Summary
Myeloid-derived suppressor cells (MDSC) play a significant role in tumor-induced immune suppression. Targeting their function could improve antitumor therapies. Previously we demonstrated that phosphodiesterase 5 (PDE5) inhibition in MDSCs augmented antitumor immunity in murine models. Here we show how the addition of the PDE5 inhibitor, tadalafil, in a patient with end-stage relapsed/refractory multiple myeloma reduced MDSC function and generated a dramatic and durable anti-myeloma immune and clinical response. Strategies targeting MDSC function with PDE5 inhibitors represent a novel approach that can augment the efficacy of tumor-directed therapies.
Introduction
A major impediment to effective cancer immunotherapy lies in the host’s inability to overcome the intrinsic immunosuppressive mechanisms associated with increasing tumor growth. A complex immunosuppressive network has been described ranging from immune editing of the tumor to the ability of the tumor to delete or anergize tumor-specific T-cell function (1). This negative immune feedback mechanism, which initially evolved to control excessive inflammation, limits the generation of effective tumor-specific immunity. Myeloid-derived suppressor cells (MDSCs) play a central role in mediating tumor-induced tolerance (2). Numerous tumor-derived factors induce MDSCs and lead to their accumulation that parallels the increasing tumor burden. MDSC-induced immune suppression is accomplished primarily through upregulation of inducible nitric oxide synthase (iNOS) and overexpression of arginase-1 (Arg-1). As such, therapies aimed at inhibiting iNOS and Arg-1 production could enhance antitumor immunity. Previously we have demonstrated the ability of phosphodiesterase-5 (PDE5) inhibitors to augment antitumor immunity through the downregulation of MDSC-dependent iNOS and Arg-1 activity in murine tumor models (3). Now we describe a patient with end-stage multiple myeloma (MM) previously refractory to lenalidomide in whom responsiveness to lenalidomide-based therapy was restored upon the addition of the PDE5 inhibitor, tadalafil.
Case Report
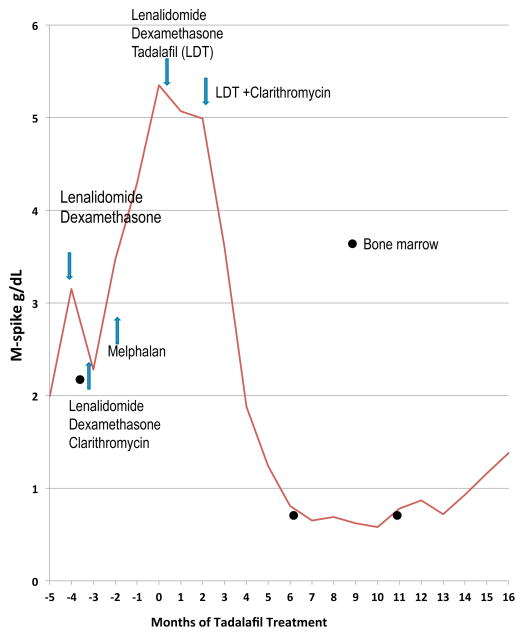
A 50 year-old male was diagnosed with IgG kappa, Durie Salmon stage IIIb myeloma in 2002. He presented with a hemoglobin level of 6 g/dL and acute renal failure (creatinine level of 4.3mg/dL). At diagnosis, his serum monoclonal (M) spike was 8g/dL and a 24-hour urine revealed a urine monoclonal spike of 11.7 g. The bone marrow showed hyperdiploidy with a 13q deletion. He received induction therapy with vincristine, adriamycin and dexamethasone (VAD) followed by autologous stem cell transplant with which he achieved a near CR but relapsed one year later. He was treated with multiple agents including interferon-α, thalidomide, bortezomib-thalidomide-dexamethasone and high dose cyclophosphamide. Five years after his initial presentation, he was started on lenalidomide and dexamethasone with a reduction in his monoclonal protein after 2 cycles. However, drug-related toxicity resulted in lenalidomide dose reductions with subsequent increases in the disease burden. Adding clarithromycin to lenalidomide and dexamethasone resulted in a slight reduction in disease burden but ultimately discontinuation of lenalidomide due to drug intolerance. This was followed by a cycle of melphalan, and subsequently bortezomib-pegylated doxorubicin-dexamethasone with progressive disease. His M-spike then rose to 5.35 g/dL with significant marrow suppression requiring one to two weekly red cell and platelet transfusions (Fig 1 and Table 1). Aware