Abstract
Hyperglycaemia and insulin resistance are associated with the increased risk of the metabolic syndrome and other severe health problems. The insulin-sensitive GLUT4 regulates glucose homoeostasis in skeletal muscle and adipose tissue. In this study, we investigated whether cacao liquor procyanidin (CLPr) extract, which contains epicatechin, catechin and other procyanidins, improves glucose tolerance by promoting GLUT4 translocation and enhances glucose uptake in muscle cells. Our results demonstrated that CLPr increased glucose uptake in a dose-dependent manner and promoted GLUT4 translocation to the plasma membrane of L6 myotubes. Oral administration of a single dose of CLPr suppressed the hyperglycaemic response after carbohydrate ingestion, which was accompanied by enhanced GLUT4 translocation in ICR mice. These effects of CLPr were independent of α-glucosidase inhibition in the small intestine. CLPr also promoted GLUT4 translocation in skeletal muscle of C57BL/6 mice fed a CLPr-supplemented diet for 7 d. These results indicate that CLPr is a beneficial food material for improvement of glucose tolerance by promoting GLUT4 translocation to the plasma membrane of skeletal muscle.
Key words: Cacao liquor, Procyanidins, Glucose tolerance, GLUT4
Abreviations: AMPK, AMP-activated protein kinase; CLPr, cacao liquor procyanidin; DMSO, dimethylsulfoxide.
Many countries are experiencing a rapid increase in the number of patients with diabetes mellitus, and type 2 diabetes mellitus in particular is a major health problem worldwide. The pathogenesis of type 2 diabetes mellitus involves progressive development of insulin resistance in peripheral tissues, combined with an insufficient pancreatic insulin secretion. It is also associated with inadequate suppression of glucagon secretion in response to ingested glucose, leading to overt hyperglycaemia( 1 ). Insulin resistance, which is usually defined as an inadequate biological response of glucose metabolism to high insulin concentrations( 2 ), is associated with increased risk of CVD( 3 , 4 ) and diabetes( 5 ). Although several studies have attempted to improve insulin sensitivity in subjects with impaired glucose tolerance by pharmacological approaches, as well as appropriate lifestyle and dietary modifications( 6 – 8 ), the success of these approaches has been limited in terms of normalisation of blood glucose levels, and novel approaches are still needed.
Epidemiological evidence and several clinical studies have demonstrated that foods rich in polyphenols, including fruits, vegetables, red wine, tea and cocoa, possess a wide range of health-promoting activities and may reduce the risk of CVD( 9 , 10 ), diabetes( 11 , 12 ) and hyperglycaemia( 13 , 14 ), for example. It was also reported that some polyphenols can normalise blood glucose levels( 13 ). Inhibition of α-glucosidase and other carbohydrate digestive enzymes is an established target of polyphenols for maintaining blood glucose levels. However, recent reports have focused on insulin-sensitive GLUT4 as a novel target of polyphenols( 15 ). GLUT4 is expressed in adipose tissue, skeletal muscle and cardiac muscle. Of these, skeletal muscle is one of a particularly therapeutic target for hyperglycaemia, because skeletal muscle accounts for approximately 80 % of insulin-stimulated glucose uptake in the postprandial state and plays a vital role in maintaining glucose homoeostasis( 16 ).
Translocation of GLUT4 from the intracellular pool to the plasma membrane in skeletal muscle is induced by insulin-dependent and insulin-independent mechanisms and is followed by glucose uptake and incorporation into the cells. Insulin promotes GLUT4 translocation via a phosphatidylinositol 3-kinase-dependent mechanism, whereas exercise and contraction promotes insulin-independent translocation of GLUT4 by activating AMP-activated protein kinase (AMPK)( 17 ). Resveratrol, a phytoalexin present in the skin of grapes and red wine, stimulates glucose uptake and translocation of GLUT4 in cultured L6 myotubes by activating both insulin- and AMPK-dependent signalling pathways( 18 ). However, we previously reported that (−)-epigallocatechin-3-gallate promotes GLUT4 translocation in skeletal muscle of rodents in vivo and in L6 myotubes in vitro by a mechanism that is at least partly independent of insulin( 19 ). (−)-Epigallocatechin-3-gallate was reported to increase AMPK phosphorylation( 20 ), indicating that the AMPK-dependent signalling pathway at least partly contributes to (−)-epigallocatechin-3-gallate -stimulated GLUT4 translocation. Moreover, intake of green and black tea retained a decrease in the GLUT4 and insulin receptor expression levels of in high-fat diet-fed C57BL/6 mice( 21 , 22 ). These results indicate that polyphenols have potential to increase translocation and/or expression of GLUT4 in peripheral tissues, including skeletal muscle, which explains their prevention of hyperglycaemia and insulin resistance.
Cacao liquor procyanidin (CLPr), extracted from cacao liquor, an ingredient of chocolate and cocoa, is rich in polyphenols( 23 – 25 ) such as monomeric epicatechin and catechin, and oligomeric procyanidins( 24 ). These polyphenols have potent antioxidant activities in vitro( 26 , 27 ). Grassi et al.( 28 – 30 ) reported that the consumption of dark chocolate increased insulin sensitivity in healthy subjects( 28 ) and hypertensive patients( 29 , 30 ). Tomaru et al.( 31 ) reported that dietary supplementation with CLPr prevents the development of hyperglycaemia in db/db mice. However, the physiological and molecular mechanism by which CLPr improves glucose tolerance is not yet fully understood. Therefore, in the present study, we investigated whether CLPr promotes GLUT4 translocation and increases glucose uptake in skeletal muscle cells in vivo and in vitro.
Materials and methods
Materials
CLPr was prepared from cacao liquor as previously described( 32 , 33 ). Polyphenol composition of CLPr was quantified by HPLC and liquid chromatography–MS, as detailed previously( 33 , 34 ). The amounts of individual procyanidins are represented as epicatechin equivalents. The total amount of polyphenol was measured by the Prussian Blue method( 35 ). The composition of polyphenols in CLPr is shown in Table 1. Glucose was measured using a commercially available kit (Labassay™ Glucose Wako kit, Wako Pure Chemical Industries, Ltd). The radiolabelled glucose analogue 2-[ 3 H]deoxy-d-glucose was purchased from American Radiolabeled Chemicals Inc. Insulin concentrations were measured using an insulin assay kit from Shibayagi Co. Dulbecco's modified Eagle's medium, penicillin G and rat intestinal acetone powder were from Sigma–Aldrich. Fetal bovine serum and streptomycin were from BioWest S.A.S. and MP Biomedicals Inc., respectively. Bovine serum albumin (fatty acid and insulin free) and polyvinylidene difluoride membranes were from Nacalai Tesque Inc. and Pall Co., respectively. Anti-GLUT1, anti-GLUT4, anti-β-actin and horseradish peroxidase-conjugated anti-goat and anti-mouse IgG antibodies were purchased from Santa Cruz Biotechnology Inc. All other reagents used were of the highest grade available from commercial sources.
Table 1.
