Abstract
Vitamin D deficiency is emerging worldwide and many studies now suggest its role in the development of several chronic diseases. Due to the low level of vitamin D naturally occurring in food there is a need for supplementation and use of vitamin D-enhanced products. The aim of the present study was to determine if daily consumption of vitamin D2-enhanced mushrooms increased vitamin D status in free-living healthy adults or affected markers of the metabolic syndrome. A total of ninety volunteers (aged 40–65 years) were randomly assigned to one of two 4-week studies: mushroom study (15 µg vitamin D2 or placebo mushroom powder) and capsule study (15 µg vitamin D3 or placebo capsules). Consumption of vitamin D2-enhanced mushrooms increased serum 25-hydroxyvitamin D2 (25(OH)D2) by 128 % from baseline (3·9 (sd 1·9) nmol/l; P < 0·05). Serum 25(OH)D3 increased significantly in the vitamin D3 capsule group (a 55 % increase from a baseline of 44.0 (sd 17·1) nmol/l; P < 0·05). Vitamin D status (25(OH)D) was affected only in the vitamin D3 group. Plasminogen activator inhibitor-1 was lowered by vitamin D2 intake. Vitamin D2 from enhanced mushrooms was bioavailable and increased serum 25(OH)D2 concentration with no significant effect on 25(OH)D3 or total 25(OH)D.
Key words: Food enhancement, Metabolic syndrome, Mushrooms, Vitamin D status
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; hsCRP, high-sensitivity C-reactive protein; MetS, metabolic syndrome; PAI-1, plasminogen activator inhibitor-1; PTH, parathyroid hormone
In recent years, a large body of evidence has emerged to support the role of vitamin D in a broad range of biological roles other than Ca absorption and bone metabolism. Though the evidence is less robust, vitamin D deficiency is now implicated in a range of diseases including psoriasis, multiple sclerosis, inflammatory bowel disease, type 1 and 2 diabetes, hypertension, CVD, the metabolic syndrome (MetS) and various cancers( 1 ). In recent years, studies have documented that hypovitaminosis D may be a risk factor for the development of the MetS( 2 , 3 ). There is an inverse relationship between serum 25-hydroxyvitamin D (25(OH)D) levels and several MetS markers, such as fasting plasma glucose, blood pressure, waist circumference, TAG and markers of systemic inflammation( 4 – 6 ). However, intervention studies have yielded contradictory results: some studies have confirmed the effect of vitamin D intake on insulin sensitivity, lipid profile and inflammation( 7 – 9 ), while others have not( 10 – 12 ). Discrepancy in the intervention results may be due to the range of populations studied, the length, frequency and the level of vitamin D supplementation used.
Vitamin D insufficiency, defined as 25(OH)D concentration below 50 nmol/l, is prevalent in nearly half of the population( 13 – 15 ) and less than a quarter of adults reach levels considered sufficient (i.e. above 75 nmol/l)( 16 ). Even during the summer months most individuals do not reach satisfactory 25(OH)D levels( 17 ). Recent work has demonstrated that daily intakes between 7·9 and 42·8 µg of vitamin D are required to maintain adequate vitamin D levels in the elderly( 18 ). Furthermore, the US Institute of Medicine has recently increased the RDA for vitamin D to 15 µg/d (600 IU)( 19 ). Since natural dietary sources are a poor provider of vitamin D with values typically ranging from 0·1 to 16·1 µg/100 g for whole milk and oil-rich fish, respectively( 20 ), the dietary intake of vitamin D is significantly below 15 µg/d in most European countries, with the highest median daily intakes of about 10 µg in the Nordic countries( 21 ). In Ireland, 72 % of men and 78 % of women had average daily vitamin D intakes of less than 5 µg/d, and over 90 % less than 10 µg/d( 22 ).
In recent years, in an attempt to combat low dietary intakes of vitamin D, attention has turned to vitamin D-enhanced foods( 23 ). In the USA, enhanced foods have been found to contribute approximately 80 % of total vitamin D intake( 24 ). A recent systematic review evaluated the efficacy of vitamin D fortification and concluded that from each 1 µg of vitamin D ingested daily a 1·2 nmol/l increase in 25(OH)D is obtained, highlighting the potential effectiveness of the approach in the prevention of vitamin D deficiency( 25 ). However, vitamin D enhancement is not straightforward and much debate exists around whether enhancement should be performed with vitamin D3 or vitamin D2( 26 , 27 ), and at what level vitamin D should be added to foods( 28 , 29 ). To date, the majority of the randomised controlled trials that have investigated the effect of intake of vitamin D-enhanced milk, yogurt or cheese at the level of 10–100 µg/d recorded an improvement in vitamin D status( 30 – 33 ). In recent years, alternatives such as fortified orange juice have been introduced and research has demonstrated that daily consumption of juice enhanced with 25 µg of vitamin D2 or D3 for 3 months resulted in improved 25(OH)D levels( 34 , 35 ). More recently, the use of UVB radiation as an effective method for increasing the concentration of vitamin D2 in mushrooms has emerged( 36 ). Mushrooms are low in energy (120–150 kJ per 100 g), a source of protein (approximately 30 % DM) and carbohydrates (47 %), as well as B vitamins and trace elements (Na, K, P, Mg, Ca and S), with eminent flavour properties( 37 , 38 ). The bioavailability of vitamin D2 from wild-grown( 39 ), as well as from UV-treated mushrooms( 40 , 41 ) has been confirmed in human subjects.
The objective of the present study was to expand our knowledge on the impact of consumption of such vitamin D2-enhanced mushrooms. With this in mind the primary objective was to examine the impact of daily consumption of 15 µg of vitamin D2-enhanced mushrooms for 4 weeks on vitamin D status in comparison with the effect of the same level of intake of vitamin D3 from capsules. The secondary objective of the study was to examine the impact of increased vitamin D2 and D3 intake on markers of the MetS.
Experimental methods
Study participants and design
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Human Research Ethics Committee in University College Dublin. Written informed consent was obtained from all subjects.
