Abstract
Study Objectives:
Functional interactions between sleep spindle activity, declarative memory consolidation, and general cognitive abilities in school-aged children.
Design:
Healthy, prepubertal children (n = 63; mean age 9.56 ± 0.76 y); ambulatory all-night polysomnography (2 nights); investigating the effect of prior learning (word pair association task; experimental night) versus nonlearning (baseline night) on sleep spindle activity; general cognitive abilities assessed using the Wechsler Intelligence Scale for Children-IV (WISC-IV).
Measurements and Results:
Analysis of spindle activity during nonrapid eye movement sleep (N2 and N3) evidenced predominant peaks in the slow (11-13 Hz) but not in the fast (13-15 Hz) sleep spindle frequency range (baseline and experimental night). Analyses were restricted to slow sleep spindles. Changes in spindle activity from the baseline to the experimental night were not associated with the overnight change in the number of recalled words reflecting declarative memory consolidation. Children with higher sleep spindle activity as measured at frontal, central, parietal, and occipital sites during both baseline and experimental nights exhibited higher general cognitive abilities (WISC-IV) and declarative learning efficiency (i.e., number of recalled words before and after sleep).
Conclusions:
Slow sleep spindles (11-13 Hz) in children age 8–11 y are associated with inter-individual differences in general cognitive abilities and learning efficiency.
Citation:
Hoedlmoser K, Heib DPJ, Roell J, Peigneux P, Sadeh A, Gruber G, Schabus M. Slow sleep spindle activity, declarative memory, and general cognitive abilities in children. SLEEP 2014;37(9):1501-1512.
Keywords: children, declarative memory, IQ, sleep spindles
INTRODUCTION
Existing relationships between sleep, learning, and memory processes are now well established in adults.1–4 Whether and how sleep similarly plays a role in the development of learning abilities and memory capacity in children remains unclear. Few studies have investigated the effect of sleep on declarative (serial digit learning test,5 English-German vocabulary list,6 word pair association,7,8 two-dimensional object-location task8) and procedural (finger tapping task,8 serial reaction time task,9 motor sequence learning task10,11) learning in children.12,13 Regarding declarative learning, it was hypothesized that the higher amounts of slow wave sleep (SWS) during childhood, known to participate in the consolidation of declarative learning in adults, would enhance the effect of sleep on memory retention.13 On the one hand, this hypothesis is unsupported by studies having compared sleep-dependent memory consolidation effects between children and adults, as retrieval rates have been found closely comparable.8 On the other hand, experimental data suggest that at variance with adults, sleep in children plays a beneficial role in the consolidation of declarative but not procedural tasks. For instance, on motor learning tasks (finger tapping, mirror tracing), performance was less improved after posttraining sleep than wakefulness8,11 or even impaired over sleep (using a serial reaction time task9), although indirect sleep-dependent learning effects have been reported using a motor adaptation task.14 It was also found that sleep related extraction of explicit knowledge after implicit motor sequence learning was more pronounced in children (8-11 y) than in adults.15 As SWS activity during sleep and hippocampal activity during recall were enhanced in children in this latter study, the authors suggested that the overnight gain in explicit knowledge could be related to a more effective SWS-driven reprocessing and transformation of hippocampal memory representations at this age.
Sleep and memory research in children has been mostly focused on “macroscopic” estimates of sleep, that is, the amount of rapid eye movement (REM) sleep or SWS.13 However, findings in adults have emphasized the importance of specific sleep features and mechanisms for different types of offline memory processing. In particular, sleep spindles16–23 have been a much-discussed topic. Sleep spindles in the nonrapid eye movement (NREM) sleep electroencephalogram (EEG), generated in thalamocortical circuits, are assumed to be involved in plastic neuronal modifications24–26 and in the coordinated transfer of information between different parts of the brain.27 Sleep stage N2, which is the richer in spindle activity (SpA), has consequently received increasing attention.23,28 Studies were mostly conducted in adult populations. First, it was shown that sleep spindle density increases after learning a declarative memory task as compared with a nonlearning control task,16 and that only subjects increasing their SpA from the control night (nonlearning control task) to the experimental night (declarative word pair task) exhibited enhanced memory performance after the experimental night.19 Furthermore, increased slow sleep spindles over the left frontocentral areas was found correlated with verbal declarative memory performance.29 However, other studies failed to replicate those findings. For instance, Lustenberger et al.30 found a relationship between SpA and general cognitive abilities (estimated by intelligence scores) as well as associations between SpA and the initial acquisition rate during the encoding session (i.e., learning efficiency). In contrast to earlier findings, SpA in this study correlated negatively with the overnight improvement in memory performance.
Maturational changes are well known to occur in terms of spindle number, density, duration, frequency, and regional distribution.31–33 Typical sleep spindles can be detected from the fourth week postterm, and appear to be well developed in the EEG by 9 w postterm.32,34–36 SpA measured at various scalp locations must be differentiated32,34,37–39 as frontal and centroparietal spindles mature at different ages. Indeed, whereas centroparietal spindles marginally change from the age of 4 to 24 y, frontal spindles become more prominent with increasing age, with a sudden increase in frequency during puberty.40 Prepubertal children exhibit more spindles in frontal than in centroparietal (or any other) regions. Frequencies also differ across locations, as demonstrated by Shibagaki et al.,36 who found more pronounced 12-Hz spindles in the frontal leads in children age 8-14 y. Finally, power is much higher for frontal than centroparietal spindles in younger children, but then progressively declines to the same level as that of centroparietal spindles around the age of 13 y.39,40 This suggests that frontal spindles in particular reflect general biological maturation.32
Although sophisticated analyses combining sleep spindle recordings and behavioral measures in children are scarce, available data suggest that SpA may be a general measure of “learning aptitude.” In this respect, several studies have found marked relationships between cognitive abilities (intelligence) and sleep spindles,41–45 drawing attention on more fundamental sleep related learning traits. Traits can be defined as a behavioral or biological attribute, which is not specific to certain situations or tasks and remain stable over time.42 The sleep EEG, and sleep spindles in particular, are characterized by traitlike aspects. Although spindle frequency power is highly variable between subjects, it is remarkably constant over different nights in individuals and shows high heritability.32,42,46–48 For instance, Geiger and colleagues42 found significant correlations between relative sigma power in NREM sleep and full-scale intelligence quotient (IQ), fluid IQ, and working memory in a sample of healthy children age 9–12 y. Contrary to findings in adults, however, spindle peak frequency was negatively associated with full-scale IQ. Similarly Chatburn and colleagues44 showed that the number of fast spindles is positively correlated with narrative memory but negatively with sensorimotor functioning in children. Like Geiger et al.,42 they also found a negative relationship between central frequency of spindles and sensorimotor functioning, planning ability, and working memory.
