Abstract
Objectives
Numerous studies have revealed the adverse health effects of acute and chronic exposure to particulate matter less than 10 μm in aerodynamic diameter (PM10). The aim of the present study was to examine the spatial distribution of PM10 concentrations and cardiovascular mortality and to investigate the spatial correlation between PM10 and cardiovascular mortality using spatial scan statistic (SaTScan) and a regression model.
Methods
From 2008 to 2010, the spatial distribution of PM10 in the Seoul metropolitan area was examined via kriging. In addition, a group of cardiovascular mortality cases was analyzed using SaTScan-based cluster exploration. Geographically weighted regression (GWR) was applied to investigate the correlation between PM10 concentrations and cardiovascular mortality.
Results
An examination of the regional distribution of the cardiovascular mortality was higher in provincial districts (gu) belonging to Incheon and the northern part of Gyeonggido than in other regions. In a comparison of PM10 concentrations and mortality cluster (MC) regions, all those belonging to MC 1 and MC 2 were found to belong to particulate matter (PM) 1 and PM 2 with high concentrations of air pollutants. In addition, the GWR showed that PM10 has a statistically significant relation to cardiovascular mortality.
Conclusions
To investigate the relation between air pollution and health impact, spatial analyses can be utilized based on kriging, cluster exploration, and GWR for a more systematic and quantitative analysis. It has been proven that cardiovascular mortality is spatially related to the concentration of PM10.
Keywords: Cardiovascular mortality, Geographically weighted regression, PM10, SaTScan, Spatial epidemiology
Introduction
Numerous studies on environmental epidemiology have reported the adverse effects of short- and long-term exposure to air pollution. Such exposure can exacerbate pre-existing heart and lung diseases, and it can lead to cardiovascular disease, respiratory disease, asthma, impaired lung function, and premature death [1,2].
Particulate matter less than 10 μm in aerodynamic diameter (PM10) increases oxidative stress and inflammatory response, and it is also associated with autonomic nervous system disorders and changes in blood coagulation, which can lead to cardiovascular disease and cardiovascular-related mortality [3-5].
Environmental epidemiology have quantitatively evaluated the adverse health effects caused by air pollution through various statistical analyses, including time-series studies and casecontrol studies [6,7]. Studies that incorporate the geospatial analysis method are actively being conducted. The geographic information system can effectively analyze spatial information by managing and analyzing spatial data obtained through a precise and systemic review of the physical space [8]. Spatial analysis has become an effective tool in the field of environmental epidemiology [9].
Health levels vary between different regions, and thus in the field of environmental health, it is important to identify regional characteristics and distribution. Health effects due to exposure to environmental pollutants are subject to spatial analysis, and it is necessary to determine the spatial correlation between them [10].
In a spatial epidemiology, spatial analyses such as measuring distance, identifying clusters, spatial smoothing and interpolation, and spatial regression were used, and such methods are widely used when researching health effects with respect to air pollution [11]. To identify the spatial correlation between potential health effects and environmental hazards, it is useful to determine cluster of health effects, assess the spatial distribution of risk for disease, and conduct a spatial analysis comparing environmental data with health data [12,13].
In this study, the spatial analyses were used to determine the correlation between PM10 and cardiovascular mortality in the Seoul metropolitan area. To identify the characteristics of spatial distribution with regard to PM10 and cardiovascular mortality, spatial analysis was conducted in each location, and cluster analysis was conducted to examine areas with high a cardiovascular mortality rate. Moreover, in order to determine the spatial correlation between PM10 and cardiovascular mortality, spatial distribution of PM10 and cluster areas of cardiovascular mortality were compared, and a spatial correlation was assessed quantitatively using regression analysis.
Materials and Methods
Study Scope and Data
The study areas were 79 provincial districts in Seoul, Incheon, and Gyeonggi-do within the metropolitan area (25 in Seoul, 11 in Incheon, and 44 in Gyeonggi-do). The metropolitan area is 11,745 km2, covering approximately 11.8% of South Korea’s land area (99,720 km2). As of 2010, the population of Seoul was 9,794,000, that of Incheon was 2,663,000, and that of Gyeonggi-do was 113,790,000. These three areas account for 49.1% of the entire South Korean population.