of our previous work, the patient initiated himself on treatment with the PDE5 inhibitor, tadalafil, while on bortezomib with no response. He was then switched to lenalidomide-dexamethasone because of lenalidomide’s immunomodulatory properties. Despite his prior intolerance to lenalidomide he was now able to tolerate the lenalidomide - dexamethasone in combination with tadalafil and demonstrated a clinical benefit with a decline in his M-spike to 4.4 g/dL. Clarithromycin was then added because of its anti-myeloma efficacy (4) and the four-drug combination resulted in a dramatic clinical response. He had a 90% reduction in his disease burden (very good partial response) and his serum M-spike nadired at 0.58 g/dL after 11 months of treatment with this combination therapy. Importantly, his quality of life improved significantly. He became transfusion-independent within 7 months of this combination, reported considerable improvement in fatigue and became a licensed scuba diver shortly thereafter. He enjoyed a progression-free interval of 14 months. He died from complications of an H1N1 infection. After 18 months on treatment he showed evidence of disease progression with an M-spike of 1.38 g/dL.
Figure 1.
M-spike graph. M-spike (g/dL) of 56 year old male patient with IgGκ Stage IIIb myeloma. The patient relapsed Month -5 and showed evidence of end-stage multiple myeloma refractory to all prior therapies. Tadalafil was added to the lenalidomide/dexamethasone treatment in Month 0. The M-spike fell from 5.35 g/dL at the start of tadalafil therapy to 4.4 g/dL. After 2 months, clarithromycin was added and the M-spike further dropped to a low of 0.58g/dL. 15 months after the addition of tadalafil, the patient began showing an increase in his M-spike. The patient eventually died due to complications that arose from an H1N1 infection and not from myeloma. Bone marrow samples collected are indicated with the filled circle and peripheral blood collections are indicated with the filled diamond shape.
Table 1.
Clinical course and transfusion requirements in response to various regimens.
| Month | M Spike (mg/dL) | RBC transfusion | Platelet transfusion | Regimen | Reason for change in regimen |
|---|---|---|---|---|---|
| −6 | 1.99 | 0 | 0 | LD | |
| −5 | 3.15 | 0 | 0 | LD | |
| −4 | 2.28 | 0 | 0 | LDC | Disease progression on Ld |
| −3 | 3.47 | 4 | 4 | Melphalan | Intolerability to LDC |
| −2 | NM | 12 | 12 | None | |
| −1 | 4.86 | 6 | 6 | VDD | Disease progression |
| 0 | 5.35 | 6 | 6 | VDDT/LDT | Tadalafil initiated by patient |
| 1 | 5.07 | 8 | 8 | LDT | Disease progression on VDD |
| 2 | 4.4 | 6 | 6 | LDT | |
| 3 | NM | 6 | 6 | LDCT | Synergy of Clarithromycin & Lenalidomide |
| 4 | 3.6 | 4 | 4 | LDCT | |
| 5 | 1.88 | 2 | 0 | LDCT | |
| 6 | 1.24 | 0 | 0 | LDCT | |
| 7 | 0.81 | 2 | 0 | LDCT | |
| 8 | 0.65 | 0 | 0 | LDCT | |
| 9 | 0.69 | 0 | 0 | LDCT | |
| 10 | 0.62 | 0 | 0 | LDCT | |
| 11 | 0.58 | 0 | 0 | LDCT | |
| 12 | 0.78 | 0 | 0 | LDCT | |
| 13 | 0.87 | 0 | 0 | LDCT | |
| 14 | 0.72 | 0 | 0 | LDCT | |
| 15 | 0.93 | 0 | 0 | LDCT | |
| 16 | 1.16 | 2 | 0 | LDCT |
LD: Lenalidomide and dexamethasone; LDC: Lenalidomide, dexamethasone and clarithromycin; VDD: Bortezomib, liposomal doxorubicin and dexamethasone; VDDT: Bortezomib, liposomal doxorubicin, dexamethasone and tadalafil; LDT: Lenalidomide, dexamethasone and tadalafil; LDCT: Lenalidomide, dexamethasone, clarithromycin and tadalafil; NM: not measured. RBC transfusion measured as units of red cells transfused per month. Platelet transfusion measured as units of single donor platelets transfused per month.