These results underline the fact that significant pieces of the “sleep and memory in children” puzzle are still missing, and that a comprehensive understanding of the functions of sleep spindles for memory consolidation and general cognitive performance in children is still needed. Based on our earlier findings in adults,19,49 we investigated in the current study the relationships among sleep spindles, declarative memory consolidation, and global cognitive abilities in children (8-11 y). Especially, we probed the hypothesis that memory consolidation processes are linked to an experience-dependent increase in NREM sleep (N2 and N3) SpA after learning. We also investigated the potential presence of sleep-trait relationships between general cognitive abilities, learning efficiency, and SpA in children.
METHODS
Subjects
Sixty-three prepubertal (Tanner I, assessed by a self-administered rating scale50) children (28 girls, 35 boys) age 8–11 y (mean age 9.56 ± 0.76 y) were recruited from public elementary schools. Nine subjects had to be excluded because of technical problems throughout one of the two polysomnography (PSG) recordings, and therefore we present data on 54 children (25 girls, 29 boys; mean age 9.48 ± 0.75 y). Children and parents were informed in detail about the project and gave their written informed consent before study inclusion. Exclusion criteria were sleep and mental disorders as assessed using standardized questionnaires, and medications that might affect sleep or alertness. Obesity (body mass index > 28kg/m2; mean [M] = 17.12, standard deviation [SD] = 2.62) and respiratory problems (e.g., asthma) are risk factors for sleep-disordered breathing in children; both factors were therefore additional exclusion criteria. In order to minimize artifacts due to co-sleeping in polysomnography (PSG) and actigraphy recordings, children who routinely co-slept with parents or siblings were not included in the study. PSG was recorded in the children's habitual environment using an ambulatory EEG system. Environmental conditions disruptive of sleep quality (e.g., light, noise, heat, cold) were controlled. Children received a gift (Professor Globus by Leap Frog Enterprise Inc., California, USA) for their participation. The experiment was conducted in accordance with the national legislation for the protection of human volunteers in nonclinical research settings and the Declaration of Helsinki.
Procedure
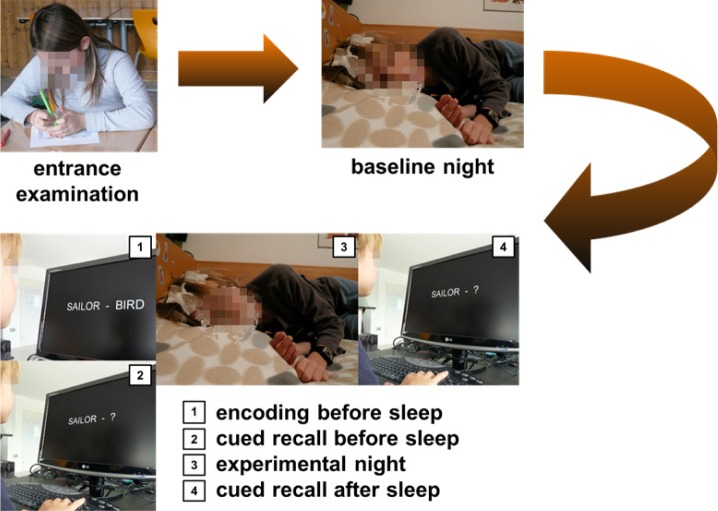
An entrance examination was carried out before starting the investigation (Figure 1), with several parts: clinical evaluation of sleep quality (Children's Sleep Habits Questionnaire, CSHQ51); emotional abilities (Strength and Difficulties Questionnaire, SDQ52); general cognitive abilities (Wechsler Intelligence Scale for Children, WISC-IV53: vocabulary, matrix reasoning, block design) and morningness-eveningness questionnaire (adapted version from Horne and Ostberg54). Subjects had a wrist actigraph and completed a sleep diary in the evening and in the morning for the duration of the study. Sleep was recorded for 2 nights (separated by 7 days) during school days. PSG recordings started between 19:30 and 20:30 (according to the habitual bedtime of the subjects) and were terminated after the subject's habitual total sleep time or after 10 h of sleep. The first baseline night also served diagnosis and adaptation purposes. On the second night, subjects performed a declarative memory task before (18:30, encoding, retrieval) and after (07:30, retrieval only) sleep. The declarative memory task consisted of 50 semantically unrelated word pairs that were presented twice (separated by a short break of approximately 5 min) in a randomized order on a computer screen. We used nonassociated (i.e., not already semantically related) lists of word pairs as the recall of non-associated word pairs is expected to more consistently rely on newly formed, hippocampus-mediated memories.55 To control for mnemonic strategies, children were instructed to visually imagine a relation between the two randomly related words (e.g., for “sailor - bird” one could imagine a sailor standing on a boat and a sea bird sitting on the railing next to him). Words were presented in white color on a black background. During the encoding session, each word pair was presented for 5 sec, followed by a fixation cross for 3 sec. The encoding session started 1-2 h prior to lights off and lasted approximately 20 min. After the encoding session, children performed a retrieval task (cued recall) with words presented in a different randomized sequence. During retrieval, only the first word of a pair was presented for a maximum of 10 sec; subjects were asked first to press a button as soon as they had the response in mind and then to report verbally the corresponding word (e.g., “bird” in response to “sailor”). They were instructed to respond as fast and as accurately as possible. No feedback was provided. The retrieval task in the morning using the same word pairs was performed 1-1.5 h after lights on.
Figure 1.
Study design. Following the entrance examination, polysomnography was recorded during a baseline night and 7 days later during the experimental night. Declarative memory was tested using a cued recall task in the evening preceding and in the morning after the experimental night.
