Abstract
Recent case-control studies have found a 7–8% increase in the serum levels of insulin-like growth factor (IGF)-I in patients with prostate cancer (CaP), the most frequently diagnosed cancer in men. We hereby review what is currently known about growth hormone (GH) and the IGF axis in CaP, take a closer inspection of the studies published to date reporting IGF-I levels in CaP patients, and derive implications for the future medical management of patients receiving trophic hormone therapies as well as those at risk for developing CaP. The role of GH in controlling prostate growth and carcinogenesis is still unclear from animal studies and human disease patterns. However, multilayered perturbations of the IGF axis, including the autocrine production of IGFs, IGF binding proteins (IGFBPs) and IGFBP proteases, such as prostate-specific antigen, have been identified in CaP cells and tissues. Interestingly, IGFBP-3 is a potent inhibitor of prostatic IGF action and also mediates prostate apoptosis via an IGF-independent mechanism. Serum IGFBP-3 levels have been identified to be negatively correlated to the risk of CaP. Notably, GH therapy raises both IGF-I and IGFBP-3 levels in serum. Conclusions based on the studies of IGF-I levels in CaP patients are affected by both the populations studied and the types of IGF-I assay employed. While the studies do indicate an association between serum IGF-I levels and CaP risk, causality has not been established. Thus, serum IGF-I level may actually be a confounding variable, serving as a marker for local prostatic IGF-I production. Increased GH levels as seen in acromegaly have been associated with benign prostatic hyperplasia but not with CaP. Thus, serum IGF-I may lead to an ascertainment bias among younger men with benign prostatic hyperplasia who are more likely to present with prostatic symptoms and have subclinical CaP diagnosed, Should serum IGF-I levels be proven to play a causal role in the pathogenesis of CaP, interpreting the risk associated with therapies such as GH must take into account both the duration of exposure and the risk magnitude associated with the degree of serum IGF-I elevation. Since GH-deficient patients often have a subnormal IGF-I serum level, which normalizes on therapy, their CaP risk on GH therapy probably does not increase substantially above that of the normal population. Until further research in the area dictates otherwise, ongoing surveillance and routine monitoring of IGF-I and IGFBP-3 levels in GH recipients must become standard of care.
Keywords: Growth hormone, prostate cancer, insulin-like growth factor-1, insulin-like growth factor-binding proteins
INTRODUCTION
There has been a long-standing query about the possible role of growth hormone (GH) in carcinogenesis, given its growth-stimulating actions and its ability to induce hepatic production of insulin-like growth factor (IGF)-I. This has been further fueled by the establishment of the paracrine and autocrine growth factor effects in the development and progression of numerous cancers. Recently, when elevated IGF-I levels were linked to a higher risk of prostate cancer (CaP), further attention was turned to the implications for GH and CaP.
EPIDEMIOLOGIC SUSPICIONS LINKING IGF-I AND CaP
Six studies to date have examined serum IGF-I levels and their relation to the incidence of CaP (Table 1). The first three were too small to achieve statistical significance (1–3). Two concurred on a 7–8% elevation in IGF-I levels among CaP patients relative to age-matched controls (4, 5). The sixth found a 28% increase in IGF-I levels, but used an artifact-prone methodology (6).
Table 1.
Synopsis of the six studies relating serum insulin-like growth factor (IGF)-I levels and prostate cancer (CaP).
| CaP patients (no.) |
Control subjects (no.) |
% Change in IGF-I levels |
Significance | IGF-I assay used |
Reference |
|---|---|---|---|---|---|
| 36 | 18 | 9 | n.s. | RIA after acid chromatography |
Cohen et al. (1) |
| 14 | 61 | −27 | n.s. | RIA | Kanety et al. (2) |
| 124 | 73 | 18 | n.s. | RIA | Ho & Baxter (3) |
| 210 | 224 | 7 | 0.02 | IRMA | Wolk et al. (4) |
| 152 | 152 | 8 | 0.03 | ELISA | Chan et al. (5) |
| 51 | 52 | 28 | 0.04 | RIA | Mantzoros et al. (6) |
RIA, radioimmunoassay; IRMA, immunoradiometric assay; ELISA, enzyme-linked immunosorbent assay.
IGF-I circulates as a component of the 150 kDa ternary complex: IGF-I, IGF binding protein (IGFBP)-3 plus the acid-labile subunit (ALS). Because hepatic production of all three complex components is dependent on GH stimulation, changes in GH levels impact IGF-I levels, and conversely, IGF-I levels are presumed to reflect modulations in GH action. Thus, the reported IGF-I/CaP associations are being extrapolated to possible implications regarding GH and CaP.
The societal significance imposed by CaP is impelling the urgency of clarifying any GH associations. CaP is the most commonly diagnosed cancer in American men, and the second most lethal. The incidence of CaP morbidity and mortality has been dramatically increasing (7), although there is some indication that it has now peaked (8, 9). At east part of the heightened prevalence is due to the enhanced identification through prostate-specific antigen (PSA) screening with the subsequently increased performance of prostate biopsies (10).
CURRENT KNOWLEDGE OF GH, IGF-I AND CaP
Cellular evidence: IGF axis disturbances in CaP
Changes in each of the IGF system levels have been reported in CaP; the IGFs and their receptors, the IGFBPs, and the IGFBP proteases (11,12). Normal prostatic epithelial cells, the originators of CaP, neither synthesize nor secrete significant amounts of IGF-I or IGF-II, yet potently respond to IGFs’ mitogenic stimulation (13); in contrast, endogenous IGF-I and IGF-II secretion has been found in several established metastatic cancer cell lines (14–18). IGF-II overexpression was also detected in prostatic stromal cells from cases of benign prostatic hyperplasia (19), Furthermore, CaP cells are responsive to the mitogenic effects of the IGF/IGF receptor signal, Evidence includes the enhanced growth of DU-145, an androgen-independent IGF receptor-positive CaP cell line, upon addition of exogenous IGF-I or IGF-II (15), and the dose-dependent inhibition of IGF receptor-negative CaP PC-3 cell growth by monoclonal anti-IGF antibodies and anti-type 1 IGF receptor antibodies (14).
