Abstract
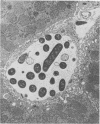
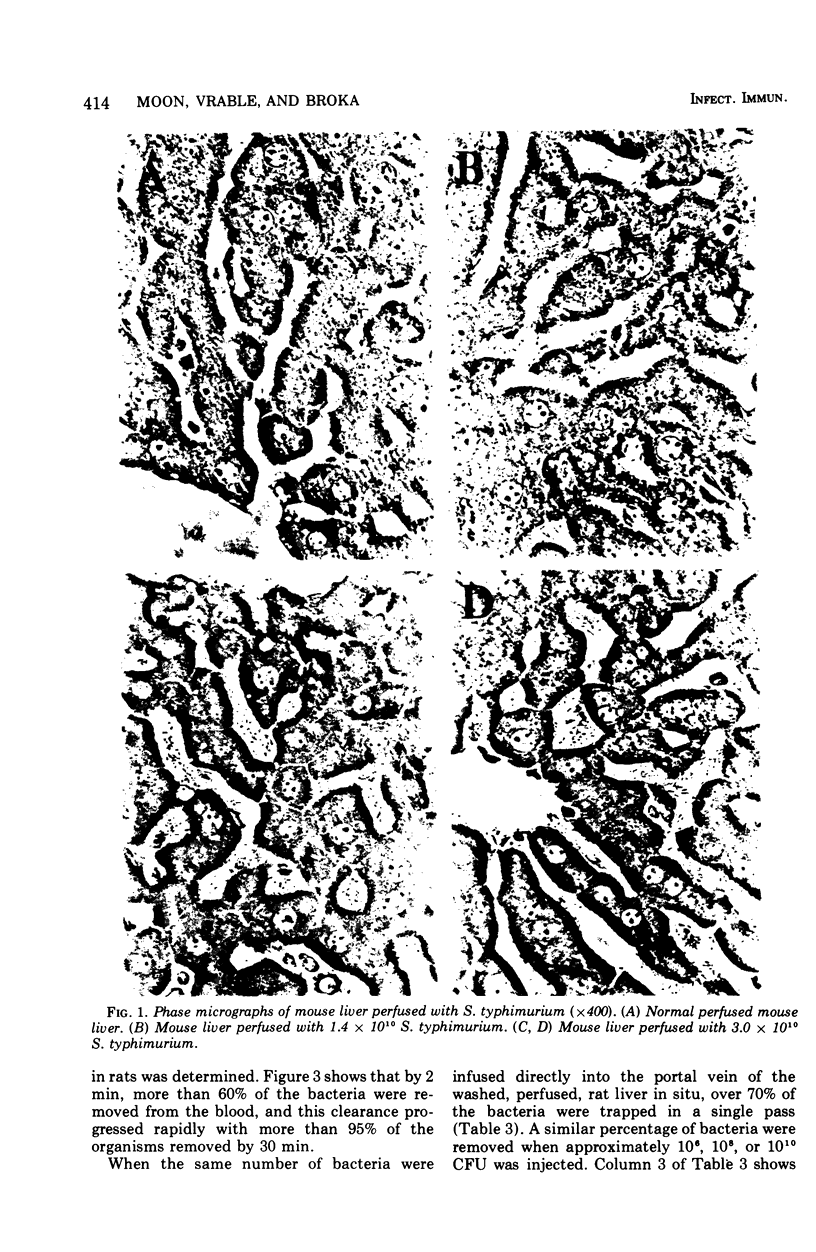
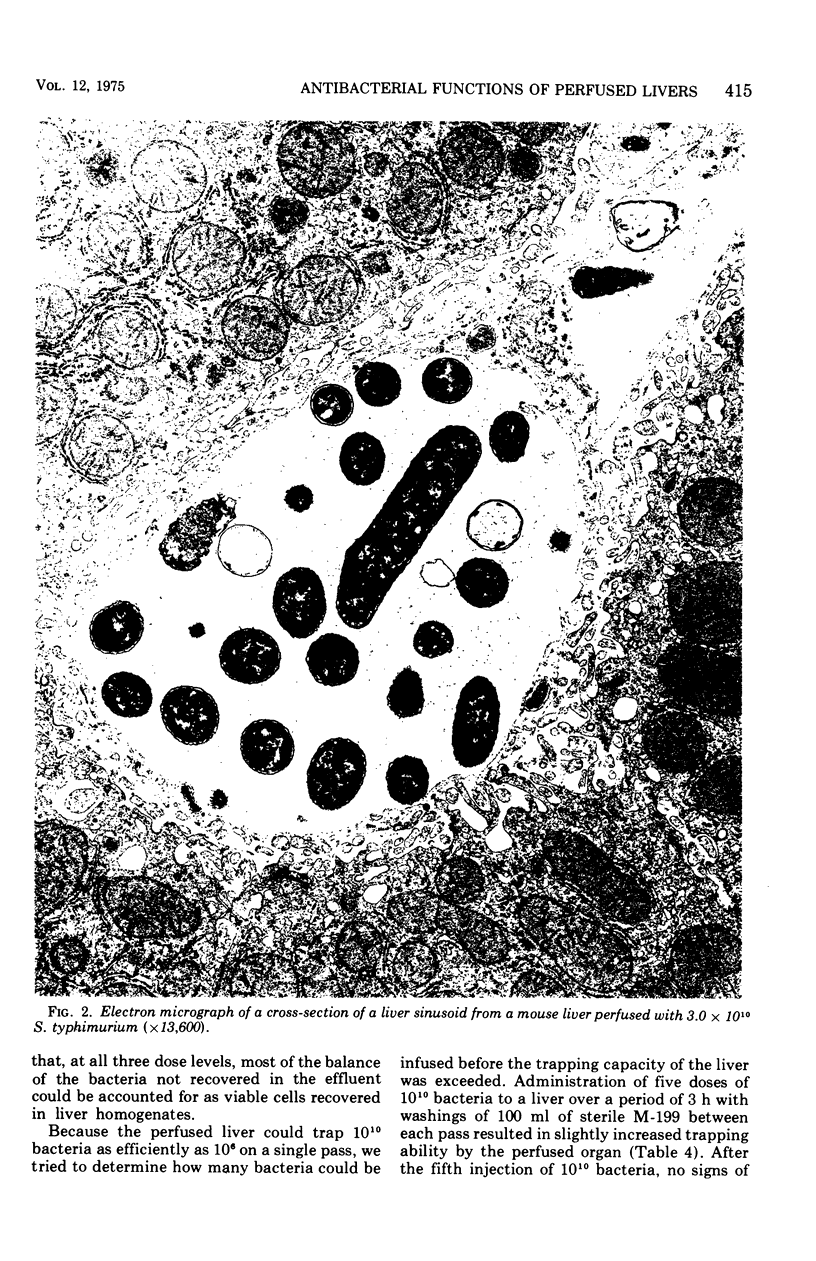
CF-1 mice cleared and killed 80% of a 1.2 x 10(9) intravenous dose of Salmonella typhimurium after 30 min. The perfused mouse liver trapped 70% of a similar dose of S. typhimurium in a single pass, but in the perfusion model no significant killing of the trapped organisms was observed. The perfused rat liver also avidly trapped bacteria. Because of its larger size, we have been able to devise techniques to experimentally distinguish between the bacterial trapping and killing functions of this organ. When the liver was washed free of blood with sterile M-199, over 70 to 80% of a 10(6) to 10(10) dose of viable S. typhimurium was trapped after a single pass, but no significant bacterial killing was observed. When blood or plasma was added to the perfusion medium, over 50% of the trapped bacteria were killed in 15 to 30 min. Phase contrast and electron micrographs of perfused livers showed extensive extracellular trapping of bacteria in the sinusoids. Our data show that humoral factors are apparently not necessary for efficient trapping of live Salmonella by the perfused rat liver but are an absolute requirement for bacterial activity of the organ.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimacopoulos-Jeannet F., Exton J. H., Jeanrenaud B. Control of gluconeogenesis and glycogenolysis in perfused livers of normal mice. Am J Physiol. 1973 Jul;225(1):25–32. doi: 10.1152/ajplegacy.1973.225.1.25. [DOI] [PubMed] [Google Scholar]
