minutes prior to activation with ACh (10 mM; 5 seconds). For experiments using α-Cbtx, bath and drug solutions were supplemented with 0.1% BSA to reduce loss of this peptide ligand by adsorption to the TEVC apparatus.
Data Analysis
The expression of [3H]Epi and [125I]-α-Bgtx receptors and the subunit contents of the [3H]Epi receptors expressed in the mouse and human samples were statistically compared using unpaired t tests. In human cerebellum samples from smokers and nonsmokers, results were compared using an unpaired t test. Statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA).
For TEVC data, EC50 and IC50 values were determined from nAChR-mediated peak currents through nonlinear least-squares curve fitting (GraphPad Prism 5.0) using unconstrained, monophasic logistic equations to fit all parameters, including Hill slopes. Desensitization/inactivation of α7*-nAChR currents in the presence of 10 mM (maximally stimulating) ACh was also analyzed by nonlinear least-squares curve fitting in Graph Pad Prism 5.0. These data were best fit by a two-phase exponential decay equation. One-way analysis of variance was used to compare parameters between multiple groups in each case. The Tukey multiple comparison test was used for post hoc analysis to compare the means of three or more groups (GraphPad Prism 5.0).
Results
α-Bgtx–Binding Sites in WT or β2 KO Mice
In preliminary experiments, we analyzed nAChR expression in 2% Triton X-100 extracts obtained from the hippocampus or basal forebrain of WT or β2 KO mice (Table 2). By two different approaches (immunoprecipitating [125I]-α-Bgtx–labeled receptors using anti-α7 subunit Abs and by [125I]-α-Bgtx binding to Triton extracts), we determined that the density of α7*-nAChRs in the mouse hippocampus is more than two times higher than that in the murine basal forebrain. During α-Bgtx binding site purification, we determined that 85–95% of those sites in Triton X-100 extracts were bound by α-Bgtx–Sepharose 4B affinity resins, whereas more than 95% of high-affinity [3H]Epi binding was recovered in the flow-through.
TABLE 2.
Levels of [3H]Epi and [125I]α-Bgtx binding to 2% Triton X-100 extracts in two different brain areas of WT and β2 KO mice
Values are the mean ± S.E.M. from three separate experiments.
| Brain Region | [3H]Epi | [125I]-αBgtx |
|---|---|---|
| fmol/mg protein | ||
| β2 WT Hippocampus | 36.3 ± 2.3 | 37.7 ± 2.3 |
| β2 KO Hippocampus | 1.0 ± 0.3* | 39.9 ± 1.0 |
| β2 WT Basal forebrain | 44.5 ± 3.5 | 15.2 ± 2.5 |
| β2 KO Basal forebrain | 0.5 ± 0.2* | 15.1 ± 2.6 |
P < 0.001 (significantly different from β2+/+ by t test).
The densities of [3H]Epi–binding nAChRs were similar between the two regions (Table 2). Elimination of β2 subunit expression dramatically reduced expression of [3H]Epi–binding nAChRs in both regions, indicating that this binding is almost entirely due to β2*-nAChRs. By contrast, [125I]-α-Bgtx (α7*-nAChR) expression was not significantly different between WT and β2 KO mice in either the hippocampus or basal forebrain.
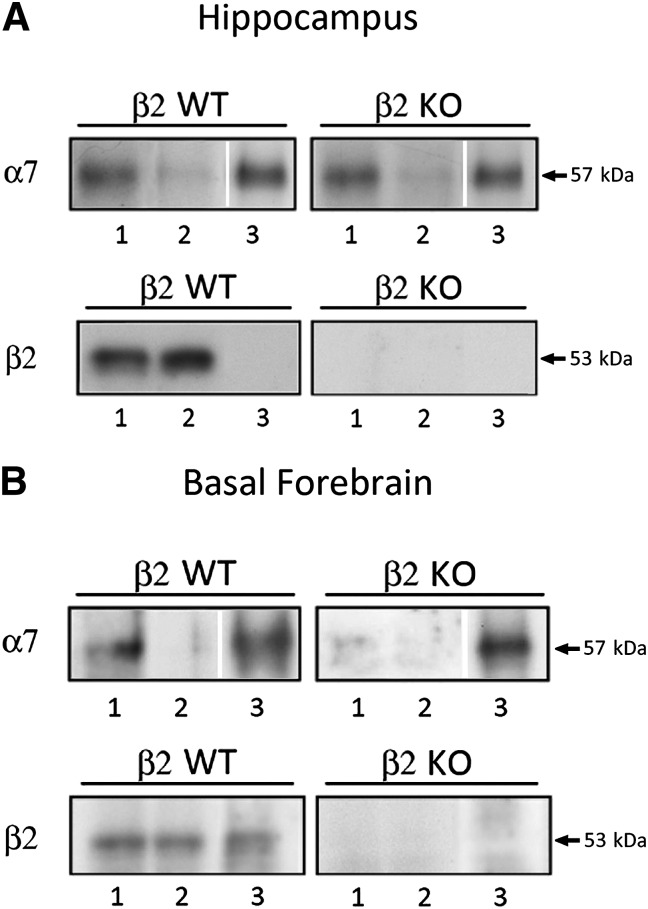
In addition, Western blot analysis was performed on α-Bgtx binding sites affinity purified from the hippocampus of WT or β2 KO mice and probed with anti-α7 (top) or anti-β2 (bottom) subunit Abs (Fig. 1A). Confirming results from the binding studies, Western blots also showed no significant differences in presumed α7*-nAChR levels (i.e., polypeptide labeled with anti-α7 subunit Abs) in whole extracts from WT or β2 KO mouse hippocampus (lane 1 in top two panels). Moreover, affinity purification on α-Bgtx affinity resins isolated comparable levels of α7*-nAChRs from WT or β2 KO mouse hippocampus (compare lanes 1 and 3 of Fig. 1A top) but did not isolate nAChRs containing β2 subunits (compare lanes 1 and 3 of Fig. 1A, bottom left), which instead were found in the flow-through fraction (lane 2; Fig. 1A, bottom left). As expected, no β2*-nAChRs were found in extracts isolated on Bgtx resins or in the flow-through from that separation for tissue taken from β2 KO mice (Fig. 1A, lower right panel lanes 1–3).
Fig. 1.
Western blot analysis of nAChR subunit content in α-Bgtx–purified receptors prepared from 2% Triton X-100 extracts of WT and β2 KO mouse hippocampi (A) and basal forebrain samples (B). (A) α-Bgtx–purified receptors were prepared from mouse hippocampi by incubating extracts with Sepharose 4B covalently bound with α-Bgtx. The bound receptors were recovered from the beads using Laemmli sample buffer. Western blot analysis of 10 µg 2% Triton X-100 extracts of the hippocampus before (lane 1) and after α-Bgtx purification (lane 2; supernatant), and 1/20 of the corresponding α-Bgtx purified receptors (lane 3; recovered from beads). The blots were probed with an anti-α7 Ab (top) or β2(1) Ab (bottom). (B) α-Bgtx–purified receptors were prepared as described above. Western blot analysis of 10 µg 2% Triton X-100 extracts of the basal forebrain before (lane 1) and after α-Bgtx purification (lane 2; supernatant), and 1/10 of the corresponding α-Bgtx–purified receptors (lane 3; recovered from beads). The blots were probed with an anti-α7 Ab (top) or β2(1) Ab (bottom).