Changes in the IGFBP profile have also been associated with CaP. Comparing RNAse protection assays of prostatectomy specimens, those with a high vs low Gleason score had significantly greater IGFBP-2 and IGFBP-5 expression but less IGFBP-3 message; there were no changes in IGFBP-4 and -6, and IGFBP-1 was not detected (20). Immunostaining of prostatectomy specimens also revealed less IGFBP-3 in neoplastic epithelial cell cytoplasm than in the normal epithelium, though the lower staining intensity did not correlate with the Gleason score (21). These findings may represent secondary phenomena, as a direct relation to CaP progression has not been demonstrated. Nonetheless, our laboratory has shown that IGFBP-3 dose-dependently induces apoptosis through an IGF/IGF receptor-independent pathway in PC-3 cells (22), suggesting a role for reduced IGFBP-3-mediat-ed apoptosis in carcinogenesis.
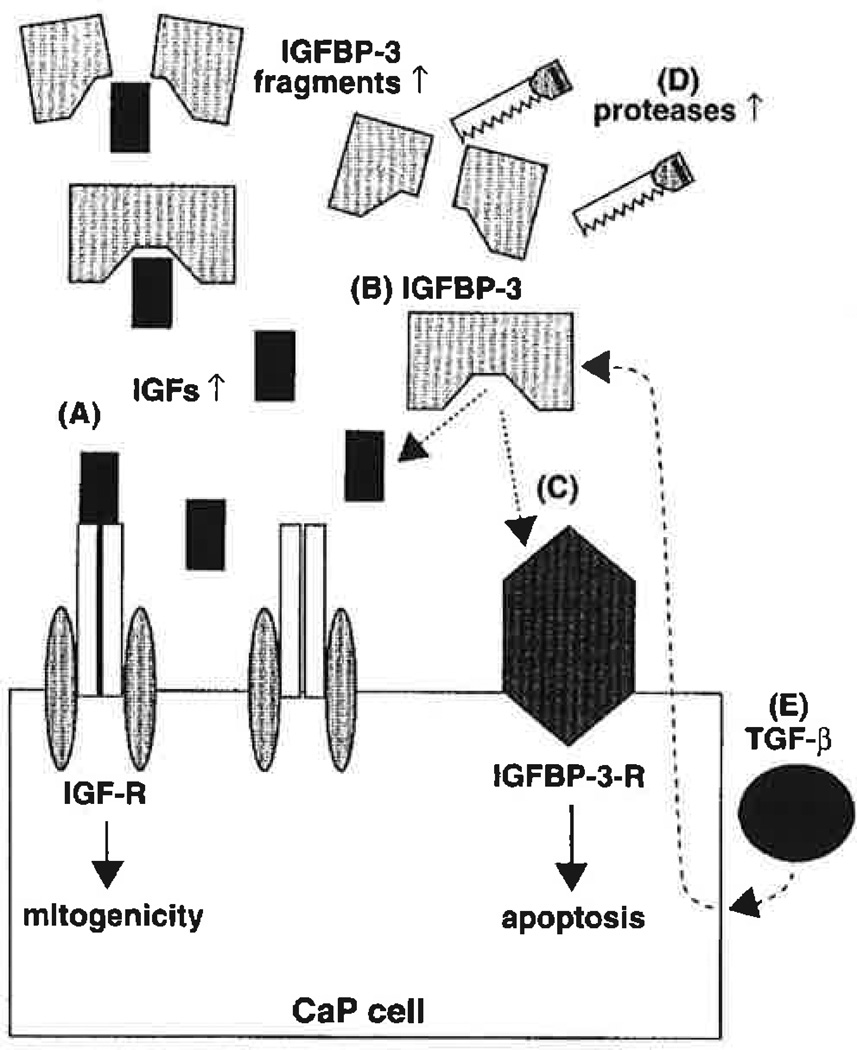
IGFBP alterations have been linked to changes in their proteases (23). The elevation in serum PSA level has been correlated with increased serum IGFBP-2 (1, 2, 24) and decreased intact IGFBP-3 (1,2); because it is also correlated to the volume of CaP, it has been used as a clinical tumor marker for CaP. Although it is tempting to attribute the augmented IGFBP-3 cleavage in CaP sera to the elevated PSA levels, serum PSA is inactivated by inhibitors (25), and the serum IGFBP-3 cleavage fragment pattern differs from that created by seminal PSA (24, 26). In contrast, PSA is active in seminal fluid and presumably in the prostate, where it is normally sequestered within the glandular lumens. Malignancy may disrupt the acinar architecture, thereby leaking PSA into the surrounding stroma and eventually the bloodstream. Thus, IGFBP-3 proteolysis by PSA (27) and cathepsin D (28) likely signifies local effects within the prostate or metastatic foci, the consequences of which contribute to the local propagation of neoplasia or metastasis. Urokinase and plasminogen-activator proteolysis of IGFBP-3 were found to impact growth of cultured PC-3 cells (14). Thus, at the cellular level, IGF, IGFBP and IGFBP protease perturbations clearly play an important role in CaP development and progression through an autocrine or paracrine mechanism (Fig. 1). Little is known about the effect of systemic IGFs and IGF-BP changes in CaP pathophysiology.
Fig. 1.
Insulin-like growth factor (IGF) axis disturbances in prostate cancer (CaP). (A) Elevated IGF levels, by binding to the IGF receptor (IGF-R), may lead to increased mitogenicity of CaP cells. (B) IGF binding proteins (IGFBPs) which block IGF action by preventing the IGF/IGF receptor binding, such as IGFBP-3, may be reduced in CaP. (C) Lower IGFBP-3 levels can also lead to diminished direct cell growth inhibition and apoptosis induction, which are normally activated by binding the putative IGFBP-3 receptor (IGFBP-3-R). (D) Augmented protease activity in CaP increases IGFBP-3 fragmentation, thereby reducing its function. (E) Transforming growth factor-β (TGF-β) induction of IGFBP-3 activates cell cycle arrest and apoptosis; this, too, may be blunted in CaP.
Animal models
Noble rats implanted with combination testosterone-estradiol caplets serve as a model for sex hormone-induced prostate carcinogenesis because they develop prostatic hyperplasia/dysplasia, carcinoma in situ and adenocarcinoma in a temporally progressive manner (29–32). Histopathological and immunohistochemical studies have revealed paracrine and autocrine roles of IGF-I in promoting prostatic epithelial cell mitogenesis. In hyperplasia, stromally produced IGF-I bound epithelial cell IGF-I receptors and stimulated epithelial cell growth, but in adenocarcinoma, IGF-I was made by the tumor epithelial cells themselves and hence stimulated their own autocrine growth (32).