Polyphenol composition of cacao liquor extract (CLPr)*

Cell culture and treatment with cacao liquor procyanidin
L6 myoblasts (passage 27–37) were maintained in Dulbecco's modified Eagle's medium supplemented with 10 % (w/v) fetal bovine serum, penicillin G (100 units/ml), and streptomycin (100 µg/ml) at 37°C under a humidified atmosphere of 5 % CO2. The L6 myoblasts were seeded on twenty-four-well plates or 60-mm dishes and grown to a semi-confluent state. After 2 d, the medium was replaced with Dulbecco's modified Eagle's medium containing 2 % fetal bovine serum. The myoblasts were cultured for a further 5 d, and the medium was replaced every 2 d. Myotubes were incubated in serum-free Dulbecco's modified Eagle's medium containing 0·2 % (w/v) bovine serum albumin for 18 h, and then treated with CLPr solution or dimethylsulfoxide (DMSO) as a vehicle control. CLPr, which was prepared by dissolving 250 mg/ml CLPr in DMSO, was added to the cells at the indicated concentrations.
Measurement of glucose uptake activity
The differentiated L6 myotubes on twenty-four-well plates were incubated with 300 µl of Krebs-Ringer-HEPES buffer (50 mmol/l HEPES, pH 7·4, 137 mmol/l NaCl, 4·8 mmol/l KCl, 1·85 mmol/l CaCl2, and 1·3 mmol/l MgSO4) at 37°C and treated with CLPr (0·05–10 μg/ml) or insulin (100 nmol/l) for 15 min. Then, the cells were incubated with 6·5 mmol/l 2-[3H]deoxy-d-glucose (18 kBq/well) for a further 5 min. Next, the cells were washed five times with ice-cold Krebs-Ringer-HEPES buffer and solubilised in 0·05 mol/l NaOH. The radioactivity of 2-[3H]deoxy-d-glucose incorporated into the cells was measured by a liquid scintillation counter with a scintillation cocktail. Non-specific 2-[3H]deoxy-d-glucose uptake was determined by treating cells with 20 µmol/l cytochalasin B before adding CLPr.
Cytotoxicity
The cytotoxicity of CLPr was determined by crystalviolet staining, following the treatment of L6 myotubes on twenty-four-well plate with DMSO or CLPr (0·1–100 mg/ml) in 0·2 % (w/v) bovine serum albumin/minimum essential medium for 15 min. Then, the cell were fixed and stained with 2 % ethanol containing 0·2 % (w/v) Crystal Violet for 10 min. The wells were washed three times with tap water, and the stained cells were extracted with 50 % ethanol containing 0·5 % w/v SDS. The absorbance at 570 nm with a reference wavelength of 630 nm was measured using the Wallac 1420 ARVOsx.
Preparation of the plasma membrane and whole protein fractions
The plasma membrane fraction was prepared from CLPr-treated myotubes as previously described( 36 ) to investigate whether CLPr stimulates GLUT4 translocation from intracellular storage vesicles to the plasma membrane. Briefly, myotubes were treated with CLPr (1–10 μg/ml), insulin (100 nmol/l) or DMSO as a vehicle control for 15 min, washed twice with ice-cold Krebs-ringer-HEPES buffer, homogenised in buffer A (50 mmol/l Tris, pH 8·0, 0·1 % (v/v) Nonidet P-40, 0·5 mmol/l dithiothreitol (DTT), protease inhibitors (1 mmol/l phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin and 5 μg/ml aprotinin) and phosphatase inhibitors (10 mmol/l NaF and 1 mmol/l Na3VO4)) using a hand-held microtube homogeniser, and passed through a twenty-seven-gauge syringe needle five times. Part of the homogenate was mixed with radio immunoprecipitation assay (RIPA) buffer (10 mmol/l Tris, pH 8·0, 150 mmol/l sodium chloride, 0·5 % (w/v) sodium deoxycholate, 0·1 % (w/v) SDS, 1 % (v/v) Nonidet P-40 and 0·5 mmol/l DTT) containing the same protease and phosphatase inhibitors and incubated on ice for 60 min with occasional mixing. The supernatant obtained after centrifugation at 16 000 g for 20 min at 4°C was referred to as the cell lysate. The remainder of the homogenate was centrifuged at 900 g for 10 min at 4°C. The resulting pellet was suspended in buffer A and centrifuged under the same conditions. The precipitate was resuspended in buffer A containing 1 % (v/v) Nonidet P-40 and the same protease and phosphatase inhibitors, incubated on ice for 60 min with occasional mixing, and centrifuged at 16 000 g for 20 min at 4°C. The resulting supernatant was used as the plasma membrane.
Animals and administration of cacao liquor procyanidin
All animal experiments were approved by the Institutional Animal Care and Use Committee (Permission No. 21-07-02) and carried out according to the guidelines for animal experiments at Kobe University. Male ICR and C57BL/6 mice (4 weeks old) were obtained from Japan SLC Inc. and maintained in a temperature-controlled room (23 ± 2°C) with a 12 h light–12 h dark cycle (lights on at 09.00 hours). The ICR mice were acclimatised for 7 d with free access to a commercial standard mouse diet consisting of 76 % carbohydrate, 15 % protein and 9 % fat (3·850 kcal/g diet; Research Diets) and tap water. These ICR mice were used for the following oral carbohydrate loading test.
To examine the effects of consecutive administration of CLPr on GLUT4 translocation, male C57BL/6 mice (4 weeks old) were used after acclimatisation for 7 d with free access to a commercial chow and tap water. The mice were randomly divided into three groups of four mice and given a diet containing 0, 0·5 or 1 % (w/w) CLPr. After 7 d of feeding, the mice were killed under anesthesia induced by an intraperitoneal injection of sodium pentobarbital. Blood samples were collected after cardiac puncture and placed in heparinised microcentrifuge tubes to prepare plasma by centrifugation at 9600 g for 10 min at 4°C. The plasma samples were used to measure glucose level. The hindlimb soleus muscle was excised, chopped into small pieces and homogenised in 10 volumes of buffer A. The plasma membrane was prepared for Western blotting analysis as previously described( 36 ).
Oral carbohydrate loading test
In Experiment 1, CLPr (50 or 250 mg/kg body weight) or water alone (5 ml/kg body weight) was orally administered to ICR mice after an 18 h fast. After the 60 min administration of CLPr, the mice in each group were orally given 1 g/kg body weight of soluble starch, maltose, sucrose or glucose. Tail vein blood samples were collected in heparinised tubes at 0 (before administration), 15, 30, 60 and 120 min after the carbohydrate load and centrifuged at 9600 g for 10 min at 4°C to prepare plasma.
In Experiment 2, mice were given an oral dose of CLPr in water (250 mg/kg body weight (C)) or water alone (5 ml/kg body weight (W)) after an 18 h fast. Then, the mice in each group were subdivided into two groups of four mice. One group was orally administered with glucose (1 g/kg body wt (G)), while the other received water alone (5 ml/kg body wt (W)). Thus, the mice used in Experiment 2 were divided into four groups designated CLPr-water, CLPr-glucose, water–water and water–glucose. The mice were killed 30 min after the glucose or water administration under anaesthesia induced by an intraperitoneal injection of sodium pentobarbital. Plasma samples were prepared from blood and used to measure the glucose and insulin levels. The plasma membrane of the soleus muscle was prepared and used for Western blotting( 36 ).