- Baine W. B., Koenig M. G., Goodman J. S. Clearance of Candida albicans from the bloodstream of rabbits. Infect Immun. 1974 Dec;10(6):1420–1425. doi: 10.1128/iai.10.6.1420-1425.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Oxman E. Phagocytosis and intracellular disposition of viable bacteria by the isolated perfused rat liver. J Reticuloendothel Soc. 1965 Nov;2(4):313–325. [PubMed] [Google Scholar]
- Folland D., Armstrong D., Seides S., Blevins A. Pneumococcal bacteremia in patients with neoplastic disease. Cancer. 1974 Mar;33(3):845–849. doi: 10.1002/1097-0142(197403)33:3<845::aid-cncr2820330333>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- HOWARD J. G. The reticulo-endothelial system and resistance to bacterial infection. Scott Med J. 1961 Feb;6:60–82. doi: 10.1177/003693306100600203. [DOI] [PubMed] [Google Scholar]
- HOWARD J. G., WARDLAW A. C. The opsonic effect of normal serum on the uptake of bacteria by the reticulo-endothelial system; perfusion studies with isolated rat liver. Immunology. 1958 Oct;1(4):338–352. [PMC free article] [PubMed] [Google Scholar]
- Jeunet F. S., Cain W. A., Good R. A. Reticuloendothelial function in the isolated perfused liver. 3. Phagocytosis of Salmonella typhosa and Brucella melitensis and the blockade of the reticuloendothelial system. J Reticuloendothel Soc. 1969 Aug;6(4):391–410. [PubMed] [Google Scholar]
- Jeunet F. S., Cain W., Good R. A. Differential recognition of Brucella organisms by Kupffer cells: studies with isolated perfused liver. Proc Soc Exp Biol Med. 1968 Oct;129(1):187–190. doi: 10.3181/00379727-129-33280. [DOI] [PubMed] [Google Scholar]
- Jeunet F. S., Good R. A. Reticuloendothelial function in the isolated perfused liver. II. Phagocytosis of heat-aggregated bovine serum albumin. Demonstration of two components in the blockade of the reticuloendothelial system. J Reticuloendothel Soc. 1969 Feb;6(1):94–107. [PubMed] [Google Scholar]