Polysomnography
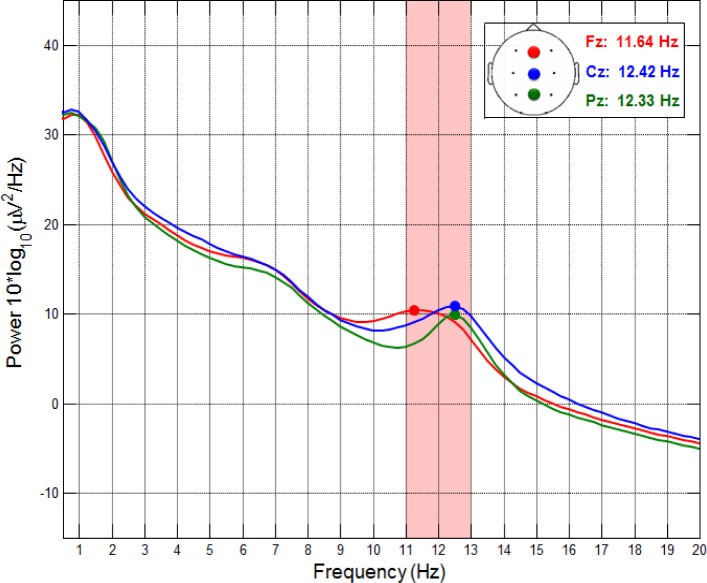
PSG recordings were obtained using an ambulatory 16-channel amplifier (Varioport, Becker Meditec, Germany). Gold electrodes (Grass Technologies, Astro-Med GmbH, Germany) were placed according to the international electrode (10-20) placement-system. Data were recorded referentially against a common reference at Cz and off-line algebraically rereferenced to averaged mastoids.37 PSG recordings including 12 EEG channels (F3, Fz, F4, C3, C4, P3, Pz, P4, O1, O2, A1, A2), two horizontal electrooculogram (HEOG) channels (right and left outer canthi; right superior; left inferior) as well as two vertical EOG (VEOG) channels above and below the right eye and two submental electromyogram (EMG) channels were obtained at a sampling rate of 512 Hz. Sleep was prescored and prestaged automatically (Somnolyzer 24 × 7, Koninklijke Philips N.V., Eindhoven, The Netherlands) according to American Academy of Sleep Medicine (AASM) criteria.56 Scoring and staging were controlled by visual inspection of an expert scorer. Sleep spindles during NREM sleep (N2 and N3) were detected automatically (ASK analyser, The Siesta Group, Vienna, Austria) using frontal (F3, Fz, F4), central (C3, Cz, C4), parietal (P3, Pz, P4) and occipital (O1, O2) electrodes. Spindle detection was based on the following criteria: (1) 11 to 15Hz band-pass filtering, (2) amplitude > 25 μV, (3) duration > 0.5 sec, and (4) controlling for muscle (30-40 Hz) and/or alpha (8-12 Hz) artefacts (for details see Anderer et al.57). Instead of the mean number of sleep spindles per 30 sec (i.e., spindle density), SpA was estimated using a measure that captures the duration as well as the amplitude of identified spindles within a 30-sec epoch, thus reflecting the activity or intensity of the spindle process (i.e., SpA = mean spindle duration * mean spindle amplitude).19 To evidence the distribution of sleep spindle peaks, we calculated EEG power spectral density for frontal (Fz), central (Cz), and parietal (Pz) leads during NREM (N2 and N3) sleep. Power spectral density was estimated using the Welch periodo-gram method58 for consecutive 4-sec segments (multiplied by a Hamming window) using Matlab (MathWorks®, Natick, MA) and the EEGLAB toolbox59 resulting in a 0.25-Hz frequency resolution. Sleep spindle peak frequency was defined as the maximal deflection between 10-16 Hz and was semiautomatically detected for each subject. As depicted in Figure 2, average peak frequency was restricted to the slow sleep spindle range between 11–13 Hz (Fz: range = 10–13, M = 11.64, SD = 0.70; Cz: range = 11–13.5, M = 12.42, SD = 0.43; Pz: range = 10.25–13.5, M = 12.33, SD = 0.56). No additional fast sleep spindle peak was evidenced between 13 and 15 Hz. Because our analyses did not yield evidence for two distinct spindle peaks, and fast spindles could not be detected at all in several children, the analyses were solely focused on slow SpA during NREM sleep.
Figure 2.
Electroencephalographic (EEG) power spectral density for frontal (Fz), central (Cz) and parietal (Pz) leads during nonrapid eye movement (NREM; N2, N3) sleep. Mean power spectral density over all subjects and both nights. Sleep spindle peak frequency is defined as the maximal deflection between 10-16 Hz and was detected semiautomatically for all three electrode sites. Note that the average sleep spindle peak frequency of all electrode sites is below 13 Hz, and thus restricted to the slow spindle frequency range.
RESULTS
Behavioral Data
On average, subjects correctly retrieved 50.81% (SD = 20.36) of the word pairs in the evening (retrieval 1 – RET1) and 48.65% (SD = 20.80) in the morning after the experimental night (retrieval 2 – RET2). Reaction times for correctly remembered word pairs improved overnight (RET1: M = 3083 ms, SD = 707 ms; RET2: M = 2404 ms, SD = 424 ms). Correlations failed to evidence an effect of age on memory performance (RET1: r(54) = 0.056, P = 0.689; RET2: r(54) = 0.062, P = 0.655). Furthermore, independent t-tests with the grouping variable sex did not show a difference between girls and boys (RET1: t(52) = -0.314, P = 0.755, Cohen d = -0.086; RET2: t(52) = -0.336, P = 0.739, Cohen d = -0.091). Correlations between IQ score and memory performance before (r(54) = 0.299, P = 0.028) and after (r(54) = 0.287, P = 0.035) sleep reached significance, whereas overnight changes in memory performance were independent of IQ score (r(54) = -0.017, P = 0.903).