In further agreement with the findings of the binding studies, Western blot analysis showed that basal forebrain extracts contained fewer α7*-nAChRs (i.e., immunoreactive α7 protein) than hippocampal extracts, whereas amounts of immunoreactive β2 subunits were very similar across brain regions. Analysis of the Western blots probed using anti-β2 subunit Abs showed clearly detectable β2 subunit presence in α7*nAChRs isolated on Bgtx resins from tissue derived from basal forebrain, but not from the hippocampus (compare lanes 3 of the bottom-left panels of Fig. 1, A and B). The percentage of β2 subunit present in the α-Bgtx purified receptor was determined by western blotting by loading on the same gel 10 μg of 2% Triton extract and 1/10 of the α-Bgtx purified receptor. We found that the immunoreactivity of the β2 subunit determined in the purified α-Bgtx was 2.25% ± 0.6% (n = 4) of the total β2 subunit immunoreactivity measured in the basal forebrain extracts of WT mice.
α-Bgtx–Binding Sites in Human Brain
The possible presence of α7β2-nAChRs in human brain was analyzed using post mortem samples of basal forebrain and cerebellum. In preliminary experiments, we characterized nAChR subtypes expressed in basal forebrain and cerebellum and their levels in 2% Triton extracts (Table 3). The average level of [125I]-α-Bgtx–labeled (α7*)-nAChRs was higher in basal forebrain than cerebellum.
TABLE 3.
Levels of [3H]Epi and [125I] α-Bungarotoxin binding to 2% Triton X-100 extracts in the two different human brain regions
Values are the mean ± S.E.M. of the four samples in each group.
| Brain Region | [3H]Epi | [125I]-α-Bgtx |
|---|---|---|
| fmol/mg protein | ||
| Basal forebrain | 31.8 ± 8.5 | 80.7 ± 7.0 |
| Cerebellum nonsmokers | 22.5 ± 2.6 | 45.7 ± 5.1 |
| Cerebellum smokers | 39.7 ± 5.3* | 48.3 ± 6.5 |
P = 0.02 (significant differences in cerebellar membrane [3H]Epi binding between smokers and nonsmokers: unpaired t test). No significant difference was seen between [125I]-α-Bgtx binding levels in cerebellar samples taken from smokers versus nonsmokers, by the same measure.
The level of [3H]Epi–binding nAChRs in cerebellum depended on smoking status. As shown in Table 3, the density of non-α7*-nAChRs measured by means of [3H]Epi binding was higher in smokers than in nonsmokers (P = 0.02). Based on immunoprecipitation using subunit-specific Abs, in both tissues the large majority of [3H]Epi–binding sites contained the β2 subunit associated with the α4 subunit (α4β2-nAChR: 75% in basal forebrain and 60 and 67% in cerebellum of smokers and nonsmokers, respectively). An additional 14% of [3H]Epi–binding sites in the basal forebrain were α2β2*-nAChRs, whereas this subtype accounted for only 7% of cerebellar [3H]Epi–binding sites. The largest region-to-region difference was for α3β2*-nAChRs: Whereas those sites accounted for 4.8% of [3H]Epi–binding sites in the basal forebrain, they represented 32% in the cerebellum.
α-Bgtx affinity-purified binding sites were obtained from three human basal forebrain (Fig. 2, lanes 1–3) or three human cerebellum (Fig. 2, lanes 5–7) samples. These sites were Western blotted and probed with anti-α7 subunit Abs (top) or two different anti-β2 subunit Abs [anti-β2(1) Abs, middle; and anti-β2(2), bottom] targeting different epitopes within the β2 subunit. Control samples were extracts from α4β2-nAChR–expressing transfected HEK cells (lane 4) or from α7-nAChR–expressing transfected SH-SY5Y cells (lane 8), also probed with the Abs. Levels of immunoreactivity for the α7 subunit were very similar in samples loaded in lanes 1, 2, 5, and 6, higher in the sample loaded in lane 3, and lower in the sample loaded in lane 7. Similar isolates from HEK-α4β2 cells were negative but SH-SY5Y-α7 cells contained immunoreactive α7 subunits (Fig. 2, upper panel, lanes 4 and 8, respectively). Isolation of α-Bgtx binding sites also yielded anti-β2 subunit Ab-labeled proteins from basal forebrain samples but not from the cerebellum, regardless of whether the cerebellum samples were obtained from smokers or nonsmokers. Such immunoreactivity was absent in extracts from SH-SY5Y-α7 cells but very evident in HEK-α4β2 cells (Fig. 2, middle and lower panels, lanes 8 and 4, respectively). Both the α7 and β2 subunits present in the human tissues show a slightly higher molecular weight then the corresponding transfected subunits. This is probably due to differences in glycosylation between native and transfected receptors.
Fig. 2.
Western blot analysis of α-Bgtx–purified nAChRs prepared from human basal forebrain and cerebellum. α-Bgtx–binding nAChRs were purified from the same volume of 2% Triton X-100 extracts of basal forebrain and cerebellum by incubating them with Sepharose 4B covalently bound with α-Bgtx. The bound receptors were eluted using sample buffer and an identical volume of purified receptors was loaded on the gel. The Western blots were probed with anti-α7 Ab (top) or anti-β2 Ab (bottom).
Since it has been shown in a heterologous expression system that an α7β4-nAChR subtype may be formed (Criado et al., 2012), we also probed human α-Bgtx–purified sites with anti-β4 subunit Abs with proven specificity (Supplemental Fig. 2, bottom). No specific labeling was observed in either the human basal forebrain or cerebellum samples, showing absence of α7β4-nAChRs. Collectively, these results clearly indicate that α7β2-nAChRs are present in the human basal forebrain but not in the cerebellum.