- Kaplan M. H., Armstrong D., Rosen P. Tuberculosis complicating neoplastic disease. A review of 201 cases. Cancer. 1974 Mar;33(3):850–858. doi: 10.1002/1097-0142(197403)33:3<850::aid-cncr2820330334>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Klastersky J., Henri A., Hensgens C., Daneau D. Gram-negative infections in cancer. Study of empiric therapy comparing carbenicillin-cephalothin with and without gentamicin. JAMA. 1974 Jan 7;227(1):45–48. doi: 10.1001/jama.227.1.45. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky H. S., Cuttner J. Fungal infection in acute leukemia. Cancer. 1972 Aug;30(2):348–352. doi: 10.1002/1097-0142(197208)30:2<348::aid-cncr2820300207>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- ROGERS D. E. Host mechanisms which act to remove bacteria from the blood stream. Bacteriol Rev. 1960 Mar;24(1):50–66. doi: 10.1128/br.24.1.50-66.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba T. M. Physiology and physiopathology of the reticuloendothelial system. Arch Intern Med. 1970 Dec;126(6):1031–1052. [PubMed] [Google Scholar]
- Sharbaugh R. J., Grogan J. B. Suppression of reticuloendothelial function in the rat with cyclophosphamide. J Bacteriol. 1969 Oct;100(1):117–122. doi: 10.1128/jb.100.1.117-122.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis (first of three parts). N Engl J Med. 1974 Mar 28;290(13):717–723. doi: 10.1056/NEJM197403282901306. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis (third of three parts). N Engl J Med. 1974 Apr 11;290(15):833–839. doi: 10.1056/NEJM197404112901506. [DOI] [PubMed] [Google Scholar]