Healthy Caucasian men and women living in Dublin and its surroundings (latitude 53·3° N) between the age of 40 and 65 years were recruited by advertisement in local and college newspapers, flyers, posters, university-based emails, website and radio advertising. The participants were initially screened and, if the inclusion criteria were met, the participants were asked to sign a consent form. Participants were excluded if they had a BMI below 18 or above 32 kg/m2, used medication or hormone replacement therapy (with the exception of the contraceptive pill), had a chronic or infectious disease, were pregnant or lactating, took part in any other dietary intervention study or had allergy to mushrooms. Abstention from taking any vitamin D supplements for 4 weeks preceding the study and during the intervention was imposed. The intervention was carried out between February and March 2011. Anthropometric measurements were performed during the screening visit. Body weight and height were measured in duplicate to the second decimal place and BMI was calculated: BMI = weight (kg)/height2 (m2). Blood pressure was measured and heart rate recorded using an Omron M6 Comfort digital automatic blood pressure monitor (Omron Healthcare Europe) in the subject's steady state. Power calculations were performed based on published results for 25(OH)D levels following consumption of vitamin D2 mushrooms and no supplementation( 39 ): twenty subjects per group were required to obtain a power of 80 % and an α of 0·05. The overall study was composed of two distinct parts: (1) supplementation with mushrooms and (2) supplementation with capsules. Both arms of these double-blind randomised placebo-controlled dietary trials were performed using identical protocols except for the treatment type. Using a computer-generated randomisation code, ninety participants were randomly assigned to receive daily: 15 µg vitamin D2-enhanced mushroom powder or placebo mushroom powder (mushroom study); 15 µg vitamin D3 capsules or placebo capsules (capsule study). Participants were given enough supplements for 7 d of treatment at the beginning of each week and compliance was assessed by reporting the non-used mushroom sachets or capsules.
The mushroom powder was manufactured by Monaghan Mushrooms. Briefly, to naturally enhance the production of vitamin D, button mushrooms (Agaricus bisporus) were treated for 3 s with a UVB dose of 1·5 J/cm2. The batch was pooled, lyophilised and analysed for vitamin D2 content. The amount of powder containing 15 µg vitamin D2 was computed. The treatment and placebo sachets were identical and weighed approximately 0·64 (sd 0·02) g. The vitamin D2 placebo sachets contained no detectable level of vitamin D2. Participants were instructed on the storage of the mushrooms (i.e. cool, dry place, hermetically closed) and were asked to consume the whole content of a sachet per d mixed with any meal. The vitamin D and placebo capsules containing 15 µg of vitamin D3 were produced specifically for the study by Banner Pharmacaps and were ingested once per d with a beverage.
Blood samples were collected by a trained phlebotomist following a 12 h overnight fast at the beginning and following the 4-week intervention. EDTA and lithium heparin tubes (BD Vacutainer) were used for plasma collection and directly placed on ice. Serum samples were collected in tubes with a clotting agent with or without a barrier gel (BD Vacutainer) and were allowed to clot for 30 min at room temperature before being placed on ice. All samples were centrifuged at 1800 g for 10 min at 4°C and samples were stored at –80°C until subsequent analysis.
Biochemical measurements
All measurements were made according to manufacturers’ instructions. Serum levels of 25(OH)D2 and 25(OH)D3 were measured using LC/MS-MS at a laboratory certified by the Vitamin D External Quality Assessment Scheme (DEQAS) at the Institute of Aging and Chronic Disease, Liverpool, UK as detailed by Tolppanen et al.( 42 ). Total 25(OH)D levels were computed by adding up the values of 25(OH)D2 and 25(OH)D3. A semi-automated biochip analyser (Metabolic Syndrome Array I, Randox Investigator; Randox) was used to determine serum levels of TNF-α, C-peptide, ferritin, IL-6, insulin, leptin, plasminogen activator inhibitor-1 (PAI-1) and resistin. Plasma hsC-reactive protein (hsCRP), glucose, TAG, NEFA, total and HDL-cholesterol, and serum Ca and albumin were measured on a RX Daytona™ automated analyser (Randox). Homeostasis model of assessment of insulin resistance (HOMA-IR) score was calculated from the glucose and insulin values as follows: HOMA-IR = fasting insulin (µU/ml) × fasting glucose (mmol/l)/22·5)( 43 ). Total Ca was adjusted for plasma albumin level using the formula of Payne et al.( 44 ): corrected Ca (mmol/l) = serum Ca (mmol/l) + 0·02 (40 – serum albumin (g/l)). Serum parathyroid hormone (PTH; MD Biosciences GmbH) and plasma adiponectin (Alpco Diagnostics) were measured using ELISA kits. Serum LDL-cholesterol was measured with enzymic colorimetric method (Wako Diagnostics).
Statistical analyses
Statistical analyses were performed using SPSS software for Windows (version 18·0; SPSS Inc.). The χ2 test was used to analyse sex distribution across the groups. The comparisons between age, BMI and waist:hip ratio were performed with independent-samples t tests. General linear model (GLM) ANOVA adjusted for sex, age and BMI was used to compare the changes in the measurements (from baseline to after the 4-week intervention) between the placebo and treatment groups. Changes in post-intervention values were assessed using a GLM ANOVA controlling for baseline values, sex, age and BMI. Linear regression analysis were performed to test the relationships between serum 25(OH)D and the measurements. The results were considered significant when P < 0·05.
Results
Characteristics of participants
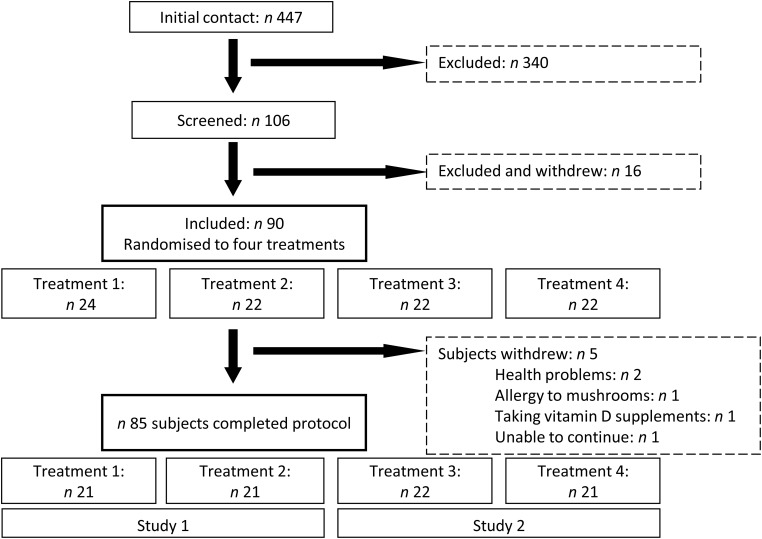
An overview of the study design and recruitment process is shown in Fig. 1. After the initial contact and screening for eligibility criteria, ninety participants aged 40 to 65 years were recruited and randomly allocated to one of four treatments that made up the mushroom and the capsule studies. In all, five participants dropped out from the study: one due to a suspected mushroom allergy, two due to health problems, one for personal reasons and one who was already taking a vitamin D supplement. No adverse effects of supplementation were observed and the average compliance was 98 %, ranging from 97 % (vitamin D2 mushroom group) to 99 % (vitamin D3 capsules). The sex distribution of participants that completed the protocol was not significantly different between groups in the studies. However, the age of the participants in the mushroom study differed between the placebo and active mushroom groups (Tables 1 and 2).