Western blotting
Proteins in the plasma membrane and cell lysate fractions of myotubes and soleus muscles were separated by SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes. After blocking with commercial Blocking one solution (Nacalai Tesque), the membranes were incubated with the specified primary antibodies overnight at 4°C, followed by the corresponding horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The proteins bands were visualised using ImmunoStar® LD (Wako) and detected with a light-Capture II (ATTO Corp.).
Measurement of α-glucosidase activity in the small intestine
α-Glucosidase activity was measured in the small intestine of mice treated with 50 or 250 mg/kg body weight CLPr after an 18 h fast. Control mice were given water alone (5 ml/kg body weight). The mice were killed 60 min after CLPr or water administration under sodium pentobarbital anaesthesia, and the small intestine between the duodenum and the caecum was removed. The small intestine was opened longitudinally with scissors and washed twice with 1·15 % (w/v) ice-cold KCl solution. The intestinal mucosa was removed by scraping with a glass slide. The mucosal scrapings were homogenised with three volumes of 1·15 % KCl solution on ice. The homogenate was centrifuged at 1000 g for 10 min at 4°C, and the resultant supernatant was collected and used to measure maltase and sucrase–isomaltase activities. The reaction mixture consisted of 100 mmol/l maltose or sucrose as a substrate in 56 mmol/l maleate buffer (pH 6·0). The reaction was initiated by adding 100 or 250 µg protein/μl homogenate, respectively. After incubation at 37°C for 0, 20, 40, 60 and 120 min, the reaction was terminated by heating the mixture in boiling water for 10 min, and then placed on ice for 10 min. After centrifugation at 1000 g for 10 min, the glucose concentration in the supernatant was measured. After the linear regression of the glucose formation from the substrates was confirmed, the enzymatic activity was determined by the slope of the linear line and represented as nmol glucose released/min per mg protein.
To estimate the inhibitory effect of CLPr on α-glucosidase in vitro, the acetone powder of rat intestine (Sigma) was used as the enzyme source of α-glucosidase. A total of 50 mg of the acetone powder was homogenised in 10 ml of 56 mmol/l ice-cold maleate buffer (pH 6·0) and centrifuged at 10 000 g at 4°C for 30 min. The resulting supernatant was used as the crude enzyme solution. Then, 100 μl of the supernatant was pre-incubated with 900 μl of 56 mmol/l maleate buffer containing 0·01, 0·03, 0·06, 0·1 or 0·15 % CLPr (final concentration) at 37°C for 10 min. The reaction was initiated by adding 1·0 ml of 2 % (w/v) maltose or sucrose solution in maleate buffer. After incubation at 37°C for 0, 60 and 120 min, the reaction was terminated, and the glucose concentration in the reaction mixture was measured. The enzymatic activity was determined as described above.
Statistical analysis
Data are presented as means with their standard errors. The statistical significance of differences among groups was determined using the Dunnett multiple comparison test (Figs. 1 and 5), or the Tukey–Kramer multiple comparison test (Figs. 2–4 and Tables 2 and 3) with the level of significance set at P < 0·05.
Fig. 1.
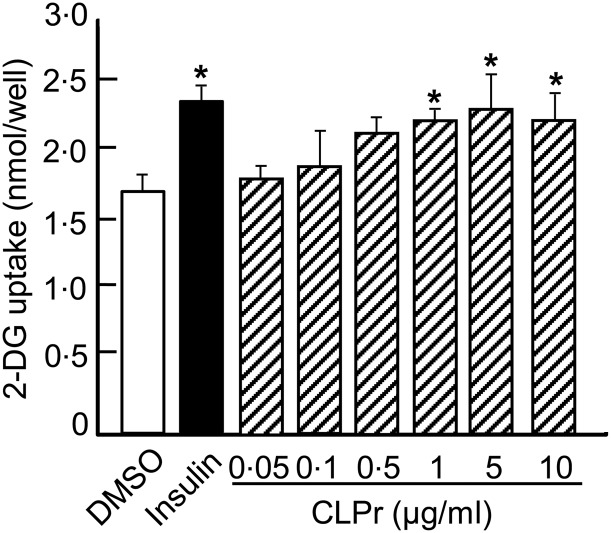
Effects of cacao liquor procyanidin (CLPr) on glucose uptake in L6 myotubes. Glucose uptake was measured in serum-starved L6 myotubes treated with 0·05–10 µg/ml CLPr for 15 min. Some cells were treated with DMSO or 100 nmol/l insulin as negative and positive controls, respectively. Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that of the control group (P < 0·05; Dunnett's test).
Fig. 5.
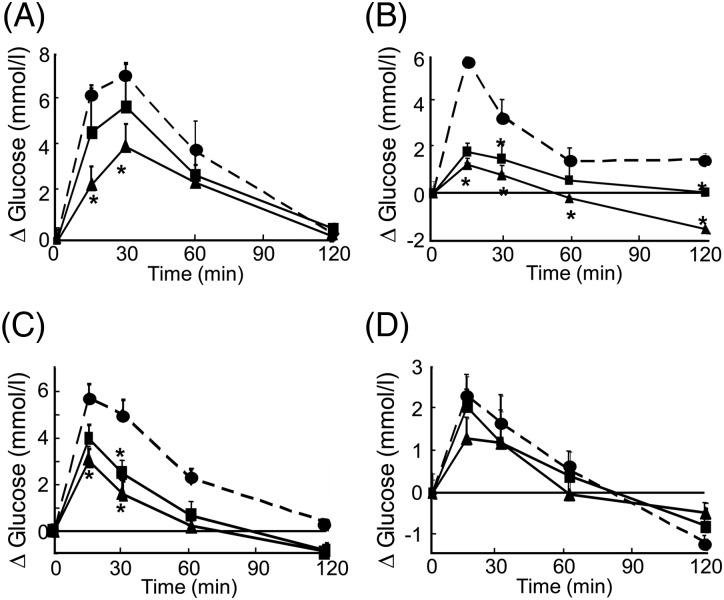
Effects of cacao liquor procyanidin (CLPr) on the plasma glucose response to an oral carbohydrate load. ICR mice were treated with 50 (■) or 250 (▲) mg/kg body weight CLPr or water (5 ml/kg body weight; (•)). At 60 min after CLPr administration, the mice in each group were then given an oral load (1 g/kg body weight) of glucose (A), soluble starch (B), maltose (C) or sucrose (D). Plasma glucose levels were measured at 0, 15, 30, 60 and 120 min after the carbohydrate load. Values are means, with standard errors represented by vertical bars, of triplicate independent experiments consisting of three mice per group. *Mean value was significantly different from the corresponding control group (P < 0·05; Dunnett's test).
Fig. 2.