Materials and Methods
Patient samples
All samples were procured under an IRB-approved informed consent at the indicated time points. Bone marrow (BM) and peripheral blood lymphocytes (PBL) samples were ficolled and frozen in 90% autologous serum and 10% DMSO.
Flow cytometry
Fluorochrome-labeled CD14, HLA-DR, CD15, IL4Rα, CD4, CD25, FOXP3 and TCRζ antibodies and isotype controls were purchased from BD Pharmingen. Reactive oxidative species (ROS) antibodies and isotype controls (Imagine-IT Live Green) were purchased from Invitrogen. BM and PBL samples were viably thawed, stained and analyzed with multicolor flow cytometry on the BD FACS Calibur. Data were acquired and analyzed utilizing Cell Quest software (BD).
Quantitative RT-PCR
CD14+ cells were selected from unfractionated BM utilizing MACS antibodies and columns from Miltenyi. The PureLink RNA Micro Kit (Invitrogen) was used to isolate total RNA from 5×105 cells per the manufacturer’s instructions. Reverse-transcription PCR was performed using the Applied Biosystems High Capacity RNA-to-cDNA as per manufacturer’s instructions. Real-time PCR was performed using the following primers: Arg-1 (Forward Primer: AAG GAA AGA TTC CCG ATG TG, Reverse Primer: CCA CGT CTC TCA AGC CAA TA) and iNOS (Forward Primer: TGC GTT ACT CCA CCA ACA AT, Reverse Primer: ATG AGC TGA GCA TTC CAC AC). The expression of iNOS and Arg-1 was determined using real-time PCR performed on an AB 7500 Real-Time PCR system (Applied Biosystems). β-actin was used as the internal reference.
Immunohistochemistry
Slides were stained using a Ventana Discovery XT automated system (Ventana Medical Systems, Tucson) as per manufacturer’s protocol. Briefly, slides were deparaffinized with EZ Prep solution (Ventana). Heat-induced antigen retrieval method was used in RiboCC(#760–107, Ventana). The slides were incubated with the murine monoclonal nitrotyrosine antibody, (#MAB5404, Millipore, Temecula, CA) for 32 min. The Ventana anti-mouse secondary antibody was used for 16 min. The detection system used was the Ventana OmniMap kit and slides were then counterstained with hematoxylin.
Tumor specificity
Unfractionated BM cells from the indicated time points were CFSE-labeled using the Cell Trace CFSE kit from Invitrogen and then pulsed for 72 hours at 37°C with either 50ug/ml of U266 and H929 myeloma cell line lysates, SW780 - a bladder carcinoma cell line, or left unpulsed. The cells were then harvested and stained for CD3 and IFNγ. Tumor-specific T cells were quantified by the IFNγ+ production of CD3+/CFSElow cells.
T-cell expansion
Dynabeads Human T –Activator CD3/CD28 beads (Life Sciences) were added to the unselected and CD14-depleted BM at a ratio of 3:1, beads: T cell and cultured for 5 days. The beads were magnetically removed and the overall CD3 expansion was calculated.