Fig. 1.
Summary of study design. Due to an expected lower number of enrolled male participants, males were assigned to treatment groups 1 to 4 in that order following the randomisation code. Treatments: 1, vitamin D2-enhanced mushrooms; 2, placebo mushrooms; 3, vitamin D3 capsules; 4, placebo capsules.
Table 1.
Characteristics of the participants assigned to the treatment and placebo groups in study 1
(Number of subjects; mean values, standard deviations and ranges)
| Vitamin D2-enhanced mushrooms | Placebo mushrooms | ||||
|---|---|---|---|---|---|
| Mean | sd | Mean | sd | P* | |
| Subjects (n) | 21 | 21 | 0·747 | ||
| Female | 14 | 13 | |||
| Male | 7 | 8 | |||
| Age (years) | 54·4 | 6·3 | 49·8 | 6·3 | 0·022 |
| Range | 40–65 | 40–63 | |||
| Height (m) | 1·68 | 0·07 | 1·70 | 0·08 | 0·388 |
| Weight (kg) | 71·6 | 13·3 | 75·3 | 12·7 | 0·359 |
| BMI (kg/m2) | 25·2 | 3·2 | 26·0 | 3·1 | 0·441 |
| Waist:hip ratio | 0·80 | 0·08 | 0·82 | 0·10 | 0·656 |
* χ2 statistics were used to test sex distribution between the groups. Independent-samples t tests were used to determine differences between subject characteristics.
Table 2.
Characteristics of the participants assigned to the treatment and placebo groups in study 2
(Number of subjects; mean values, standard deviations and ranges)
| Vitamin D3 capsules | Placebo capsules | ||||
|---|---|---|---|---|---|
| Mean | sd | Mean | sd | P* | |
| Subjects (n) | 22 | 21 | 0·835 | ||
| Female | 14 | 15 | |||
| Male | 8 | 7 | |||
| Age (years) | 49·1 | 6·7 | 52·9 | 7·2 | 0·083 |
| Range | 40–63 | 40–64 | |||
| Height (m) | 1·69 | 0·10 | 1·72 | 0·10 | 0·351 |
| Weight (kg) | 74·7 | 15·0 | 76·5 | 14·6 | 0·691 |
| BMI (kg/m2) | 25·9 | 3·5 | 25·7 | 3·2 | 0·791 |
| Waist:hip ratio | 0·83 | 0·09 | 0·81 | 0·08 | 0·285 |
* χ2 statistics were used to test sex distribution between the groups. Independent-samples t tests were used to determine differences between subject characteristics.
At baseline, 56·5 % of the participants were vitamin D deficient (total serum 25(OH)D concentration less than 50 nmol/l), 11·8 % had sufficient total 25(OH)D levels above 75 nmol/l, while the remaining 31·8 % were classified as insufficient (total 25(OH)D levels between 50 and 75 nmol/l). Linear regression analysis indicated a negative relationship between baseline total 25(OH)D concentration and PTH levels (β coefficient –0·184; P = 0·017; R 2 0·260).
Serum 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and total 25-hydroxyvitamin D concentrations
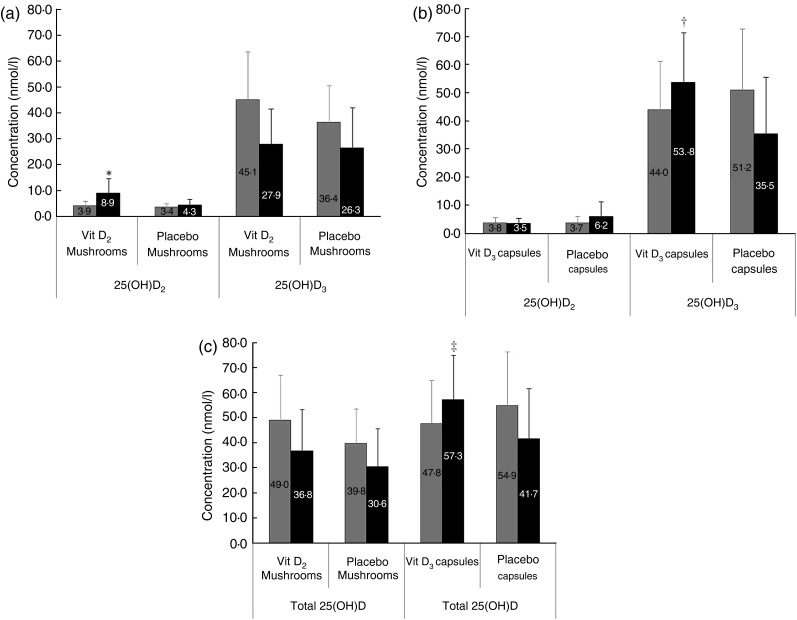
Following daily supplementation with vitamin D-enhanced mushrooms the change in 25(OH)D2 concentration was significantly higher than for the placebo mushroom group (P = 0·007) (Fig. 2(a)). The final 25(OH)D2 concentrations were 8·9 (sd 5·8) and 4·3 (sd 2·2) nmol/l for the enhanced mushroom and the placebo group, respectively. There was no significant change in serum 25(OH)D3 and total 25(OH)D following supplementation with vitamin D-enhanced mushrooms compared with placebo mushrooms. The post-intervention total 25(OH)D concentration was 36·8 (sd 16·6) and 30·6 (sd 15·1) nmol/l for enhanced and placebo mushrooms (Fig. 2(a) and 2(c)), respectively.
Fig. 2.
Serum levels of 25-hydroxyvitamin D2 (25(OH)D2) and
25(OH)D3 at baseline ( ) and after a 4-week
intervention (■) in (a) the mushroom study and (b) capsule study. (c) Serum levels
of total 25(OH)D in the mushroom and capsule studies. Vit D2, vitamin
D2; Vit D3, vitamin D3. Values are means, with
standard deviations represented by vertical bars. A general linear model ANOVA was
used, adjusted for sex, age and BMI (P < 0·05). *
Significant difference in the change of 25(OH)D2 was observed in the
vitamin D2-enhanced mushroom group v. placebo mushroom
group (P = 0·007). There was no difference in the change of
25(OH)D3. † Significant difference in the change of 25(OH)D3
was observed in the vitamin D3 capsule group v. placebo
capsule group (P < 0·0003). There was no difference in the
change of 25(OH)D2. ‡ Significant difference in the change of total
25(OH)D between the supplemented and placebo groups in the capsule study
(P = 0·0003). No difference in total 25(OH)D was observed in the
mushroom study. Total 25(OH)D was computed as a sum of 25(OH)D2 and
25(OH)D3 forms.