Sleep Spindle Activity
SpA-Dependent Declarative Learning and Memory Consolidation
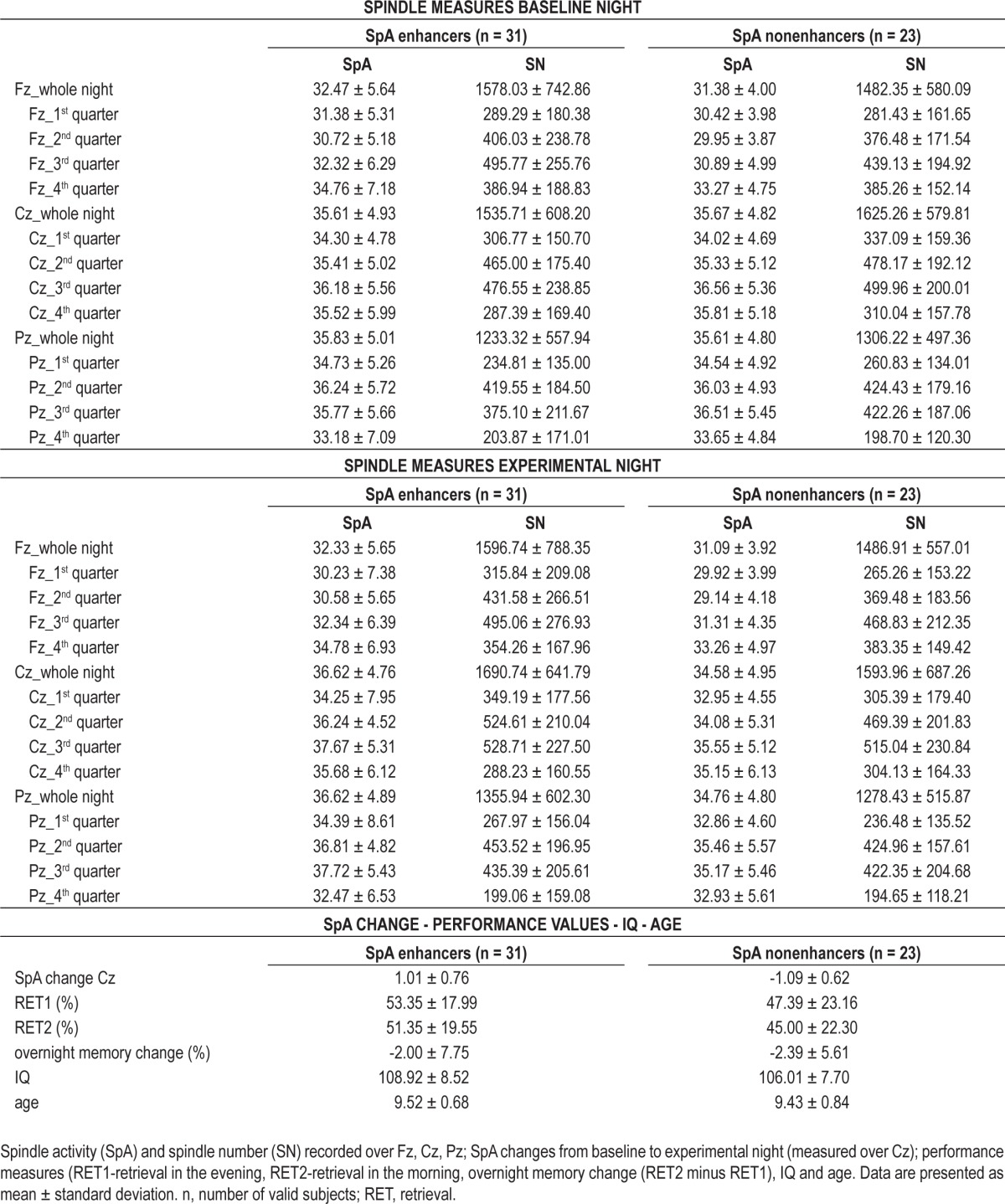
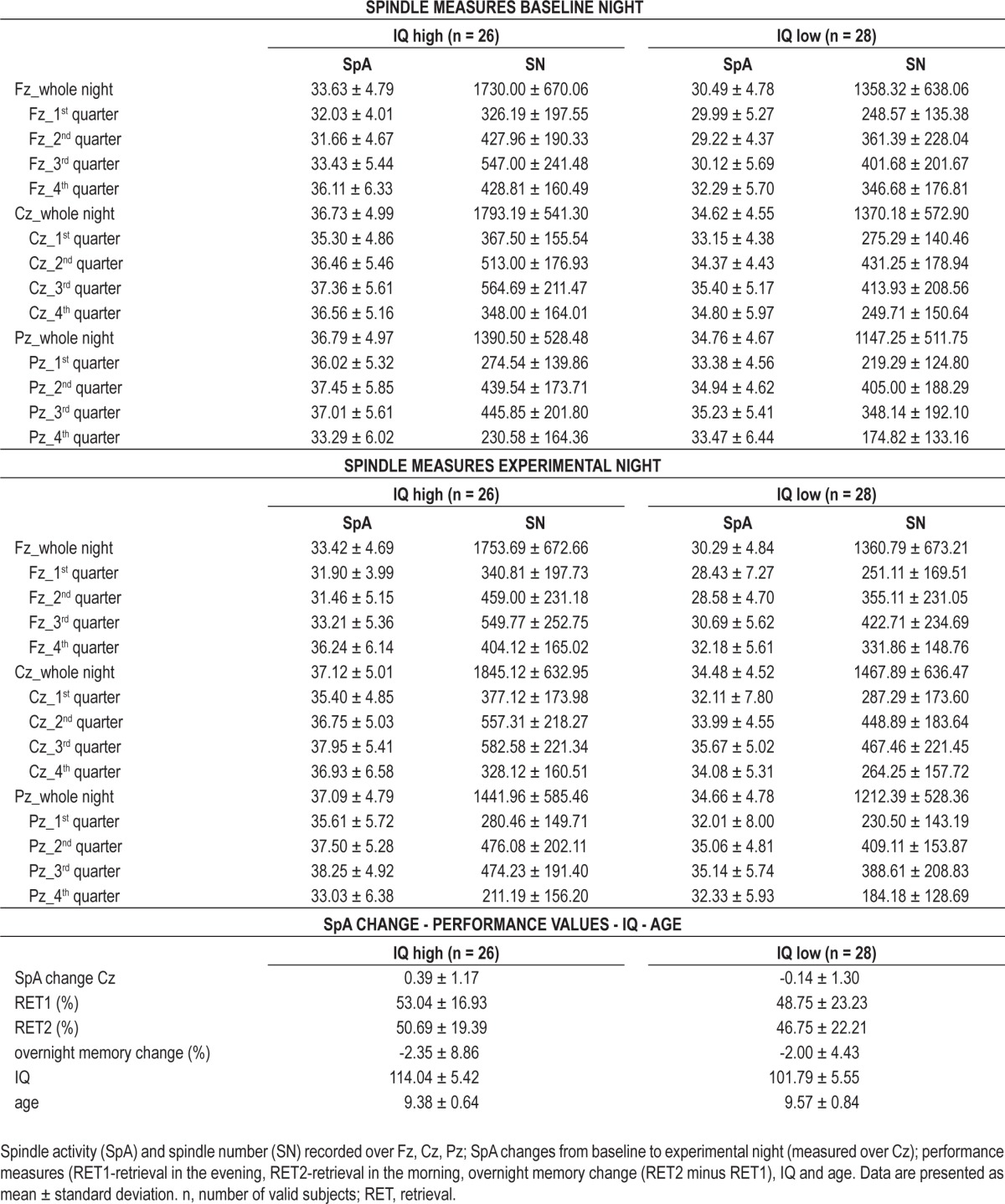
Based on our prior findings in adults,19 subjects were divided into two groups: children showing enhanced central SpA (SpA enhancers; n = 31; range of SpA change from baseline to experimental night: 0.01 to 2.90, M = 1.01, SD = 0.76), and children without enhanced SpA (SpA nonenhancers; n = 23; range of SpA change from baseline to experimental night: -2.46 to -0.11, M = -1.09, SD = 0.62). Changes in central (Cz) SpA from the baseline to the experimental night (SpA experimental minus SpA baseline) were computed using 0 as a cutoff score (i.e., no change in SpA from baseline to experimental night). Descriptive data for SpA enhancers versus SpA nonenhancers are provided in Table 1. In addition to differences in SpA changes from baseline to experimental night, analyses showed that SpA enhancers and SpA nonenhancers did not differ with respect to other possibly confounding variables, such as IQ (t(52) = 1.293, P = 0.202, Cohen d = 0.359) or age (t(52) = 0.393, P = 0.696, Cohen d = 0.118).
