Abstract
The development of non-viral methods for efficient gene transfer to the lung is highly desired for the treatment of a number of pulmonary diseases. We have developed a non-invasive procedure using electroporation to transfer genes to the lungs of rats. Purified plasmid (100 to 600 μg) was delivered to the lungs of anesthetized rats through an endotracheal tube and a series of square wave pulses were delivered via electrodes placed on the chest. Relatively uniform gene expression was observed in multiple cell types and layers throughout the lung, including airway and alveolar epithelial cells, airway smooth muscle cells, and vascular endothelial cells and was dose- and pulse length-dependent. Most importantly, no inflammatory response was detected. To demonstrate efficacy of this approach, the β1 subunit of the Na+,K+-ATPase was transferred to the lungs of rats with or without electroporation, and three days later, alveolar fluid clearance was measured. Animals electroporated with the β1 subunit plasmid showed a two-fold increase in alveolar fluid clearance and Na+,K+-ATPase activity as compared to animals receiving all other plasmids, with or without electroporation. These results demonstrate that electroporation is an effective method to increase clearance by introducing therapeutic genes (Na+,K+-ATPase) into the rat lung.
Keywords: plasmid, electroporation, acute lung injury, edema
INTRODUCTION
Over the past decade, numerous viral and non-viral approaches have been proposed and developed for transferring genes to the lung but most have significant limitations. Inefficiency of gene transfer, immunological responses and non-specificity of cell targeting are just a few of the problems. For example, while adenovirus appears to be the vector of choice for pulmonary gene therapy in the laboratory, their use can cause inflammation and cell damage (1). Further, immune responses developed against the viral vector limit the success of repeated administration. These drawbacks make its clinical use doubtful. In contrast, much less inflammation and immune response are generated against DNA, either as naked plasmid or when complexed with liposomes or other polymers such as polyethylenimine (2). Plasmid production is simple and yields high levels of contaminant-free, pure vector. Unfortunately, the efficiency of nonviral gene transfer remains low (2). Thus, for non-viral vectors to be of use in the lung, their ability to transfect cells in vivo must be increased.
Recent research from our laboratory and others has demonstrated that electroporation can be used to efficiently deliver genes to various tissues in vivo with no damage and yields high level gene expression (3). The application of electric fields to tissues transiently opens pores in the plasma membrane for the lifetime of the pulse, allowing exogenous DNA or other extracellular molecules to enter the cell (4). Electroporation causes up to a 1000-fold increase in gene expression compared to DNA injection alone (5–9). At the appropriate field strengths, electroporation has proven to be a safe and effective method.
In the current study, we have developed a relatively non-invasive procedure to transfer genes to the lungs of rats using electric fields. The procedure is safe and non-traumatic and results in levels of reporter gene expression that approach those obtained with the latest generation adenoviruses. Moreover, this technique is appropriate for the delivery of therapeutic genes without associated inflammatory responses that are common to most other methods for pulmonary gene delivery. Some of the results of these studies have been previously reported in the form of abstracts (10, 11).
METHODS
Plasmids
pEGFP-N1 (Clontech, Palo Alto, CA), pCMV-lux-DTS, pCMV-lacZDTS, and pCMV-β1 express green fluorescent protein (GFP), firefly luciferase, β-galactosidase, and the rat Na+,K+-ATPase β1 subunit, respectively, from the CMV immediate early promoter/enhancer (9, 12). pGFP-β1 expresses a GFP-rat Na+,K+-ATPase β1 subunit fusion protein from the CMV promoter. Plasmids were purified using Qiagen Giga-prep kits (Qiagen, Chatsworth CA) and suspended in 10 mM Tris, pH 8.0, 1 mM EDTA, and 140 mM NaCl.
In vivo Gene Transfer to the Lung
Male Sprague-Dawley rats (200–350 g) were anesthetized and hair under the forelimbs was removed. Pediatric cutaneous pacemaker electrodes (Quik-Combo RTS, Medtronic Physio-Control Corp., Redmond, WA) were cut to 3 × 4 cm and placed on either side of the chest under the forelimbs. A small amount of KY jelly (Johnson & Johnson) was placed on the skin and the electrodes were held in place using surgical tape. A solution of 500 μl of plasmid in saline was administered between breaths over a 2 second period via a 16 gauge Angiocath catheter used as an endotracheal tube and the animal was allowed to recover breathing for 20 seconds. Immediately following this, a series of 8 square wave electric pulses were administered using an ECM830 electroporator (Gentronics, San Diego, CA). Unless otherwise stated, a field strength of 200 volts/cm (V/cm) was applied (calculated as through the chest, electrode center to electrode center). At later times, the lungs were removed and analyzed. All experiments were conducted in accordance with institutional guidelines in compliance with the recommendations of the Guide for Care and Use of Laboratory Animals.
Adenoviral gene transfer was performed as previously described, using 5 × 108 pfu of either a null virus (InVivogen, San Diego, CA) or a virus expressing the rat Na+,K+-ATPase β1 subunit (Ad-β1), delivered with surfactant (“Survanta”, Abbott Laboratories, Columbus, OH) (13).
Measurement of lung liquid clearance
The isolated, fluid-filled, perfused lung preparation was performed immediately following brief ventilation of rats as previously described (14–16). Changes in concentration of Evan's blue tagged-albumin instilled into the airspace were used to estimate the volume of fluid cleared from the alveolar airspaces. The unidirectional flux of Na+ from the alveolar space, (i.e. active transport and passive movement), was calculated from the rate of loss of 22Na+ from the airspaces. Passive Na+ flux was calculated by subtracting the active Na+ flux (calculated from the rate of net fluid clearance) from total Na+ flux (16). Similarly, the flux of mannitol was calculated from the rate of loss of 3H-mannitol from the airspaces (16). Albumin flux from the pulmonary circulation into the alveolar space was determined from the fraction of FITC-labeled albumin, placed in the perfusate, that appeared in the alveolar instillate during the experimental protocol.
