Abstract
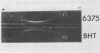
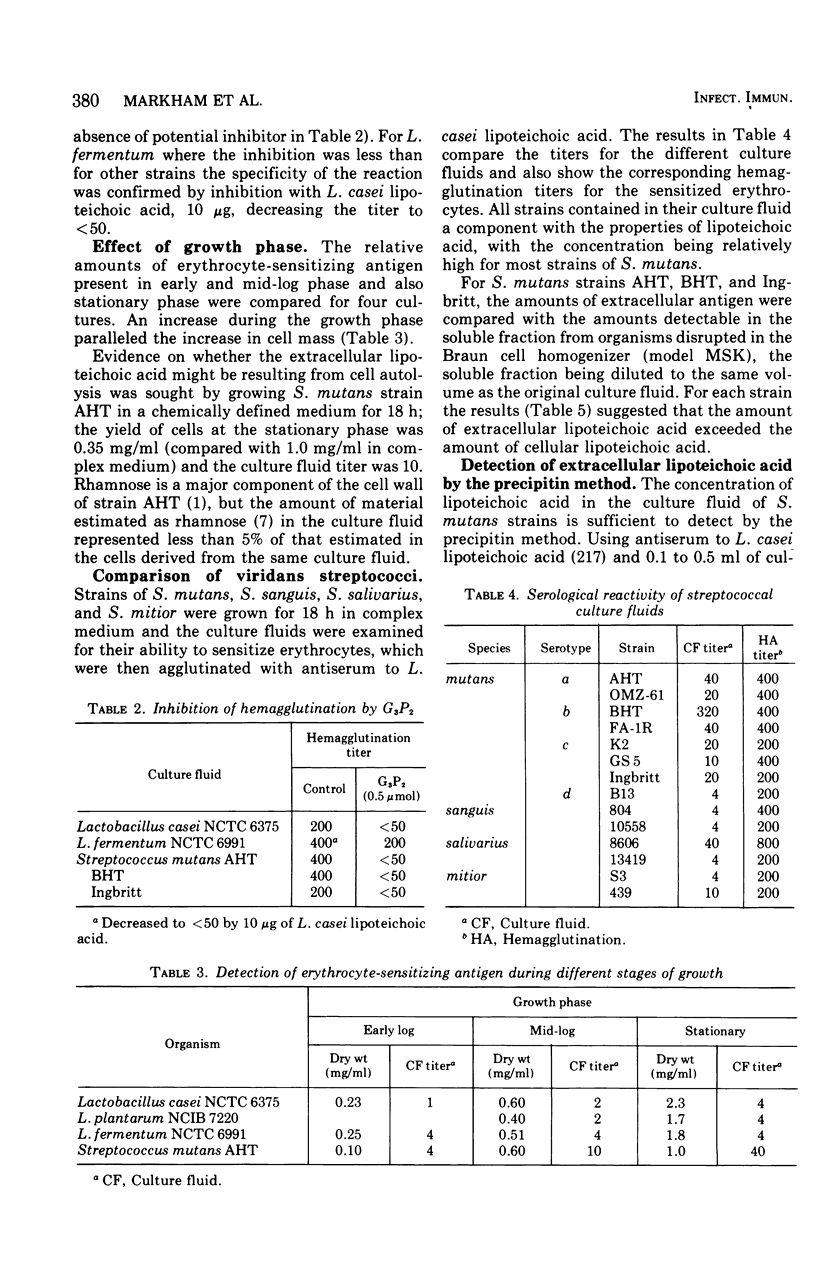
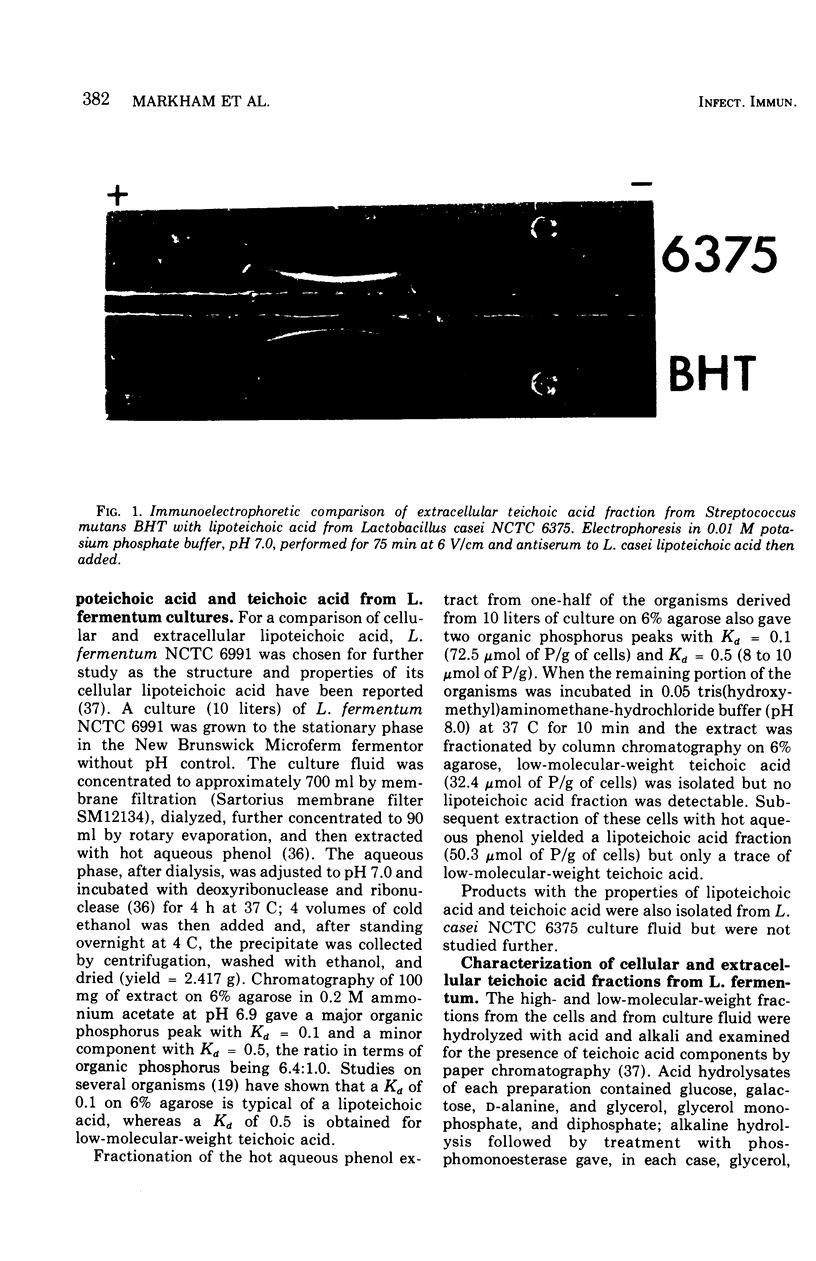
Examination of the culture fluids from a number of strains of oral streptococci and latobacilli has shown the presence of an erythrocyte-sensitizing antigen with the properties of lipoteichoic acid. The antigen was isolated from the culture fluids of Lactobacillus casei and Lactobacillus fermentum and characterized chemically and serologically, For other strains, serological evidence for the presence of lipoteichoic acid depends on the reactivity with antiserum specific for the glycerol phosphate backbone. The relative concentrations of the antigen in culture fluids from different organisms, in culture fluids from different stages of growth, and in extracts of organisms was estimated by determining the maximum dilution that fully sensitized erythrocytes; the culture fluid titer, which is the reciprocal of the dilution, varied from 4 to 320. Strains of Streptococcus mutans were generally characterized by a high level of extracellular lipoteichoic acid, the amount being greater than that detectable in cell extracts; this conclusion was confirmed by using the quantitative precipitin method. A high-molecular-weight fraction obtained from S. mutans BHT culture fluid was effective in sensitizing erythrocytes at a concentration of 1 mug/ml, compared with 2 mug/ml required for cellular lipoteichoic acid from L. casei. The detecting procedure depends on the teichoic acid sensitizing erythrocytes but, as shown with L. fermentum, low-molecular-weight nonsensitizing teichoic acid may also be present in culture fluid.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleiweis A. S., Craig R. A., Zinner D. D., Jablon J. M. Chemical composition of purified cell walls of cariogenic streptococci. Infect Immun. 1971 Jan;3(1):189–191. doi: 10.1128/iai.3.1.189-191.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAPRIOTTI A. Production of soluble melanoid pigments by Streptomyces in gelatin and glucose media. Nature. 1961 Apr 29;190:464–465. doi: 10.1038/190464b0. [DOI] [PubMed] [Google Scholar]