Animal studies of GH, on the other hand, have failed to elicit such direct effects. GH injections alone had little or no impact on prostate tissue growth, although they could cooperatively affect androgen-mediated growth (33, 34). However, GH releasing hormone (GHRH) antagonist treatment of nude mice containing human androgen-independent CaP xenografts slowed prostate tumor growth; serum IGF-I levels were also reduced following treatment, presumably related to blunted GH release (35, 36). Of note, GHRH antagonist treatment was shown to markedly diminish IGF-II expression by the tumors themselves (35, 36). Thus, it is unclear whether the tumor growth inhibition was due to changes in serum IGF-I levels or, more likely, to blunting of autocrine/paracrine tumor overexpression of IGF-II.
Human clinical experience with systemic alterations in GH levels and CaP
The relationship of GH excess to an increased incidence of neoplasia has been debated in the literature (37). Various carcinomas comprise the second leading cause of death, behind cardiovascular disease, in men with acromegaly (38). Some studies found a higher rate of cancer-related deaths than expected in the local population (39, 40), whereas others did not find any increase (41). A recent retrospective review revealed a greater relative risk of malignancy in female but not male acromegaly patients (42). Of the various neoplasms, the strongest association has been with colonic polyps and adenocarcinoma (38). Again, finding an increased incidence of gastric and colonic cancers among acromegalics has been inconsistent (43–45). Even in a study reporting a higher frequency of colonic polyps in patients with acromegaly, there was no correlation between that frequency and serum GH or IGF-I levels (46). CaP has not been linked to acromegaly, although accentuated and early benign prostatic hyperplasia has recently been reported (47). In a comparison of 10 untreated acromegalic men under age 40 with 10 age- and body mass index-matched healthy controls, the former group had significantly increased prostate volume, median lobe, transitional zone diameter and prevalence of periurethral calcifications. One year’s treatment with octreotide to normalize circulating GH and IGF-I levels was found to reduce prostate volume, although it did not change the transitional zone; a significant increase in serum testosterone levels was also found with treatment (47).
The use of recombinant human GH (rhGH) in treating children, and now adults, with GH deficiency has also formed a population for evaluating the effects of GH on cancer incidence. A 1988 report from Japan linked GH therapy to an increased incidence of leukemia (48), but subsequent studies identified the higher presence of leukemic risk factors, such as previous neoplasms and radiotherapy, among GH-deficient children as a confounding variable (49, 50). Likewise, the 10 new cases of extracranial neoplasms among 12,209 rhGH recipients did not differ significantly from the incidence predicted by general population rates (51). Physicians are encouraged to report the occurrence of cancer in their GH patients via the FDA’s MED-WATCH system, the National Cooperative Growth Study, KABI International Growth Study and other non-governmental monitoring bodies (52, 53), so additional data are continuously being accumulated. A retrospective evaluation of 333 hypopituitary patients diagnosed between 1956 and 1987 and treated with routine replacements (not including rhGH) revealed a shortened life-expectancy associated with about double the expected rate of cardiovascular deaths; conversely, fewer men died from malignancies than expected (54).
SUSPICIONS RE-EXAMINED
IGFBP interference
The marked discrepancy between the changes shown in Cap patient IGF-I levels in the various studies (7– 8% vs 28%) can be explained by artifactual IGFBP effects (55). IGF-I radioimmunoassay results following acid-ethanol extraction, such as the reported 28% increase in the study by Mantzoros et al. (6), can be influenced by IGFBP-2 competition with the radiolabelled IGF-I trace (56). Thus, the higher IGFBP-2 levels found in CaP (1–3) can falsely elevate the IGF-I values measured by this technique. On the other hand, the two recent studies employed two-antibody methods, such as immunoradiometric assay (4) and enzyme-linked immunosorbent assay (5), which are less susceptible to IGFBP interference. The 7–8% increase in serum IGF-I levels in CaP patients is, therefore, more accurate.
Association vs causation
The two largest and most recent studies of IGF-I levels and CaP used more reliable IGF-I assays and concurred on a 7–8% greater IGF-I level in patients than in matched controls. This significant association does not necessarily mean causation, however. There are two possible alternatives (55).
As indicated by cellular studies and the animal experiments, growth factors acting in a paracrine or autocrine fashion likely play an integral role in cancer progression. Modifications in local IGF production and handling may, in fact, constitute the primary phenomenon, with altered circulating levels merely a reflection of local growth factor spillage. Furthermore, small changes in serum IGF-I concentrations may actually require much larger local amplification to become apparent. For example, the enhanced IGFBP-2 expression by prostate intraepithelial neoplasia and prostatic adenocarcinoma cells (20) may bind the circulating IGF-I and thereby make the locally active free IGF-I pool much smaller. Similarly, prostate tissue androgen concentrations, which also change with prostatic disease, are not reflected by serum hormone levels (57).
Marked ethnic differences in CaP incidence and mortality have been observed (7, 58). Genetic susceptibility to CaP may run in families (59), and there seems to be a hereditary form of CaP (60). Non-GH regulators of tissue-specific IGF-I expression are also being identified. For example, polymorphism in a cytosine-adenine repeat upstream of the IGF-I gene transcription start site has been linked to both serum IGF-I levels and bone mineral density (61); since GH secretion was not changed, the altered serum IGF-I levels likely resulted from alterations in tissue IGF-I production. Such alterations may underlie the hereditary and ethnic variance in CaP risk, and need to be further examined.
The second possible alternative is that elevated IGF-I levels may be indirectly causing an ascertainment bias for CaP diagnosis. As seen in patients with acromegaly, increased serum IGF-I levels may cause benign prostate proliferation rather than carcinogenesis. Symptoms from the benign prostatic growth may prompt these men to seek medical attention, which invariably includes a PSA level and a subsequent prostate biopsy if the PSA is even modestly elevated, as is frequently the case in benign prostatic hyperplasia. Thus, detection of subclinical CaP is enhanced in this population. One of the IGF-I studies did not reveal a significant interaction between age and IGF-I levels relative to CaP risk (5), but the other found a much stronger IGF-I/CaP association in men younger than 70 (4). Since younger men are less likely to obtain routine PSA screening, prostate symptoms may become more important for the detection of subclinical CaP.
What if association equals causation?
The possibility remains, of course, that increased serum IGF-I levels do indeed raise the risk for developing CaP. Polymorphism in genes conferring modest CaP risk increases could explain a larger portion of the ethnic CaP differences than could variations in the prevalence of rare germline mutations causing substantially increased risk (62). What if variations in serum IGF-I levels constitute one of these hereditary, modest risk raisers?