Histological Analysis
Paraffin-embedded thin sections were cut from lungs inflated to total lung capacity with 3% paraformaldehyde immediately following euthanasia. Lungs were X-gal stained as described (9). Immunohistochemistry was performed using the Vectastain ABC-AP system (Vector Laboratories, Foster City, CA). Hematoxylin and eosin-stained sections (3 sections each from 3 animals per condition) were blinded and reviewed by a pathologist for lung injury.
Measurement of Luciferase Expression
Lungs were frozen in liquid nitrogen immediately after removal and extracts were prepared as previously described (9). Luciferase activity was measured in duplicate using the Luciferase Assay System (Promega, Madison WI) in a Turner luminometer. Purified recombinant luciferase (Promega) was used to produce a standard curve for each experiment.
Measurement of IL6
IL6 was measured in lung extracts by ELISA (R & D Systems, Minneapolis, MN).
Isolation of alveolar type II cells
Three days after lung electroporation, alveolar epithelial type II cells (ATII) were isolated as previously described (17–20). Productively transfected cells were scored for GFP-β1 expression by counting GFP+ cells in multiple fields by fluorescence microscopy.
Measurement of Rubidium uptake in isolated cells
Ouabain-sensitive 86Rb+ uptake was used to estimate Na+,K+-ATPase activity in ATII cells isolated from treated animals as previously described (21).
Quantitation of Na+,K+-ATPase β1 subunit
Western blots were performed on basolateral membranes isolated from the lungs of treated animals as previously described (21).
RESULTS
In vivo gene transfer to the rat lung using electroporation
We have developed a non-invasive electroporation procedure for gene transfer to rat lungs for in vivo studies (Fig. 1A). Briefly, DNA was delivered to the lungs via an endotracheal tube, the electric field was applied transthoracically using electrodes placed directly on the chest, and gene expression was assessed three days later. In our initial studies, we attempted to apply 10 msec square wave pulses at a field strength of 200 V/cm, as we did ex vivo and in previous in vivo studies in other tissues. However, because the size of the rats necessitated a large gap (4 cm between electrodes requiring 800 V), we were unable to administer the field for greater than 10 μsec using the BTX830 electroporator, due to instrument constraints. The BTX830 will allow the administration of up to 500 V at long pulse lengths (msec range), but above this voltage, only shorter pulse lengths can be used (μsec range). Thus, we investigated the ability of this reduced pulse length to mediate gene transfer (Fig. 1B). Even at a pulse length of 10 μsec, statistically significant gene transfer and expression was observed over DNA delivered in the absence of an electric field. Electroporation-mediated gene transfer was dose-dependent, with 170 ± 9 pg of luciferase per g wet weight (mean ± sem, n=6) being expressed when 600 μg of DNA was electroporated into the lungs. Expression was detected as early as 6 hours post-electroporation (but not at 1 hour) and persisted for 3 days; by 5 and 7 days, gene expression could no longer be detected (not shown).
Figure 1. Electroporation-mediated reporter gene transfer to the rat lung.
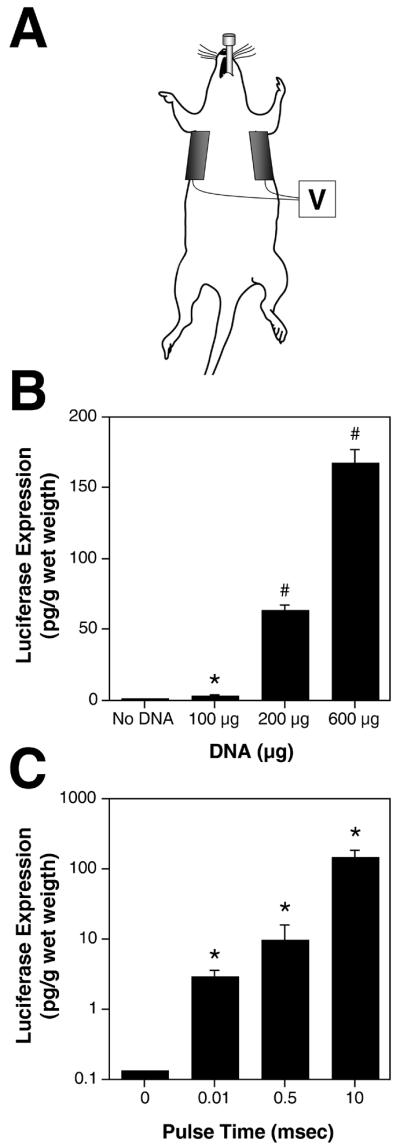
A. Cartoon for in vivo plasmid delivery and electroporation for the lungs showing endotracheal tube and electrodes. B. Dose-dependent gene transfer and expression in vivo. Five hundred microliters of DNA or saline was delivered to the lungs of anesthetized animals at the indicated doses, followed by electroporation (200 V/cm; 8 pulses; 10 μsec each). Three days later, luciferase gene expression was measured (mean ± sem, n=6). *, p < 0.01 and #, p < 0.001 versus no DNA using the non-parametric Mann-Whitney U-test. C. Effects of pulse length on in vivo gene transfer. One hundred micrograms of plasmid was delivered to the lungs of anesthetized rats which were immediately electroporated at 200 V/cm using 8 pulses at 10 μsec or 1 msec or at 100 V/cm using 8 pulses at 10 msec. Three days later luciferase expression was measured (mean ± sem, n=6). *, p < 0.01 compared to DNA only (0 msec) by Mann-Whitney U-test.