Functional Expression of Concatemeric α7*-nAChRs from Human Subunits
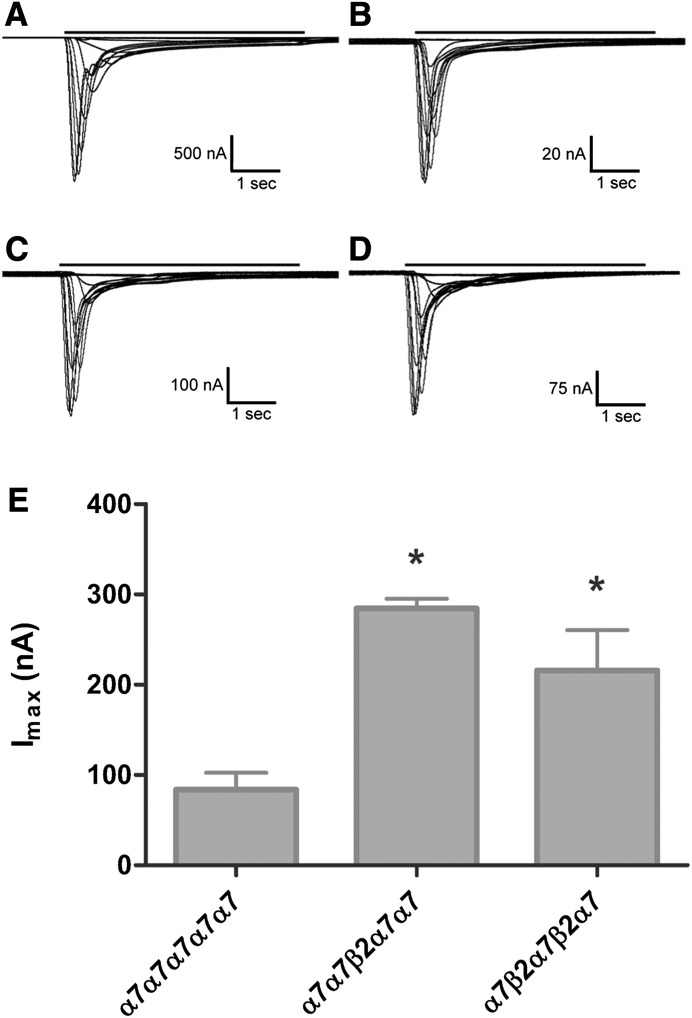
Heterologous expression has shown assembly of functional α7β2*-nAChRs (see the Introduction), but the way(s) in which α7 and β2 subunits might combine from individual, unlinked, subunits could not be defined. Accordingly, we used a linked-subunit approach to produce α7*-nAChRs with defined subunit ratios and assembly orders. Each of the three concatemeric constructs [(α7)5-nAChR homopentamer, (α7)4(β2)1-nAChR, and (α7)3(β2)2-nAChR] showed concentration-dependent ACh-evoked function (representative traces shown in Fig. 3, A–D). This function, although smaller than that measured in Xenopus oocytes expressing homomeric α7-nAChRs from unlinked human α7 subunits (typically > 1 µA at 7 days after mRNA injection) was easily measurable (approximately 100–300 nA peak current response, depending on the construct). The time course of desensitization/inactivation after a peak response stimulated by 10 mM ACh (maximally stimulating concentration) was also measured for each construct. For each construct, desensitization/inactivation was best fit by a double-exponential decay model. As detailed in the legend to Fig. 3, no significant differences were seen between the fast desensitization/inactivation time constants calculated for each group. This is not surprising since the apparent time constants will likely reflect the relatively slow kinetics of agonist application in the apparatus, rather than the much faster kinetics of α7*-nAChR desensitization (Papke, 2010). Indeed, the apparent τfast values are very similar to those measured for solution exchange in our apparatus (Eaton et al., 2014). However, the τslow value calculated for the (α7)3(β2)2 construct was significantly slower than those associated with the other groups. Thus, despite the admitted disadvantages of measuring kinetic parameters in the Xenopus oocyte expression system, there is some evidence that α7β2*-nAChR desensitization may be slower than that of homomeric α7-nAChRs.
Fig. 3.
Representative traces and maximum function (Imax) comparison for α7*-nAChR pentameric concatemer constructs. Oocytes were injected with mRNA encoding unlinked α7-nAChR subunit monomers (A), concatenated α7 homopentamers (B), α7β2-nAChRs with the β2 subunit in position 3 (C), or α7β2-nAChRs with the β2 subunit in positions 2 and 4 (D). Representative TEVC recordings are shown in each case for ACh concentration-response determinations (see Materials and Methods for details). Black bars above each trace represent 5-second applications of ACh at a range of concentrations. The time course of receptor desensitization/inactivation during stimulation with a maximally effective dose of ACh (10 mM) was also investigated for each nAChR construct using additional groups of oocytes. In each case, the time course was best fit by a double-exponential decay. The fast time constants (τfast) for desensitization/inactivation were statistically indistinguishable by one-way ANOVA across all four groups (unlinked α7, 436 ± 85 milliseconds; α7-only concatemer, 214 ± 80 milliseconds; α7β2(p3), 312 ± 55 milliseconds; α7β2(p2,4), 247 ± 35 milliseconds; F[3,11] = 2.06, P = 0.16; and n = 3 in each group). By contrast, the slow time constant (τslow) for desensitization/inactivation of the α7β2(p2,4) construct was significantly longer that of the other groups. No other differences were detected by a Tukey post hoc comparison (P < 0.05). Values were as follows: unlinked α7, 5109 ± 800 milliseconds; α7-only concatemer, 3130 ± 585 milliseconds; α7β2(p3), 5073 ± 638 milliseconds; α7β2(p2,4), 6318 ± 365 millisecond; F[3,11] = 5.29, P = 0.02; and n = 3 in each group. (E) Summary of maximal function (Imax) measured in each concatemeric nAChR group by stimulation with the full agonist ACh (10 mM). Bars represent mean ± S.E.M. (n = 3). Imax values were as follows: α7 only, 83.9 ± 18.6 nA; α7β2(p3), 285 ± 11 nA; and α7β2(p2,4), 216 ± 45 nA. Analysis using one-way ANOVA with a Tukey post hoc comparison showed that incorporation of β2 subunits resulted in a statistically significant increase in Imax (F[2,6] = 12.7, P = 0.007; denoted by the asterisk). The Imax values obtained from the two α7β2-nAChR constructs were statistically indistinguishable from each other. ANOVA, analysis of variance.
Agonist and Antagonist Pharmacology of Concatemeric Human α7*-nAChRs
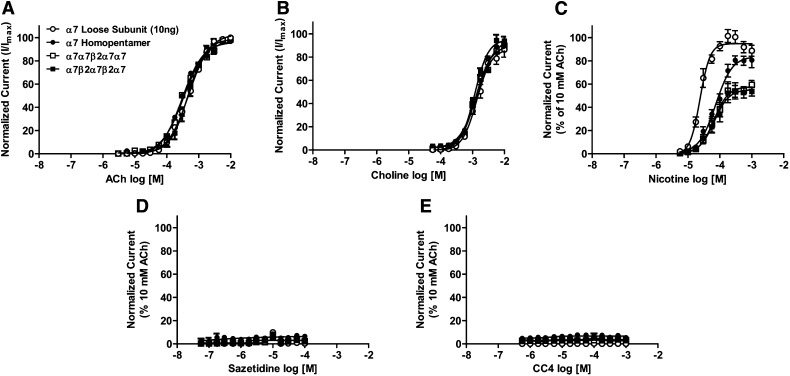
Pharmacological parameters of selected ligands were determined at concatenated α7*-nAChRs. Compounds chosen included the prototypical agonists, ACh and nicotine, choline (which is a relatively selective agonist of α7-nAChR; Alkondon et al., 1997), and two further agonists with established selectivity for other β2*-nAChR subtypes (sazetidine-A and CC4; Xiao et al., 2006; Kozikowski et al., 2009; Sala et al., 2013). Agonist pharmacological profiles for (α7)5-, (α7)4(β2)1-, and (α7)3(β2)2-nAChR subtypes were largely indistinguishable from each other, and from that for nonconcatemeric (loose-subunit), homomeric α7nAChRs (Fig. 4; Table 4). The only exception is that nicotine has significantly lower efficacy (normalized to that of ACh) at both α7β2*-nAChR subtypes than at concatemeric (α7)5-nAChRs or unlinked α7- nAChRs (which are statistically indistinguishable on this measure). There was also a trend toward lower nicotine potency across all concatemeric α7*-nAChR constructs, but this did not reach statistical significance (see Table 4). The observed slight trend toward lower choline efficacy, although not significant, is suggestive of the previous observation of 50–70% efficacy of choline versus ACh at putative α7β2-nAChRs expressed from nonlinked subunits (Khiroug et al., 2002; Zwart et al., 2014). Strikingly, both sazetidine-A and CC4 were very weak agonists (<10% efficacy normalized to that of ACh) at all α7*-nAChR subtypes tested, including both α7β2-nAChRs, making it impossible to reliably calculate EC50 or Hill slope values from the resulting concentration-response data.
Fig. 4.