Table 1.
Descriptive data for spindle activity enhancers (SpA+) and SpA nonenhancers (SpA−)
A two-way analysis of variance (ANOVA) was computed on declarative memory performance (i.e., correctly recalled word pairs [%]) with the within-subject factor RETRIEVAL (RET1 in the evening versus RET2 in the morning) and the between-subjects factor SpA-ENHANCEMENT (SpA enhancers versus SpA nonenhancers). Results revealed a main effect of RETRIEVAL (F(1,52) = 5.310, P = 0.025, partial eta2 = 0.093). Independently of SpA enhancement there was a general decrease in memory performance over sleep (Figure 3). No significant group (SpA-ENHANCEMENT: F(1,52) = 1.221, P = 0.274, partial eta2 = 0.023) or interaction effects (RETRIEVAL × SpA-ENHANCEMENT: F(1,52) = 0.042, P = 0.838, partial eta2 = 0.001) were found. Changes in SpA (experimental minus baseline night) were not related to overnight changes in memory performance between the evening and the next morning (r(54) = 0.105, P = 0.449).
Figure 3.
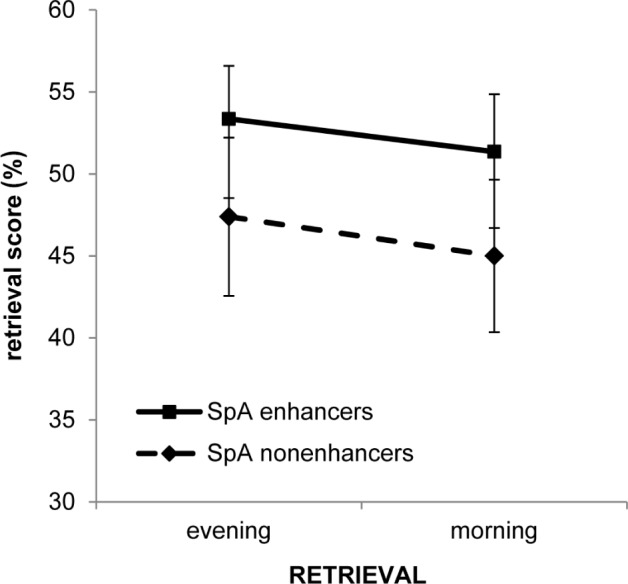
Overnight memory changes (evening [RET1] versus morning [RET2]) in word-pair cued recall performance for spindle activity (SpA) enhancers and SpA nonenhancers. Note that memory performance is decreased in the morning [RET2] after sleep in comparison to the evening [RET1] independently whether children could enhance SpA from the baseline to the experimental night (SpA enhancers) or not (SpA nonenhancers). Error bars indicate standard error of means. RET, reaction time.
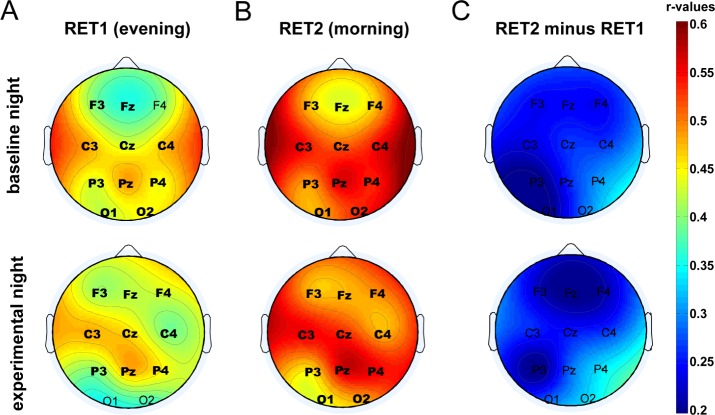
Furthermore and contrary to our prior results in adults,19 we found highly significant correlations between SpA during both nights and absolute memory performance, both before (baseline night: r(54) = 0.479, P < 0.001; experimental night: r(54) = 0.478, P < 0.001) and after (baseline night: r(54) = 0.547, P < 0.001; experimental night: r(54) = 0.555, P < 0.001) sleep. Also, correlations between SpA and changes in memory performance (RET2 minus RET1) were close to significance for both the experimental (r(54) = 0.262, P = 0.056) and the baseline (r(54) = 0.238, P = 0.083) night. The relationship between SpA and absolute memory performance was not only restricted to electrode Cz but was found over all 11 recording sites (Figure 4).
Figure 4.
Topographical maps representing Pearson correlation coefficients (color scaled) between recall performance (A) in the evening (RET1), (B) in the morning (RET2) as well as (C) overnight memory change (RET2 minus RET1) and slow spindle activity (SpA) both at baseline (upper row) and experimental (lower row) nights. Correlation values at electrode positions printed in bold remained significant after Bonferroni correction for multiple comparisons (P < 0.05/11 = 0.0045). RET, retrieval.