To determine whether longer pulse durations or lower field strengths could be used to increase gene delivery and expression, a set of experiments was performed using small rats (200 to 225 g) whose chests were small enough to electroporate with higher pulse times (Fig. 1C). Pulse times of 10 μsec, 0.5 msec, and 10 msec were used. The mortality rate using all conditions was very low (less than 5%). In all cases, death appeared to be due to fluid delivery and upon necropsy, there was no evidence hemorrhaging or bleeding. Essentially no gene expression was detected in the absence of an electric field when 100 μg of DNA was delivered to the lungs, but the application of an electric field with all pulse durations promoted gene transfer and expression. Maximal gene transfer and expression was detected using a field strength of 100 V/cm with 8 pulses at 10 msec each. This amount of expression was similar to that obtained using higher doses of DNA but with the 10 μsec pulse duration (compare Fig. 1B and 1C). Consequently, either approach (i.e., high dose DNA with low pulse length or low dose DNA with long pulse length) promotes high level gene transfer to the rat lung in vivo with very little mortality. However, for all subsequent studies, the low pulse length method (10 μsec) was used in order to be able to use rats of all sizes.
Localization of gene transfer and expression
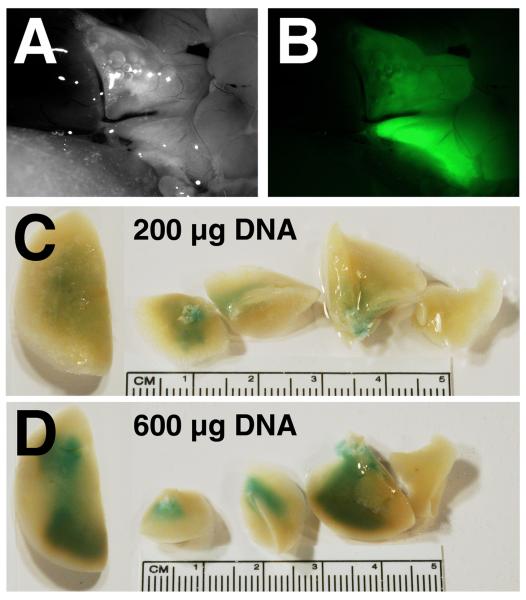
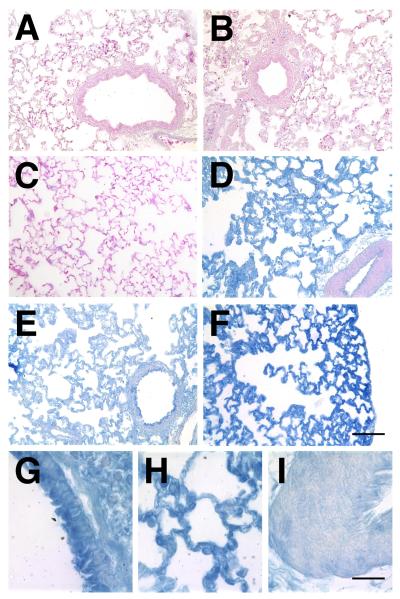
To determine the distribution of gene transfer and expression in the lungs, several different reporter plasmids were used. When a plasmid expressing a fusion protein between GFP and the Na+,K+-ATPase β1 subunit was electroporated into the rat lung, GFP-β1 expression could be detected throughout multiple lobes of the lung, but was absent from surrounding tissues (Fig 2A and B). As can be seen, GFP-β1 expression is relatively uniform throughout the lungs. When alveolar type II epithelial cells were isolated from the lungs of electroporated animals 3 days post-electroporation and observed by fluorescence microscopy, 54.7 ± 7.4% of cells were GFP-β1+. Similarly, 54% of macrophages that were panned during isolation were GFP-β1+ as well. To better distinguish what cell types received DNA in the intact lung, plasmids expressing β-galactosidase were electroporated into the lungs of rats and three days later, the lungs were removed and reacted with X-gal to visualize gene expression (Fig 2C and D). β-galactosidase expression was dose dependent, with increasing amounts of X-gal reactive tissue in all lobes of the lungs as the dose of DNA was increased. Lungs treated with 600 μg of pCMV-lacZ DNA in the absence of an electric field and naïve lungs showed no X-gal reactivity (not shown in whole lungs). To determine which cells in the lungs expressed the gene product, lungs were paraffin embedded, sectioned, and stained for β-galactosidase expression by immunohistochemistry (Fig. 3). In naïve lungs (Fig. 3A), lungs electroporated without added DNA (200 V/cm, 10 μsec; Fig. 3B), and lungs receiving DNA without electroporation (Fig. 3C), no β-galactosidase expression was detected in any cell type. By contrast, electroporation of the reporter plasmid gave significant gene transfer and expression (Fig. 3D – I). At high magnification, expression is detectable in airway epithelial and smooth muscle cells (Fig. 3G), alveolar epithelial type I and type II cells (H), and vascular endothelial and smooth muscle cells (I). However, less gene expression was consistently detected in the smooth muscle layers of large blood vessels (Fig. 3D). This expression pattern was present throughout the lung in all lobes, although some areas showed greater expression than others. Taken together, these results demonstrate that electroporation can be used effectively to target genes to multiple cell types throughout the lung.
Figure 2. Distribution of gene delivery and expression in electroporated rat lungs.
A and B. GFP-β1 expressing plasmids (600 μg) were administered to the lungs and electroporated (200 V/cm, 8 pulses at 10 μsec each). Three days later, the lungs were visualized in situ (A) and GFP-β1 expression was detected by fluorescence microscopy (B). C and D. Dose-dependency of gene transfer and expression. Two hundred micrograms (C) or 600 μg (D) of a β-galactosidase-expressing plasmid was transferred to lungs and electroporated (200 V/cm, 8 pulses at 10 μsec each). Three days later, the lungs were removed and reacted with X-gal to visualize β-galactosidase expression.
Figure 3. Localization of gene expression in electroporated lungs.