- Chiu T. H., Emdur L. I., Platt D. Lipoteichoic acids from Streptococcus sanguis. J Bacteriol. 1974 May;118(2):471–479. doi: 10.1128/jb.118.2.471-479.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorpenning F. W., Dodd M. C. Heterogenetic antigens of gram-positive bacteria. J Bacteriol. 1966 Apr;91(4):1440–1445. doi: 10.1128/jb.91.4.1440-1445.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdur L. I., Chiu T. H. Turnover of phosphatidylglycerol in Streptococcus sanguis. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1137–1144. doi: 10.1016/s0006-291x(74)80097-5. [DOI] [PubMed] [Google Scholar]
- GORZYNSKI E. A., NETER E., COHEN E. Effect of lysozyme on the release of erythrocyte-modifying antigen from staphylococci and Micrococcus lysodeikticus. J Bacteriol. 1960 Aug;80:207–211. doi: 10.1128/jb.80.2.207-211.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Hausmann E. Potential pathways for bone resorption in human periodontal disease. J Periodontol. 1974 May;45(5):338–343. doi: 10.1902/jop.1974.45.5.338. [DOI] [PubMed] [Google Scholar]
- Hausmann E., Weinfeld N., Miller W. A. Effects of lipopolysaccharides on bone resorption in tissue culture. Calcif Tissue Res. 1972;9(4):272–282. doi: 10.1007/BF02061967. [DOI] [PubMed] [Google Scholar]
- Hewett M. J., Knox K. W., Wicken A. J. Studies on the group F antigen of lactobacilli: detection of antibodies by haemagglutination. J Gen Microbiol. 1970 Mar;60(3):315–322. doi: 10.1099/00221287-60-3-315. [DOI] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Synthesis and excretion of glycerol teichoic acid during growth of two streptococcal species. Infect Immun. 1975 Aug;12(2):333–338. doi: 10.1128/iai.12.2.333-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelstrup J., Funder-Nielsen T. D. Molecular interactions between the extracellular polysaccharides of Streptococcus mutans. Arch Oral Biol. 1972 Dec;17(12):1659–1670. doi: 10.1016/0003-9969(72)90228-2. [DOI] [PubMed] [Google Scholar]