Results
Effect of PDE5 Inhibition on Myeloid-Derived Suppressor Cells
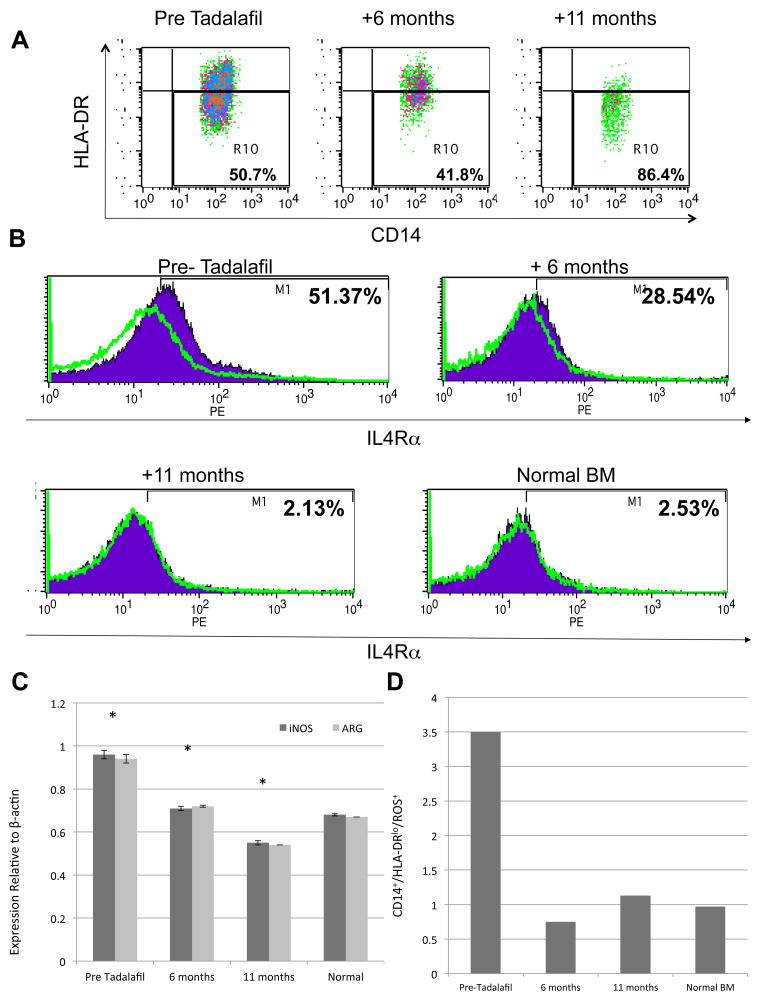
While no clear phenotype exists for describing human MDSCs, IL4Rα expression has been associated with the suppressive phenotype of MDSCs (5). In this study, MDSCs were identified as IL14+ or IL15+/HLA-DRlow/IL4Rα+. With tadalafil treatment, the actual number of CD14+ cells decreased from 7.5% prior to treatment to 1.9% at 11 months. While the percentage of CD14+/HLA-DRlow cells increased on treatment (Fig 2A), IL4Rα expression went from 51.37% of the CD14+/HLA-DRlow cells pre-treatment to 2.13% at 11 months, which corresponded to the same percentage found in the bone marrow of normal donors (Fig 2B). We then sought to determine whether this reduction in the percentage of “suppressive” MDSCs correlated with changes in their functional characteristics. Both Arg-1 and iNOS expression were reduced with tadalafil treatment (Fig 2C). Reactive oxygen species (ROS) also plays a critical role in suppressing antigen-specific CD8+ T cell responses (6,7). A reduction of ROS expression would be critical to both reducing the inhibitory effect of MDSCs and increasing T cell-mediated antitumor immunity. We thus examined ROS expression on the CD14+/HLA-DRlow cells (Fig 2D). ROS levels were reduced to baseline by 6 months. Of note, these changes were significantly more pronounced in the bone marrow (the tumor site) than in the blood.
Figure 2.
Characterization of bone marrow (BM) MDSC. (a) Total number of BM CD14+ cells decreased over time when staining the Pre Tadalafil, +6 month and +11 month timepoints (b) IL4Rα (purple fill) and isotype control (green line) staining of CD14+/HLA-DRlo cells over time. IL4Rα expression showed a decrease from Pre Tadalafil, +6 months, to near normal expression at +11 months of therapy. (c) iNOS and Arg-1 expression levels relative to β-actin in Pre Tadalafil, +6 months, +11 months and a normal BM sample. (d) ROS staining of CD14+/HLA-DRlo cells at the Pre Tadalafil, +6 month and +11 month time points as well as a normal BM for comparison.