Agonist concentration-response profiles for α7- and α7β2-nAChRs. Oocytes were injected with mRNA encoding unlinked α7 subunits (○), concatenated α7 homopentamers (●), or concatenated α7β2 pentameric concatemers (□ indicates α7β2-nAChR with the β2 subunit in position 3; ▪ indicates α7β2-nAChR with the β2 subunit in positions 2 and 4). Oocytes were perfused with the following nAChR agonists: ACh (10−5.5 to 10−2; n = 6) (A), choline (10−4.25 to 10−2; n = 3) (B), nicotine (10−5.5 to 10−3; n = 3) (C), sazetidine-A (10−7.5 to 10−4; n = 3) (D), or CC4 (10−6.5 to 10−3; n = 3) (E). All responses within each group were normalized to an initial control stimulation with 10 mM ACh. Data points represent the mean ± S.E.M. Drug potency and efficacy parameters were calculated by nonlinear least-squares curve fitting to the Hill equation (see Materials and Methods). The resulting pharmacological parameters and statistical analyses are summarized in Table 4.
TABLE 4.
α7 and α7β2-nAChR agonist pharmacological parameters
Values are the mean ± S.E.M. of the number of indicated replicates (n=).
| Subtype | ACh |
Choline |
Nicotine |
Sazetidine-A |
CC4 |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n= | log(EC50/M) | nH | Efficacy | n= | log(EC50/M) | nH | Efficacy | n= | log(EC50/M) | nH | Efficacy | n= | log(EC50/M) | nH | Efficacy | n= | log(EC50/M) | nH | Efficacy | |
| α7 (unlinked) | 6 | −3.3 ± 0.2 | 1.3 ± 0.1 | 103 ± 3 | 3 | −2.9 ± 0.04 | 1.9 ± 0.3 | 87 ± 4 | 3 | −4.6 ± 0.14 | 2.7 ± 0.5 | 95 ± 2 | 3 | ND | ND | 3.0 ± 0.5 | 3 | ND | ND | 3.0 ± 1.0 |
| α7-α7-α7-α7-α7 | 6 | −3.5 ± 0.3 | 1.3 ± 0.1 | 96 ± 2 | 3 | −2.9 ± 0.17 | 1.6 ± 0.4 | 96 ± 8 | 3 | −4.1 ± 0.15 | 1.7 ± 0.3 | 84 ± 4 | 3 | ND | ND | 7.0 ± 3.0 | 3 | ND | ND | 6.7 ± 1.6 |
| α7-α7-β2-α7-α7 | 6 | −3.5 ± 0.03 | 1.3 ± 0.1 | 97 ± 2 | 3 | −2.9 ± 0.03 | 1.8 ± 0.2 | 90 ± 3 | 3 | −4.2 ± 0.15 | 1.9 ± 0.5 | 58 ± 3* | 3 | ND | ND | 3.5 ± 0.5 | 3 | ND | ND | 3.2 ± 0.6 |
| α7-β2-α7-β2-α7 | 6 | −3.3 ± 0.3 | 1.6 ± 0.3 | 99 ± 3 | 3 | −2.9 ± 0.12 | 2.0 ± 0.2 | 95 ± 2 | 3 | −4.2 ± 0.08 | 1.9 ± 0.6 | 55 ± 4* | 3 | ND | ND | 3.4 ± 0.4 | 3 | ND | ND | 3.5 ± 1.0 |
Agonist logEC50, Hill slope (nH), and efficacy values relative to a maximally effective (10 mM) concentration of ACh were derived by nonlinear least-squares curve fitting of the data shown in Fig. 4 to the Hill model. α7-only nAChR expressed in Xenopus oocytes from unlinked subunits were used as a control group, to which the functional properties of α7-nAChR concatemeric constructs were compared (N-to-C-terminal subunit orders are shown). Pharmacological parameters measured for each agonist were generally indistinguishable between all four groups of oocytes, with one exception: The relative efficacy of nicotine was lower for both α7β2 subtypes tested compared with the α7 unlinked control group (although the α7-only concatemer group was not different to the control). One-way analysis of variance: F[3,8] = 34.2, P < 0.001, followed by Dunnett’s post hoc test (*P < 0.05). ND, not determinable (reliable curve fitting is not possible for very low-efficacy compounds).
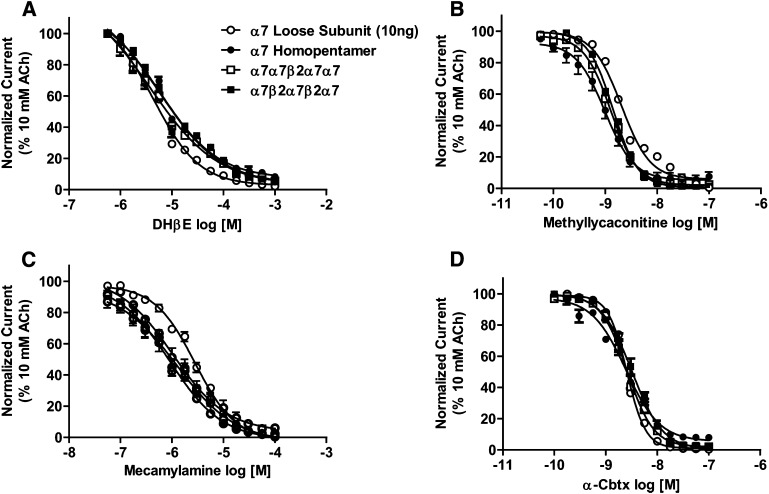
Concentration-response relationships were also explored for archetypal α7 antagonists (MLA and the snake venom α-toxin, α-Cbtx), together with the β2-selective antagonist DHβE and the noncompetitive antagonist mecamylamine (Fig. 5). The resulting pharmacological parameters are summarized in Table 5. Similar to the agonist pharmacology, antagonist responses were statistically indistinguishable between the α7* subtypes (including between α7-only nAChRs expressed from either unlinked subunits, or from the concatenated α7 homopentameric construct).
Fig. 5.
Antagonist concentration response profiles for α7- and α7β2-nAChRs. Oocytes were injected with mRNA encoding unlinked α7 subunits (○), concatenated α7 homopentamers (●), or concatenated α7β2 pentameric concatemers (□ indicates α7β2 nAChR with the β2 subunit in position 3; ▪ indicates α7β2 nAChR with the β2 subunit in positions 2 and 4). Before antagonists were applied to each oocyte, a control 10 mM ACh-evoked response was measured. Oocytes were preperfused with the following nAChR antagonists: DHβE (10−6.25 to 10−3; n = 3) (A), methyllycaconitine (10−10.5 to 10−7; n = 3) (B), mecamylamine (10−7.25 to 10−4; n = 3) (C), or α-Cbtx (10−10 to 10−7; n = 3) (D). The magnitudes of subsequent 10 mM ACh stimulations were compared with that of the initial control. Data points represent the mean ± S.E.M. Drug potency and efficacy parameters were calculated by nonlinear least-squares curve fitting to the Hill equation (see Materials and Methods). The resulting pharmacological parameters and statistical analyses are summarized in Table 5.
TABLE 5.