As of 2010, the National Institute of Environmental Research has been operating 102 urban air monitoring stations in the metropolitan area in order to assess the level of air quality in the city. Data from the urban air monitoring network were used for PM10, and the β-ray absorption method is being used to measure PM10 on an hourly basis.
We obtained daily counts of deaths between January 1, 2008 and December 31, 2010 from the National Statistical Office, South Korea. This study focused on deaths caused by cardiovascular-related diseases (International Classification of Diseases 10th revision [ICD-10], code I00-I99) within the metropolitan area, based on the time of death.
Spatial Distribution of PM10
To investigate the spatial distribution of PM10, kriging, which is widely used to estimate the spatial distribution of environmental factors, was applied. To estimate the concentration of PM10 unobserved in this study, ordinary kriging was applied by using the spherical theoretical variogram model as the weight. Compared to the Gaussian model or the exponential model, the calculated error value is minimal in the spherical model [14]. In general, the formula for ordinary kriging is as follows:
Here, z* is the critical value of the given point, zi is the value from the established point, γi is the weight for each of the neighboring data used, and n is the number of data used for the kriging calculation. Weight is a function of distance, and it should be determined so that there is minimal variance of estimation between the predicted value and the observed value.
To assess the concentration of PM10 in the Seoul metropolitan area between 2008 and 2010, the concentration of PM10 in the 79 provincial districts was calculated using kriging and zonal statistics via ArcGIS 10.1 (ESRI Inc., Redlands, CA, USA).
Software for The Spatial and Space-Time Scan Statistic
To assess the overall spatial correlation, Moran’s index (I) was calculated for the distribution of cardiovascular mortality [15]. Moran’s I ranges from -1 to +1, with a positive/negative sign representing positive/negative spatial autocorrelation [16]. OpenGeoDa was used to determine the spatial autocorrelation of cardiovascular mortality.
Cluster detection was performed using software for the spatial and space-time scan statistic (SaTScan) version 9.0 (Martin Kulldorff, Boston, MA, USA) with a Poisson model. Statistical cluster areas of hot spots are mapped based on SaTScan’s likelihood ratio [17]. This study used SaTScan’s Poisson model, and the likelihood ratio γ of the Poisson model is as follows:
Here, C is the entire value and c is the observed value. E[c] is the predicted value of the observed value and I() is the index factor.
Geographically Weighted Regression
Geographically weighted regression (GWR) was performed in order to analyze the spatial correlation between PM10 and cardiovascular mortality. Compared to general regression analysis, which represents the study area with one regression coefficient, GWR estimates regression coefficients regionally, thereby explaining the changes in the independent variable depending on the location of the spatial unit [18].
GWR uses the weighted matrix (W) between regions to calculate βji, each of the region’s regression coefficients, with regard to variable j, which depends on the geographical location i. GWR’s regression analysis is as follows:
Here, i is the location of an area, Y represents cardiovascular mortality, the independent variable is the PM10 concentration, and the regression coefficients and error term are represented as β and ϵ, respectively. The geographical weighted estimate of regression coefficient () is calculated by the weighted least square, and Wi is the local degree of the neighborhood i [19]. The GWR used ArcGIS version 10.1 to determine the relationship between PM10 and cardiovascular mortality.
Results
Table 1 displays the basic statistics of the number of cardiovascular mortality and PM10 concentration from 2008 to 2010, which were identified through kriging. In 2010, the annual average concentration of PM10 was 52.06 μg/m3, showing considerable improvement in contrast to 2008 and 2009. The number of cardiovascular mortality was maintained at a constant level during the research period, and the number of cases over three years was 778.34.
Table 1.