) and after a 4-week
intervention (■) in (a) the mushroom study and (b) capsule study. (c) Serum levels
of total 25(OH)D in the mushroom and capsule studies. Vit D2, vitamin
D2; Vit D3, vitamin D3. Values are means, with
standard deviations represented by vertical bars. A general linear model ANOVA was
used, adjusted for sex, age and BMI (P < 0·05). *
Significant difference in the change of 25(OH)D2 was observed in the
vitamin D2-enhanced mushroom group v. placebo mushroom
group (P = 0·007). There was no difference in the change of
25(OH)D3. † Significant difference in the change of 25(OH)D3
was observed in the vitamin D3 capsule group v. placebo
capsule group (P < 0·0003). There was no difference in the
change of 25(OH)D2. ‡ Significant difference in the change of total
25(OH)D between the supplemented and placebo groups in the capsule study
(P = 0·0003). No difference in total 25(OH)D was observed in the
mushroom study. Total 25(OH)D was computed as a sum of 25(OH)D2 and
25(OH)D3 forms.
For participants consuming a daily vitamin D3 capsule there was no significant change in 25(OH)D2 (Fig. 2(b)). Both serum 25(OH)D3 and total 25(OH)D increased significantly in the supplemented group (P = 10·5 × 10−6 and P = 8·1 × 10−5, respectively) compared with the placebo group (Fig. 2(b) and 2(c)). Following the intervention total 25(OH)D levels were 57·3 (sd 17·7) and 41·7 (sd 20·1) nmol/l for the supplemented and placebo groups, respectively.
Comparison of the concentrations across the four groups revealed that the 25(OH)D2 concentration post-intervention was significantly higher in the vitamin D2-enhanced mushroom compared with the placebo mushroom and the vitamin D3 capsule group. The post-intervention values for 25(OH)D3 and total 25(OH)D were significantly higher in the vitamin D3 capsule group compared with all other groups (Table 3).
Table 3.
Concentrations (nmol/l) of 25-hydroxyvitamin D2 (25(OH)D2), 25(OH)D3 and total 25(OH)D across the four treatment groups following the intervention
(Mean values and standard deviations)
| Vitamin D2-enhanced mushrooms | Placebo mushrooms | Vitamin D3 capsules | Placebo capsules | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | Mean | sd | Mean | sd | P∥ | |
| 25(OH)D2 | 8·9†‡ | 5·8 | 4·3* | 2·2 | 3·5* | 1·8 | 6·2 | 5·2 | 0·001 |
| 25(OH)D3 | 27·9‡ | 13·5 | 26·3‡ | 15·7 | 53·8*†§ | 18·0 | 35·5‡ | 18·4 | <0·001 |
| 25(OH)D | 36·8‡ | 16·6 | 30·6‡ | 15·1 | 57·3*†§ | 17·7 | 41·7‡ | 20·1 | <0·001 |
* Mean value was significantly different from that of the vitamin D2-enhanced mushroom treatment group (P < 0·05).
† Mean value was significantly different from that of the placebo mushroom treatment group (P < 0·05).
‡ Mean value was significantly different from that of the vitamin D3 capsule treatment group (P < 0·05).
§ Mean value was significantly different from that of the placebo capsule treatment group (P < 0·05).
║ P values are based on analysis of group effect for post-values adjusted for baseline values, sex, age and BMI (P < 0·05).
Blood pressure and markers of the metabolic syndrome
Analysis of the markers of the MetS was performed across the four groups and significant group effects were found for hsCRP and PAI-1. A decrease in hsCRP was observed in the vitamin D2 mushroom group compared with the vitamin D3 capsule group. Subsequent analysis was performed separately for the mushroom and the capsule groups (Tables 4 and 5). Following 4 weeks of daily supplementation with mushrooms enhanced with 15 µg of vitamin D2 a decrease in plasma PAI-1 concentration was observed (P = 0·038). Comparison of the capsule groups revealed significant differences only in C-peptide. However, there was no change in the supplemented group and a decrease in the placebo group.
Table 4.
Metabolic syndrome markers pre- and post-intervention in the vitamin D2-enhanced and placebo mushroom groups
(Mean values and standard deviations)
| Vitamin D2-enhanced mushrooms | Placebo mushrooms | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||||
| Mean | sd | Mean | sd | Mean | sd | Mean | sd | P* | |
| Systolic BP (mmHg) | 117 | 13 | 115 | 13 | 121 | 18 | 122 | 18 | 0·143 |
| Diastolic BP (mmHg) | 79 | 8 | 77 | 10 | 83 | 11 | 84 | 14 | 0·101 |
| HR (bpm) | 72 | 11 | 70 | 9 | 75 | 12 | 72 | 14 | 0·702 |
| Glucose (mmol/l) | 5·73 | 0·45 | 5·65 | 0·41 | 5·95 | 0·69 | 5·68 | 0·56 | 0·753 |
| Insulin (µIU/ml) | 3·94 | 2·79 | 3·55 | 2·87 | 4·53 | 1·99 | 4·41 | 3·07 | 0·719 |
| HOMA-IR | 1·01 | 0·73 | 0·89 | 0·73 | 1·22 | 0·60 | 1·14 | 0·95 | 0·617 |
| C-peptide (ng/ml) | 1·50 | 1·38 | 1·29 | 1·06 | 1·21 | 0·68 | 1·34 | 0·89 | 0·307 |
| TAG (mmol/l) | 0·83 | 0·27 | 0·90 | 0·35 | 1·23 | 0·79 | 1·13 | 0·75 | 0·420 |
| Total cholesterol (mmol/l) | 5·33 | 1·42 | 5·61 | 1·06 | 5·86 | 1·20 | 6·07 | 1·41 | 0·195 |
| HDL-cholesterol (mmol/l) | 1·71 | 0·49 | 1·72 | 0·45 | 1·61 | 0·41 | 1·63 | 0·44 | 0·484 |
| LDL-cholesterol (mmol/l) | 3·77 | 0·64 | 3·84 | 0·63 | 3·73 | 0·72 | 3·99 | 0·79 | 0·107 |
| NEFA (mmol/l) | 0·59 | 0·32 | 0·55 | 0·31 | 0·73 | 0·27 | 0·53 | 0·19 | 0·398 |
| PTH (pg/ml) | 61·6 | 32·2 | 59·1 | 32·0 | 60·1 | 21·4 | 57·1 | 16·6 | 0·659 |
| Corrected Ca (mmol/l) | 2·36 | 0·10 | 2·36 | 0·13 | 2·34 | 0·12 | 2·32 | 0·12 | 0·750 |
| Adiponectin (µg/ml) | 8·28 | 3·17 | 7·36 | 2·48 | 6·76 | 4·01 | 5·67 | 4·00 | 0·210 |
| Ferritin (ng/ml) | 48·2 | 44·4 | 52·4 | 45·8 | 75·0 | 62·0 | 61·8 | 51·5 | 0·650 |
| Leptin (ng/ml) | 5·17 | 13·13 | 3·55 | 9·16 | 2·25 | 2·70 | 1·77 | 1·59 | 0·774 |
| Resistin (ng/ml) | 3·29 | 0·88 | 3·00 | 1·03 | 3·06 | 0·96 | 3·39 | 1·11 | 0·135 |
| hsCRP (mg/l) | 2·15 | 2·37 | 1·06 | 0·72 | 1·78 | 2·48 | 1·23 | 1·48 | 0·498 |
| TNFα (pg/ml) | 4·62 | 1·27 | 4·34 | 1·24 | 4·47 | 1·48 | 4·14 | 1·27 | 0·333 |
| IL-6 (pg/ml) | 0·92 | 0·63 | 0·90 | 0·47 | 1·36 | 1·38 | 0·77 | 0·47 | 0·066 |
| PAI-1 (ng/ml) | 15·43 | 4·07 | 11·46 | 4·83 | 14·39 | 3·75 | 15·19 | 6·33 | 0·038 |
BP, blood pressure; HR, heart rate; bpm, beats per min; HOMA-IR, homeostasis model of assessment of insulin resistance; PTH, parathyroid hormone; hsCRP, high-sensitivity C-reactive protein; PAI-1, plasminogen activator inhibitor-1.