α7 and α7β2-nAChR antagonist pharmacological parameters
Values are the mean ± S.E.M. of the number of indicated replicates (n=).
| Subtype | DHβE |
MLA |
Mecamylamine |
α-Cbtx |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n= | log(IC50/M) | nH | n= | log(IC50/M) | nH | n= | log(IC50 / M) | nH | n= | log(IC50/M) | nH | |
| α7 (unlinked) | 3 | −5.2 ± 0.05 | −1.0 ± 0.1 | 3 | −8.7 ± 0.1 | −1.6 ± 0.2 | 3 | −5.6 ± 0.2 | 3 | −8.6 ± 0.1 | 2.4 ± 0.5 | |
| α7-α7-α7-α7-α7 | 3 | −5.3 ± 0.07 | −0.8 ± 0.1 | 3 | −9.0 ± 0.1 | −1.6 ± 0.2 | 3 | −6.0 ± 0.2 | 3 | −8.6 ± 0.1 | 1.3 ± 0.8 | |
| α7-α7-β2-α7-α7 | 3 | −5.4 ± 0.10 | −0.6 ± 0.1 | 3 | −8.9 ± 0.1 | −1.7 ± 0.1 | 3 | −6.0 ± 0.1 | 3 | −8.6 ± 0.1 | 1.8 ± 0.5 | |
| α7-β2-α7-β2-α7 | 3 | −5.4 ± 0.10 | −0.7 ± 0.1 | 3 | −8.8 ± 0.1 | −1.8 ± 0.1 | 3 | −6.0 ± 0.2 | 3 | −8.5 ± 0.2 | 1.5 ± 0.6 | |
Antagonist log IC50 and Hill slope (nH) values were derived by nonlinear least-squares curve fitting of the data shown in Fig. 5 to the Hill model. Pharmacological parameters obtained for each antagonist were statistically indistinguishable between all four groups of oocytes according to analysis with one-way analysis of variance.
Discussion
This study provides the first direct evidence that α7β2-nAChRs are expressed in the mammalian central nervous system. This is demonstrated by isolation of Bgtx-binding or α7 subunit–containing complexes also shown to contain β2 subunits from human or mouse forebrain samples. In addition, we have demonstrated for the first time that multiple human α7β2-nAChR isoforms of defined subunit composition have pharmacological profiles similar to each other and to homopentameric α7-nAChRs.
Our findings indicate that α7β2-nAChRs are found in post mortem, human basal forebrain but not in the cerebellum. Note that total amounts of α7*-nAChRs are <2-fold different in the two brain regions. Specificity of the anti-α7 or anti-β2 Abs used in Western blot analysis of these nAChRs is demonstrated by control studies using cell lines transfected with specific nAChR subunits, and by studies using WT and subunit-null mice. We also found α7β2-nAChR expression in mouse basal forebrain but not hippocampus. Our results agree with earlier findings of α7β2-nAChR expression in mouse basal forebrain (Liu et al., 2009) but not with the same investigators’ study in mouse hippocampus (Liu et al., 2012). There could be several explanations for these seemingly discrepant observations. nAChR α7 and β2 subunit mRNAs are coexpressed in both basal forebrain and hippocampal cholinergic neurons (Azam et al., 2003). However, fewer than 3% of β2*-nAChRs in WT mouse basal forebrain extracts (this study) were associated with the α7 subunit. This indicates that the large majority of α-Bgtx–binding sites are homomeric α7-nAChRs. Accordingly, we feel that the most likely explanation for the lack of an immunochemically detectable α7β2-nAChR in mouse hippocampus is that it is even less prevalent than in basal forebrain. The previous electrophysiology experiments (Liu et al., 2012) used brain slices from very young mice, whereas our work used tissue from 4- to 6-month-old mice. Therefore, it is also possible that mouse hippocampal α7β2-nAChR expression levels fall from early life into adulthood. Multiple examples of developmental modulation of nAChR subunit expression (including of α7) have previously been seen (Zoli et al., 1995; Conroy and Berg, 1998; Zhang et al., 1998; Balestra et al., 2000; Flora et al., 2000).
The use of a linked-subunit approach allowed us, for the first time, to directly assess the effects of defined β2-nAChR subunit incorporation on α7*-nAChR function. Of critical importance, no significant differences in EC/IC50 values or efficacy relative to ACh were seen between concatenated or unlinked-subunit homomeric α7-nAChRs. This indicates that, as has previously been shown for α3β4*-nAChR (George et al., 2012; Stokes and Papke, 2012), α4β2-nAChR (Zhou et al., 2003; Carbone et al., 2009; Mazzaferro et al., 2011; Eaton et al., 2014), and α6β2*-nAChR (Kuryatov and Lindstrom, 2011) subtypes, introduction of appropriately sized linkers can be performed without altering nAChR functional pharmacology. Several of these previous studies also showed that concatemeric constructs were assembled correctly. To further confirm correct that concatemers were being assembled correctly and not fragmenting and rearranging into unanticipated functional forms, we also coinjected unlinked β2 subunits containing a gain-of-function mutation (L9’S) in the second transmembrane domain. This additional control has previously been used by us and others (Carbone et al., 2009; Eaton et al., 2014). If concatemer fragments were contributing to the functional nAChR population, the β2-gain-of-function subunit would assemble into resulting α7*-nAChR as previously shown (Khiroug et al., 2002; Murray et al., 2012; Zwart et al., 2014). Therefore, if fragments containing α7 were present, this would result in appearance of a novel α7β2-gain-of-function population with distinctive (more agonist-sensitive) properties. No such effect was seen.
It is noted, however, that overall function was reduced when α7-nAChR homopentamers were expressed from a concatemeric construct as opposed to unlinked subunits. This relative diminution in function of concatenated nAChR constructs has been noted in the previous publications cited above and appears to be a regular feature of using concatemeric nAChR constructs. Importantly, both (α7)4(β2)1-and (α7)3(β2)2-nAChR concatemeric constructs expressed more function than did the (α7)5-nAChR concatemer. This is the opposite of the situation in which loose β2-nAChR subunits are coexpressed with α7 subunits (Murray et al., 2012) and replicates an earlier finding in which coexpression of unlinked α5, α3, and β4 nAChR subunits reduced function compared with expression of loose α3 and β4 subunits alone, but incorporation of the α5 subunit into a concatemeric construct actually increased observed function of an α3β4*-nAChR pentameric concatemer (George et al., 2012). As in the previous publication, we suspect that uncontrolled assembly of an unlinked additional subunit (in this case β2) may be deleterious, but directed assembly may result in greater functional expression of the new nAChR subtype. Certainly, this study provides direct evidence that β2 subunit incorporation into α7*-nAChRs is compatible with agonist-induced function.
The pharmacological profiles of α7β2-nAChRs were very similar to those of homopentameric α7-nAChRs. Even agonists (sazetidine-A, CC4) and an antagonist (DHβE) previously shown to have significant β2*-nAChR selectivity had indistinguishable pharmacology across homomeric α7-nAChRs and the two different α7β2-nAChR isoforms. Each of these findings matches those very recently published using Xenopus oocytes expressing α7 and β2 subunits at a 1:10 ratio (Zwart et al., 2014). The only statistically significant difference in this study was a diminution of nicotine’s efficacy relative to that of ACh in the two α7β2-nAChR isoforms (also seen by Zwart et al., 2014). This nicotine partial agonism further confirms that β2 was incorporated into α7β2-nAChR concatemers as planned and may represent a pharmacological marker for the presence of α7β2-nAChRs. The same may be true of the slower desensitization kinetics measured for the (α7)3(β2)2 (Fig. 3), although it is important to note the limitations of measuring receptor kinetics in a Xenopus oocyte system (Papke, 2010). We note that the similar α7-nAChR versus α7β2-nAChR potency of DHβE observed by us and by Zwart et al. (2014) does not match the observations made in two previous studies (Liu et al., 2009; Murray et al., 2012). The reason for this discrepancy between the pairs of studies is not clear, but two possible explanations occur. First, the differences previously measured are relatively subtle, so they may be hard to reproduce. Related to this point, we note that the Hill slopes of the α7β2-nAChR DHβE concentration response curves (Fig. 5A) are shallower than those measured for other competitive antagonists (≤1, as opposed to significantly >1 for MLA and α-Cbtx). This would tend to obscure fine differences in IC50 values. Second, other α7 and β2 subunit associations are possible, in addition to those used in the α7β2-nAChR concatemers deployed in this study. It is possible that an α7β2*-nAChR population expressed from unlinked subunits may assemble differently, giving rise to the slightly different DHβE sensitivity previously measured. This would match the previous experience in which α3β4α5-nAChR pharmacology perfectly matched between concatenated and unlinked-subunit nAChRs, but that of loose-subunit α3β4-only nAChRs was close, but not identical, between loose-subunit and concatemeric constructs (George et al., 2012). Further work may be needed to understand the (admittedly subtle) pharmacological differences between alternative α7β2-nAChR subunit stoichiometries and association orders.
