Abstract
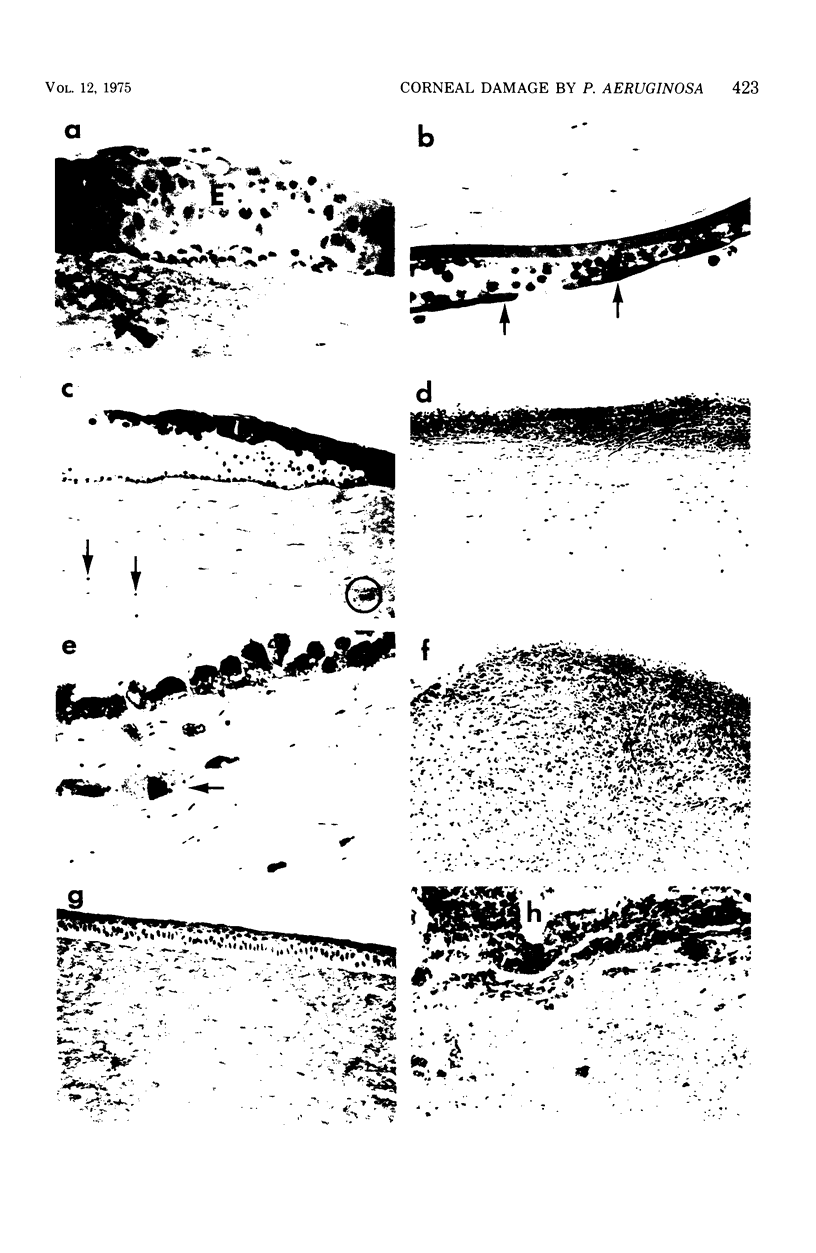
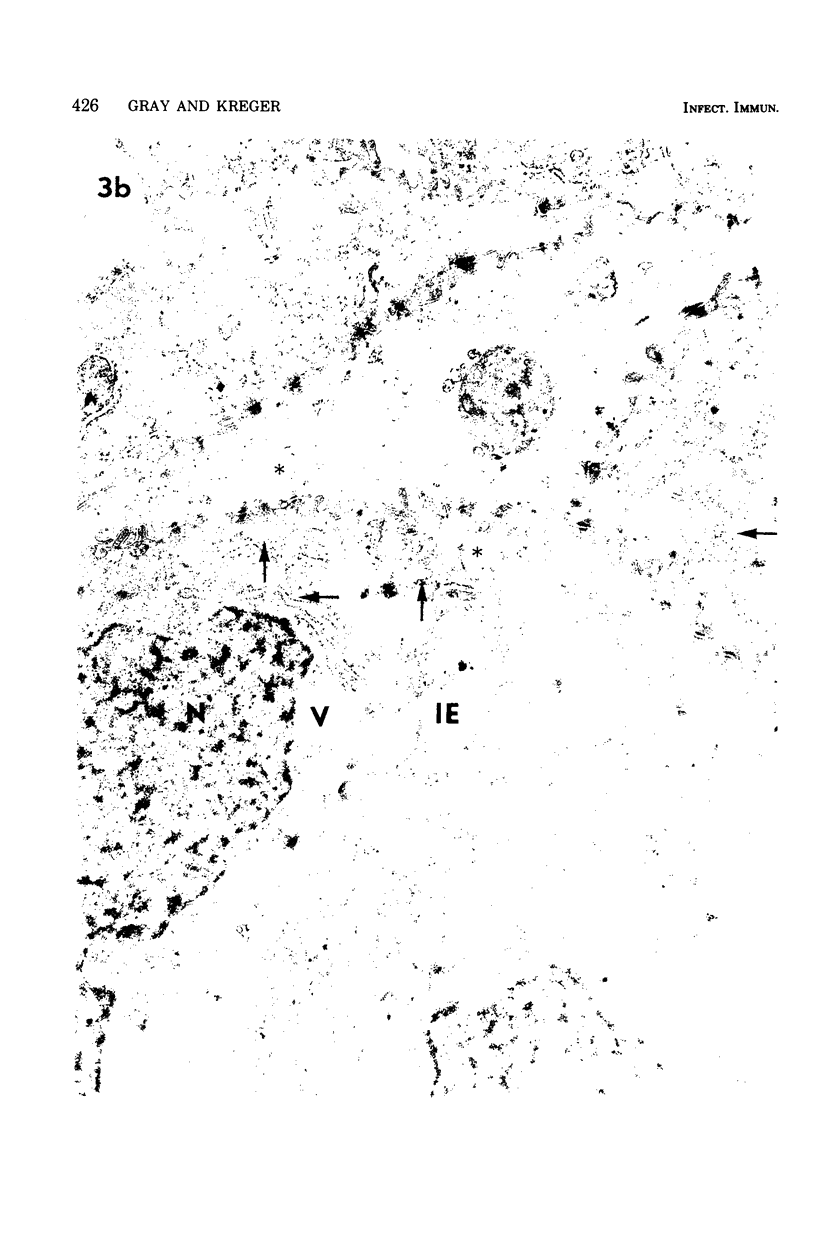
Gross, light microscopic, and electron microscopic examination of the rabbit corneal destruction produced by experimental Pseudomonas aeruginosa infections revealed a combination of acute inflammation and liquefaction necrosis of the cornea. Degeneration of the epithelial cells and the start of polymorphonuclear leukocyte infiltration of the cornea occurred initially. These changes were followed by loss of the epithelium, degeneration and loss of the keratocytes and endothelium, loss of the characteristic weblike pattern of the proteoglycan ground substance, dispersal of ultrastructurally normal collagen fibrils, extensive accumulation followed by degeneration of polymorphonuclear leukocytes, and accumulation of plasma proteins and fibrin in the necrotic cornea. Histochemical examination of the cornea suggested a loss of the proteoglycan ground substance but not of collagen. Rabbit corneas injected with Clostridium histolyticum collagenase showed gross and cellular changes similar to those observed during the pseudomonal infections; however, histochemical examination suggested a loss of collagen, and electron microscopy revealed ultrastructurally abnormal collagen fibrils. The results support the idea (i) that a bacterial or host-derived collagenase is not required for extensive corneal damage during a P. aeruginosa corneal infection, and (ii) that a P. aeruginosa corneal infection may severly damage the cornea by producing extensive corneal edema and by causing the loss of the corneal proteoglycan ground substance, thus resulting in dispersal of undamaged collagen fibrils, weakening of the cornea, and subsequent descemetocele formation and corneal perforation by the anterior chamber pressure.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown S. I., Bloomfield S. E., Tam W. The cornea-destroying enzyme of Pseudomonas aeruginosa. Invest Ophthalmol. 1974 Mar;13(3):174–180. [PubMed] [Google Scholar]