β-galactosidase-expressing plasmids were transferred to rat lungs (n=6), electroporated (200 V/cm, 8 pulses at 10 μsec each), and three days later, the lungs were removed, inflated to total lung capacity, fixed, and paraffin-embedded for sections. Immunohistochemistry was performed with antibodies against β-galactosidase using the Vector-Blue ABC reagent and sections were counterstained with eosin (A – F). A. Naïve lung. B. Electroporation only (no plasmid). C. DNA only (no electroporaiton). D – I. Plasmid with electroporation. At high magnification, airway epithelial and smooth muscle cells (G), alveolar type I and type II cells (H), and vascular smooth muscle cells (I) can be seen to express transgene. A – F, Bar = 100 μm; G – I, Bar = 20 μm.
Inflammatory response and histological analysis of electroporated lungs
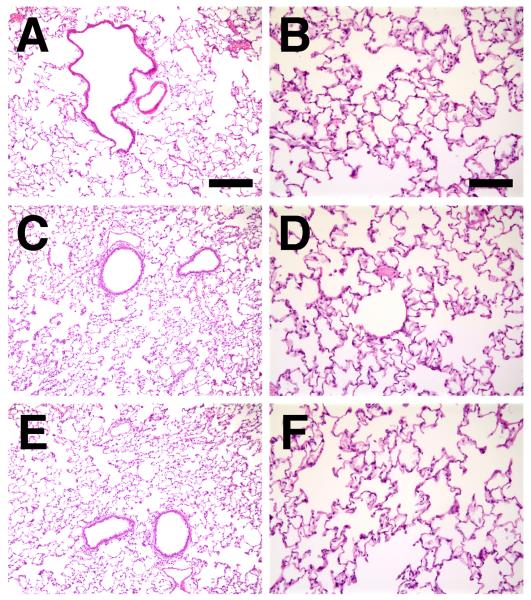
To assess whether electroporation elicited any damage in the rat lung, sections of treated and naïve lungs were examined three days post-treatment for histological changes and cytokine responses due to the procedure (Fig. 4 and Table 1). Additional animals were examined at 1, 24, and 72 hours following treatment (Table 1). Blinded pathological examination could not distinguish between naïve lungs (Fig. 4A and B), lungs from animals that received Tris-EDTA-saline with electroporation (Fig. 4C and D) or those that received DNA (600 μg of pcDNA3) with electroporation using four criteria (vascular congestion, hyaline membranes, polymorphonuclear cell infiltrates, and interstitial infiltrates) on a 5 point scale. Further, there was no difference between lungs from animals that were electroporated using 10 μsec (not shown) or 10 msec pulses (Fig. 4E and F). By contrast, animals receiving 5 × 108 pfu of a control null first-generation adenovirus showed profound vascular congestion, polymorphonuclear cell and interstitial infiltrates, as has been previously described (14). As another indicator of inflammation, levels of IL-6 were also measured in treated and naïve lungs. Again, no differences in levels of this cytokine were noted between animals that were treated under any of the above conditions, with the exception of the adenovirus-treated animals (Table 1).
Figure 4. Histological analysis of electroporated lungs.
Lungs from naive animals (A), or those that were electroporated (100 V/cm, 8 pulses at 10 msec each) without added DNA (C and D) or with 600 μg of pcDNA3 (empty vector; E and F) were harvested three days post-electroporation, inflated to total lung capacity, fixed, paraffin-embedded, sectioned and stained with hematoxylin and eosin. Panels A, C, and E were taken at the same magnification (bar = 200 μm), and panels B, D, and F were taken at a higher magnification (bar = 100 μm). Representative sections from one of 3 animals at each condition are shown.
Table 1.
Histological analyses and measurement of IL-6 levels in electroporated and control lungs.
| Condition | DNA | time | Vascular congestion | Hyaline membranes | Alveolar infiltrates | Interstitial infiltrates | IL-6 (pg/ml) |
|---|---|---|---|---|---|---|---|
| Naïve | − | 0.14 ± 0.1 | 0 | 0.43 ± 0.2 | 0.14 ± 0.14 | 29 ± 14 | |
| 100 V/cm 10 ms | − | 1 h | 0 | 0 | 0 | 0.33 ± 0.33 | 25 ± 25 |
| − | 24 h | 0 | 0 | 0.33 ± 0.33 | 0.33 ± 0.33 | 87 ± 56 | |
| − | 72 h | 0 | 0 | 0.67 ± 0.33 | 1 ± 0 | 0 | |
| 200 V/cm 10 us | − | 1 h | 0 | 0 | 0.33 ± 0.33 | 0.67 ± 0.33 | 0 |
| − | 24 h | 0 | 0 | 0.67 ± 0.33 | 0.67 ± 0.33 | 31 ± 31 | |
| − | 72 h | 0.2 ± 0.2 | 0 | 0.80 ± 0.49 | 1.2 ± 0.37* | 19 ± 19 | |
| 100 V/cm 10 ms | + | 1 h | 0 | 0 | 0 | 0.67 ± 0.33 | 44 ± 44 |
| + | 24 h | 0 | 0 | 1 ± 0 | 1.33 ± 0.33‡ | 132 ± 24 | |
| + | 72 h | 0.33 ± 0.33 | 0 | 0.83 ± 0.39 | 1 ± 0.26* | 73 ± 25 | |
| 200 V/cm 10 us | + | 1 h | 0 | 0 | 0 | 0 | 22 ± 22 |
| + | 24 h | 0 | 0 | 1 ± 0 | 1 ± 0 | 0 | |
| + | 72 h | 0.33 ± 0.33 | 0 | 1 ± 0.4 | 1.16 ± 0.33* | 0 | |
| Adenovirus | 72 h | 1 ± 0‡ | 0 | 3 ± 0.58 ‡ | 2 ± 0‡ | 161 ± 42‡ |
Results are presented as mean ± sem. Scale for histopathological analysis of lung injury is from 0 (healthy) to 5 (severly injured).