Overall, however, the functional pharmacology of α7-nAChR and α7β2-nAChR subtypes is remarkably similar. This observation indirectly supports the concept that activation of α7β2-nAChRs may be predominantly or exclusively mediated only through agonist binding sites at α7/α7 (not α7/β2) interfaces (Murray et al., 2012). If this is true, it seems unlikely that any competitive agonist could exhibit a significantly different potency between the α7-nAChR and α7β2-nAChR. However, antagonists capable of disrupting the allosteric transitions required for nAChR activation (Celie et al., 2005), and of selectively binding to α7/β2 interfaces, could be valuable in this regard as could other noncompetitive ligands. In the concatemeric (α7)4(β2)1-nAChR construct (subunit order α7-α7-β2-α7-α7), only three α7/α7 subunit interfaces will be retained (between the first two subunits, the last two subunits, and between the first and last subunits that will assemble together to complete the pentameric nAChR structure). In the (α7)3(β2)2-nAChR construct, only the α7/α7 interface formed between the first and last subunits will be retained. At first glance, it may seem remarkable that an α7*-nAChR containing such a diminished complement of putative agonist binding sites could be effectively activated. However, elegant recent work indicates that the nAChR, including α7-nAChR, can be activated effectively by as few as one agonist binding site (Rayes et al., 2009; Williams et al., 2011; Andersen et al., 2013).
That α7β2-nAChRs are relatively scarce in basal forebrain does not imply that their role is necessarily insignificant. For example, α6β2*-nAChR expression on substantia nigra/ventral tegmental area dopamine projections comprises <10% of all β2*-nAChRs in dopamine terminal regions (Whiteaker et al., 2000; Gotti et al., 2005), but this subtype is extremely important in controlling local neuronal behavior and signal processing (Exley and Cragg, 2008; Exley et al., 2008). Cholinergic neurons constitute only a fraction (10–15%) of basal forebrain neurons (Semba, 2000) and the proportion of a α7β2-nAChRs in these neurons may therefore be relatively large. The basal forebrain cholinergic system provides primary cholinergic innervations to limbic and cortical brain structures, and expresses nAChRs that participate in the cholinergic transmission and cognitive processes associated with learning and memory (Voytko et al., 1994; Hernandez et al., 2010). One of the most marked pathologic changes in the brain in Alzheimer disease is the degeneration of this cholinergic projection and the consequent reduction in the number of nAChRs (Dumas and Newhouse, 2011; Pinto et al., 2011). A number of studies have found that the β-amyloid (Aβ) peptide (a hallmark of Alzheimer disease) plays a critical role in neuronal degeneration and subsequent memory deficits (Price et al., 1985; Holtzman et al., 1992; Wenk, 1993; Fraser et al., 1997; Capsoni et al., 2000; Dolga et al., 2009). Furthermore, a recent electrophysiological study has demonstrated that Aβ binds with higher affinity to α7β2-nAChR than to α7-nAChR, and that this can produce hippocampal neuronal hyperexcitation (through α7-nAChR upregulation) and subsequent neurodegeneration (Liu et al., 2013).
Post mortem tissue is an underused substrate for genetic and/or preclinical studies, and provides a translational element that is difficult to recapitulate in animal models alone (McCullumsmith et al., 2014). This study’s definitive evidence that α7β2*-nAChRs are found in human as well as mouse basal forebrain provides valuable support for the concept that this subtype may be relevant to the study and etiology of Alzheimer disease. The similarities in human- and mouse-brain basal forebrain α7β2*-nAChR expression are also supportive of the use of mouse models in this context.
Supplementary Material
Acknowledgments
The authors thank Dr. Emanuele Sher and Dr. Rudolf Zwart (Eli Lilly Company) for their continuous support and for supply of human basal forebrain samples. They also thank Dr. Jenny Court (Newcastle upon Tyne General Hospital) for supplying human cerebellum tissue. Cerebellum tissue for this study was provided by the Newcastle Brain Tissue Resource.
Abbreviations
- α-Bgtx
α-bungarotoxin
- Ab
antibody
- α-Cbtx
α-cobratoxin
- ACh
acetylcholine
- BSA
bovine serum albumin
- CC4
1,2-bis-N-cystinylethane
- CYT
intracytoplasmic loop
- DHβE
dihydro-β-erythroidine
- Epi
epibatidine
- KO
subunit-null knock out mutants
- MLA
methyllycaconitine
- nAChR
nicotinic acetylcholine receptor
- TEVC
two-electrode voltage clamp
- WT
wild-type
Authorship Contributions
Participated in research design: Zoli, George, Lukas, Whiteaker, Gotti.
Conducted experiments: Moretti, George, Pistillo.
Contributed new reagents or analytic tools: Pistillo, Maskos, Whiteaker.
Performed data analysis: Moretti, George, Pistillo, Whiteaker.
Wrote or contributed to the writing of the manuscript: Zoli, Lukas, Whiteaker, Gotti.