SpA-Dependent General Cognitive Abilities
In a second step, we investigated whether slow SpA differs as a function of general cognitive abilities. To do so, subjects were divided into two groups based on their general cognitive abilities as measured by performance on the WISC-IV, using a median split (median IQ = 106.67). This resulted in a group of highly gifted (IQ high: n = 26; M = 114.04, SD = 5.42) and a group of moderately gifted (IQ low: n = 28; M = 101.79, SD = 5.55) individuals. The IQ categorizing factor was independent of the SpA enhancement factor. An independent t-test showed that SpA enhancement was similar in subjects with high and moderate cognitive abilities (t(52) = 1.573, P = 0.122, Cohen d = 0.429). Furthermore, highly and moderately gifted subjects did not differ with respect to other possibly confounding variables, such as declarative memory performance (RET1: t(52) = 0.770, P = 0.445, Cohen d = 0.214; RET2: t(52) = 0.692, P = 0.492, Cohen d = 0.189), overnight memory performance change (RET2 minus RET1: t(52) = -0.184, P = 0.855, Cohen d = -0.053) or age (t(52) = -0.918, P = 0.363, Cohen d = -0.258). Descriptive data for IQ low versus IQ high are provided in Table 2.
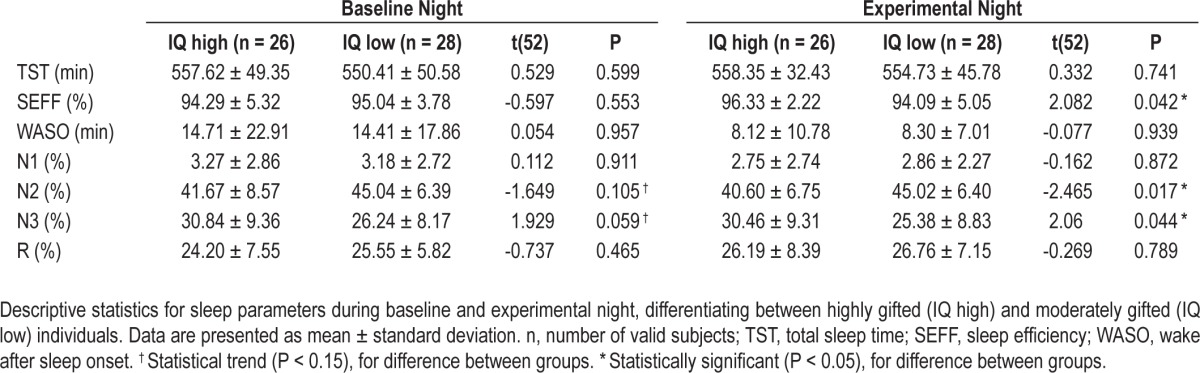
Table 2.
Descriptive data for highly gifted (IQ high) and moderately (IQ low) subjects
A three-way repeated-measures ANOVA was computed on mean SpA during baseline and experimental night with the within-subject factors ELECTRODE (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4, O1, O2) and NIGHT QUARTER (first, second, third, fourth) and the between-subjects factor IQ (IQ low, IQ high). Results disclosed a significant main effect of within-subject factors ELECTRODE (F(10,520) = 82.960, P < 0.001, partial eta2 = 0.615) and NIGHT QUARTER (F(3,156) = 5.543, P = 0.005, partial eta2 = 0.096). A significant effect for the between-subjects factor IQ (F(1,52) = 4.302, P = 0.043, partial eta2 = 0.076) showed that highly gifted children have higher SpA than moderately gifted children over all 11 electrodes, during both nights and all four quarters of the night. Furthermore, the interaction between ELECTRODE × NIGHT QUARTER (F(30,1560) = 35.310, P < 0.001, partial eta2 = 0.404) and the interaction between ELECTRODE × NIGHT QUARTER × IQ (F(30,1560) = 3.316, P = 0.005, partial eta2 = 0.060) reached significance (Figure 5 and Figure S1, supplemental material).
Figure 5.
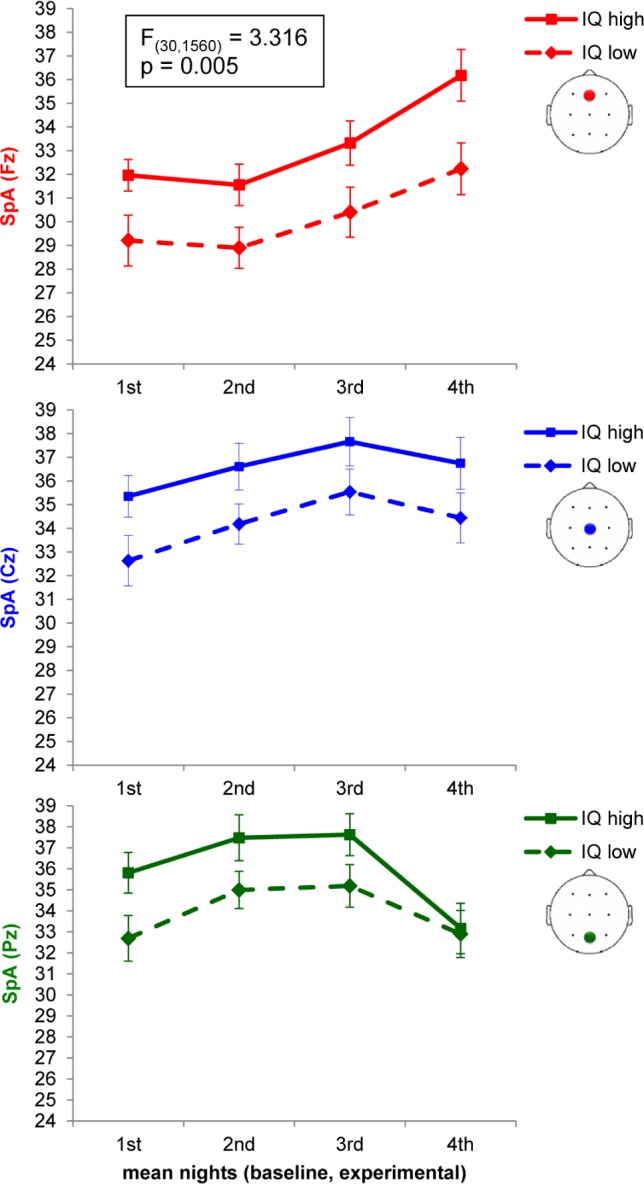
Three-way interaction between ELECTRODE × NIGHT QUARTER × IQ. Mean spindle activity (SpA) during baseline and experimental night over three electrodes (Fz, Cz, Pz) are illustrated. Please refer to Figure S1 (supplemental material) including all 11 electrodes. Group means ± standard error of means are depicted.
Correlations between SpA during NREM sleep and IQ scores reached significance for all 11 electrodes during baseline (r(54) range 0.296–0.391, P values range 0.03–0.003) and experimental night (r(54) range 0.291–0.399, P values range 0.033–0.003; Figure 6). After Bonferroni correction for multiple comparisons (P < 0.05/11 = 0.0045) correlations for Fz, Pz, and O1 during baseline night and those for Fz, O1, and O2 during experimental night remained significant.