* P values are based on analysis of group effect for post values adjusted for baseline values, sex, age and BMI (P < 0·05).
Table 5.
Metabolic syndrome markers pre- and post-intervention in the vitamin D3 and placebo capsule groups
(Mean values and standard deviations)
| Vitamin D3 capsules | Placebo capsules | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||||
| Mean | sd | Mean | sd | Mean | sd | Mean | sd | P* | |
| Systolic BP (mmHg) | 119 | 12 | 115 | 11 | 113 | 13 | 117 | 13 | 0·073 |
| Diastolic BP (mmHg) | 85 | 11 | 80 | 10 | 78 | 8 | 79 | 9 | 0·507 |
| HR (bpm) | 76 | 14 | 73 | 13 | 71 | 9 | 69 | 10 | 0·583 |
| Glucose (mmol/l) | 5·92 | 0·43 | 5·68 | 0·43 | 5·76 | 0·48 | 5·59 | 0·46 | 0·830 |
| Insulin (µIU/ml) | 4·57 | 2·35 | 4·23 | 2·99 | 4·32 | 3·59 | 3·17 | 1·63 | 0·081 |
| HOMA-IR | 1·21 | 0·63 | 1·09 | 0·81 | 1·14 | 1·05 | 0·80 | 0·44 | 0·064 |
| C-peptide (ng/ml) | 1·39 | 1·05 | 1·33 | 0·91 | 1·46 | 1·18 | 1·01 | 0·70 | 0·020 |
| TAG (mmol/l) | 1·30 | 0·69 | 1·24 | 0·72 | 1·07 | 0·43 | 0·95 | 0·39 | 0·153 |
| Total cholesterol (mmol/l) | 5·66 | 1·12 | 5·43 | 1·24 | 6·47 | 1·16 | 6·06 | 1·40 | 0·496 |
| HDL-cholesterol (mmol/l) | 1·57 | 0·33 | 1·51 | 0·35 | 1·81 | 0·63 | 1·76 | 0·66 | 0·957 |
| LDL-cholesterol (mmol/l) | 3·57 | 0·48 | 3·73 | 0·60 | 4·08 | 0·48 | 4·12 | 0·42 | 0·736 |
| NEFA (mmol/l) | 0·62 | 0·27 | 0·58 | 0·21 | 0·51 | 0·19 | 0·49 | 0·19 | 0·650 |
| PTH (pg/ml) | 59·9 | 25·3 | 54·0 | 20·1 | 54·5 | 25·8 | 57·0 | 21·3 | 0·588 |
| Corrected Ca (mmol/l) | 2·31 | 0·09 | 2·31 | 0·09 | 2·34 | 0·10 | 2·36 | 0·12 | 0·178 |
| Adiponectin (µg/ml) | 6·23 | 2·96 | 5·73 | 2·12 | 7·28 | 2·93 | 6·98 | 2·68 | 0·528 |
| Ferritin (ng/ml) | 68·4 | 47·0 | 63·5 | 46·6 | 70·0 | 47·9 | 64·4 | 46·0 | 0·712 |
| Leptin (ng/ml) | 2·21 | 2·12 | 2·51 | 2·99 | 1·70 | 1·35 | 1·81 | 1·44 | 0·466 |
| Resistin (ng/ml) | 3·34 | 0·96 | 3·24 | 0·90 | 3·40 | 1·94 | 3·59 | 1·44 | 0·326 |
| hsCRP (mg/l) | 1·92 | 1·61 | 2·08 | 2·44 | 1·54 | 2·18 | 1·68 | 1·94 | 0·434 |
| TNFα (pg/ml) | 4·59 | 1·14 | 4·24 | 1·02 | 4·85 | 1·49 | 4·40 | 1·59 | 0·970 |
| IL-6 (pg/ml) | 1·06 | 1·30 | 0·83 | 0·71 | 1·12 | 1·28 | 1·65 | 2·94 | 0·536 |
| PAI-1 (ng/ml) | 15·29 | 5·62 | 14·19 | 6·26 | 14·35 | 5·80 | 11·09 | 4·51 | 0·082 |
BP, blood pressure; HR, heart rate; bpm, beats per min; HOMA-IR, homeostasis model of assessment of insulin resistance; PTH, parathyroid hormone; hsCRP, high-sensitivity C-reactive protein; PAI-1, plasminogen activator inhibitor-1.
* P values are based on analysis of group effect for post values adjusted for baseline values, sex, age and BMI (P < 0·05).
Discussion
In the present study the effect of a daily intake for 4 weeks of 15 µg of vitamin D2-enhanced mushrooms or vitamin D3 in capsule form on serum 25(OH)D2 and 25(OH)D3 concentrations and markers of the MetS was investigated. The intake of each form of the vitamin significantly increased serum concentration of its own hydroxylated form, but only vitamin D3 supplements positively affected vitamin D status, i.e. total 25(OH)D levels.