Footnotes
This research was supported by the European Union Seventh Framework Programme [Grant HEALTH-F2-2008-20208 (to C.G. and M.Z.)], CNR Research Project on Aging, Regione Lombardia Project NUTEC [Grant 30263049], Italian Ministry of Health [Grant RF2009-1549619 (to M.Z.)], and the National Institutes of Health National Institute on Drug Abuse [Grant R21-DA026627], and Barrow Neurologic Foundation (to P.W.). The Newcastle Brain Tissue Resource is supported by the Research Councils UK Medical Research Council [Grant G0400074], National Institute for Health Research Newcastle Biomedical Research Centre, Newcastle upon Tyne National Health Service Foundation Trust, Newcastle University, and the Alzheimer’s Society and Alzheimer’s Research Trust [Brains for Dementia Research Project].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Albuquerque EX, Alkondon M, Pereira EFR, Castro NG, Schrattenholz A, Barbosa CTF, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A. (1997) Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther 280:1117–1136 [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EFR, Alkondon M, Rogers SW. (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89:73–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EFR, Cortes WS, Maelicke A, Albuquerque EX. (1997) Choline is a selective agonist of α7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci 9:2734–2742 [DOI] [PubMed] [Google Scholar]
- Andersen N, Corradi J, Sine SM, Bouzat C. (2013) Stoichiometry for activation of neuronal α7 nicotinic receptors. Proc Natl Acad Sci USA 110:20819–20824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan U, Leslie FM. (2003) Co-expression of α7 and β2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience 119:965–977 [DOI] [PubMed] [Google Scholar]
- Balestra B, Vailati S, Moretti M, Hanke W, Clementi F, Gotti C. (2000) Chick optic lobe contains a developmentally regulated alpha2alpha5beta2 nicotinic receptor subtype. Mol Pharmacol 58:300–311 [DOI] [PubMed] [Google Scholar]
- Capsoni S, Ugolini G, Comparini A, Ruberti F, Berardi N, Cattaneo A. (2000) Alzheimer-like neurodegeneration in aged antinerve growth factor transgenic mice. Proc Natl Acad Sci USA 97:6826–6831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone AL, Moroni M, Groot-Kormelink PJ, Bermudez I. (2009) Pentameric concatenated (alpha4)(2)(beta2)(3) and (alpha4)(3)(beta2)(2) nicotinic acetylcholine receptors: subunit arrangement determines functional expression. Br J Pharmacol 156:970–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celie PHN, Kasheverov IE, Mordvintsev DY, Hogg RC, van Nierop P, van Elk R, van Rossum-Fikkert SE, Zhmak MN, Bertrand D, Tsetlin V, et al. (2005) Crystal structure of nicotinic acetylcholine receptor homolog AChBP in complex with an alpha-conotoxin PnIA variant. Nat Struct Mol Biol 12:582–588 [DOI] [PubMed] [Google Scholar]
- Conroy WG, Berg DK. (1998) Nicotinic receptor subtypes in the developing chick brain: appearance of a species containing the alpha4, beta2, and alpha5 gene products. Mol Pharmacol 53:392–401 [DOI] [PubMed] [Google Scholar]
- Court JA, Johnson M, Religa D, Keverne J, Kalaria R, Jaros E, McKeith IG, Perry R, Naslund J, Perry EK. (2005) Attenuation of Abeta deposition in the entorhinal cortex of normal elderly individuals associated with tabacco smoking. neuropathol Appl Neurobiol 31:522–535 [DOI] [PubMed] [Google Scholar]
- Criado M, Valor LM, Mulet J, Gerber S, Sala S, Sala F. (2012) Expression and functional properties of α7 acetylcholine nicotinic receptors are modified in the presence of other receptor subunits. J Neurochem 123:504–514 [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S. (2004) Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci 25:317–324 [DOI] [PubMed] [Google Scholar]
- Dolga AM, Granic I, Nijholt IM, Nyakas C, van der Zee EA, Luiten PG, Eisel UL. (2009) Pretreatment with lovastatin prevents N-methyl-D-aspartate-induced neurodegeneration in the magnocellular nucleus basalis and behavioral dysfunction. J Alzheimers Dis 17:327–336 [DOI] [PubMed] [Google Scholar]
- Drisdel RC, Green WN. (2000) Neuronal α-bungarotoxin receptors are α7 subunit homomers. J Neurosci 20:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Newhouse PA. (2011) The cholinergic hypothesis of cognitive aging revisited again: cholinergic functional compensation. Pharmacol Biochem Behav 99:254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JB, Lucero LM, Stratton H, Chang Y, Cooper JF, Lindstrom JM, Lukas RJ, Whiteaker P. (2014) The unique α4+/-α4 agonist binding site in (α4)3(β2)2 subtype nicotinic acetylcholine receptors permits differential agonist desensitization pharmacology versus the (α4)2(β2)3 subtype. J Pharmacol Exp Ther 348:46–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. (2008) Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology 33:2158–2166 [DOI] [PubMed] [Google Scholar]
- Exley R, Cragg SJ. (2008) Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol 153 (Suppl 1):S283–S297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora A, Schulz R, Benfante R, Battaglioli E, Terzano S, Clementi F, Fornasari D. (2000) Neuronal and extraneuronal expression and regulation of the human alpha5 nicotinic receptor subunit gene. J Neurochem 75:18–27 [DOI] [PubMed] [Google Scholar]
- Fraser SP, Suh YH, Djamgoz MB. (1997) Ionic effects of the Alzheimer’s disease beta-amyloid precursor protein and its metabolic fragments. Trends Neurosci 20:67–72 [DOI] [PubMed] [Google Scholar]
- George AA, Lucero LM, Damaj MI, Lukas RJ, Chen X, Whiteaker P. (2012) Function of human α3β4α5 nicotinic acetylcholine receptors is reduced by the α5(D398N) variant. J Biol Chem 287:25151–25162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod R, Crabtree G, Ernstrom G, Ramirez-Latorre J, McGehee D, Turner J, Role L. (1999) Heteromeric complexes of alpha 5 and/or alpha 7 subunits. Effects of calcium and potential role in nicotine-induced presynaptic facilitation. Ann N Y Acad Sci 868:578–590 [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F. (2004) Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol 74:363–396 [DOI] [PubMed] [Google Scholar]
- Gotti C, Hanke W, Maury K, Moretti M, Ballivet M, Clementi F, Bertrand D. (1994) Pharmacology and biophysical properties of α 7 and α 7-α 8 α-bungarotoxin receptor subtypes immunopurified from the chick optic lobe. Eur J Neurosci 6:1281–1291 [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Bohr I, Ziabreva I, Vailati S, Longhi R, Riganti L, Gaimarri A, McKeith IG, Perry RH, et al. (2006) Selective nicotinic acetylcholine receptor subunit deficits identified in Alzheimer’s disease, Parkinson’s disease and dementia with Lewy bodies by immunoprecipitation. Neurobiol Dis 23:481–489 [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Clementi F, Riganti L, McIntosh JM, Collins AC, Marks MJ, Whiteaker P. (2005) Expression of nigrostriatal alpha 6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by beta 3 subunit gene deletion. Mol Pharmacol 67:2007–2015 [DOI] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C. (2009) Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci 29:2272–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griguoli M, Cherubini E. (2012) Regulation of hippocampal inhibitory circuits by nicotinic acetylcholine receptors. J Physiol 590:655–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevi S, McKay J, Palfreyman M, Yassin L, Eshel M, Jorgensen E, Treinin M. (2002) The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J 21:1012–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Kayed R, Zheng H, Sweatt JD, Dineley KT. (2010) Loss of alpha7 nicotinic receptors enhances beta-amyloid oligomer accumulation, exacerbating early-stage cognitive decline and septohippocampal pathology in a mouse model of Alzheimer’s disease. J Neurosci 30:2442–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Li YW, DeArmond SJ, McKinley MP, Gage FH, Epstein CJ, Mobley WC. (1992) Mouse model of neurodegeneration: atrophy of basal forebrain cholinergic neurons in trisomy 16 transplants. Proc Natl Acad Sci USA 89:1383–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst R, Rollema H, Bertrand D. (2013) Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther 137:22–54 [DOI] [PubMed] [Google Scholar]