Even if higher IGF-I levels raise the risk of developing CaP, two important factors must be considered when interpreting the importance of this risk. Firstly, the magnitude of relative risks does not always translate equally into differences in absolute risk. A doubling of relative risk imparts a much smaller increment in absolute risk in a population with a low baseline incidence than in a population with a higher baseline. Secondly, the height of incurred risk is influenced by the duration of exposure. Thus, since the 7–8% higher CaP risk quoted in the studies was found in men of average age 60 or greater, the elevated CaP risk reflects a lifetime of exposure to elevated IGF-I levels. A more practical, generalizable risk definition would be the calculated risk increment per unit time (i.e. year) of exposure to greater IGF-I.
When extrapolating IGF-I-mediated CaP risk to GH-induced CaP risk, one must do so within the context of the entire GH/IGF axis. GH leads to increases in both IGF-I and IGFBP-3 production. Since IGFBP-3 binds circulating IGF-I, thereby lowering the amount of free bioactive growth factor, and can IGF-independently induce apoptosis, any increase in IGFBP-3 may actually serve as a protective role against cancer development. This is paralleled by the cellular studies identifying a reduction in IGFBP-3 production by CaP cells (20, 21). In fact, Chan et al, (5) found an inverse relation between plasma IGFBP-3 levels and CaP risk after controlling for IGF-I levels. Thus, one must consider not only GH’s elevation of IGF-I levels, but GH’s differential augmentation of IGF-I relative to IGFBP-3. Should IGF-I prove to be an independent CaP risk factor, GH may not necessarily follow if its effects on IGFBP-3 counterbalance those on IGF-I.
IMPLICATIONS FOR MEDICAL MANAGEMENT
Using IGF-I levels as a predictor or screen for CaP risk
Serum IGF-I levels should not be routinely used as a CaP predictor until direct causality is proven. Even then, identifying individuals with greater CaP risk may not be beneficial unless specific interventions which can lower the IGF-I level and, hence, the CaP risk, are developed. Otherwise, IGF-I screening would only serve as a marker for individuals requiring more screening (e.g. PSA, digital rectal examinations). For example, the Cancer Genetics Studies Consortium warned against the unknown efficacy of cancer surveillance or other measures to reduce risk in individuals carrying cancer-predisposing mutations; their consensus statement recommended informing men who carry BRCA1 mutations (and therefore have a threefold higher CaP risk, with a cumulative 8% risk by age 70) (63) of screening options but counselling them that benefits of screening have not been proven (64).
Lessons can also be learned from the experience with PSA screening. Markedly elevated PSA levels serve as a useful marker for CaP. The significance of modest increases in PSA levels is less clear. PSA screening has increased detection of localized CaP and, hence, led to changes in the prevalent management decisions (65). Unfortunately, there are few data on the impact on cause-specific survival by the early treatment of clinically localized CaP (66). PSA screening has increased overall health care costs, however, by both stage migration and cost-discounting (67). Stage migration has increased utilization of early treatment options, which are more expensive, and earlier CaP detection leads to initiating treatment sooner, which incurs cost-discounting.
In a 1994 conference, The College of American Pathologists proposed criteria to evaluate prognostic markers (68) which were later adopted at a consensus conference of the American Cancer Society, the American Urological Association and the National Cancer Institute (69). These criteria were: clinical importance, independence of known prognostic markers, and significance. It is currently not known if IGF-I level fulfills any of these criteria for CaP. In fact, the only two parameters which to date fulfill these criteria are pathologic stage and histologic grade (70). Although clearly elevated PSA levels do meet these requirements, modest elevations in PSA have unclear clinical importance and significance. Further research may establish IGF-I levels as a future potential screen, assuming an appropriate intervention will also be available.
Ramifications for patients receiving rhGH therapy
The risk-benefit analysis of therapies which raise systemic IGF-I levels (i.e. rhGH and rhIGF-I) must be balanced for each individual patient. Withholding treatment due to fear of CaP entails risks of its own: increased risk for osteoporosis and cardiovascular disease as well as, for children, significant short stature. In contrast, the incremental risk elevation for CaP must be calculated in terms of absolute risk, duration of exposure, and degree of exposure (i.e. rhGH dosing) (Fig. 2 and 3). This is analogous to decision-making in estrogen therapy for postmenopausal women: estrogen’s protective effects for cardiovascular and bone health must be weighed against the possibility of higher breast cancer risk. rhGH replacement in GH-deficient adults would, of course, entail a much more prolonged exposure and, hence, greater CaP risk than pediatric rhGH therapy. The risk augmentation of prolonged exposure can be offset by the fact that normal adult serum IGF-I levels are lower than those in children, and if hormone replacement strives to normalize the growth factor levels, then lower doses of rhGH can be used in adults. Thus, whereas pediatric rhGH therapy may aim for increases in the growth factors to the highest quartile, adult therapy may target the middle quartile and, thereby, diminish the CaP risk incurred. When more information about CaP etiology and epidemiology is known, we may be able to use other parameters, such as family history of CaP, in better assessing the positive predictive value of IGF-I levels for any given patient (71).
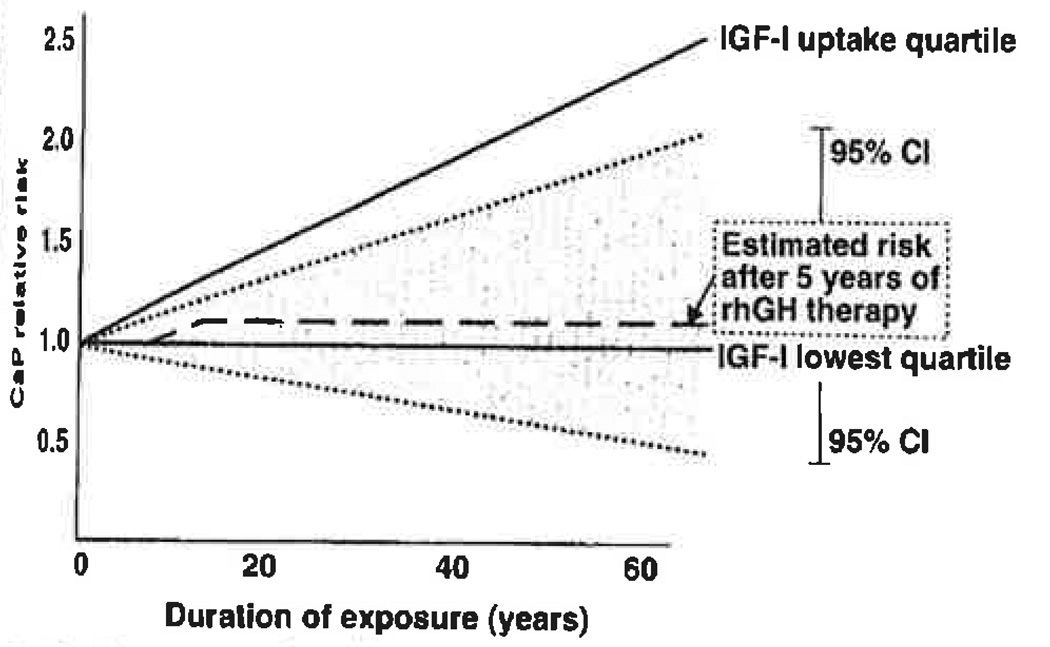
Fig. 2.