p < 0.05 compared with naive by Mann-Whitney U-test
p < 0.01 compared with naive by Mann-Whitney U-test
Because transgene expression was detected throughout the lung in cells within and below the epithelial layer (Fig. 3), DNA must be gaining access to cells across the epithelial layer. Thus, we asked whether the process of electroporation caused a transient increase in epithelial barrier permeability. When FITC-labeled albumin was instilled into the lungs along with DNA and electroporated, there was no significant accumulation of the alveolar tracer in the blood of the rats within 15 minutes of the procedure, compared with animals that were instilled with the tracer and DNA but not electroporated (0.011% ± 0.01% vs 0.09% ± 0.01% of the instilled tracer in the blood, mean ± sem, n=5). These results suggest that although the DNA may be gaining access to the subepithelial cells through the epithelial tight junctions, there is no significant increase in epithelial barrier permeability.
Transfer of the Na+,K+-ATPase β1 subunit to rat lungs
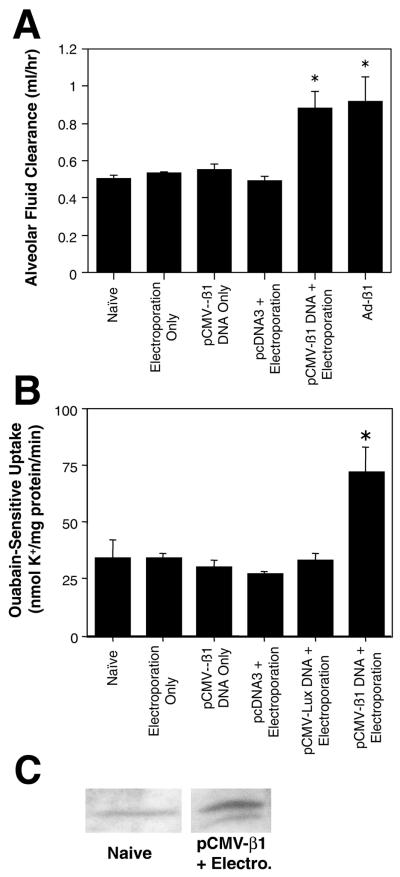
Based on the results that reporter genes could be transferred efficiently to the lungs with little or no inflammation or trauma to the tissue, we evaluated electroporation as a means to transfer the rat Na+,K+-ATPase β1 subunit to the lung and measured alveolar fluid clearance (AFC) following gene transfer. Plasmids were transferred to the lungs of animals with or without electroporation, and three days later, the animals were euthanized and AFC was measured in the isolated lungs (Fig. 5A). Neither electroporation in the absence of DNA, transfer of the β subunit-expressing plasmid (pCMV-β1 subunit) without electroporation, nor electroporation of an “empty” plasmid that does not code for a gene product (pcDNA3), increased AFC over that seen in naïve animals. By contrast, electroporation of pCMV-β1 subunit into lungs increased AFC by 74%. Similarly, a recombinant adenovirus expressing the Na+,K+-ATPase β1 subunit (Ad-β1) also increased AFC by almost a factor of two. However, fluid clearance could not be assessed in the adenovirus treated lungs until 10 days post-transfer due to severe inflammation between days 2 and 7 (14). Furthermore, electroporation did not alter the permeability of FITC-albumin, mannitol, or sodium across the alveolar-capillary barrier under any of the conditions tested (Table 2).
Figure 5. Alveolar fluid clearance and Na+,K+-ATPase activity in electroporated rat lungs.
A. Alveolar fluid clearance was measured in isolated lungs, three days after the indicated non-viral treatment or 10 days after recombinant adenoviral infection. Plasmids were administered at 600 μg per animal and electroporations were carried out at 200 V/cm using 8 pulses of 10 μsec each. Ad-β1 was administered in surfactant at 5 × 108 pfu per animal. Alveolar fluid clearance was measured as described in Methods (mean ± sem, n=6). *, p < 0.003 versus all conditions except Ad-β1 and pCMV-β1 + electroporation, using the non-parametric Mann-Whitney U test. B. Na+,K+-ATPase activity in ATII cells isolated from treated lungs, three days post-treatment. Plasmids (600 μg) were transferred to rat lungs with or without electroporation (200 V/cm, 8 pulses at 10 μsec each). Ouabain-sensitive 86Rb+ uptake was measured (mean ± sd, n=3) as an indicator of Na+,K+-ATPase activity. *, p < 0.005 versus all other conditions using the non-parametric Mann-Whitney U test. C. Western blot of β1 protein abundance in basolateral membranes of naïve rats and those electroporated with pCMV-β1 as in B (n=5).
Table 2. Non-specific permeability measurements in isolated rat lungs following electroporation.
The non-specific permeability of solutes was measured in the isolated lungs from electroporated, control, or Ad-β1-treated animals shown in Figure 5A. All measurements are mean ± sem (n=6).