Figure 6.
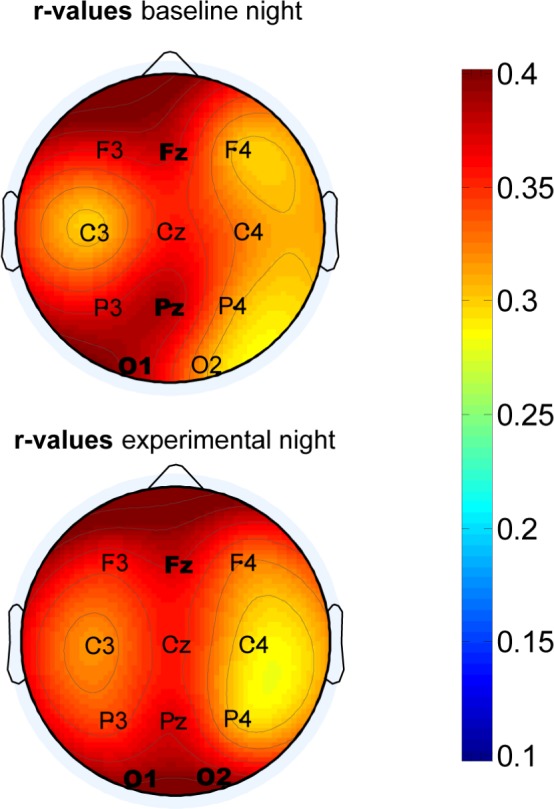
Topographical maps representing Pearson correlation coefficients (color scaled) between IQ scores and slow spindle activity (SpA) both for baseline (upper row) and experimental (lower row) nights. Correlation values at electrode positions printed in bold remain significant after Bonferroni correction for multiple comparisons (P < 0.05/11 = 0.0045).
Sleep Architecture
Independent sample t-tests (Table 3) failed to disclose significant (Bonferroni correction for multiple comparisons P < 0.05/7 = 0.0056) differences in sleep architecture between SpA enhancers and SpA nonenhancers, either during the baseline or the experimental night. However, there was a statistical trend (t(52) = -1.923, P = 0.06, Cohen d = -0.524) for SpA nonenhancers to have more N3 sleep than SpA enhancers during the baseline night. Paired sample t-tests were computed further to control for differences in the sleep architecture between baseline and experimental nights within both groups. Again, no significant differences were found, but SpA enhancers showed a marginally longer wake after sleep onset time during the baseline night compared to the experimental night (t(30) = 1.855, P = 0.073, Cohen d = 0.380) whereas SpA nonenhancers exhibited a statistical trend for reduced total sleep time (t(22) = -1.970, P = 0.062, Cohen d = -0.461), more N3 (t(22) = 2.201, P = 0.039, Cohen d = 0.459) and less REM (t(22) = -1.964, P = 0.062, Cohen d = -0.435) during the baseline night compared to the experimental night.
Table 3.
Sleep architecture in SpA enhancers versus SpA nonenhancers
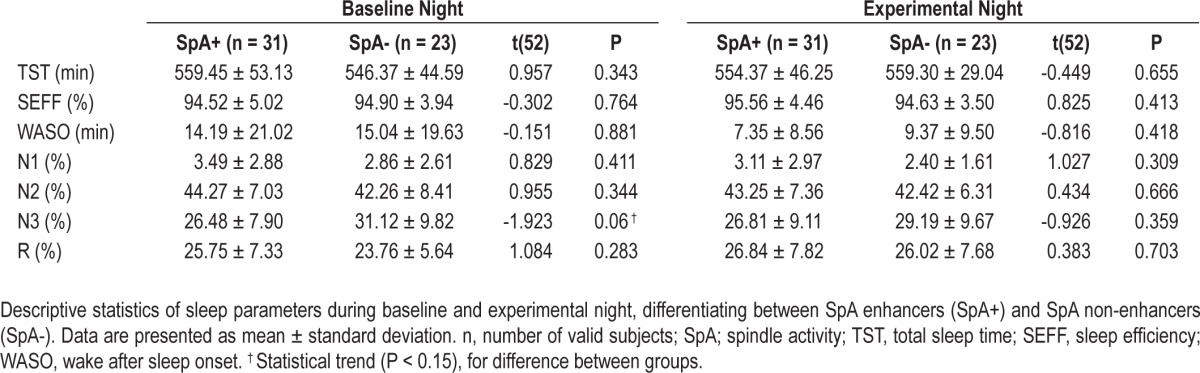
Similarly, independent t-tests conducted with the between-subjects variable cognitive abilities (IQ high versus IQ low; Table 4) revealed a statistical trend (Bonferroni correction for multiple comparisons P < 0.05/7 = 0.0056) for highly gifted children spending less time in stage N2 (baseline night: t(52) = -1.649, P = 0.105, Cohen d = -0.451; experimental night: t(52) = -2.465, P = 0.017, Cohen d = -0.672) and more time in stage N3 (baseline night: t(52) = 1.929, P = 0.059, Cohen d = 0.525; experimental night: t(52) = 2.06, P = 0.044, Cohen d = 0.560) sleep, as compared to moderately gifted children. Additionally, highly gifted children showed a trend for higher sleep efficiency during the experimental night than moderately gifted children (t(52) = 2.082, P = 0.042, Cohen d = 0.616). To control for differences in the sleep architecture between baseline and experimental nights within both groups, further paired sample t-tests were computed. A statistical trend for reduced sleep efficiency during the experimental night in comparison to the baseline night in highly gifted children (t(25) = -1.846, P = 0.077, Cohen d = -0.396) was disclosed.
Table 4.
Sleep architecture in IQ high versus IQ low individuals
DISCUSSION
In a prepubertal sample of children, we found that sleep spindle peak frequency is restricted to the slow (11-13 Hz) spindle range; no additional fast (13-15 Hz) sleep spindle peak could be detected (Figure 2). Therefore, we focused our analyses investigating relationships between SpA, declarative memory, and general cognitive abilities (intelligence score) on the frequency range of slow sleep spindles during NREM sleep. Our results failed to support the hypothesis that increased SpA after learning during an experimental night (in comparison with a nonlearning baseline night) is involved in overnight memory consolidation. However, we found SpA during both nights related to declarative memory performance before and after the experimental night (i.e., learning efficiency). This finding indicates that more efficient learning is associated with a general higher SpA whether or not learning occurred before sleep. Additionally, our findings show that SpA in children is strongly associated with general cognitive abilities as measured by the WISC-IV.