Summaries of PM10 and cardiovascular death in the Seoul metropolitan area of South Korea, 2008-2010
| Percentiles |
Mean | Standard deviation | ||||||
|---|---|---|---|---|---|---|---|---|
| Minimum | 25th | 50th | 75th | Maximum | ||||
| PM10 (μg/m3) | 2008 | 49.32 | 54.09 | 56.63 | 58.52 | 61.54 | 56.29 | 2.67 |
| 2009 | 51.28 | 54.14 | 56.53 | 59.84 | 63.76 | 57.21 | 3.49 | |
| 2010 | 43.55 | 48.99 | 52.25 | 55.91 | 59.03 | 52.06 | 4.32 | |
| 2008-2010 | 48.41 | 52.36 | 55.22 | 57.94 | 61.12 | 55.19 | 3.29 | |
| Cardiovascular death | 2008 | 26 | 168 | 241 | 359 | 564 | 262.47 | 118.48 |
| 2009 | 34 | 162 | 236 | 348 | 526 | 252.77 | 112.04 | |
| 2010 | 42 | 182 | 240 | 339 | 539 | 263.10 | 111.31 | |
| 2008-2010 | 34 | 180 | 235 | 345 | 536 | 259.45 | 112.58 | |
PM10, particulate matter less than 10 μm in aerodynamic diameter.
The global Moran’s I showed that the reported rates were 0.36 in 2008, 0.29 in 2009, 0.46 in 2010, and 0.44 between 2008 and 2010, all of which proved to be statistically significant. The Moran’s I was 0.2 or higher, indicating that the cardiovascular mortality presented a spatial autocorrelation, and in particular, Moran’s I was higher than 0.4 in the case of cardiovascular mortality in 2010, along with the period between 2008 and 2010, signifying a strong spatial autocorrelation.
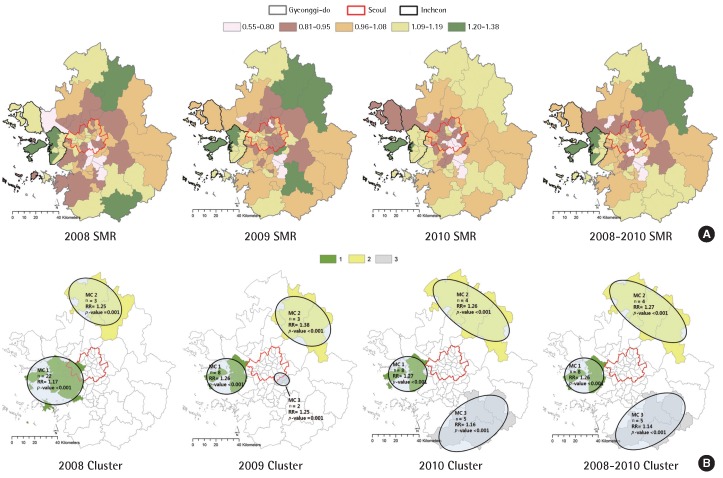
Standardized mortality ratio (SMR) and the results of spatial scan statistics for cardiovascular mortality are shown in Figure 1. The SMR for each provincial district (gu) of the Seoul metropolitan area was 0.55–1.38 in 2008, 0.51–1.48 in 2009, and 0.70–1.69 in 2010. The SMR was higher within Incheon and the northern part of Gyeonggi-do compared to other regions between 2008 and 2010. In contrast, the SMR of Seoul and the neighboring Gyeonggi-do area was lower than that in other regions.
Figure 1.
Standardized mortality ratio (SMR) (A) and mapping of cardiovascular mortality clusters (MC) (B) with higher mortality rates from cardiovascular disease. RR, relative risk.
The difference in the likelihood ratio determined the cluster region, and mortality cluster (MC) 1 indicates the likelihood ratio within the highest risk cluster. Regions MC 1 and MC 2 were categorized as the provincial district within Incheon and the northern part of Gyeonggi-do, respectively.
Table 2 displays the results of SaTScan such as the population exposed to risk within the cluster as a hot spot for cardiovascular mortality between 2008 and 2010, incidences of cardiovascular mortality as well as relative risk, and data regarding the cluster, which were statistically significant.
Table 2.