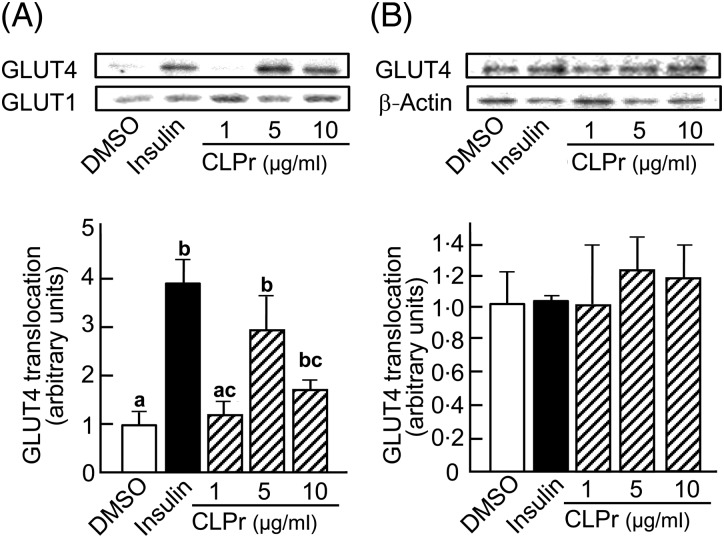
Effects of cacao liquor procyanidin (CLPr) on GLUT4 translocation in L6 myotubes. Serum-starved L6 myotubes were treated with 1, 5 or 10 µg/ ml CLPr for 15 min, or with dimethylsulfoxide (DMSO) or 100 nmol/l insulin as negative and positive controls, respectively. The abundance of GLUT4 and GLUT1 protein in the plasma membrane of L6 myotubes (A) and GLUT4 and β-actin proteins in cell lysate (B) was determined by Western blotting. Each panel shows representative data from triplicate experiments. The density of each band was analysed and normalised to that of β-actin for the cell lysate or GLUT1 for the plasma membrane. Values are means, with standard errors represented by vertical bars (n 3).a,b,c Mean values with unlike letters were significantly different (P < 0·05; Tukey–Kramer multiple comparison test).
Fig. 4.
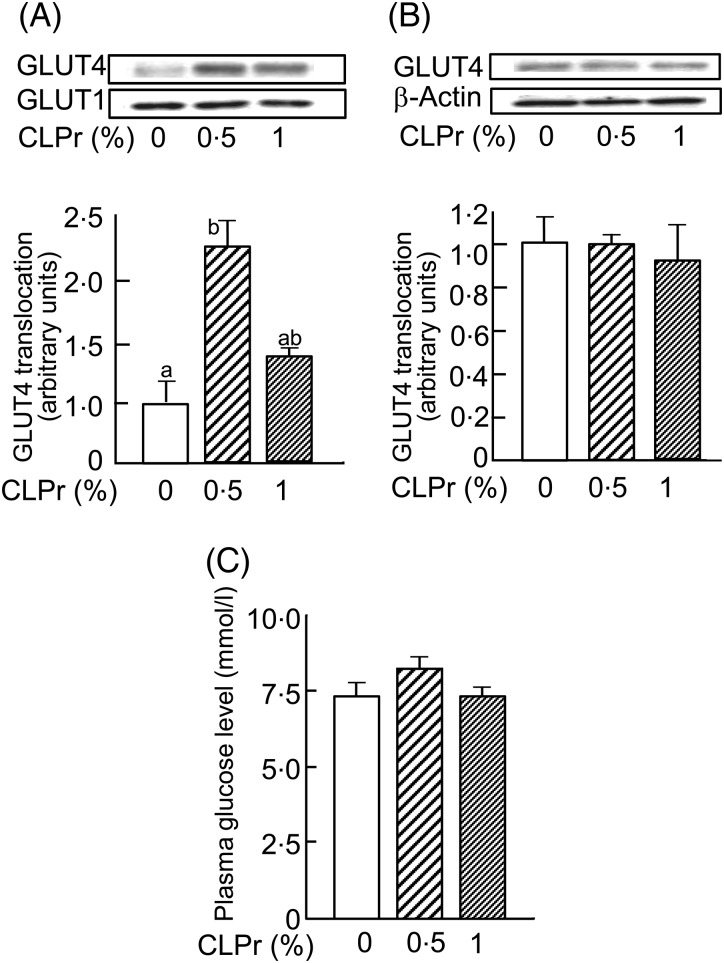
Effects of 7 d of cacao liquor procyanidin (CLPr) administration on GLUT4 translocation in skeletal muscle and plasma glucose level. C57BL/6 mice were given a diet containing 0, 0.5 or 1 % (w/w) CLPr for 7-d, after which skeletal muscle was dissected. The abundance of GLUT4 and GLUT1 proteins in the plasma membrane of skeletal muscle (A) and GLUT4 and β-actin proteins in the tissue lysate (B) was determined by Western blotting. Each panel shows representative data from four mice. The density of each band was analysed and normalised to that of β-actin for the tissue lysate or GLUT1 for the plasma membrane. (C) Plasma glucose levels. Values are means, with standard errors represented by vertical bars (n 4).a,b Mean values with unlike letters were significantly different (P < 0·05; Tukey–Kramer multiple comparison test).
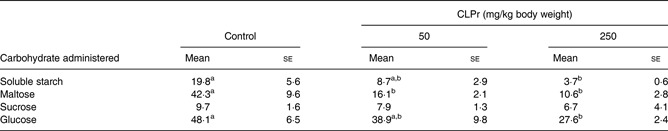
Table 2.
Area under the curve (AUC; mmol/l×120 min×10−2) of plasma glucose level* (Mean values with their standard errors of triplicate independent experiments consisting of three mice per group)
CLPr, cacao liquor procyanidin.
AUC were calculated using the trapezoidal rule from the data presented in Fig. 5.
a,bMean values within a row with unlike superscript letters with significantly different (P < 0·05; Tukey–Kramer multiple comparison test).
Table 3.
Effects of cacao liquor procyanidin (CLPr) on α-glucosidase in vivo and in vitro* (Mean values with their standard errors; n 3)
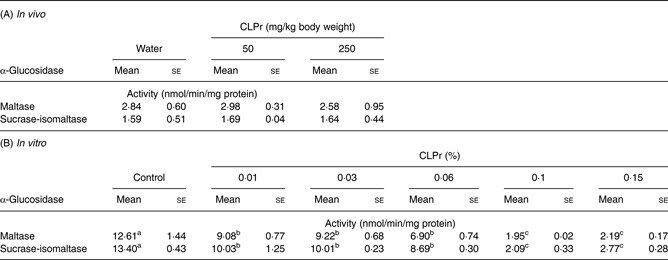
α-Glucosidase activity was measured in the jejunum of CLPr-treated mice (A) or in the CLPr-treated homogenate of rat intestinal acetone powder (B).
a,b,cMean values within a row with unlike superscript letters were significantly different (P<0·05; Tukey–Kramer multiple comparison test).
Results
Effects of cacao liquor procyanidin on glucose uptake and GLUT4 translocation in L6 myotubes
We first investigated the effects of CLPr on glucose uptake in L6 myotubes, since CLPr contains polyphenols, such as catechins and epicatechin (Table 1), which are known to modulate glucose uptake in L6 myotubes( 19 ). When L6 myotubes were treated with 100 nmol/l insulin for 15 min, glucose uptake increased by approximately 1·4-fold compared with the DMSO-treated negative control (Fig. 1). In the absence of insulin, CLPr increased glucose uptake in a dose-dependent manner except for 10 µg/ml. The maximum effect was observed at a CLPr dose of 5 µg/ml, which elicited a response similar to 100 nmol/l insulin. Therefore, the following experiments were carried out using 1, 5 and 10 µg/ml CLPr.