Effect of PDE5 Inhibition on Nitrosylation
It has been shown previously that Arg-1 and iNOS together can generate peroxynitrites capable of inducing protein tyrosine nitrosylation (8), which induce CD8 tolerance through the disruption of binding of specific peptide – major histocompatibility complex dimers (9) and that inhibiting these enzymes can reverse this process (10). In light of our previous work demonstrating the ability of PDE5 inhibitors to functionally impair both Arg1 and iNOs, we sought to determine whether this pharmacologic inhibition could reverse tyrosine nitrosylation in the bone marrow of our patient. Data supporting the role of this pathway in myeloma shows that prior to initiation of PDE5 inhibition, the vast majority of the bone marrow demonstrated significant tyrosine nitrosylation (Fig 3A). In parallel with the decrease of MDSCs as well as both Arg1 and iNOS activity, the late bone marrow biopsy showed minimal amounts of nitrosylation (Fig 3B), thus demonstrating a direct correlation between reductions in iNOS/Arg-1 levels and tyrosine nitrosylation.
Figure 3.

Bone Marrow Nitrosylation. Bone marrow obtained prior to initiation of tadalafil treatment (panel A) and at +11 months (panel B) was stained with nitro-tyrosine.
Restoring T-cell Function with PDE5 Inhibition
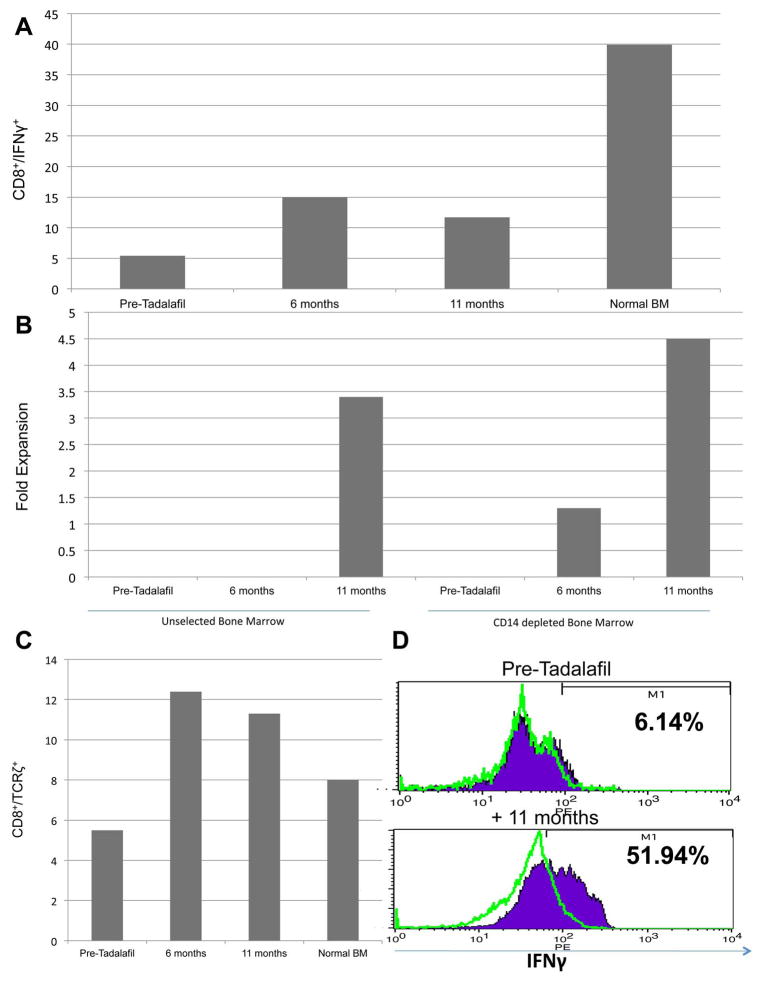
Extensive murine data support the notion that abrogating MDSC function can effectively restore tumor-specific immunity (11). As a measure of endogenous T-cell activity, IFNγ expression increased during treatment although never to the level seen in normal donors (Fig 4A). We had shown previously that elimination of the MDSC population either physically or through PDE5 blockade resulted in enhanced T-cell proliferation to anti-CD3/CD28 beads (3). We thus repeated this assay in our patient. While the later time points showed increased proliferation in both groups, CD14 depletion further increased T-cell proliferation at 6 and 11 months (Fig 4B). Interestingly, despite CD14 depletion, no proliferation was observed at baseline. One mechanism mediating T-cell dysfunction is the extracellular depletion of arginine resulting from the upregulation of arginase-1 by MDSCs. This downregulates the TCRζ chain resulting in decreased T-cell proliferation and function (12). TCRζ chain expression was initially downregulated and significantly increased upon tadalafil treatment (Fig 4C). Lastly, we sought to determine whether our clinical findings and data demonstrating enhanced immune function correlated with increased tumor-specific immunity. As shown in Fig 4D, the reduction in tumor burden was associated with a dramatic increase in tumor-specific immunity of the marrow-infiltrating T cells after 11 months of treatment with 51.9% of the divided T cells demonstrating anti-myeloma specificity. Taken together, these data demonstrate the ability of PDE5 inhibition to restore T-cell function and augment antitumor immunity.