Significant clusters found by the spatial scan statistic software for cardiovascular mortality in the Seoul metropolitan area of South Korea, 2008-2010
| Year | Cluster label (n) | Population at risk | No. of case | Relative risk | Log likelihood ratio | p-value |
|---|---|---|---|---|---|---|
| 2008 | MC 1 (22) | 7,456,536 | 6,774 | 1.17 | 55.68 | <0.001 |
| MC 2 (3) | 268,308 | 472 | 1.25 | 11.06 | 0.001 | |
| 2009 | MC 1 (8) | 2,561,111 | 2,057 | 1.26 | 56.73 | <0.001 |
| MC 2 (3) | 277,285 | 521 | 1.38 | 23.96 | <0.001 | |
| MC 3 (2) | 477,862 | 484 | 1.25 | 10.78 | 0.001 | |
| 2010 | MC 1 (8) | 2,561,111 | 2,613 | 1.27 | 60.25 | <0.001 |
| MC 2 (4) | 318,580 | 600 | 1.26 | 14.65 | <0.001 | |
| MC 3 (5) | 1,127,049 | 1,265 | 1.16 | 12.73 | <0.001 | |
| 2008-2010 | MC 1 (8) | 2,561,111 | 7,683 | 1.26 | 166.86 | <0.001 |
| MC 2 (4) | 318,580 | 1,780 | 1.27 | 46.18 | <0.001 | |
| MC 3 (5) | 1,127,049 | 3,660 | 1.14 | 27.34 | <0.001 |
MC, mortality cluster.
The concentration of PM10 was categorized in four equal intervals in order to compare the distribution of regional clusters regarding PM10 and cardiovascular mortality. Particulate matter (PM) 1 signifies a region with PM10 concentration that is higher than 75%, PM 2 50–75%, PM 3 25–50%, and PM 4 lower than 25%. Figure 2 indicates overlapping spatial distribution regions that are the concentration range of PM10 and hot spots of cardiovascular mortality.
Figure 2.
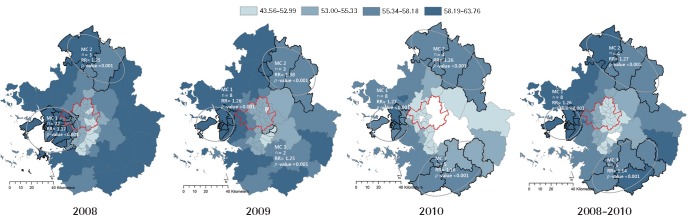
Spatial distribution of PM10 concentrations and cardiovascular mortality clusters (MC). PM10, particulate matter less than 10 μm in aerodynamic diameter; RR, relative risk.
Table 3 presents the relationships between MCs and PM10 concentration. The results of a comparison of the average PM10 range and the cluster as hot spots of cardiovascular mortality accumulated throughout 2008–2010 show that the eight provincial districts of the MC 1 area all coincided with the PM 1 area, which are regions with a high concentration of PM10. A comparison of the corresponding ratio regarding the cardiovascular MC regions by the level of PM10 concentration indicates that cluster of cardiovascular mortality correspond mostly with areas of high PM10 concentration.
Table 3.
Relationship between cardiovascular mortality clusters (MC) and PM10 concentration
| MC | PM10 concentration | |||
|---|---|---|---|---|
| 2008 | PM 1 (26) | PM 2 (21) | PM 3 (22) | PM 4 (10) |
| MC 1 (22) | 12 (54.5) | 10 (45.5) | ||
| MC 2 (3) | 1 (33.3) | 2 (66.7) | ||
| 2009 | PM 1 (29) | PM 2 (18) | PM 3 (28) | PM 4 (4) |
| MC 1 (8) | 8 (100.0) | |||
| MC 2 (3) | 1 (33.3) | 2 (66.7) | ||
| MC 3 (2) | 1 (50.0) | 1 (50.0) | ||
| 2010 | PM 1 (4) | PM 2 (21) | PM 3 (9) | PM 4 (45) |
| MC 1 (8) | 3 (37.5) | 5 (62.5) | ||
| MC 2 (4) | 4 (100.0) | |||
| MC 3 (5) | 3 (60.0) | 2 (40.0) | ||
| 2008-2010 | PM 1 (17) | PM 2 (22) | PM 3 (16) | PM 4 (24) |
| MC 1 (8) | 8 (100.0) | |||
| MC 2 (4) | 1 (25.0) | 3 (75.0) | ||
| MC 3 (5) | 1 (20.0) | 4 (80.0) | ||
Values are presentd as number (%).
PM10, particulate matter less than 10 μm in aerodynamic diameter; PM, particulate matter.