As would be expected, 100 nmol/l insulin significantly (P = 0·011) enhanced GLUT4 translocation in L6 myotubes from the intracellular storage vesicles to the plasma membrane as compared with DMSO-treated control cells (Fig. 2(A)). In the absence of insulin, 5 and 10 µg/ml CLPr significantly (P = 0·023 and P = 0·043, respectively) stimulated GLUT4 translocation to the plasma membrane. In contrast, the abundance of GLUT1 in the plasma membrane was unchanged. Moreover, CLPr did not affect the expression of GLUT4 in the cell lysate (Fig. 2(B)). These results indicate that CLPr stimulates GLUT4 translocation to the plasma membrane, and thus enhances glucose uptake capacity in muscle cells. In addition, CLPr did not show any cytotoxic effects by 100 µg/ml (data not shown).
Effect of a single oral administration of cacao liquor procyanidin on GLUT4 translocation and plasma glucose and insulin levels
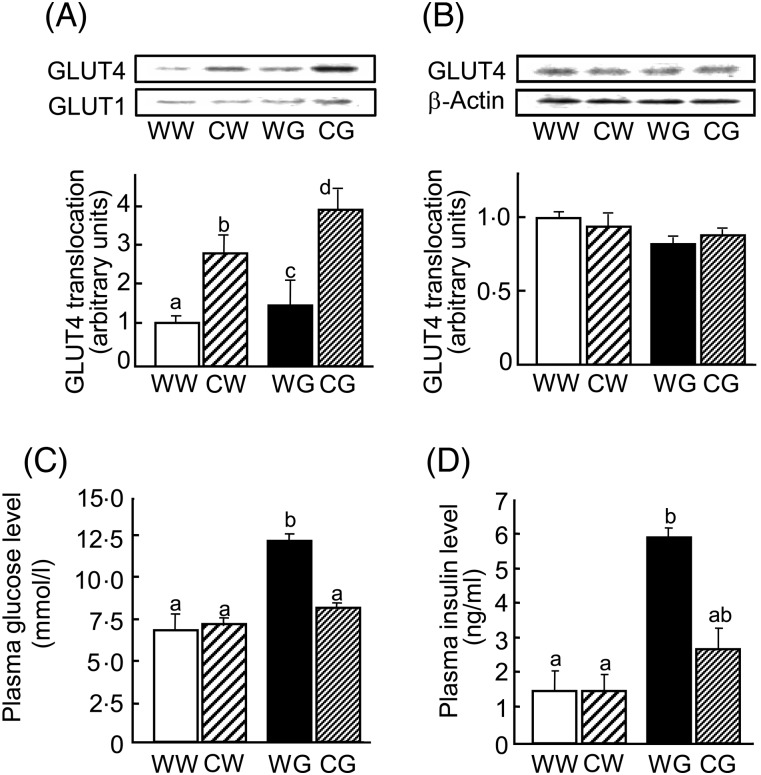
Next, we investigated the effect of single oral administration of CLPr on GLUT4 translocation in the soleus muscle of ICR mice after a glucose load. As shown in Fig. 3(A), GLUT4 translocation in the water–glucose group was increased by approximately 1·4-fold compared with the water–water group. Interestingly, CLPr alone (CLPr-water group) stimulated GLUT4 translocation without a glucose load; GLUT4 translocation in the CLPr-water group was approximately 2·9-fold higher than that in the water–water group. Moreover, CLPr showed additive effects with glucose because GLUT4 translocation in the CLPr-glucose group was approximately 3·9-fold higher when compared with the water–water group. In contrast, CLPr did not affect the plasma membrane expression of GLUT1 (Fig. 3(A)) or tissue lysate GLUT4 expression (Fig. 3(B)).
Fig. 3.
Effects of cacao liquor procyanidin (CLPr) on GLUT4 translocation in skeletal muscle and plasma glucose and insulin levels in mice following a glucose load. CLPr (250 mg/kg body weight; C) or water alone (5 ml/kg body weight; W) were orally administered to ICR mice. Mice in each group were then subdivided into two groups and given glucose (1 g/kg body weight; G) or water (5 ml/kg body weight; W). CW, CLPr-water; CG, CLPr-glucose; WW, water–water; WG, water–glucose. Skeletal muscle tissue (soleus) was removed 30 min after the glucose load. The abundance of GLUT4 and GLUT1 proteins in the plasma membrane of the muscle (A) and GLUT4 and β-actin proteins in the tissue lysate (B) was determined by Western blotting. Each panel shows representative data from four mice. The density of each band was analysed and normalised to that of β-actin for the tissue lysate or GLUT1 for the plasma membrane. (C, D) Plasma glucose (C) and insulin (D) levels. Values are means, with standard errors represented by vertical bars (n 4).a,b Mean values with unlike letters were significantly different (P < 0·05; Tukey–Kramer multiple comparison test).
In the same experiment, the plasma glucose level in the water–glucose group was significantly higher than that in the other groups (Fig. 3(C)). This indicates that pre-administration of CLPr suppressed the acute elevation in plasma glucose, with levels similar to that in the control group. Similar results were observed for plasma insulin levels as CLPr suppressed hyperinsulinaemia induced by the glucose load (Fig. 3(D)). Taken together, these results indicate that pre-administration of CLPr inhibited the hyperglycaemic and hyperinsulinaemic responses to a glucose load by promoting GLUT4 translocation to the plasma membrane of skeletal muscle.
Effects of consecutive administration of cacao liquor procyanidin on GLUT4 translocation and plasma glucose levels
GLUT4 translocation induced by CLPr was also examined in the skeletal muscle of mice treated with 0·5 or 1 % CLPr for 7 d. Dietary supplementation of CLPr did not affect food or water intake during the experimental period (data not shown). The final body weight of mice was also not affected by CLPr, being 21·1 (se 0·22) g, 21·3 (se 0·26) g and 20·7 (se 0·75) g in mice fed 0, 0·5 and 1 % CLPr, respectively. However, CLPr, particularly at a dose of 0·5 %, significantly enhanced GLUT4 translocation to the plasma membrane of skeletal muscle (Fig. 4(A)) without affecting plasma glucose levels (Fig. 4(C)). The expression of GLUT1 in the plasma membrane (Fig. 4(A)) and GLUT4 in the tissue lysate (Fig. 4(B)) was unchanged.