Figure 4.
Characterization of T-cell response over time. (a) BM was stimulated with PMA/Ionomycin for 4 hours and the overall intracellular expression of IFNγ on CD8+ cells was determined for the Pre-Tadalafil, +6 month, +11 month and normal BM samples. (b) PBL from two Post Tadalafil time points (+5 months and +8 months) as well as a normal PBL were stained for CD4+/CD25+ and intracellular expression of FOXP3 was determined. (c) TCRζ expression was determined on BM CD8+ cells at the Pre-Tadalafil, +6 month, +11 month and normal BM samples. (d) BM samples from the Pre-Tadalafil and +11 month Tadalafil timepoints were CFSE labeled and then pulsed with either H929/U266 (purple fill) or SW780 lysates (data not shown/ no increase from unpulsed) or left unpulsed (green line). After a five-day stimulation the cells were harvested and stained with CD3 and intracellularly for IFNγ. CD3+/CFSElo/IFNγ+ cells are shown in this graph. (e) CD3/CD28 T-cell stimulations of either unselected BM or CD14+ depleted BM. CD3/CD28 beads were added at a 3 bead to 1 T cell ratio in both groups for a 5-day stimulation. On D+5 the cells were harvested, beads removed and the overall expansion of CD3+ cells was determined.
Discussion
This is the first demonstration in humans that PDE5 inhibitors can effectively block MDSC function and restore immune responsiveness. Specifically, we observed a reduction in the expression of IL4Rα - a protein shown to be responsible for the MDSC-mediated immune suppression (5) and reductions in iNOS, arginase-1, and ROS expression. This further translated into increased expression of IFNγ and TCRζ upregulation (13). Lastly, enhanced T-cell immunity correlated with the development of a clinically measurable antitumor response in a patient with multiply relapsed/refractory myeloma.
The exact phenotype of MDSCs has become increasingly complex with the recent identification of monocytic (CD14+) or granulocytic (CD15+) subpopulations (14). The dominant population in myeloma remains to be clearly elucidated. We had demonstrated previously that depletion of CD14+ monocytes in myeloma effectively restored T-cell responsiveness to anti-CD3/CD28 stimulation (3). More recently, others have shown that CD15+ MDSCs may play a more prominent role in other malignancies (11). The presence of both populations could possibly explain why CD14-depletion only partially restored T-cell function in this patient.
PDE5 inhibitors have been described as exerting a direct effect on CLL through caspase-3-induced apoptosis likely through the inhibition of PDE-4 (15). However, we have been unable to detect a direct antitumor effect of PDE-5 inhibitors on myeloma cell lines or primary samples. In contrast, our preclinical studies demonstrated a therapeutic effect of PDE5 inhibition on the CD11+/Gr-1+ MDSCs in mice and a CD14+ population in patients with both head and neck cancer and myeloma (3). The data presented here demonstrate a role of MDSCs in myeloma and underscore how inhibiting MDSC function with a non-cytotoxic agent can generate clinically meaningful antitumor immunity. This therapeutic effect was achieved through the down-regulation of both iNOS and arginase-1. Interestingly, while not considered to have a direct cytotoxic effect on the MDSCs, we observed a significant reduction in MDSC numbers in addition to the expected reduction in IL4Rα expression. This is likely explained by the presence of a positive feedback loop between the tumor and the MDSCs. Abrogation of MDSC function altered the tumor microenvironment, which augmented the tumor-specific T-cell response. This reduced the tumor burden and the secretion of tumor-derived factors such as GM-CSF, IL-6 and VEGF (2), which, in turn, generated fewer MDSCs thereby reducing MDSC numbers.