Vitamin D2-enhanced mushrooms could serve as an alternative vegetarian source of vitamin D, which could be added to any meal, giving a broad choice for consumers. To date only four randomised controlled trials have investigated the impact of vitamin D2-enhanced foods on vitamin D status and, contradictory to the present results, three of these showed a significant increase in total 25(OH)D concentration by 50–67 %( 34 , 39 , 40 ). However, Stephensen et al.( 41 ) found no effect of supplementation with vitamin D2-enhanced mushrooms on vitamin D status. Three of these studies used vitamin D2-enriched mushrooms with levels of vitamin D2 ranging from an equivalent daily dose of 8·8 to 100 µg. In addition to this, the baseline values of total 25(OH)D were dramatically different in the various studies. In the study group used by Stephensen et al.( 41 ), 94 % of participants had 25(OH)D concentrations > 50 nmol/l at the start of the study with a significant contribution from cutaneous synthesis, making it difficult to improve status via a dietary source. In contrast, in the study by Outila et al.( 39 ), a younger population with low baseline vitamin D status (25(OH)D < 60 nmol/l) was selected for the intervention. This contrasts with our older population with no pre-selection based on vitamin D status. In the present study, despite no changes in total 25(OH)D there was a significant increase in 25(OH)D2 levels in the enhanced mushroom group, similarly to the results obtained by Stephensen et al.( 41 ). A number of reasons may explain the lack of changes in total 25(OH)D: first, 25(OH)D2 only contributed to approximately 8 % of the total 25(OH)D at baseline, making it difficult for any change in 25(OH)D2 levels to alter the overall status. Second, there was a large decrease in 25(OH)D3 (38 %) due to the time of year, again making it very difficult to overcome this drop with supplementation of the minor contributor to total 25(OH)D. Although daily consumption of 15 µg of vitamin D2-enhanced mushrooms did not significantly make an impact on vitamin D status, we have clearly demonstrated that the vitamin was well absorbed, since it significantly increased 25(OH)D2 levels.
In the current literature a debate exists as to whether vitamin D3 or vitamin D2 is more potent in improving vitamin D status. Recent systematic reviews have indicated that vitamin D3 is more efficacious at increasing total 25(OH)D( 45 , 46 ). Previous work has suggested that vitamin D2 supplementation may cause a reduction of serum 25(OH)D3 levels( 41 , 47 ). One of the potential mechanisms by which this may occur is due to a competition of both forms of the vitamin for 25-hydroxylase or/and increased degradation of 25(OH)D3( 48 ). The results of the present study in conjunction with other studies( 27 , 34 , 49 ) do not concur with this hypothesis. Total serum 25(OH)D was higher in vitamin D2 supplement users compared with the unsupplemented group without any change in 25(OH)D3 levels in the study of Rapuri et al.( 49 ) and Holick et al.( 27 ). Biancuzzo et al.( 34 ) observed a similar increase in total 25(OH)D after supplementation with vitamin D2 or D3. The present study demonstrated a depletion of 25(OH)D3 levels in all three groups not supplemented with vitamin D3 and this decrease was not greater in the vitamin D2-enhanced mushroom group. Winter depletion and seasonal fluctuation of total 25(OH)D levels have been previously shown in northern latitudes. We also found, in agreement with several earlier studies( 40 , 50 ), no changes in Ca levels and a negative relationship between total 25(OH)D and PTH levels, with no response of PTH to vitamin D supplementation, underlying the safety of the supplementation.
A secondary objective of the study was to investigate the effect of vitamin D supplementation on MetS markers. In the present study the 4-week intake of vitamin D2-enhanced mushrooms led to a decrease in PAI-1 concentration. To date, no clear consensus regarding the impact of vitamin D supplementation on MetS markers has emerged in the literature. Some intervention studies failed to report any changes in MetS biomarkers( 10 ), while others found positive changes in the components of MetS in vitamin D-supplemented groups, such as insulin secretion or sensitivity, β-cell function, inflammation markers and lipid profile( 7 – 9 , 11 , 12 ). In our randomised controlled trial, only PAI-1 was lowered by the intake of vitamin D2. However, it is important to consider that the study population was healthy and the intervention was only for 4 weeks, which may be too short to see any changes in markers of the MetS. Several authors have suggested that a high dose of vitamin D that increases total 25(OH)D to levels above 75 nmol/l may be needed to produce significant changes in disease biomarkers( 51 , 52 ): in the present study the final circulating 25(OH)D concentration was below this threshold. PAI-1, which is a risk marker for coronary artery disease, was altered following mushroom supplementation( 53 ). Despite no changes in vitamin D status, a significant decrease in PAI-1 in the vitamin D2-supplemented group was observed. In a recent study PAI-1 activity was associated with serum 2(OH)D levels( 54 ). Furthermore, mushroom extracts have been shown to alter PAI-1 levels in vitro( 55 ). To the best of our knowledge, this is the first report of alterations in PAI-1 following vitamin D supplementation. Further work will be needed to validate this finding and ascertain whether the changes are due to alterations in 25(OH)D2 levels or due to other bioactives present in the mushrooms.
In agreement with other studies, no inflammatory markers were affected by vitamin D supplementation( 56 , 57 ). A unique aspect of the present study was the use of vitamin D2-enhanced mushrooms as a vehicle for supplementation; all of the previous studies examining these biomarkers used only vitamin D3. Mushrooms contain several antioxidant and anti-inflammatory compounds( 58 ) and an interesting result with respect to hsCRP was observed in the present study. Analysis across the groups revealed a significant reduction in hsCRP in the vitamin D2-enhanced mushroom group. However, more studies are needed to confirm prolonged mushroom and/or vitamin D2 intake on the systemic inflammation marker hsCRP. In conclusion, consumption of vitamin D2-enhanced mushrooms did not alter total 25(OH)D levels but significantly increased serum 25(OH)D2 levels, indicating that the vitamin was bioavailable in this form. Furthermore, supplementation with vitamin D2 did not negatively affect the levels of 25(OH)D3. Further studies are warranted to ascertain the effects of supplementation with vitamin D2-enhanced mushrooms at higher levels of vitamin D2 and for longer durations. These studies will be critical for the future development of vitamin D-enhanced mushrooms as a vitamin D food source.
Acknowledgements
The authors would like to thank the study participants for their involvement in the study. The authors would like to acknowledge Tracey Claxton for her help with the autoanalyser.
The present study was supported by a research grant from The Mushroom Council in the USA and the Australian Mushroom Growers Association. The Mushroom Council in the USA and the Australian Mushroom Growers Association had no role in the design, analysis or writing of this article. Monaghan Mushrooms supplied the mushrooms.
M. S., L. B. and A. P. N. prepared the manuscript. L. B., A. P. N. and M. J. G. designed the research. M. S., L. O. M. and A. O. S. performed the research. M. S., L. B. and W. D. F. performed the analysis. J. C. prepared and analysed the mushrooms for the study.