| Passive Na+ Flux (ml/hr) | Mannitol Flux (ml/hr) | Albumin Flux (ml/hr) | |
|---|---|---|---|
| Control | 1.665 ± 0.195 | 1.230 ± 0.294 | 0.044 ± 0.012 |
| Electroporation Alone | 1.274 ± 0.250 | 1.152 ± 0.138 | 0.040 ± 0.033 |
| pCMV-β1 Alone | 1.253 ± 0.374 | 0.936 ± 0.218 | 0.054 ± 0.025 |
| pcDNA3 + Electroporation | 1.220 ± 0.415 | 0.966 ± 0.212 | 0.072 ± 0.007 |
| pCMV-β1 + Electroporation | 1.439 ± 0.262 | 1.299 ± 0.148 | 0.025 ± 0.013 |
| Ad-β1 | 1.500 ± 0.400 | 0.965 ± 0.060 | 0.070 ± 0.020 |
To study whether the increases in alveolar fluid clearance were due to the functional expression of the β1 subunit, the activity of the Na+,K+-ATPase was measured in cells isolated from treated lungs. Three days after gene transfer by electroporation, alveolar type II epithelial cells were isolated from the lungs and the uptake of 86Rb+ was measured as an assay for Na+,K+-ATPase activity (Fig. 5B). Cells isolated from naïve lungs, lungs electroporated without DNA, lungs receiving pCMV-β1 subunit DNA alone (no electroporation), or lungs electroporated with the empty vector or a Luciferase-expressing plasmid showed essentially the same levels of ouabain-sensitive 86Rb+uptake. However, cells isolated from lungs electroporated with the pCMV-β1 subunit plasmid showed a 2-fold increase in ouabain-sensitive 86Rb+ transport, confirming that the increased fluid clearance paralleled the increased Na+ pump activity. Finally, as shown in Figure 5C, the levels of the β1 subunit, quantified by Western blot, from the lungs of rats that were electroporated with the pCMV-β1 subunit plasmid showed a 2-fold increase in the Na+,K+-ATPase β1 subunit protein abundance at the basolateral membranes isolated from the lungs at three days-post-treatment relative to naïve lungs. Taken together, these results demonstrate that electroporation can effectively deliver the Na+,K+-ATPase β1 subunit, resulting in increased lung liquid clearance.
DISCUSSION
One obstacle to the use of in vivo gene delivery, is the level of technical difficulty associated with many delivery methods. The production of high quality, helper-free recombinant viral vectors can be difficult, costly, and time consuming. Although polyplex and lipoplex formulations are easier to create, as are the plasmid constructs that are delivered with them, effective formulation of these agents can be cumbersome. By contrast, electroporation offers an alternative that only requires purified plasmids and an electric field generator. Large scale plasmid production and purification is both easy and inexpensive. Further, the application of this approach to the lung is fast and relatively non-invasive. Once the animal is anesthetized, the entire procedure takes less than 10 minutes, and recovery is rapid. The use of an endotracheal tube circumvents any incision being made to deliver the DNA to the lungs, as we have reported in mice (9).
Perhaps the major limitation of viral vectors is the potent pro-inflammatory response. The application of an electric field (200 V/cm) to the lungs, either to animals receiving vehicle or DNA, caused no increases in IL-6 levels, and showed no histopathological changes compared to naïve animals, either immediate following electroporation (1 hour) or 1 and 3 days later. By contrast, lungs from animals treated with recombinant adenoviruses show increases in all of these criteria between days 2 and 7 compared to naïve lungs.
We report here that electroporation achieves high levels of gene expression. Indeed, the physiological effects following electroporation-mediated delivery of the Na+,K+-ATPase β1 subunit were indistinguishable from those using recombinant adenoviruses expressing the β1 subunit, which have been very effective for pulmonary gene transfer (14).
One additional drawback to most viral and non-viral methods of gene delivery is that they are limited in delivery to only those cells with which they come into contact. Thus, delivery from the airways results overwhelmingly in gene transfer to the airway and alveolar epithelial cells only, while systemic delivery targets only endothelial cells in the lung (22–26). However, electroporation mediates gene delivery to multiple cell layers within the lung following DNA delivery via the airways. Indeed, we detected relatively uniform gene expression throughout the periphery and parenchyma in airway and alveolar epithelial cells, airway smooth muscle cells, vascular endothelial cells, and some vascular smooth muscle cells. Similar distributions of gene transfer and expression were observed using GFP and lacZ genes with detection by direct fluorescence and immunohistochemistry, respectively. When lungs that had been electroporated with a lacZ construct were reacted with X-gal, gene expression did not appear as evenly distributed as it did in sections from the same lungs using immunohistochemistry. A similar observation was made in the mouse lung where the majority of X-gal reactivity congregated in type I and type II epithelial cells, although the gene product appeared uniformly distributed throughout all cell types when immunohistochemistry was performed using antibodies against β-galactosidase (9). The likely reason for this apparent discrepancy is that the use X-gal reactivity to measure β-galactosidase expression resulting from gene transfer routinely underestimates transfection efficiency during transient transfections (27). By contrast, GFP fluorescence or immunohistochemistry using an amplification system such as the Vector ABC system are much more sensitive and are more reliable indicators of real transfection efficiency (28). Thus, it is possible that more DNA is delivered to the alveolar epithelium or that these cells may express more protein than other pulmonary cells, but all cells in the lung do receive and express the transgene.
How the DNA is gaining access to the subepithelial cells in the electroporated lungs is currently unclear. Electroporation has both electrophoretic and electropermeabilization components that aid DNA delivery to cells within tissues (4). Thus, the DNA is electrophoresed to and into cells via membrane pores that exist for the lifetime of the electric pulse (29). However, it is unlikely that the DNA moves transcellularly by electrophoresis to access cells on the other side of the epithelium. Rather, it is more likely that the DNA moves across and/or through the epithelial tight junctions with the applied electric field. This would imply that a transient increase in epithelial barrier permeability would be coincident with the electric field. Although we did not detect changes in alveolar epithelial permeability, nor did we see histological evidence of pulmonary edema, we cannot rule out the possibility of a very transient, but physiologically insignificant, barrier permeability. Indeed, if electrophoresis and membrane permeabilization occur only during the duration of the electric field which is between 10 μsec and 10 msec in our experiments, it is unlikely that any significant permeability changes would be detected or be detrimental.
It has been demonstrated previously that transfer of the β1 or α2 subunit of the Na+,K+-ATPase using recombinant adenoviruses can increase expression of the Na+,K+-ATPase in lungs of rats and result in increased alveolar fluid clearance (13, 14, 21, 30). A drawback to these studies is that the effects of gene transfer could only be experimentally measured 7 days post-infection, due to the induction of inflammatory responses by the viral vector. As such, the timing of this therapeutic response corresponds more to the subacute or “proliferative” phase of acute lung injury, rather than the acute phase when therapy may be more meaningful (31). The fact that gene transfer using electroporation causes no inflammatory response greatly widens the therapeutic window of gene expression from between 1 and 5 days post-electroporation to cover the entire acute phase of lung injury (9).