Effects of cacao liquor procyanidin on plasma glucose response to oral ingestion of carbohydrates
Fig. 5 shows the effect of oral administration of CLPr on the plasma glucose response to an oral load of soluble starch, maltose, sucrose or glucose. Plasma glucose levels in the control group (carbohydrate alone) increased in response to oral carbohydrate loading, reaching a peak at 15 min (soluble starch, maltose and sucrose) or 30 min (glucose), and then decreased with time. Following the glucose load, 250 mg/kg body weight CLPr significantly suppressed the transient increases in plasma glucose levels at 15 and 30 min (P < 0·05; Fig. 5(A)). The area under the curve of the plasma glucose levels showed similar results (Table 2), and was significantly reduced by 250 mg/kg body weight CLPr. CLPr dose-dependently suppressed the acute elevations in the plasma glucose levels after the soluble starch and maltose loads (P < 0·05; Fig. 5(B, C) and Table 2). On the other hand, CLPr tended to decrease the plasma glucose levels in sucrose-loaded mice (Fig. 5(D) and Table 2), although not significantly.
Effects of cacao liquor procyanidin on intestinal α-glucosidase activity
Oligomeric procyanidins from grape seed were reported to inhibit the enzymatic activity of intestinal α-glucosidases, including maltase and sucrase in vitro( 37 ). Therefore, it is possible that a similar effect may be responsible for the decreases in postprandial blood glucose levels as described earlier. Therefore, we measured the inhibitory effects of CLPr on α-glucosidase activity in vivo and in vitro (Table 3). These experiments revealed that CLPr did not inhibit maltase or sucrose–isomaltase activities in vivo; although it did inhibit these enzymes in a dose-dependent manner in vitro (P < 0·05).
Discussion
Diabetes mellitus is characterised by chronic hyperglycaemia, which is involved in the development of obesity, CVD and hypertension( 1 – 3 ). Controlling the postprandial blood glucose excursions can prevent hyperglycaemia and improve insulin resistance( 1 , 3 ). As a strategy for preventing and treating hyperglycaemia, we focused on GLUT, which play important roles in glucose homoeostasis by regulating cellular glucose uptake. GLUT4 is a major GLUT expressed in skeletal and cardiac muscle, and in adipose tissue specifically GLUT4 is responsible for the uptake of large amounts of glucose into cells following its translocation from intracellular storage vesicles to the plasma membrane in response to insulin stimulation and muscle contraction( 38 ). In skeletal muscle, stimulation of glucose uptake is mostly attributed to increased translocation and redistribution of the GLUT4 to the plasma membrane( 2 , 38 ). In the present study, we showed that CLPr reduces postprandial glucose tolerance by stimulating GLUT4 translocation to the plasma membrane in cultured L6 myotubes in vitro (Figs. 1 and 2) and in murine skeletal muscle in vivo (Figs. 3 and 4). Moreover, CLPr prevented postprandial glucose tolerance following a carbohydrate load (Fig. 5) without inhibiting small intestinal α-glucosidase (Table 3). To our knowledge, this is the first report showing that CLPr promotes GLUT4 translocation in skeletal muscle, although a previous report showed that CLPr prevented the development of hyperglycaemia in db/db mice( 31 ). Since GLUT4 is a key molecule involved in regulating glucose levels, CLPr is an attractive and beneficial food material for preventing hyperglycaemia.
The most important finding in this study was that CLPr promoted the translocation of GLUT4 in the absence of insulin in L6 myotubes and in skeletal muscle in mice. Moreover, we detected an additive effect of CLPr and glucose on GLUT4 translocation in vivo. These findings indicate that the molecular effects of CLPr are independent of insulin. The CLPr used in this study contained 4·28 % catechins, 6·12 % epicatechin and 7·64 % procyanidins (dimer to tetramer). These compounds probably contribute to the enhanced GLUT4 translocation because catechins and procyanidins were previously reported to suppress hyperglycaemia( 39 – 41 ). In fact, we previously reported that catechins, particularly (−)-epigallocatechin-3-gallate, increased glucose uptake and enhanced GLUT4 translocation in L6 myotubes and in the skeletal muscle of rodents in vivo and ex vivo( 19 ). In 3T3-L1 cells, non-gallate-type catechins increased glucose uptake and GLUT4 translocation, whereas gallate-type catechins inhibited insulin-stimulated glucose uptake( 42 ). Similarly, the intake of green and black tea increased GLUT4 translocation in skeletal muscle and maintained GLUT4 and insulin receptor expression in high-fat diet-fed C57BL/6 mice( 3 , 21 , 22 ). Although the effects of epicatechin and catechin on glucose uptake were weaker than those of other catechins in L6 myotubes( 19 ), a recent report demonstrated that epicatechin treatment conferred diabetic mice with a healthier and longer lifespan, and also improved skeletal muscle stress output and AMPKα activity in skeletal muscle( 40 ). Regarding procyanidins, it was reported that grape-seed procyanidin extract prevented the development of hyperglycaemia in streptozotocin-induced diabetic rats and high-fructose diet-induced insulin resistance rat( 39 , 43 ). Similarly, black soyabean seed extract, which also contains abundant procyanidins, also suppressed hyperglycaemia and obesity in high-fat diet-fed C57BL/6 mice( 44 ). In vitro experiments revealed that grape-seed procyanidin extract also stimulated glucose uptake in 3T3-L1 adipocytes and L6E9 myotubes( 39 , 41 ). In 3T3-L1 adipocytes, the molecular mechanisms seemed to involve phosphorylation of the insulin receptor and phosphorylation of downstream signalling components, including Akt, p44/42 and p38 mitogen-activated protein kinase( 39 , 41 ). However, the molecular mechanisms by which procyanidins promote GLUT4 translocation are still unclear, because the cellular machinery controlling its translocation in muscle cells differs from that in adipose cells( 15 , 45 , 46 ). Indeed, gallate-type catechins elicited different effects on glucose uptake between L6 myotubes and 3T3-L1 adipocytes( 19 , 42 ), and GLUT4 translocation increased in skeletal muscle but decreased in adipose tissue of rats fed green tea( 47 ), Moreover, the composition of procyanidins differs between plants, suggesting that the functions of the procyanidin-rich plant extracts will differ. Regarding the bioavailability of procyanidins, several in vivo and in vitro studies are reported. For example, catechins and procyanidin dimers are detected in human plasma after ingestion of cocoa( 48 , 49 ); free form of procyanidin dimers and trimers were detected in rat plasma after oral intake of a grape seed extract( 50 ); and procyanidin dimers, trimers and tetramers were transported across Caco-2 cells( 51 ). Further studies are needed to clarify the bioavailability and molecular mechanisms underlying the effects of CLPr in muscle cells.
CLPr prevented the transient increase in plasma glucose levels after a carbohydrate load. This preventive effect was observed not only for glucose loading but also for soluble starch and maltose loading. This result suggests that inhibition of α-glucosidase is involved in the preventive effects of CLPr on hyperglycaemia in vivo. Inhibition of intestinal α-glucosidase activity is a well-documented mechanism for the prevention of hyperglycaemia. However, our results clearly showed that CLPr did not inhibit intestinal α-glucosidase activity in vivo, even though it did inhibit the enzyme in vitro. Many reports have shown that certain polyphenols, including anthocyanins, catechins, quercetin and luteolin, can inhibit intestinal α-glucosidase activity in vitro( 52 – 56 ). It was also reported that rutin inhibited α-glucosidase activity in vivo and in vitro( 53 ). Meanwhile, Schäfer & Högger( 37 ) demonstrated that the inhibitory effects of oligomeric procyanidins on α-glucosidase activity were dependent on their molecular weight as tetrameric and hexameric procyanidins were more potent inhibitors than dimeric and trimeric procyanidins in vitro. However, we previously reported that long-term intake of green or black tea suppressed hyperglycaemia by modulating the expression and translocation of GLUT4 without inhibition of α-glucosidase activity( 21 ). Taken together, these findings indicate that polyphenols have a potential to prevent hyperglycaemia by inhibiting α-glucosidase, although the evidence in vivo is less convincing. Our present findings provide strong evidence to support that CLPr improves glucose tolerance mainly by enhancing GLUT4 translocation and glucose uptake in skeletal muscle.