Assessing the effects of recombinant human growth hormone (rhGH) therapy on prostate cancer (CaP) risk: duration of treatment. This hypothetical model is based on the following assumptions; 1) individuals maintain their insulin-like growth factor(IGF)-I percentiles throughout life; 2) CaP risk is the result of cumulative IGF-I exposure; 3) CaP relative risks are based on data from the paper by Chan et al. (5); 4) these risks were based on lifetime exposure to the various IGF-I levels, with median baseline age 60 years; 5) we ignore the contribution of IGF binding protein-3 levels to CaP risk. The rhGH therapy-mediated increase in CaP risk must proportionally consider the number of years of therapy relative to the baseline median of 60 years of exposure. Any increment would then be added to the patient’s own baseline IGF-I level risk, assuming risk acquisition is permanent and does not regress with time after treatment cessation. Thus, as shown in the figure, 5 years of pediatric rhGH therapy, which raise the IGF-I levels from the lowest quartile to the highest quartile, increase the CaP risk during the duration of rhGH therapy in parallel to that seen for individuals within the highest IGF-I quartile at baseline. Since most children are Treated with rhGH for an average of 5–7 years, this additional risk becomes very small in adulthood and clearly remains within the confidence intervals (CI) for the lowest risk group.
Fig. 3.
Assessing the effects of recombinant human growth hormone (rhGH) therapy on prostate cancer (CaP) risk: treatment dose. This hypothetical model is based on the same assumptions as Fig. 2. In this illustrative figure, a GH-deficient adult receives lifetime rhGH therapy starting at age 25. Line (A) represents an adult receiving lifetime rhGH replacement to bring insulin-like growth factor (IGF)-I to the highest quartile; line (B) is the same adult whose rhGH therapy corrects IGF-I levels to the third quartile. Case (A) does incur a substantial CaP risk, but the lower rhGH dosing in (B) allows CaP risk to remain acceptable.
Even with better understanding of the impact of systemic IGF-I levels on CaP, this may not translate directly into GH-mediated risk acquisition. GH stimulates hepatic production of both IGF-I and IGFBP-3. IGFBP-3 binds circulating IGF-I, reducing the pool of free growth factor available to bind its receptors, and can mediate apoptosis in an IGF-in-dependent manner. Thus, increasing IGFBP-3 can lower the carcinogenic risk. Unfortunately, the only clear conclusion at this time is that regular monitoring of rhGH recipients for normalization of their growth factor levels must become standard of care for rhGH therapy (Table 2). Ongoing surveillance of these patients for development of cancer must proceed, and specific research be designed, in order to accumulate sufficient data to better understand the significance of serum IGF-I levels as they pertain to CaP.
Table 2.
Strategies to optimize recombinant human growth hormone (rhGH) use regarding possible prostate cancer (CaP) risk
|
IGF-I, insulin-like growth factor-I; IGFBP-3, insulin-like growth factor binding protein-3.
Footnotes
This work was supported in part by the Lawson Wilkins Pediatric Endocrine Society Research Fellowship Award and fellowship grants from Eli Lilly and Pharmacia-Upjohn (A.G.), and grants 2RO1DK47591 from the NIH and an American Cancer Society Idea Development Award (P.C.).
REFERENCES
- 1.Cohen P, Peehl DM, Stamey TA, Wilson KF, Clemmons DR, Rosenfeld RG. Elevated levels of insulin-like growth factor binding protein-2 in the serum of prostate cancer patients. J. Clin. Endocrinol. Metab. 1993;76:1031. doi: 10.1210/jcem.76.4.7682560. [DOI] [PubMed] [Google Scholar]
- 2.Kanety H, Madjar Y, Dagan Y, Levi J, Papa MZ, Pariente C, Goldwasser B, Karasik A. Serum insulin-like growth factor-binding protein-2 (IGFBP-2) is increased and IGFBP-3 is decreased in patients with prostate cancer: correlation with serum pro state-specific antigen. J. Clin. Endocrinol. Metab. 1993;77:229. doi: 10.1210/jcem.77.1.7686915. [DOI] [PubMed] [Google Scholar]
- 3.Ho PJ, Baxter RC. Insulin-like growth factor-binding protein-2 in patients with prostate carcinoma and benign prostatic hyperplasia. Clin. Endocrinol. 1997;46:333. [PubMed] [Google Scholar]
- 4.Wolk A, Mantzoros CS, Andersson SO, Bergstrom R, Signorello LB, Lagiou P, Adami HO, Trichopouios D. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J. Natl, Cancer Inst. 1998;90:911. doi: 10.1093/jnci/90.12.911. [DOI] [PubMed] [Google Scholar]
- 5.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollack M. Plasma insulin-like growth factor-l and prostate cancer risk: a prospective study. Science. 1998;279:563. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 6.Mantzoros CS, Tzonou A, Signorello LB, Stampfer A, Trichopouios D, Adami HO. Insulin-like growth factor 1 in relation to prostate cancer and benign prostatic hyperplasia. Br. J. Cancer. 1997;76:1115. doi: 10.1038/bjc.1997.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mettlin C. Recent developments in the epidemiology of prostate cancer. Eur. J. Cancer. 1997;33:340. doi: 10.1016/s0959-8049(97)89003-x. [DOI] [PubMed] [Google Scholar]