- Brown S. I., Hook C. W., Tragakis M. P. Presence, significance, and inhibition of lysosomal proteoglycanases. Invest Ophthalmol. 1972 Mar;11(3):149–152. [PubMed] [Google Scholar]
- Brown S. I., Tragakis M. P., Pearce D. B. Treatment of the alkali-burned cornea. Am J Ophthalmol. 1972 Aug;74(2):316–320. doi: 10.1016/0002-9394(72)90552-1. [DOI] [PubMed] [Google Scholar]
- Dohlman C. H. The function of the corneal epithelium in health and disease. The Jonas S. Friedenwald Memorial Lecture. Invest Ophthalmol. 1971 Jun;10(6):383–407. [PubMed] [Google Scholar]
- FISHER E., Jr, ALLEN J. H. Corneal ulcers produced by cell-free extracts of Pseudomonas aeruginosa. Am J Ophthalmol. 1958 Jul;46(1 Pt 2):21–27. doi: 10.1016/0002-9394(58)90030-8. [DOI] [PubMed] [Google Scholar]
- FISHER E., Jr, ALLEN J. H. Mechanism of corneal destruction by pseudomonas proteases. Am J Ophthalmol. 1958 Nov;46(5 Pt 2):249–255. doi: 10.1016/0002-9394(58)90804-3. [DOI] [PubMed] [Google Scholar]
- Gerke J. R., Magliocco M. V. Experimental Pseudomonas aeruginosa Infection of the Mouse Cornea. Infect Immun. 1971 Feb;3(2):209–216. doi: 10.1128/iai.3.2.209-216.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook C. W., Bull F. G., Iwanij V., Brown S. I. Purification of corneal collagenases. Invest Ophthalmol. 1972 Sep;11(9):728–734. [PubMed] [Google Scholar]
- Huber J. D., Parker F., Odland G. F. A basic fuchsin and alkalinized methylene blue rapid stain for epoxy-embedded tissue. Stain Technol. 1968 Mar;43(2):83–87. doi: 10.3109/10520296809115048. [DOI] [PubMed] [Google Scholar]