- Kelstrup J., Gibbons R. J. Induction of dental caries and alveolar bone loss by a human isolate resembling Streptococcus salivarius. Caries Res. 1970;4(4):360–377. doi: 10.1159/000259657. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Hewett M. J., Wicken A. J. Studies on the group F antigen of lactobacilli: antigenicity and serological specificity of teichoic acid preparations. J Gen Microbiol. 1970 Mar;60(3):303–313. doi: 10.1099/00221287-60-3-303. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Serological properties of the wall and membrane teichoic acids from Lactobacillus helveticus NCIB 8025. J Gen Microbiol. 1970 Oct;63(2):237–248. doi: 10.1099/00221287-63-2-237. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Serological studies on the teichoic acids of Lactobacillus plantarum. Infect Immun. 1972 Jul;6(1):43–49. doi: 10.1128/iai.6.1.43-49.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson J. W. Growth of cariogenic streptococci in chemically defined medium. Arch Oral Biol. 1971 Mar;16(3):339–342. doi: 10.1016/0003-9969(71)90025-2. [DOI] [PubMed] [Google Scholar]
- Magrill D. S. The reduction of the solubility of hydroxyapatite in acid by adsorption of phytate from solution. Arch Oral Biol. 1973 May;18(5):591–600. doi: 10.1016/0003-9969(73)90097-6. [DOI] [PubMed] [Google Scholar]
- Markham J. L., Knox K. W., Schamschula R. G., Wicken A. J. Antibodies to teichoic acids in man. Arch Oral Biol. 1973 Mar;18(3):313–319. doi: 10.1016/0003-9969(73)90153-2. [DOI] [PubMed] [Google Scholar]
- McCARTY M. The occurrence of polyglycerophosphate as an antigenic component of various gram-positive bacterial species. J Exp Med. 1959 Apr 1;109(4):361–378. doi: 10.1084/jem.109.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvaer K. L., Helgeland K., Rölla G. A charged component in purified polysaccharide preparations from Streptococcus mutans and Streptococcus sanguis. Arch Oral Biol. 1974 Jul;19(7):589–595. doi: 10.1016/0003-9969(74)90077-6. [DOI] [PubMed] [Google Scholar]
- PAKULA R., WALCZAK W. An erythrocyte-sensitising factor common to staphylococci and haemolytic streptococci. Acta Microbiol Pol. 1955;4(4):235–243. [PubMed] [Google Scholar]
- RANTZ L. A., RANDALL E., ZUCKERMAN A. Hemolysis and hemagglutination by normal and immune serums of erythrocytes treated with a nonspecies specific bacterial substance. J Infect Dis. 1956 Mar-Apr;98(2):211–222. doi: 10.1093/infdis/98.2.211. [DOI] [PubMed] [Google Scholar]