In conclusion, the results of this study indicate that CLPr enhances GLUT4 translocation in muscle cells in vitro and in vivo. GLUT4 translocation to the plasma membrane will facilitate glucose uptake and improve postprandial glucose tolerance. Therefore, CLPr offers a beneficial food for the prevention of hyperglycaemia and potentially diabetes mellitus.
Acknowledgements
This work was supported in part by Special Coordination Funds for Promoting Science and Technology and the Creation of Innovation Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction Kobe) from MEXT, Japan. All authors approved the submission of the manuscript and contributed as follows. Y. Y. carried out experiments, statistical analysis and wrote the manuscript; M. O. prepared the CLPr extract and proof read the manuscript; M. N. analysed the polyphenol composition of the extract and proof read the manuscript; and H. A. conducted the experimental design, wrote and proof read the manuscript. All authors read and approved the final manuscript. There is no conflict of interest associated with the present study.
References
- 1. American Diabetes Association ( 2010. Diagnosis and classification of diabetes mellitus Diabetes Care 33 Suppl. 1 S62 S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hauner H ( 2002. Insulin resistance and the metabolic syndrome – a challenge of the new millennium Eur J Clin Nutr 56 Suppl. 1 S25 S29 [DOI] [PubMed] [Google Scholar]
- 3. Alderman MH Cohen H & Madhavan S ( 1999. Diabetes and cardiovascular events in hypertensive patients Hypertension 33 1130 1134 [DOI] [PubMed] [Google Scholar]
- 4. The DECODE Study Group ( 1999. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria Lancet 354 617 662 [PubMed] [Google Scholar]
- 5. Osei K Rhinesmith S Gaillard T et al. ( 2004. Impaired insulin sensitivity, insulin secretion, and glucose effectiveness predict future development of impaired glucose tolerance and type 2 diabetes in pre-diabetic African Americans Diabetes Care 27 1439 1446 [DOI] [PubMed] [Google Scholar]
- 6. Dagogo-Jack S Egbuonu N & Edeoga C ( 2010. Principles and practice of nonpharmacological interventions to reduce cardiometabolic risk Med Princ Pract 19 167 175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bolen S Feldman L Vassy J et al. ( 2007. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus Ann Intern Med 147 386 399 [DOI] [PubMed] [Google Scholar]
- 8. Distefano JK & Watanabe RM ( 2010. Pharmacogenetics of anti-diabetes drugs Pharmaceuticals (Basel) 3 2610 2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ding EL Hutfless SM Ding X et al. ( 2006. Chocolate and prevention of cardiovascular disease: a systematic review Nutr Metab 3 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deka A & Vita JA ( 2011. Tea and cardiovascular disease Pharmacol Res 64 136 145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolfram S ( 2007. Effects of green tea and EGCG on cardiovascular and metabolic health J Am Coll Nutu 26 373S 388S [DOI] [PubMed] [Google Scholar]
- 12. Gin H Rigalleau V Caubet O et al. ( 1999. Effects of red wine, tannic acid, or ethanol on glucose tolerance in non-insulin-dependent diabetic patients and on starch digestibility in vitro Metabolism 48 1179 1183 [DOI] [PubMed] [Google Scholar]
- 13. Hanhineva K Törrönen R Bondia-Pons I et al. ( 2010. Impact of dietary polyphenols on carbohydrate metabolism Int J Mol Sci 11 1365 1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsui T Tanaka T Tamura S et al. ( 2007. α-Glucosidase inhibitory profile of catechins and theaflacins J. Agric Food Chem 55 99 105 [DOI] [PubMed] [Google Scholar]
- 15. Bryan NJ Govers R & James DE ( 2002. Regulated transport of the glucose transporter GLUT4 Nat Rev Mol Cell Biol 3 267 277 [DOI] [PubMed] [Google Scholar]
- 16. Saltiel AR & Kahn CR ( 2001. Insulin signaling and the regulation of glucose and lipid metabolism Nature 414 799 806 [DOI] [PubMed] [Google Scholar]
- 17. Hardie DG ( 2004. AMP-activated protein kinase: a key system mediating metabolic responses to exercise Med Sci Sports Exerc 36 28 34 [DOI] [PubMed] [Google Scholar]
- 18. Minakawa M Kawano A Miura Y et al. ( 2011. Hypoglycemic effect of resveratrol in type 2 diabetic model db/db mice and its actions in cultured L6 myotubes and RIN-5F pancreatic β-cells J Clin Biochem Nutr 48 237 244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ueda M Nishiumi S Nagayasu H et al. ( 2008. Epigallocatechin gallate promotes GLUT4 translocation in skeletal muscle Biochem Biophys Res Commun 377 286 290 [DOI] [PubMed] [Google Scholar]
- 20. Ahang ZF Li Q Liang J et al. ( 2010. Epigallocatechin-3-O-gallate (EGCG) protects the insulin sensitivity in rat L6 muscle cells exposed to dexamethasone condition Phytomedicine 17 14 18 [DOI] [PubMed] [Google Scholar]
- 21. Nishiumi S Bessyo H Kubo M et al. ( 2010. Green and black tea suppress hyperglycemia and insulin resistance by retaining the expression of glucose transporter 4 in muscle of high-fat diet-fed C57BL/6J mice J Agric Food Chem 58 12916 12923 [DOI] [PubMed] [Google Scholar]
- 22. Imada S Tanaka S Nishiumi S et al. ( 2011. Concentration of catechins and caffeine in black tea affects suppression of fat accumulation and hyperglycemia in high-fat diet-fed mice Food Sci Technol Res 17 353 359 [Google Scholar]
- 23. Crozier SJ Preston AG Hurst JW et al. ( 2011. Cacao seeds are a “Super Fruit”: A comparative analysis of various fruit powders and products Chem Cent J 5 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Urpi-Sarda M Monagas M Khan N et al. ( 2009. Epicatechin, procyanidins, and phenolic microbial metabolites after cocoa intake in humans and rats Anal Bioanal Chem 394 1545 1556 [DOI] [PubMed] [Google Scholar]
- 25. Adamson GE Lazarus SA Mitchell AE et al. ( 1999. HPLC method for the quantification of procyanidins in cocoa and chocolate samples and correlation to total antioxidant capacity J Agric Food Chem 47 4184 4188 [DOI] [PubMed] [Google Scholar]
- 26. Gu L House SE Wu X et al. ( 2006. Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products J Agric Food Chem 54 4057 4061 [DOI] [PubMed] [Google Scholar]