Tadalafil increased IFNγ production and TCRζ chain expression on marrow-infiltrating T cells obtained from the tumor microenvironment. While only these two parameters of T-cell function were examined, other MDSC-induced factors leading to T-cell anergy include the depletion of extracellular cystine and cysteine (16), nitration of the T-cell receptor and CD8 molecules (9), and the induction of regulatory T cells (Tregs) (17). We also observed a decrease in Tregs in the blood over time (data not shown). The major effect of PDE5 blockade in this patient was the ability to increase the tumor-specific immune response and to generate a meaningful and durable anti-myeloma response using a regimen to which he was previously refractory. It is also worth noting that tumor-specific T-cell responses were increased with PDE5 inhibition despite the presence of chronic corticosteroid therapy given to treat the myeloma. Taken together, these results underscore the critical role of MDSCs within the complex immunosuppressive pathway found in the tumor microenvironment but also suggest the presence of additional inhibitory mechanisms.
These data would suggest that a non-cytotoxic, non-tumoricidal agent may be capable of targeting MDSC function and generating a potent antitumor immune response with an associated clinical benefit. Despite being refractory and intolerant to lenalidomide in the past, the addition of tadalafil enabled the patient to tolerate lenalidomide-based therapy and lead to a significant clinical response with associated transfusion-independence and improvement in the quality of life. Tadalafil alone is unlikely to generate a measurable clinical response. One possible explanation for this synergy is that the immune-mediated efficacy of lenalidomide was augmented by tadalafil inhibition of MDSC function, which would justify combination of PDE5 inhibitors with other immunotherapeutic approaches. A clinical trial in myeloma is underway examining the therapeutic efficacy of PDE5 inhibitors in conjunction with a lenalidomide-based regimen.
Acknowledgments
This project was funded by R01CA124996 from the NIH.
We would also like to thank the Nosoff Foundation for their support that they provided for this project to whom we are forever grateful.
Footnotes
Competing Interests Statement
The authors declare that they have no competing financial interests.
References
- 1.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 2.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niesvizky R, Jayabalan DS, Christos PJ, Furst JR, Naib T, Ely S, et al. BiRD (Biaxin [clarithromycin]/Revlimid [lenalidomide]/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naive symptomatic multiple myeloma. Blood. 2008;111:1101–9. doi: 10.1182/blood-2007-05-090258. [DOI] [PubMed] [Google Scholar]
- 5.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–99. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 7.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brito C, Naviliat M, Tiscornia AC, Vuillier F, Gualco G, Dighiero G, et al. Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J Immunol. 1999;162:3356–66. [PubMed] [Google Scholar]
- 9.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–35. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–68. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zea AH, Rodriguez PC, Culotta KS, Hernandez CP, DeSalvo J, Ochoa JB, et al. L-Arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cell Immunol. 2004;232:21–31. doi: 10.1016/j.cellimm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Ezernitchi AV, Vaknin I, Cohen-Daniel L, Levy O, Manaster E, Halabi A, et al. TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. J Immunol. 2006;177:4763–72. doi: 10.4049/jimmunol.177.7.4763. [DOI] [PubMed] [Google Scholar]
- 14.Tadmor T, Attias D, Polliack A. Myeloid-derived suppressor cells--their role in haemato-oncological malignancies and other cancers and possible implications for therapy. Br J Haematol. 2011;153:557–67. doi: 10.1111/j.1365-2141.2011.08678.x. [DOI] [PubMed] [Google Scholar]
- 15.Sarfati M, Mateo V, Baudet S, Rubio M, Fernandez C, Davi F, et al. Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood. 2003;101:265–9. doi: 10.1182/blood-2002-01-0075. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–49. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]