In order to quantitatively analyze the correlation between the SMR of cardiovascular mortality and PM10, spatial regression analysis using the GWR was conducted. This study excluded the 1% region with the highest level of PM10 concentration (one provincial district) as an outlier among the 79 provincial districts of the Seoul metropolitan area.
Table 4 presents the results of the correlation of cardiovascular mortality and PM10 using the GWR, which signifies a significant relevance between PM10 and cardiovascular mortality. The minimum, maximum, and average regression coefficient of PM10 and cardiovascular mortality by region in the GWR were 0.799, 1.562, and 0.956, respectively and a difference in the correlation between PM10 and cardiovascular mortality was reported. The R2adj of the GWR was 0.205, and the result of analyzing the residual of Moran’s I was 0.039, which indicates that the spatial autocorrelation of the residual shows an arbitrary pattern and satisfies the assumption of the basic GWR.
Table 4.
Geographically weighted regression (GWR) of PM10 and cardiovascular mortality in the Seoul metropolitan area of South Korea, 2008-2010
| GWR |
||||
|---|---|---|---|---|
| Min β | Max β | Mean β | SE | |
| PM10 | 0.799 | 1.562 | 0.956 | 0.102 |
| Local R2 | 0.157 | 0.249 | 0.198 | 0.017 |
| R2adj | 0.205 | |||
| AIC | 639.324 | |||
| Moran’s index of residual | 0.039** | |||
Min, minimum; Max, maximum; SE, standard error; PM10, particulate matter less than 10 μm in aerodynamic diameter; AIC, Akaike information criterion.
p<0.01.
Discussion
In this research, various spatial epidemiological analyses were applied to the Seoul metropolitan area between 2008 and 2010 in order to determine the correlation between PM10 and cardiovascular mortality. Kriging was performed on PM10 concentration for to determine spatial distribution, and spatial autocorrelation was determined by using Moran’s I. Disease mapping of cardiovascular mortality was conducted with use of SaTScan in order to explore the geographical distribution, and cluster of cardiovascular mortality were investigated. Also, the spatial correlation between PM10 and cardiovascular mortality was identified quantitatively using the GWR.
Disease surveillance researches pursuing the temporal spatial distribution of disease using SaTScan have been actively conducted. Fukuda et al. [20] have investigated clusters of mortality cases due to colon cancer, lung cancer, and breast cancer using SaTScan throughout Japan as the subject matter, and the relation between mortality clusters and social clusters (socio-economic indicators and population density) was analyzed. The results of the analysis revealed that the mortality cluster due to lung cancer and breast cancer deaths were the urban areas. Horst and Coco [21] used SaTScan to analyze clusters of patients visiting basic health services and emergency rooms due to respiratory and gastrointestinal diseases. Su et al. [22] used SaTScan to spatially analyze clusters of cancer patients based on gender, type of cancer, and ethnicity in order to identify the range and characteristics of cancer center service areas.
Also, the following five categories were examined for the purpose of disease surveillance: data processing, analysis methods, technical issues, analysis output, and user’s facility regarding the four spatiotemporal analysis programs (SaTScan, ClusterSeer, GeoSurveillance, and R-Surveillance); results showed that SaTScan was the most robust program for searching clusters of health effects [23].
Disease surveillance entails collecting, analyzing, and interpreting data in order to identify the frequency and distribution of diseases within the population [24]. It is an important process that supplies valuable information for policy decisions and implementation. Utilizing spatial cluster analysis enables basic data to be used for preventative activities by identifying areas with relatively high disease risk as well as disease clusters.