- Kreger A. S., Griffin O. K. Physicochemical fractionation of extracellular cornea-damaging proteases of Pseudomonas aeruginosa. Infect Immun. 1974 May;9(5):828–834. doi: 10.1128/iai.9.5.828-834.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laibson P. R. Cornea and sclera. Arch Ophthalmol. 1972 Nov;88(5):553–574. doi: 10.1001/archopht.1972.01000030555018. [DOI] [PubMed] [Google Scholar]
- MORIHARA K., TSUZUKI H., OKA T., INOUE H., EBATA M. PSEUDOMONAS AERUGINOSA ELASTASE. ISOLATION, CRYSTALLIZATION, AND PRELIMINARY CHARACTERIZATION. J Biol Chem. 1965 Aug;240:3295–3304. [PubMed] [Google Scholar]
- McCulley J. P., Slansky H. H., Pavan-Langston D., Dohlman C. H. Collagenolytic activity in experimental herpes simplex keratitis. Arch Ophthalmol. 1970 Oct;84(4):516–519. doi: 10.1001/archopht.1970.00990040518023. [DOI] [PubMed] [Google Scholar]
- RICHARDSON K. C., JARETT L., FINKE E. H. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 1960 Nov;35:313–323. doi: 10.3109/10520296009114754. [DOI] [PubMed] [Google Scholar]
- Scharmann W., Balke E. Untersuchungen über eine Protease (Elastase) von Pseudomonas aeruginosa, I. Bildung und Reinigung der Protease. Hoppe Seylers Z Physiol Chem. 1974 Apr;355(4):443–450. [PubMed] [Google Scholar]
- Smith J. W., Frame J. Observations on the collagen and proteinpolysaccharide complex of rabbit cornea stroma. J Cell Sci. 1969 Mar;4(2):421–436. doi: 10.1242/jcs.4.2.421. [DOI] [PubMed] [Google Scholar]
- TRUMP B. F., SMUCKLER E. A., BENDITT E. P. A method for staining epoxy sections for light microscopy. J Ultrastruct Res. 1961 Aug;5:343–348. doi: 10.1016/s0022-5320(61)80011-7. [DOI] [PubMed] [Google Scholar]
- Wilson L. A. Chelation in experimental Pseudomonas keratitis. Br J Ophthalmol. 1970 Sep;54(9):587–593. doi: 10.1136/bjo.54.9.587. [DOI] [PMC free article] [PubMed] [Google Scholar]