The present study was supported by a research grant from The Mushroom Council in the USA and the Australian Mushroom Growers Association. L. B., M. J. G. and A. P. N. received research funding from The Mushroom Council. M. S., L. O. M., A. O. S. and W. D. F. have no conflicts of interest. J. C. was employed by Monahan Mushrooms Ireland.
References
- 1.Norman AW & Bouillon R (2010) Vitamin D nutritional policy needs a vision for the future. Exp Biol Med (Maywood) 235, 1034–1045 [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Ajani UA, McGuire LC, et al. (2005) Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care 28, 1228–1230 [DOI] [PubMed] [Google Scholar]
- 3.Pacifico L, Anania C, Osborn JF, et al. (2011) Low 25(OH)D3 levels are associated with total adiposity, metabolic syndrome, and hypertension in Caucasian children and adolescents. Eur J Endocrinol 165, 603–611 [DOI] [PubMed] [Google Scholar]
- 4.Kayaniyil S, Vieth R, Harris SB, et al. (2011) Association of 25(OH)D and PTH with metabolic syndrome and its traditional and nontraditional components. J Clin Endocrinol Metab 96, 168–175 [DOI] [PubMed] [Google Scholar]
- 5.Salekzamani S, Neyestani TR, Alavi-Majd H, et al. (2011) Is vitamin D status a determining factor for metabolic syndrome? A case–control study. Diabetes Metab Syndr Obes 4, 205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jorde R, Figenschau Y, Emaus N, et al. (2010) Serum 25-hydroxyvitamin D levels are strongly related to systolic blood pressure but do not predict future hypertension. Hypertension 55, 792–798 [DOI] [PubMed] [Google Scholar]
- 7.Major GC, Alarie F, Dore J, et al. (2007) Supplementation with calcium + vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. Am J Clin Nutr 85, 54–59 [DOI] [PubMed] [Google Scholar]
- 8.Borissova AM, Tankova T, Kirilov G, et al. (2003) The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract 57, 258–261 [PubMed] [Google Scholar]
- 9.Zittermann A, Frisch S, Berthold HK, et al. (2009) Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr 89, 1321–1327 [DOI] [PubMed] [Google Scholar]
- 10.Muldowney S, Lucey AJ, Hill TR, et al. (2012) Incremental cholecalciferol supplementation up to 15 µg/d throughout winter at 51–55° N has no effect on biomarkers of cardiovascular risk in healthy young and older adults. J Nutr 142, 1519–1525 [DOI] [PubMed] [Google Scholar]
- 11.Mitri J, Dawson-Hughes B, Hu FB, et al. (2011) Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr 94, 486–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jorde R, Sneve M, Torjesen P, et al. (2010) No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med 267, 462–472 [DOI] [PubMed] [Google Scholar]
- 13.Forrest KY & Stuhldreher WL (2011) Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res 31, 48–54 [DOI] [PubMed] [Google Scholar]
- 14.Langlois K, Greene-Finestone L, Little J, et al. (2010) Vitamin D status of Canadians as measured in the 2007 to 2009 Canadian Health Measures Survey. Health Rep 21, 47–55 [PubMed] [Google Scholar]
- 15.Cashman KD, Muldowney S, McNulty B, et al. (2013) Vitamin D status of Irish adults: findings from the National Adult Nutrition Survey. Br J Nutr 109, 1248–1256 [DOI] [PubMed] [Google Scholar]
- 16.Hill TR, O'Brien MM, Lamberg-Allardt C, et al. (2006) Vitamin D status of 51–75-year-old Irish women: its determinants and impact on biochemical indices of bone turnover. Public Health Nutr 9, 225–233 [DOI] [PubMed] [Google Scholar]
- 17.Hall LM, Kimlin MG, Aronov PA, et al. (2010) Vitamin D intake needed to maintain target serum 25-hydroxyvitamin D concentrations in participants with low sun exposure and dark skin pigmentation is substantially higher than current recommendations. J Nutr 140, 542–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cashman KD, Wallace JM, Horigan G, et al. (2009) Estimation of the dietary requirement for vitamin D in free-living adults ≥64 y of age. Am J Clin Nutr 89, 1366–1374 [DOI] [PubMed] [Google Scholar]
- 19.Institute of Medicine (2011) Table 5-3. Vitamin D dietary reference intakes (DRIs) for adequacy (amount/day) In Dietary Reference Intakes for Calcium and Vitamin D, p. 363. Washington, DC: The National Academies Press [Google Scholar]
- 20.United States Department of Agriculture (2011) USDA National Nutrient Database for Standard Reference, Release 24, Nutrient data 01211 & 15046. 2011; Nutrient Data Laboratory Home Page. http://www.ars.usda.gov/ba/bhnrc/ndl
- 21.Brustad M, Sandanger T, Aksnes L, et al. (2004) Vitamin D status in a rural population of northern Norway with high fish liver consumption. Public Health Nutr 7, 783–789 [DOI] [PubMed] [Google Scholar]
- 22.Irish Universities Nutrition Alliance (2011) National Adult Nutrition Survey. Ireland: Irish Universities Nutrition Alliance [Google Scholar]
- 23.O'Mahony L, Stepien M, Gibney MJ, et al. (2012) The potential role of vitamin D enhanced foods in improving vitamin D status. Nutrients 3, 1023–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore CE, Murphy MM & Holick MF (2005) Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr 135, 2478–2785 [DOI] [PubMed] [Google Scholar]
- 25.Black LJ, Seamans KM, Cashman KD, et al. (2012) An updated systematic review and meta-analysis of the efficacy of vitamin D food fortification. J Nutr 142, 1102–1108 [DOI] [PubMed] [Google Scholar]
- 26.Heaney RP, Recker RR, Grote J, et al. (2011) Vitamin D3 is more potent than vitamin D2 in humans. J Clin Endocrinol Metab 96, E447–E452 [DOI] [PubMed] [Google Scholar]
- 27.Holick MF, Biancuzzo RM, Chen TC, et al. (2008) Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 93, 677–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bischoff-Ferrari H (2009) Vitamin D: what is an adequate vitamin D level and how much supplementation is necessary? Best Pract Res Clin Rheumatol 23, 789–795 [DOI] [PubMed] [Google Scholar]
- 29.Cashman KD, Hill TR, Lucey AJ, et al. (2008) Estimation of the dietary requirement for vitamin D in healthy adults. Am J Clin Nutr 88, 1535–1542 [DOI] [PubMed] [Google Scholar]
- 30.Chee WS, Suriah AR, Chan SP, et al. (2003) The effect of milk supplementation on bone mineral density in postmenopausal Chinese women in Malaysia. Osteoporos Int 14, 828–834 [DOI] [PubMed] [Google Scholar]