- RANTZ L. A., ZUCKERMAN A., RANDALL E. Hemolysis of red blood cells treated by bacterial filtrates in the presence of serum and complement. J Lab Clin Med. 1952 Mar;39(3):443–448. [PubMed] [Google Scholar]
- Schwartz J., Stinson F. L., Parker R. B. The passage of tritiated bacterial endotoxin across intact gingival crevicular epithelium. J Periodontol. 1972 May;43(5):270–276. doi: 10.1902/jop.1972.43.5.270. [DOI] [PubMed] [Google Scholar]
- Sharpe M. E., Brock J. H., Knox K. W., Wicken A. J. Glycerol teichoic acid as a common antigenic factor in lactobacilli and some other gram-positive organisms. J Gen Microbiol. 1973 Jan;74(1):119–126. doi: 10.1099/00221287-74-1-119. [DOI] [PubMed] [Google Scholar]
- Van Driel D., Wicken A. J., Dickson M. R., Knox K. W. Cellular location of the lipoteichoic acids of Lactobacillus fermenti NCTC 6991 and Lactobacillus casei NCTC 6375. J Ultrastruct Res. 1973 Jun;43(5):483–497. doi: 10.1016/s0022-5320(73)90025-7. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Gibbens J. W., Knox K. W. Comparative studies on the isolation of membrane lipoteichoic acid from Lactobacillus fermenti. J Bacteriol. 1973 Jan;113(1):365–372. doi: 10.1128/jb.113.1.365-372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. A serological comparison of the membrane teichoic acids from lactobacilli of different serological groups. J Gen Microbiol. 1971 Aug;67(2):251–254. doi: 10.1099/00221287-67-2-251. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Studies on the group F antigen of lactobacilli: isolation of a teichoic acid-lipid complex from Lactobacillus fermenti NCTC 6991. J Gen Microbiol. 1970 Mar;60(3):293–301. doi: 10.1099/00221287-60-3-293. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G. Glycosyl diglycerides from Pseudomonas rubescens. Biochim Biophys Acta. 1968 Oct 22;164(2):148–156. doi: 10.1016/0005-2760(68)90141-0. [DOI] [PubMed] [Google Scholar]
- van Houte J., Saxton C. A. Cell wall thickening and intracellular polysaccharide in microorganisms of the dental plaque. Caries Res. 1971;5(1):30–43. doi: 10.1159/000259730. [DOI] [PubMed] [Google Scholar]