- 8.Krongrad A, Lai S, Vidal EM. The significance of changing trends in prostate cancer incidence and mortality. Semin. Urol. Oncol. 1998;16:30. [PubMed] [Google Scholar]
- 9.Stephenson RA, Stanford JL. Population-based prostate cancer trends in the United States: patterns of change in the era of prostate-specific antigen. World J. Urol. 1997;75:331. doi: 10.1007/BF01300179. [DOI] [PubMed] [Google Scholar]
- 10.Potosky AL, Miller BA, Albertsen PC, Kramer BS. The role of increasing detection in the rising incidence of prostate cancer. J. Am. Med, Assoc. 1995;273:548. [PubMed] [Google Scholar]
- 11.Grimberg A, Rajah R, Zhao H, Cohen P. Takano K, Hizuka N, Takahashi SI, editors. The prostatic IGF system: new levels of complexity. Molecular mechanisms to regulate the activities of insulin-like growth factors. Elsevier, Amsterdam. 1998:205. [Google Scholar]
- 12.Cohen P, Peehl DM, Bhala A, Dong G, Hintz RL, Rosenfeld RG. Baxter RC, Gluckman PD, Rosenfeld RG, editors. The IGF axis in prostatic disease. Elsevier, Amsterdam. 1994:369. [Google Scholar]
- 13.Cohen P, Peehl DM, Lamson G, Rosenfeld RG. Insulin-like growth factors (IGFs), IGF receptors and IGF binding proteins in primary cultures of prostate epithelial cells. J. Clin. Endocrinol. Metab. 1991;73:491. doi: 10.1210/jcem-73-2-401. [DOI] [PubMed] [Google Scholar]
- 14.Angelloz-Nicoud P, Binoux M. Autocrine regulation of cell proliferation by the insulin-like growth factor (IGF) and IGF binding pro-tein-3 protease system in a human prostate carcinoma cell line (PC-3) Endocrinology. 1995;736:5485. doi: 10.1210/endo.136.12.7588299. [DOI] [PubMed] [Google Scholar]
- 15.Figueroa JA, Lee AV, Jackson JG, Yee D. Proliferation of cultured human prostate cancer cells is inhibited by insulin-like growth factor (IGF) binding protein-1 : evidence for an IGF-II autocrine growth loop. J. Clin. Endocrinol. Metab. 1995;80:3476. doi: 10.1210/jcem.80.12.8530586. [DOI] [PubMed] [Google Scholar]
- 16.Pietrzkowski 2, Mulholland G, Gomella L, Jameson BA, Wernicke D, Baserga R. Inhibition of growth of prostatic cancer cell lines by peptide analogues of insulin-like growth factor 1. Cancer Res. 1993;53:1102. [PubMed] [Google Scholar]
- 17.Plymate SR, Tennant M, Birnbaum RS, Thrasher JB, Chatta G, Ware JL. The effect on the insulin-like growth factor system in human prostate epithelial cells of immortalization and transformation by simian virus-40 T antigen. J. Clin. Endocrinol. Metab. 1996;81:3709. doi: 10.1210/jcem.81.10.8855827. [DOI] [PubMed] [Google Scholar]
- 18.Tennant MK, Thrasher JB, Twomey PA, Drivdahl RH, Birnbaum RS, Plymate SR. Protein and messenger ribonucleic acid (mRNA) for the type 1 insulin-like growth factor (IGF) receptor is decreased and IGF-II mRNA is increased in human prostate carcinoma compared to benign prostate epithelium. J. Clin. Endocrinol. Metab. 1996;81:3774. doi: 10.1210/jcem.81.10.8855837. [DOI] [PubMed] [Google Scholar]
- 19.Cohen P, Peehl DM, Maker B, Liu F, Hintz RL, Rosenfeld RG. Insulin-like growth factor axis abnormalities in prostatic stromal cells from patients with benign prostatic hyperplasia. J. Clin. Endocrinol. Metab. 1994;79:1410. doi: 10.1210/jcem.79.5.7525636. [DOI] [PubMed] [Google Scholar]
- 20.Figueroa JA, De Raad S, Tadlock L, Speights VO, Rinehart JJ. Differential expression of insulin-like growth factor binding proteins in high versus low Gleason score prostate cancer. J.Urol. 1998;159:1379. [PubMed] [Google Scholar]
- 21.Hampel OZ, Kattan MW, Yang G, Haidacher SJ, Saleh GY, Thompson TC, Wheeler TM, Marcelli M. Quantitative immunohistochemical analysis of insulin-like growth factor binding protein-3 in human prostatic adenocarcinoma: a prognostic study. J.Urol. 1998;759:2220. doi: 10.1016/S0022-5347(01)63309-3. [DOI] [PubMed] [Google Scholar]
- 22.Rajah R, Valentis B, Cohen P. Insulin-like growth factor (IGF)-binding protein-3 induces apoptosis and mediates effects of transforming growth factor-beta1 on programmed cell death through a p53- and IGF-independent mechanism. J. Biol. Chem. 1997;272:12181. doi: 10.1074/jbc.272.18.12181. [DOI] [PubMed] [Google Scholar]
- 23.Nunn SE, Gibson TB, Rajah R, Cohen P. Regulation of prostate cell growth by the insulin-like growth factor binding proteins and their proteases. Endocrine. 1997;7:115. doi: 10.1007/BF02778077. [DOI] [PubMed] [Google Scholar]
- 24.Stamey TA, Kabalin JN, McNeil JE, Johnstone IM, Freiha F, Redwine EA, Yang N. Prostate specific antigen in the diagnosis, treatment of adenocarcinoma of the prostate. II. Radical prostatectomy treated patients. J.Urol. 1989;741:1076. doi: 10.1016/s0022-5347(17)41175-x. [DOI] [PubMed] [Google Scholar]
- 25.Christensson A, Laurell CB, Lilja H. Enzymatic activity of prostate-specific antigen and its reactions with extracellular serine protease inhibitors. Eur. J. Biochem. 1990;794:755. doi: 10.1111/j.1432-1033.1990.tb19466.x. [DOI] [PubMed] [Google Scholar]
- 26.Cohen P, Graves HC, Peehl DM, Kamarei M, Giudice LC, Rosenfeld RG. Prostate-specific antigen (PSA) is an insulin-like growth factor binding protein-3 protease found in seminal plasma. J. Clin. Endocrinol. Metab. 1992;75:1046. doi: 10.1210/jcem.75.4.1383255. [DOI] [PubMed] [Google Scholar]
- 27.Cohen P, Peehl DM, Graves HCB, Rosenfeld RG. Biological effects of prostate specific antigen (PSA) as an IGFBP-3 protease. J. Endocrinol. 1994;742:407. doi: 10.1677/joe.0.1420407. [DOI] [PubMed] [Google Scholar]