- Keyser KT, Britto LRG, Schoepfer R, Whiting P, Cooper J, Conroy W, Brozozowska-Prechtl A, Karten HJ, Lindstrom J. (1993) Three subtypes of alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors are expressed in chick retina. J Neurosci 13:442–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug SS, Harkness PC, Lamb PW, Sudweeks SN, Khiroug L, Millar NS, Yakel JL. (2002) Rat nicotinic ACh receptor α7 and β2 subunits co-assemble to form functional heteromeric nicotinic receptor channels. J Physiol 540:425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozikowski AP, Eaton JB, Bajjuri KM, Chellappan SK, Chen Y, Karadi S, He R, Caldarone B, Manzano M, Yuen PW, et al. (2009) Chemistry and pharmacology of nicotinic ligands based on 6-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]hex-5-yn-1-ol (AMOP-H-OH) for possible use in depression. ChemMedChem 4:1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Lindstrom J. (2011) Expression of functional human α6β2β3* acetylcholine receptors in Xenopus laevis oocytes achieved through subunit chimeras and concatamers. Mol Pharmacol 79:126–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendvai B, Vizi ES. (2008) Nonsynaptic chemical transmission through nicotinic acetylcholine receptors. Physiol Rev 88:333–349 [DOI] [PubMed] [Google Scholar]
- Lewis AS, Picciotto MR. (2013) High-affinity nicotinic acetylcholine receptor expression and trafficking abnormalities in psychiatric illness. Psychopharmacology (Berl) 229:477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Huang Y, Shen JX, Steffensen S, Wu J. (2012) Functional α7β2 nicotinic acetylcholine receptors expressed in hippocampal interneurons exhibit high sensitivity to pathological level of amyloid β peptides. BMC Neurosci 13:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Huang Y, Xue F, Simard A, DeChon J, Li G, Zhang J, Lucero L, Wang M, Sierks M, et al. (2009) A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J Neurosci 29:918–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Xie X, Lukas RJ, St John PA, Wu J. (2013) A novel nicotinic mechanism underlies β-amyloid-induced neuronal hyperexcitation. J Neurosci 33:7253–7263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novère N, Albuquerque EX, Balfour DJK, Berg DK, Bertrand D, Chiappinelli VA, Clarke PBS, Collins AC, et al. (1999) International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev 51:397–401 [PubMed] [Google Scholar]
- Mazzaferro S, Benallegue N, Carbone A, Gasparri F, Vijayan R, Biggin PC, Moroni M, Bermudez I. (2011) Additional acetylcholine (ACh) binding site at α4/α4 interface of (α4β2)2α4 nicotinic receptor influences agonist sensitivity. J Biol Chem 286:31043–31054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullumsmith RE, Hammond JH, Shan D, Meador-Woodruff JH. (2014) Postmortem brain: an underutilized substrate for studying severe mental illness. Neuropsychopharmacology 39:65–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray TA, Bertrand D, Papke RL, George AA, Pantoja R, Srinivasan R, Liu Q, Wu J, Whiteaker P, Lester HA, et al. (2012) α7β2 nicotinic acetylcholine receptors assemble, function, and are activated primarily via their α7-α7 interfaces. Mol Pharmacol 81:175–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Urtreger A, Göldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, Dani JA, Patrick JW, Beaudet AL. (1997) Mice deficient in the α7 neuronal nicotinic acetylcholine receptor lack α-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci 17:9165–9171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Maggi L, Barabino B, Eusebi F, Ballivet M. (1999) Nicotinic acetylcholine receptors assembled from the α7 and β3 subunits. J Biol Chem 274:18335–18340 [DOI] [PubMed] [Google Scholar]
- Papke RL. (2010) Tricks of perspective: insights and limitations to the study of macroscopic currents for the analysis of nAChR activation and desensitization. Mol Neurosci J 401:77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ, Mineur YS. (2012) Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76:116–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Léna C, Bessis A, Lallemand Y, Le Novère N, Vincent P, Pich EM, Brûlet P, Changeux JP. (1995) Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature 374:65–67 [DOI] [PubMed] [Google Scholar]
- Pinto T, Lanctôt KL, Herrmann N. (2011) Revisiting the cholinergic hypothesis of behavioral and psychological symptoms in dementia of the Alzheimer’s type. Ageing Res Rev 10:404–412 [DOI] [PubMed] [Google Scholar]
- Price DL, Cork LC, Struble RG, Whitehouse PJ, Kitt CA, Walker LC. (1985) The functional organization of the basal forebrain cholinergic system in primates and the role of this system in Alzheimer’s disease. Ann N Y Acad Sci 444:287–295 [DOI] [PubMed] [Google Scholar]
- Rayes D, De Rosa MJ, Sine SM, Bouzat C. (2009) Number and locations of agonist binding sites required to activate homomeric Cys-loop receptors. J Neurosci 29:6022–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riganti L, Matteoni C, Di Angelantonio S, Nistri A, Gaimarri A, Sparatore F, Canu-Boido C, Clementi F, Gotti C. (2005) Long-term exposure to the new nicotinic antagonist 1,2-bisN-cytisinylethane upregulates nicotinic receptor subtypes of SH-SY5Y human neuroblastoma cells. Br J Pharmacol 146:1096–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Braida D, Pucci L, Manfredi I, Marks MJ, Wageman CR, Grady SR, Loi B, Fucile S, Fasoli F, et al. (2013) CC4, a dimer of cytisine, is a selective partial agonist at α4β2/α6β2 nAChR with improved selectivity for tobacco smoking cessation. Br J Pharmacol 168:835–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K. (2000) Multiple output pathways of the basal forebrain: organization, chemical heterogeneity, and roles in vigilance. Behav Brain Res 115:117–141 [DOI] [PubMed] [Google Scholar]
- Stokes C, Papke RL. (2012) Use of an α3β4 nicotinic acetylcholine receptor subunit concatamer to characterize ganglionic receptor subtypes with specific subunit composition reveals species-specific pharmacologic properties. Neuropharmacology 63:538–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML, Olton DS, Richardson RT, Gorman LK, Tobin JR, Price DL. (1994) Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J Neurosci 14:167–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL. (1993) A primate model of Alzheimer’s disease. Behav Brain Res 57:117–122 [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Christensen S, Yoshikami D, Dowell C, Watkins M, Gulyas J, Rivier J, Olivera BM, McIntosh JM. (2007) Discovery, synthesis, and structure activity of a highly selective alpha7 nicotinic acetylcholine receptor antagonist. Biochemistry 46:6628–6638 [DOI] [PubMed] [Google Scholar]
- Whiteaker P, McIntosh JM, Luo S, Collins AC, Marks MJ. (2000) 125I-alpha-conotoxin MII identifies a novel nicotinic acetylcholine receptor population in mouse brain. Mol Pharmacol 57:913–925 [PubMed] [Google Scholar]
- Williams DK, Stokes C, Horenstein NA, Papke RL. (2011) The effective opening of nicotinic acetylcholine receptors with single agonist binding sites. J Gen Physiol 137:369–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ. (2006) Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol 70:1454–1460 [DOI] [PubMed] [Google Scholar]
- Yakel J. (2013) Cholinergic receptors: functional role of nicotinic ACh receptors in brain circuits and disease. Pflugers Arch 465:441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Liu C, Miao H, Gong ZH, Nordberg A. (1998) Postnatal changes of nicotinic acetylcholine receptor alpha 2, alpha 3, alpha 4, alpha 7 and beta 2 subunits genes expression in rat brain. Int J Dev Neurosci 16:507–518 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J. (2003) Human alpha4beta2 acetylcholine receptors formed from linked subunits. J Neurosci 23:9004–9015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Le Novère N, Hill JA, Jr, Changeux JP. (1995) Developmental regulation of nicotinic ACh receptor subunit mRNAs in the rat central and peripheral nervous systems. J Neurosci 15:1912–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Strotton M, Ching J, Astles PC, Sher E. (2014) Unique pharmacology of heteromeric α7β2 nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. Eur J Pharmacol 726:77–86 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.