- 27. Hatano T Miyatake H Natsume M et al. ( 2002. Proanthocyanidin glycosides and related polyphenols from cacao liquor and their antioxidant effects Phytochemistry 59 749 758 [DOI] [PubMed] [Google Scholar]
- 28. Grassi D Lippi C Necozione S et al. ( 2004. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons Am J Clin Nutr 81 611 614 [DOI] [PubMed] [Google Scholar]
- 29. Grassi D Necozione S Lippi C et al. ( 2005. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives Hypertension 46 398 405 [DOI] [PubMed] [Google Scholar]
- 30. Grassi D Desideri G Necozione S et al. ( 2008. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate J Nutr 138 1671 1676 [DOI] [PubMed] [Google Scholar]
- 31. Tomaru M Takano H Osakabe N et al. ( 2007. Dietary supplementation with cacao liquor proanthocyanidins prevents elevation of blood glucose levels in diabetic obese mice Nutrition 23 351 355 [DOI] [PubMed] [Google Scholar]
- 32. Osakabe N Yamagishi M Sanbongi C et al. ( 1998. The antioxidative substances in cacao liquor J Nutr Sci Vitaminol 44 313 321 [DOI] [PubMed] [Google Scholar]
- 33. Natsume M Osakabe N Yamagishi M et al. ( 2000. Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase HPLC Biosci Biotechnol Biochem 64 2581 2587 [DOI] [PubMed] [Google Scholar]
- 34. Yasuda A Natsume M Sasaki K et al. ( 2008. Cacao procyanidins reduce plasma cholesterol and increase fecal steroid excretion in rats fed a high-cholesterol diet Biofactors 33 211 223 [DOI] [PubMed] [Google Scholar]
- 35. Price ML & Butler LG ( 1977. Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain J Agric Food Chem 25 1268 1273 [Google Scholar]
- 36. Nishiumi S & Ashida H ( 2007. Rapid preparation of a plasma membrane fraction from adipocytes and muscle cells: application to detection of translocated glucose transporter 4 on the plasma membrane Biosci Biotechnol Biochem 7 2343 2346 [DOI] [PubMed] [Google Scholar]
- 37. Schäfer A & Högger P ( 2007. Oligomeric procyanidins of French maritime pine bark extract (Pycnogenol®) effectively inhibit α-glucosidase Diabetes Res Clin Pract 77 41 46 [DOI] [PubMed] [Google Scholar]
- 38. Huang S & Czech MP ( 2007. The GLUT4 glucose transporter Cell Metab 5 237 252 [DOI] [PubMed] [Google Scholar]
- 39. Pinent M Blay M Bladé MC et al. ( 2004. Grape seed-derived procyanidins have an antihyperglycemic effect in streptozotocin-induced diabetic rats and insulinomimetic activity in insulin-sensitive cell lines Endocrinology 145 4985 4990 [DOI] [PubMed] [Google Scholar]
- 40. Si H Fu Z Babu PV et al. ( 2011. Dietary epicatechin promotes survival of obese diabetic mice and Drosophila melanogaster J Nutr 141 1095 1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Montagut G Onnockx S Vaqué M et al. ( 2010. Oligomers of grape-seed procyanidin extract activate the insulin receptor and key targets of the insulin signaling pathway differently from insulin J Nutr Biochem 21 476 481 [DOI] [PubMed] [Google Scholar]
- 42. Ueda M Furuyashiki T Yamada K et al. ( 2010. Tea catechins modulate the glucose transport system in 3T3-L1 adipocytes Food Funct 1 167 173 [DOI] [PubMed] [Google Scholar]
- 43. Meeprom A Sompong W Suwannaphet W et al. ( 2011. Grape seed extract supplementation prevents high-fructose diet-induced insulin resistance in rats by improving insulin and adiponectin signaling pathways Br J Nutr 106 1173 1181 [DOI] [PubMed] [Google Scholar]
- 44. Kanamoto Y Yamashita Y Nanba F et al. ( 2011. A black soybean seed coat extract prevents obesity and glucose intolerance by up-regulating uncoupling proteins and down-regulating inflammatory cytokines in high-fat diet-fed mice J Agric Food Chem 59 8985 8993 [DOI] [PubMed] [Google Scholar]
- 45. Stöckli J Fazakerley DJ Coster AC et al. ( 2010. Muscling in on GLUT4 kinetics Commun Integr Biol 3 260 362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rowland AF Fazakerley DJ & James DE ( 2011. Mapping insulin/GLUT4 circuitry Traffic 12 672 681 [DOI] [PubMed] [Google Scholar]
- 47. Ashida H Furuyashiki T Nagayasu H et al. ( 2004. Anti-obesity actions of green tea: possible involvements in modulation of the glucose uptake system and suppression of the adipogenesis-related transcription factors Biofactors 22 135 140 [DOI] [PubMed] [Google Scholar]
- 48. Holt RR Lazarus SA Sullards MC et al. ( 2002. Procyanidin dimer B2 (epicatechin-(4beta-8)-epicatechin) in human plasma after the consumption of a flavanol-rich cocoa Am J Clin Nutr 76 798 804 [DOI] [PubMed] [Google Scholar]
- 49. Tomas-Barberan FA Cienfuegos-Jovellanos E Marín A et al. ( 2007. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans J Agric Food Chem 55 3926 3935 [DOI] [PubMed] [Google Scholar]
- 50. Serra A Macià A Romero MP et al. ( 2010. Bioavailability of procyanidin dimers and trimers and matrix food effects in in vitro and in vivo models Br J Nutr 103 944 952 [DOI] [PubMed] [Google Scholar]
- 51. Ou K Percival SS Zou T et al. ( 2012. Transport of cranberry A-type procyanidin dimers, trimers, and tetramers across monolayers of human intestinal epithelial Caco-2 cells J Agric Food Chem 60 1390 1396 [DOI] [PubMed] [Google Scholar]
- 52. Matsui T Ebuchi S Kobayashi M et al. ( 2002. Anti-hyperglycemic effect of diacylated anthocyanin derived from Ipomoea batatas cultivar ayamurasaski can be achieved through the α-glucosidase inhibitory action J Agric Food Chem 50 7244 7248 [DOI] [PubMed] [Google Scholar]
- 53. Fontana PD Cazarolli LH Lavado C et al. ( 2011. Effects of flavonoids on α-glucosidase activity: Potential targets for glucose homeostasis Nutrition 27 1161 1167 [DOI] [PubMed] [Google Scholar]
- 54. Ishikawa A Yamashita H Hiemori M et al. ( 2007. Characterization of inhibitor of postprandial hyperglycemia from the leaves of Nerium indicum J Nutr Sci Vitaminol 53 166 173 [DOI] [PubMed] [Google Scholar]
- 55. Tadera K Minami Y Takamatsu K et al. ( 2006. Inhibition of α-glucosidase and α-amylase by flavonoids J Nutr Sci Vitaminol 52 149 153 [DOI] [PubMed] [Google Scholar]
- 56. Kim JS Kwon CS & Son KH ( 2000. Inhibition of alpha-glucosidase and amylase by luteolin, a falconoid Biosci Biotechnol Biochem 64 2458 2461 [DOI] [PubMed] [Google Scholar]