- 28.Nunn SE, Peehl DM, Cohen P. Acid-activated insulin-like growth factor binding protein protease activity of cathepsin D in normal and malignant prostatic epithelial cells and seminal plasma. J. Cell. Physiol. 1997;171:196. doi: 10.1002/(SICI)1097-4652(199705)171:2<196::AID-JCP10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 29.Noble RL. Prostate carcinoma of the Noble rat in relation to hormones. Int. Rev. Exp. Pathol. 1982;23:113. [PubMed] [Google Scholar]
- 30.Drago JR. The induction of Noble rat prostatic carcinomas. Anticancer Res. 1984;4:255. [PubMed] [Google Scholar]
- 31.Leav I, Ho SM, Ofner P, Merk FB, Kwan PWL, Damassa D. Biochemical alterations in sex hormone-induced hyperplasia and dysplasia of the dorsolateral prostates of Noble rats. J. Natl. Cancer Inst. 1988;80:1045. doi: 10.1093/jnci/80.13.1045. [DOI] [PubMed] [Google Scholar]
- 32.Wang YZ, Wong YC. Sex hormone-induced prostatic carcinogenesis in the Noble rat: the role of insulin-like growth factor-l (IGF-I) and vascular endothelial growth factor (VEGF) in the development of prostate cancer. Prostate. 1998;35:165. doi: 10.1002/(sici)1097-0045(19980515)35:3<165::aid-pros2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 33.Sjogren I, Jonsson M, Madej A, Johansson HE, Ploen L. Effects of very high doses of human growth hormone (hGH) on the male reproductive system in the dog. Andrologia. 1998;30:37. doi: 10.1111/j.1439-0272.1998.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 34.Keenan EJ, Thomas JA. Effects of testosterone and prolactin or growth hormone on the accessory sex organs of castrated mice. J.Endocrinol. 1975;64:111. doi: 10.1677/joe.0.0640111. [DOI] [PubMed] [Google Scholar]
- 35.Jungwirth A, Schally AV, Pinski J, Halmos G, Groot K, Armatis P, Vadillo-Buentil M. Inhibition of in vivo proliferation of androgen-independent prostate cancers by an antagonist of growth hormone-releasing hormone. Br. J. Cancer. 1997;75:1585. doi: 10.1038/bjc.1997.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamharzi N, Schally AV, Koppan M, Groot K. Growth hormone-releasing hormone antagonist MZ-5-156 inhibits growth of DU-145 human androgen-in-dependent prostate carcinoma in nude mice and suppresses the levels and mRNA expression of insulin-like growth factor-II in tumors. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8864. doi: 10.1073/pnas.95.15.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bengtsson B-A. Acromegaly and neoplasia. J. Pediatr. Endocrinol. 1993;6:73. doi: 10.1515/jpem.1993.6.1.73. [DOI] [PubMed] [Google Scholar]
- 38.Ezzat S, Melmed S. Clinical review 18: are patients with acromegaly at increased risk for neoplasia? J. Clin. Endocrinol. Metab. 1991;72:245. doi: 10.1210/jcem-72-2-245. [DOI] [PubMed] [Google Scholar]
- 39.Alexander L, Appleton D, Hall R, Ross WM, Wilkinson R. Epidemiology of acromegaly in the Newcastle region. Clin. Endocrinol. (Oxf.) 1980;72:71. doi: 10.1111/j.1365-2265.1980.tb03135.x. [DOI] [PubMed] [Google Scholar]
- 40.Bengtsson B-Å, Edén S, Ernest I, Oden A, Sjögren B. Epidemiology and long-term survival in acromegaly. Acta Med. Scand. 1988;223:327. doi: 10.1111/j.0954-6820.1988.tb15881.x. [DOI] [PubMed] [Google Scholar]
- 41.Wright AD, Hill DM, Lowry C, Fraser TR. Mortality in acromegaly. Q.J. Med. 1970;39:1. [PubMed] [Google Scholar]
- 42.Cheung NW, Boyages SC. Increased incidence of neoplasia in females with acromegaly. Clin. Endocrinol. 1997;47:323. doi: 10.1046/j.1365-2265.1997.2561053.x. [DOI] [PubMed] [Google Scholar]
- 43.Ron E, Gridley G, Hrubec Z, Page W, Arora S, Fraumeni JF., Jr Acromegaly and gastrointestinal cancer. Cancer. 1991;68:1673. doi: 10.1002/1097-0142(19911015)68:8<1673::aid-cncr2820680802>3.0.co;2-0. [erratum in Cancer 1992, 69: 549] [DOI] [PubMed] [Google Scholar]
- 44.Barzilay J, Heatley GJ, Cushing GW. Benign and malignant tumors in patients with acromegaly. Arch. Intern. Med. 1991;151:1629. [PubMed] [Google Scholar]
- 45.Ladas SD, Thalassinos NC, Toannides G, Raptis SA. Does acromegaly really predispose to an increased prevalence of gastrointestinal tumours? Clin. Endocrinol. (Oxf.) 1994;41:597. doi: 10.1111/j.1365-2265.1994.tb01824.x. [DOI] [PubMed] [Google Scholar]
- 46.Klein I, Parveen G, Gavoeler JS, Vanthiel DH. Colonic polyps in patients with acromegaly. Ann. Intern. Med. 1982;97:27. doi: 10.7326/0003-4819-97-1-27. [DOI] [PubMed] [Google Scholar]
- 47.Colao A, Marzullo P, Ferone D, Spiezia S, Cerbone G, Marino V, Di Sarno A, Merola B, Lombardi G. Prostatic hyperplasia: an unknown feature of acromegaly. J. Clin. Endocrinol. Metab. 1998;83:775. doi: 10.1210/jcem.83.3.4645. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe S, Tsunematsu Y, Fujimoto J, Komiyama A for the Leukaemia Study Group, Foundation for Growth Science in Japan. Leukaemia in patients treated with growth hormone. Lancet. 1988;27:1159. [Google Scholar]
- 49.Blethen SL. Complications of growth hormone therapy in children. Curr. Opin. Pediatr. 1995;7:466. doi: 10.1097/00008480-199508000-00020. [DOI] [PubMed] [Google Scholar]
- 50.Allen DB. Safety of human growth hormone therapy: current topics. J. Pediatr. 1996;128:S8. doi: 10.1016/s0022-3476(96)70003-3. [DOI] [PubMed] [Google Scholar]