Internationally, research analysis on the relationship between air pollution and health effects is being conducted using various spatial analyses, and a significant spatial correlation between health and air pollution is being reported. Corburn et al. [25] used SaTScan to identify asthma cluster in New York City, and they analyzed the correlation between land use, housing type, and air pollution. The analysis results revealed a quantitative correlation of 69% between asthma cluster and air pollution facilities. Jephcote and Chen [26] utilized the GWR in order to examine the relationship between respiratory hospitalizations of children under the age of 15, socio-economic indicators, and PM10 from automobile emissions in Leicester, UK. The results of their study indicated that respiratory hospitalization of children is related to socio-economic indicators and PM10 from automobile emissions, and this relationship differed between regions. In this research, a comparison of the PM10 and the hot spot of cardiovascular mortality identified through SaTScan showed that in most cases, the cardiovascular mortality cluster was included within the area with high levels of PM10. Also, a significant correlation was identified as a result of analyzing the PM10 concentration and cardiovascular mortality using the GWR, and the spatial correlation appeared differently in the result of the regional regression coefficient calculation, which indicates a similar result as the previous research results. The reason that the regional spatial correlation between PM10 and cardiovascular mortality appears differently is due to various factors, such as concentration levels and components of the PM10, demographic characteristics, and sensitivity of the population exposed to the PM10, as well as socio-economic factors [27,28].
The spatial analysis using the GWR scientifically suggests regional differences between air pollution and health effects, and conducting policy intervention based on this information can help in the effective distribution of limited resources by prioritizing the policy statement and the target area.
Through the development of a geographic information system and the joining of statistical techniques, a more scientific analysis regarding the relationship between health risks due to environmental exposure and risks within the spatial epidemiology is made possible. Spatial epidemiology involves integrated analysis, such as visualization, exploratory analysis, and modeling based on spatial data regarding environmental exposure and health effects, and it examines the analysis of the spatial correlation and patterns in order to predict and prevent health risk [10,29]. This research utilized distance interpolation, disease mapping, and GWR analysis among various spatial methods in order to identify the correlation between air pollution and health effects, and it produced significant results of the correlation between air pollution and health effects through systematic and quantitative analysis.
However, there are certain limitations to this research. First, it is an ecological study that uses population data, and it does not integrate personalized data due to the limitations of the data. Therefore, the research does not consider personal characteristics or dynamic factors such as population movement because the analysis is based on population data. Second, cardiovascular mortality can occur due to factors other than air pollution, including environmental factors, socio-economic factors, medical factors, and dietary factors. This study only analyzed health effects caused by PM10. In the future, it will be necessary to configure the model to consider a number of factors.
The spatial analyses and findings of the research on the relationship between air pollution and health effects can be utilized as a scientific method to identify vulnerable areas for evidencebased public health policies.
Acknowledgments
This study is part of the research ‘Evaluation of Impacts of Climate Change on Air Pollution and Health (III)’ conducted by the Korean Environmental Institute, funded by the Ministry of Environment and the National Institute of Environmental Research.
Footnotes
The authors have no conflicts of interest with material presented in this paper.
References
- 1.Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329(24):1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 2.Puett RC, Hart JE, Yanosky JD, Paciorek C, Schwartz J, Suh H, et al. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ Health Study. Environ Health Perspect. 2009;117(11):1697–1701. doi: 10.1289/ehp.0900572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pope CA, 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect. 2009;117(6):898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cesaroni G, Badaloni C, Gariazzo C, Stafoggia M, Sozzi R, Davoli M, et al. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect. 2013;121(3):324–331. doi: 10.1289/ehp.1205862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meister K, Johansson C, Forsberg B. Estimated short-term effects of coarse particles on daily mortality in Stockholm, Sweden. Environ Health Perspect. 