While several groups have reported that gene transfer of either the Na+,K+-ATPase β1 or α2 subunit can protect from lung injury initiated after gene delivery, in the clinical setting, this is not the way that therapy would be administered (13, 14, 21, 30, 32). Rather, the ideal therapy would be administered after lung injury and edema have developed. However, the injured lung presents many additional barriers to gene transfer that are not encountered in the normal lung (33). It has been shown recently that recombinant adenoviruses can be used effectively in edematous lungs following hyperoxia-induced acute lung injury (34). It remains to be seen whether electroporation will work as efficiently in the injured lung as it does in the healthy lung, but based on the fact that electroporated DNA moves through mucous and multiple cell and extracellular matrix layers to target cells beyond the pulmonary epithelium, it could be effective and warrants further research. Thus, based on the ease, efficiency, and non-traumatic nature of this electroporation method for pulmonary gene transfer, its use may have clinical potential.
ACKNOWLEDGEMENTS
We would like to thank Drs. Karen Ridge, Laura Dada, and Emilia Lecuona for helpful discussions, and Dr. Phil Factor for the gift of the Na+,K+-ATPase β1 plasmid.
Funding: Sandler Program for Asthma Research (DAD), HL71643 (DAD & JIS), HL48129 (JIS), and HL67835 (GRSB)
REFERENCES
- 1.Liu Q, Muruve DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003;10:935–40. doi: 10.1038/sj.gt.3302036. [DOI] [PubMed] [Google Scholar]
- 2.Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9:1647–52. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- 3.Trezise AE. In vivo DNA electrotransfer. DNA Cell Biol. 2002;21:869–77. doi: 10.1089/104454902762053837. [DOI] [PubMed] [Google Scholar]
- 4.Somiari S, Glasspool-Malone J, Drabick JJ, Gilbert RA, Heller R, Jaroszeski MJ, Malone RW. Theory and in vivo application of electroporative gene delivery. Mol Ther. 2000;2:178–187. doi: 10.1006/mthe.2000.0124. [DOI] [PubMed] [Google Scholar]
- 5.Mathiesen I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Therapy. 1999;6:508–514. doi: 10.1038/sj.gt.3300847. [DOI] [PubMed] [Google Scholar]
- 6.Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud J-M, Delaere P, Branellec D, Schwartz B, Scherman D. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc. Natl. Acad. Sci. USA. 1999;96:4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin JB, Young JL, Benoit JN, Dean DA. Gene transfer to intact mesenteric arteries by electroporation. J Vasc Res. 2000;37:372–80. doi: 10.1159/000025753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blair-Parks K, Weston BC, Dean DA. Gene delivery to the cornea by plasmid injection and electroporation. J. Gene Med. 2002;4:92–100. doi: 10.1002/jgm.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL. Electroporation as a method for high-level non-viral gene transfer to the lung. Gene Ther. 2003;10:1608–1615. doi: 10.1038/sj.gt.3302053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machado-Aranda D, Adir Y, Sznajder JI, Dean DA. Electroporaiton-mediated transfer of the Na+/K+-ATPase Beta-1 subunit safely increases alveolar fluid clearance in rat lungs. Mol Ther. 2003;7:S381. [Google Scholar]
- 11.Machado-Aranda D, Adir Y, Sznajder JI, Dean DA. Efficient Gene Transfer Of The Na+/K+-ATPase Beta-1 Subunit In Rat Lungs Using Electroporation. Am J Respir Crit Care Med. 2003;167:A122. doi: 10.1164/rccm.200403-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Factor P, Senne C, Dumasius V, Ridge K, Jaffe HA, Uhal B, Gao Z, Sznajder JI. Overexpression of the Na+,K+-ATPase alpha1 subunit increases Na+,K+-ATPase function in A549 cells. Am J Respir Cell Mol Biol. 1998;18:741–9. doi: 10.1165/ajrcmb.18.6.2918. [DOI] [PubMed] [Google Scholar]
- 13.Factor P, Dumasius V, Saldias F, Brown LA, Sznajder JI. Adenovirus-mediated transfer of an Na+/K+-ATPase beta1 subunit gene improves alveolar fluid clearance and survival in hyperoxic rats. Hum Gene Ther. 2000;11:2231–42. doi: 10.1089/104303400750035753. [DOI] [PubMed] [Google Scholar]
- 14.Factor P, Saldias F, Ridge K, Dumasius V, Zabner J, Jaffe HA, Blanco G, Barnard M, Mercer R, Perrin R, Sznajder JI. Augmentation of lung liquid clearance via adenovirus-mediated transfer of a Na,K-ATPase beta1 subunit gene. J Clin Invest. 1998;102:1421–30. doi: 10.1172/JCI3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lecuona E, Saldiás F, Comellas A, Ridge K, Guerrero C, Sznajder JI. Ventillator-associated lung injury decreases lung ability to clear edema in rats. Am. J. Respir. Crit. Care Med. 1999;159:603–609. doi: 10.1164/ajrccm.159.2.9805050. [DOI] [PubMed] [Google Scholar]