- 31.Daly RM, Brown M, Bass S, et al. (2006) Calcium- and vitamin D3-fortified milk reduces bone loss at clinically relevant skeletal sites in older men: a 2-year randomized controlled trial. J Bone Miner Res 21, 397–405 [DOI] [PubMed] [Google Scholar]
- 32.Nikooyeh B, Neyestani TR, Farvid M, et al. (2011) Daily consumption of vitamin D- or vitamin D + calcium-fortified yogurt drink improved glycemic control in patients with type 2 diabetes: a randomized clinical trial. Am J Clin Nutr 93, 764–771 [DOI] [PubMed] [Google Scholar]
- 33.Wagner D, Sidhom G, Whiting SJ, et al. (2008) The bioavailability of vitamin D from fortified cheeses and supplements is equivalent in adults. J Nutr 138, 1365–1371 [DOI] [PubMed] [Google Scholar]
- 34.Biancuzzo RM, Young A, Bibuld D, et al. (2010) Fortification of orange juice with vitamin D2 or vitamin D3 is as effective as an oral supplement in maintaining vitamin D status in adults. Am J Clin Nutr 91, 1621–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tangpricha V, Koutkia P, Rieke SM, et al. (2003) Fortification of orange juice with vitamin D: a novel approach for enhancing vitamin D nutritional health. Am J Clin Nutr 77, 1478–1483 [DOI] [PubMed] [Google Scholar]
- 36.Ko JA, Lee BH, Lee JS, et al. (2008) Effect of UV-B exposure on the concentration of vitamin D2 in sliced shiitake mushroom (Lentinus edodes) and white button mushroom (Agaricus bisporus). J Agric Food Chem 56, 3671–3674 [DOI] [PubMed] [Google Scholar]
- 37.Kalac P (2009) Chemical composition and nutritional value of European species of wild growing mushrooms: a review. Food Chem 113, 9–16 [DOI] [PubMed] [Google Scholar]
- 38.Mattila P, Konko K, Eurola M, et al. (2001) Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J Agric Food Chem 49, 2343–2348 [DOI] [PubMed] [Google Scholar]
- 39.Outila TA, Mattila PH, Piironen VI, et al. (1999) Bioavailability of vitamin D from wild edible mushrooms (Cantharellus tubaeformis) as measured with a human bioassay. Am J Clin Nutr 69, 95–98 [DOI] [PubMed] [Google Scholar]
- 40.Urbain P, Singler F, Ihorst G, et al. (2011) Bioavailability of vitamin D2 from UV-B-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D: a randomized controlled trial. Eur J Clin Nutr 65, 965–971 [DOI] [PubMed] [Google Scholar]
- 41.Stephensen CB, Zerofsky M, Burnett DJ, et al. (2012) Ergocalciferol from mushrooms or supplements consumed with a standard meal increases 25-hydroxyergocalciferol but decreases 25-hydroxycholecalciferol in the serum of healthy adults. J Nutr 142, 1246–1252 [DOI] [PubMed] [Google Scholar]
- 42.Tolppanen AM, Sayers A, Fraser WD, et al. (2012) Serum 25-hydroxyvitamin D3 and D2 and non-clinical psychotic experiences in childhood. PLOS ONE 7, e41575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matthews DR, Hosker JP, Rudenski AS, et al. (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 [DOI] [PubMed] [Google Scholar]
- 44.Payne RB, Little AJ, Williams RB, et al. (1973) Interpretation of serum calcium in patients with abnormal serum proteins. BMJ 4, 643–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tripkovic L, Lambert H, Hart K, et al. (2012) Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr 95, 1357–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Autier P, Gandini S & Mullie P (2012) A systematic review: influence of vitamin D supplementation on serum 25-hydroxyvitamin D concentration. J Clin Endocrinol Metab 97, 2606–2613 [DOI] [PubMed] [Google Scholar]
- 47.Logan VF, Gray AR, Peddie MC, et al. (2013) Long-term vitamin D3 supplementation is more effective than vitamin D2 in maintaining serum 25-hydroxyvitamin D status over the winter months. Br J Nutr 109, 1082–1088 [DOI] [PubMed] [Google Scholar]
- 48.Armas LA, Hollis BW & Heaney RP (2004) Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 89, 5387–5391 [DOI] [PubMed] [Google Scholar]
- 49.Rapuri PB, Gallagher JC & Haynatzki G (2004) Effect of vitamins D2 and D3 supplement use on serum 25OHD concentration in elderly women in summer and winter. Calcif Tissue Int 74, 150–156 [DOI] [PubMed] [Google Scholar]
- 50.Seamans KM, Hill TR, Wallace JM, et al. (2010) Cholecalciferol supplementation throughout winter does not affect markers of bone turnover in healthy young and elderly adults. J Nutr 140, 454–460 [DOI] [PubMed] [Google Scholar]
- 51.de Boer IH, Tinker LF, Connelly S, et al. (2008) Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women's Health Initiative. Diabetes Care 31, 701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. (2007) The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 85, 649–650 [DOI] [PubMed] [Google Scholar]
- 53.Nordt TK, Peter K, Ruef J, Kubler W, et al. (1999) Plasminogen activator inhibitor type-1 (PAI-1) and its role in cardiovascular disease. Thromb Haemost 82, Suppl. 1, 14–18 [PubMed] [Google Scholar]
- 54.Deleskog A, Piksasova O, Silveira A, et al. (2012) Serum 25-hydroxyvitamin D concentration, established and emerging cardiovascular risk factors and risk of myocardial infarction before the age of 60 years. Atherosclerosis 223, 223–229 [DOI] [PubMed] [Google Scholar]
- 55.Yang HL, Kuo YH, Tsai CT, et al. (2011) Anti-metastatic activities of Antrodia camphorata against human breast cancer cells mediated through suppression of the MAPK signaling pathway. Food Chem Toxicol 49, 290–298 [DOI] [PubMed] [Google Scholar]
- 56.Barnes MS, Horigan G, Cashman KD, et al. (2011) Maintenance of wintertime vitamin D status with cholecalciferol supplementation is not associated with alterations in serum cytokine concentrations among apparently healthy younger or older adults. J Nutr 141, 476–481 [DOI] [PubMed] [Google Scholar]
- 57.Jorde R, Sneve M, Torjesen PA, et al. (2010) No effect of supplementation with cholecalciferol on cytokines and markers of inflammation in overweight and obese subjects. Cytokine 50, 175–180 [DOI] [PubMed] [Google Scholar]
- 58.Yu S, Weaver V, Martin K, et al. (2009) The effects of whole mushrooms during inflammation. BMC Immunol 10, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]