2012;120(3):431–436. doi: 10.1289/ehp.1103995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostro B, Tobias A, Querol X, Alastuey A, Amato F, Pey J, et al. The effects of particulate matter sources on daily mortality: a casecrossover study of Barcelona, Spain. Environ Health Perspect. 2011;119(12):1781–1787. doi: 10.1289/ehp.1103618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auchincloss AH, Gebreab SY, Mair C, Diez Roux AV. A review of spatial methods in epidemiology, 2000-2010. Annu Rev Public Health. 2012;33:107–22. doi: 10.1146/annurev-publhealth-031811-124655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han D, Hwang SS. Spatial epidemiology and environmental health: on the use of spatially referenced health and environment data. J Environ Health Sci. 2011;37(1):1–11. (Korean) [Google Scholar]
- 10.Kim J, Kang D. Spatial analysis methods for asbestos exposure research. J Environ Health Sci. 2012;38(5):369–379. (Korean) [Google Scholar]
- 11.Beale L, Hodgson S, Abellan JJ, Lefevre S, Jarup L. Evaluation of spatial relationships between health and the environment: the rapid inquiry facility. Environ Health Perspect. 2010;118(9):1306–1312. doi: 10.1289/ehp.0901849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beale L, Abellan JJ, Hodgson S, Jarup L. Methodologic issues and approaches to spatial epidemiology. Environ Health Perspect. 2008;116(8):1105–1110. doi: 10.1289/ehp.10816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott P, Wartenberg D. Spatial epidemiology: current approaches and future challenges. Environ Health Perspect. 2004;112(9):998–1006. doi: 10.1289/ehp.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SY, Kim Y. Spatial analysis of ground level ozone in Seoul, Korea. J Korean Urban Geogr Soc. 2012;15(2):39–50. (Korean) [Google Scholar]
- 15.Shin HS, Lee SH. Regional disparity of ambulatory health care utilization. J Korean Assoc Geogr Inf Stud. 2012;15(4):138–150. (Korean) [Google Scholar]
- 16.Kim G. Detecting spatial autocorrelation and using spatial regression. Korean J Policy Anal Eval. 2003;13(1):273–306. (Korean) [Google Scholar]
- 17.Kulldorffa M. A spatial scan statistic. Commun Stat Theory Methods. 1997;26(6):1481–1496. [Google Scholar]
- 18.Charlton M, Fotheringham AS. Geographically weighted regression white paper. Kildare: National University of Ireland Maynooth; 2009. pp. 1–14. [Google Scholar]
- 19.Lee H, Shim J. GIS geomatics: theory and practice. Seoul: Bobmensa; 2011. pp. 404–423. (Korean) [Google Scholar]
- 20.Fukuda Y, Umezaki M, Nakamura K, Takano T. Variations in societal characteristics of spatial disease clusters: examples of colon, lung and breast cancer in Japan. Int J Health Geogr. 2005;4:16. doi: 10.1186/1476-072X-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horst MA, Coco AS. Observing the spread of common illnesses through a community: using Geographic Information Systems (GIS) for surveillance. J Am Board Fam Med. 2010;23(1):32–41. doi: 10.3122/jabfm.2010.01.090137. [DOI] [PubMed] [Google Scholar]
- 22.Su SC, Kanarek N, Fox MG, Guseynova A, Crow S, Piantadosi S. Spatial analyses identify the geographic source of patients at a National Cancer Institute Comprehensive Cancer Center. Clin Cancer Res. 2010;16(3):1065–1072. doi: 10.1158/1078-0432.CCR-09-1875. [DOI] [PubMed] [Google Scholar]
- 23.Robertson C, Nelson TA. Review of software for space-time disease surveillance. Int J Health Geogr. 2010;9:16. doi: 10.1186/1476-072X-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ball W, LeFevre S, Jarup L, Beale L. Comparison of different methods for spatial analysis of cancer data in Utah. Environ Health Perspect. 2008;116(8):1120–1124. doi: 10.1289/ehp.10815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corburn J, Osleeb J, Porter M. Urban asthma and the neighbourhood environment in New York City. Health Place. 2006;12(2):167–179. doi: 10.1016/j.healthplace.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Jephcote C, Chen H. Environmental injustices of children’s exposure to air pollution from road-transport within the model British multicultural city of Leicester: 2000-09. Sci Total Environ. 2012;414:140–151. doi: 10.1016/j.scitotenv.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 27.Katsouyanni K, Samet JM, Anderson HR, Atkinson R, Le Tertre A, Medina S, et al. Air pollution and health: a European and North American approach (APHENA) Res Rep Health Eff Inst. 2009;(142):5–90. [PubMed] [Google Scholar]
- 28.Hajat A, Diez-Roux AV, Adar SD, Auchincloss AH, Lovasi GS, O’Neill MS, et al. Air pollution and individual and neighborhood socioeconomic status: evidence from the Multi-Ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2013;121(11-12):1325–1333. doi: 10.1289/ehp.1206337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfeiffer DU, Robinson TP, Stevenson M, Stevens KB, Rogers DJ, Clements AC. Spatial analysis in epidemiology. Oxford: Oxford University Press; 2008. p. 3. [Google Scholar]