- 16.Rutschman DH, Olivera W, Sznajder JI. Active transport and passive liquid movement in isolated perfused rat lungs. J Appl Physiol. 1993;75:1574–80. doi: 10.1152/jappl.1993.75.4.1574. [DOI] [PubMed] [Google Scholar]
- 17.Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis. 1986;134:141–5. doi: 10.1164/arrd.1986.134.1.141. [DOI] [PubMed] [Google Scholar]
- 18.Ridge K, Olivera W, Rutschman DH, Mercer RW, Uhal B, Horowitz S, Hughes F, Factor P, Barnard ML, Sznajder JI. Alpha-2 Na,K-ATPase contributes to lung liquid clearance. Ann N Y Acad Sci. 1997;834:651–2. doi: 10.1111/j.1749-6632.1997.tb52340.x. [DOI] [PubMed] [Google Scholar]
- 19.Pesce L, Guerrero C, Comellas A, Ridge KM, Sznajder JI. beta-agonists regulate Na,K-ATPase via novel MAPK/ERK and rapamycin-sensitive pathways. FEBS Lett. 2000;486:310–4. doi: 10.1016/s0014-5793(00)02298-5. [DOI] [PubMed] [Google Scholar]
- 20.Bertorello AM, Ridge KM, Chibalin AV, Katz AI, Sznajder JI. Isoproterenol increases Na+-K+-ATPase activity by membrane insertion of alpha-subunits in lung alveolar cells. Am J Physiol. 1999;276:L20–7. doi: 10.1152/ajplung.1999.276.1.L20. [DOI] [PubMed] [Google Scholar]
- 21.Ridge KM, Olivera WG, Saldias F, Azzam Z, Horowitz S, Rutschman DH, Dumasius V, Factor P, Sznajder JI. Alveolar type 1 cells express the alpha2 Na,K-ATPase, which contributes to lung liquid clearance. Circ Res. 2003;92:453–60. doi: 10.1161/01.RES.0000059414.10360.F2. [DOI] [PubMed] [Google Scholar]
- 22.Canonico AE, Conary JT, Meyrick BO, Brigham KL. Aerosol and intravenous transfection of human alpha 1-antitrypsin gene to lungs of rabbits. Am J Respir Cell Mol Biol. 1994;10:24–9. doi: 10.1165/ajrcmb.10.1.8292378. [DOI] [PubMed] [Google Scholar]
- 23.Uyechi LS, Gagne L, Thurston G, Szoka FC., Jr Mechanism of lipoplex gene delivery in mouse lung: binding and internalization of fluorescent lipid and DNA components. Gene Ther. 2001;8:828–36. doi: 10.1038/sj.gt.3301461. [DOI] [PubMed] [Google Scholar]
- 24.Song YK, Liu F, Chu S, Liu D. Characterization of cationic liposome-mediated gene transfer in vivo by intravenous administration. Hum. Gene Ther. 1997;8:1585–1594. doi: 10.1089/hum.1997.8.13-1585. [DOI] [PubMed] [Google Scholar]
- 25.Yonemitsu Y, Kitson C, Ferrari S, Farley R, Griesenbach U, Judd D, Steel R, Scheid P, Zhu J, Jeffery PK, Kato A, Hasan MK, Nagai Y, Masaki I, Fukumura M, Hasegawa M, Geddes DM, Alton EW. Efficient gene transfer to airway epithelium using recombinant Sendai virus. Nat Biotechnol. 2000;18:970–3. doi: 10.1038/79463. [DOI] [PubMed] [Google Scholar]
- 26.Weiss DJ, Bonneau L, Liggitt D. Use of perfluorochemical liquid allows earlier detection of gene expression and use of less vector in normal lung and enhances gene expression in acutely injured lung. Mol Ther. 2001;3:734–45. doi: 10.1006/mthe.2001.0321. [DOI] [PubMed] [Google Scholar]
- 27.Bebok Z, Abai AM, Dong JY, King SA, Kirk KL, Berta G, Hughes BW, Kraft AS, Burgess SW, Shaw W, Felgner PL, Sorscher EJ. Efficiency of plasmid delivery and expression after lipid-mediated gene transfer to human cells in vitro. J. Pharmacol. Exp. Ther. 1996;279:1462–1469. [PubMed] [Google Scholar]
- 28.Zhang G, Gurtu V, Kain SR. An enhanced green fluorescent protein allows sensitive detection of gene transfer in mammalian cells. Biochem Biophys Res Commun. 1996;227:707–11. doi: 10.1006/bbrc.1996.1573. [DOI] [PubMed] [Google Scholar]
- 29.Zaharoff DA, Barr RC, Li CY, Yuan F. Electromobility of plasmid DNA in tumor tissues during electric field-mediated gene delivery. Gene Ther. 2002;9:1286–90. doi: 10.1038/sj.gt.3301799. [DOI] [PubMed] [Google Scholar]
- 30.Adir Y, Factor P, Dumasius V, Ridge KM, Sznajder JI. Na,K-ATPase gene transfer increases liquid clearance during ventilation-induced lung injury. Am J Respir Crit Care Med. 2003;168:1445–8. doi: 10.1164/rccm.200207-702OC. [DOI] [PubMed] [Google Scholar]
- 31.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 32.Stern M, Ulrich K, Robinson C, Copeland J, Griesenbach U, Masse C, Cheng S, Munkonge F, Geddes D, Berthiaume Y, Alton EWFW. Pretreatment with cationic lipid-mediated transfer of the Na+ K+ -ATPase pump in a mouse model in vivo augments resolution of high permeability pulmonary oedema. Gene Ther. 2001;7:960–966. doi: 10.1038/sj.gt.3301193. [DOI] [PubMed] [Google Scholar]
- 33.Weiss D. Delivery of gene transfer vectors to lung: obstacles and the role of adjunct techniques for airway administration. Mol Ther. 2002;6:148–152. doi: 10.1006/mthe.2002.0662. [DOI] [PubMed] [Google Scholar]
- 34.Factor P, Mendez M, Mutlu GM, Dumasius V. Acute hyperoxic lung injury does not impede adenoviral-mediated alveolar gene transfer. Am J Respir Crit Care Med. 2002;165:521–6. doi: 10.1164/ajrccm.165.4.2101016. [DOI] [PubMed] [Google Scholar]