- 51.Tuffli GA, Johanson A, Rundle AC, Allen DB. Lack of increased risk for extracranial, nonleukemic neoplasms in recipients of recombinant deoxyribonucleic acid growth hormone. J. Clin. Endocrinol. Metab. 1995;80:1416. doi: 10.1210/jcem.80.4.7714117. [DOI] [PubMed] [Google Scholar]
- 52.Furlanetto RW, Allen DB, Gertner JM, Klingensmith GJ, Lee MM, Lightner ES, Moshang T, Oberfield SE, Polychronakos C, Rubin K, Silverstein J, Sklar C, Van Vliet G, Zimmerman D, Foley TP, MacGillivray MH, August GP, Plotnick L, Pescovitz O, Hopwood NJ. Guidelines for the use of growth hormone in children with short stature: a report by the drug and therapeutics committee of the Lawson Wilkins Pediatric Endocrine Society. J. Pediatr. 1995;727:857. doi: 10.1016/s0022-3476(95)70019-6. [DOI] [PubMed] [Google Scholar]
- 53.Blethen SL, Allen DB, Graves D, August G, Moshang T, Rosenfeld R on behalf of the National Cooperative Growth Study. Safety of recombinant deoxyribonucleic acid-derived growth hormone: the National Cooperative Growth Study experience. In: Growth hormone: science, research, and the NCGS −10 years of research. Gardiner-Caldwell SynerMed, Califon, NJ. 1996:232. doi: 10.1210/jcem.81.5.8626820. [DOI] [PubMed] [Google Scholar]
- 54.Rosén T, Bengtsson BÅ. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet. 1990;336:285. doi: 10.1016/0140-6736(90)91812-o. [DOI] [PubMed] [Google Scholar]
- 55.Cohen P. Serum insulin-like growth factor-l levels and prostate cancer risk - interpreting the evidence. J. Natl. Cancer Inst. 1998;90:876. doi: 10.1093/jnci/90.12.876. [DOI] [PubMed] [Google Scholar]
- 56.Rosenfeld RG, Gargosky SE. Assays for insulin-like growth factors and their binding proteins: practicalities and pitfalls. J. Pediatr. 1996;728:552. doi: 10.1016/s0022-3476(96)70012-4. [DOI] [PubMed] [Google Scholar]
- 57.Habib FK. Steroid hormones and cancer. IV. Prostate cancer. Eur. J. Surg. Oncol. 1997;23:264. doi: 10.1016/s0748-7983(97)92604-4. [DOI] [PubMed] [Google Scholar]
- 58.Powell IJ. Prostate cancer and African-American men. Oncology. 1997;11:599. [PubMed] [Google Scholar]
- 59.Bishop DT, Kiemeney LA. Family studies and the evidence for genetic susceptibility to prostate cancer. Semin. Cancer Biol. 1997;8:45. doi: 10.1006/scbi.1997.0053. [DOI] [PubMed] [Google Scholar]
- 60.Rowley KH, Mason MD. The aetiology and pathogenesis of prostate cancer, Clin. Oncol. (R. Coll. Radiol.) 1997;9:213. doi: 10.1016/s0936-6555(97)80003-9. [DOI] [PubMed] [Google Scholar]
- 61.Rosen CJ, Kurland ES, Vereault D, Adler RA, Rackoff PJ, Craig WY, Witte S, Rogers J, Bilezikian JP. Association between serum insulin-like growth factor-l (IGF-I) and a simple sequence repeat in IGF-I gene: implications for genetic studies of bone mineral density. J. Clin. Endocrinol. Metab. 1998;83:2286. doi: 10.1210/jcem.83.7.4964. [DOI] [PubMed] [Google Scholar]
- 62.Shibata A, Whittemore AS. Genetic predisposition to prostate cancer: possible explanations for ethnic differences in risk. Prostate. 1997;32:65. doi: 10.1002/(sici)1097-0045(19970615)32:1<65::aid-pros9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 63.Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE the Breast Cancer Linkage Consortium. Risks of cancer in BRCA1-mutation carriers. Lancet. 1994;343:692. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- 64.Burke W, Daly M, Garber J, Botkin J, Kahn MJE, Lynch P, McTiernan A, Offit K, Perlman J, Peterson G, Thomson E, Varricchio C for the Cancer Genetics Studies Consortium. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. J, Am. Med. Assoc. 277:997. [PubMed] [Google Scholar]
- 65.Mettlin C. Changes in patterns of prostate cancer care in the United States: results of American College of Surgeons Commission on Cancer Studies, 1974–1993. Prostate. 1997;32:221. doi: 10.1002/(sici)1097-0045(19970801)32:3<221::aid-pros9>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 66.Carter HB, Epstein JI. Prediction of significant cancer in men with stage T1c adenocarcinoma of the prostate. World J. Urol. 1997;75:359. doi: 10.1007/BF01300183. [DOI] [PubMed] [Google Scholar]
- 67.Benoit RM, Naslund MJ. The socioeconomic implications of prostate-specific antigen screening. Urol. Clin. North Am. 1997;24:451. doi: 10.1016/s0094-0143(05)70392-x. [DOI] [PubMed] [Google Scholar]
- 68.Grignon DJ, Hammond EH. Clinical relevance of prognostic markers in solid tumors: report of prostate cancer subcommittee, 26th CaP Conference, 1994. Arch. Pathol. Lab. Med. 1995;119:1122. [PubMed] [Google Scholar]
- 69.Hutter RV, Montie JE, Busch C, Grignon DJ, Lieber M, Logothetis C, Ragde H, Reuter VE. Current prognostic factors and their relevance to staging. Cancer. 1996;78:369. doi: 10.1002/(SICI)1097-0142(19960715)78:2<369::AID-CNCR30>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 70.Sakr WA, Grignon DJ. Prostate cancer: indicators of aggressiveness. Eur. Urol. 1997;32:15. [PubMed] [Google Scholar]
- 71.Coley CM, Barry MJ, Fleming C, Mulley AG. Clinical guideline, Part 1: early detection of prostate cancer. Part I: prior probability and effectiveness of tests (position paper) Ann. Intern. Med. 1997;126:394. doi: 10.7326/0003-4819-126-5-199703010-00010. [DOI] [PubMed] [Google Scholar]