SpA-Dependent Declarative Learning and Memory Consolidation
Similar to our earlier investigations in adults,19 participants have been categorized into SpA enhancers and SpA nonenhancers based on the observed increase in postlearning central (Cz) SpA. Our results indicate that children generally exhibit reduced memory performance after sleep in the morning, whether or not SpA was enhanced from the baseline to the experimental night (Figure 3). Hence, the relationship between SpA enhancement and overnight improvement in memory performance found in adults16,19,29 could not be replicated in this school-aged sample. This lack of effect might be caused by various factors. First, task difficulty might have been too high. Here we used 50 unrelated word pairs presented twice at the encoding phase in the evening, and children performed a cued recall task in the evening and in the morning after sleep. Children only correctly retrieved 50.81% (SD = 20.36) of the word pairs in the evening and 48.65% (SD = 20.80) in the morning. Even if we instructed the children to learn to the best of their ability, we did not use a criteria-learning protocol (i.e., testing until a specific amount of correct responses; e.g., 60% criterion) and no feedback (i.e., displaying the correct word pair after each response) was given during cued recall. Prior studies reporting enhancement of declarative memory after sleep in children (e.g., Wilhelm et al.8) used only 20 related word-pairs, which were presented until a criterion of 60% correct responses was reached at the encoding phase. Backhaus et al.7 presented 40 related word-pairs until children correctly recalled at least 20 words (50% criterion), and additionally gave feedback during learning. Second, for organizational reasons children did not perform a control task during the nonlearning baseline night as done previously in earlier adult populations.16,19 Therefore, it cannot be ruled out that changes in SpA in this current study reflect use-dependent (i.e., restoration of an optimal neuronal function after the sustained waking neuronal activity) rather than experience-dependent (i.e., exposure to a new environment, expansion of the behavioral repertoire) changes.60 Third, and again for cost efficiency reasons, the order of the baseline and experimental nights was not balanced across subjects. We had to limit as much as possible the already extensive schedule proposed to our children participants, and therefore decided to use the baseline night as the nonlearning control night, instead of adding a third recording night in the protocol (which would have made the investment much heavier for the children). This caveat makes it difficult to rule out whether differences in SpA between the 2 successive nights are not the result of habituation instead of being related to processes of memory consolidation. However, it should be considered also that sleep architecture was similar in SpA enhancers and SpA nonenhancers in the current study, which does not support a first night effect. Fourth, because we have not measured performance changes over either daytime or a night without sleep, we cannot ascertain whether overnight memory change was sleep dependent in this study. Finally, at the methodological level, effects of regression to the mean (RTM) may have influenced the current design. RTM is a ubiquitous characteristic of repeated measurements that should always be considered as a possible cause for observed changes. Indeed, because of the nature of normally distributed random variables, cases with high values at pretest will on average be slightly lower on a second measurement, and cases with low values in the pretest will be slightly higher on the second measurement. Consequently, a practical problem caused by RTM is the need to distinguish a real change from the expected change because of the natural variation.61 These confounds make it difficult to disentangle trait (general SpA) and state (change in SpA after learning) influences on performance. Further studies should investigate this issue in a specially designed protocol.
SpA-Dependent General Cognitive Abilities
To investigate a possible trait-related effect of sleep SpA on general cognitive abilities, we split the children into two groups regarding their Wechsler Intelligence Scale scores: highly (IQ high) and moderately (IQ low) gifted subjects. Our results are in agreement with earlier findings in adults49,62 and children.42,43 Subjects with higher general cognitive abilities, as measured by means of intelligence scores, exhibit higher SpA over all electrodes during both baseline and experimental night throughout all quarters of the night (Figure 5). These findings seem to be in line with our results showing an association between SpA and declarative memory performance before and after the experimental night, irrespective of whether learning occurred before sleep (Figure 4). These results highlight a triangle of SpA, learning efficiency, and intelligence,30 indicating that SpA shows robust relations to both of these measures constituting “general cognitive abilities.” In a population of children of similar age as the presented sample, Geiger et al.42 found a positive relationship between sigma power and full scale IQ scores, fluid IQ scores, and individual relative sigma power, but not with verbal IQ. However, spindle peak frequency was negatively related to full-scale IQ. Additionally, they showed a striking individual stability of sleep related oscillations (including alpha, sigma, and beta range) across several nights, further supporting the view that these (sleep) EEG invariances indicate a traitlike “fingerprint” characteristic, probably reflecting traits of the underlying brain anatomy. Our data support these previous findings by showing robust associations between slow SpA and general cognitive abilities (WISC-IV subtest scores vocabulary, matrix reasoning, block design). The fact that SpA is positively related to general cognitive ability irrespective of whether learning occurred before sleep further indicates the general nature of this association. Hence, our results are in line with earlier developmental studies reporting that sleep spindles reflect important aspects of the central nervous system34,39 and more specifically of thalamocortical networks,63 which might be associated with neocorticohippocampal processes. Furthermore, Bruni et al.64 found that slow SpA in children with dyslexia is associated with the extent of dyslexic impairment. They consequently suggested that increased slow SpA in this specific population might be related to a genetically more efficient thalamocortical system, to an obligatory early adoption of strategies aimed at compensating this specific learning disability, or to the intensive stimulation linked to cognitive rehabilitation.
Interestingly, we further found a statistical trend that highly gifted children generally (baseline and experimental night) tend to spend less time in N2 sleep and more time in N3 sleep in comparison with moderately gifted children (Table 4). It is well known that children sleep longer and more deeply in comparison with adults.65,66 Additionally, they spend a larger amount of time in N3, a sleep stage known to contribute to the consolidation of declarative memories.67,68 Not only sleep spindles but also slow wave oscillations play a key role in neuronal plasticity in the hippocampal-neocortical dialogue,69 and slow oscillations in prepubertal children present higher amplitudes and steeper slopes in comparison with adults.70 Therefore, it seems to be a promising research direction to further focus on possible relationships between N3 sleep, including its predominating slow wave oscillations, which are higher in amplitude and steeper in slope in children than in adults.63,66,70
In summary, our current findings together with previous studies suggest an association between slow SpA, learning efficiency, and general cognitive abilities in school-aged children. We provide further evidence that sleep spindles may be a useful marker for the development and integrity of the central nervous system during early life,34,39 and indeed promote the formation of thalamocortical networks by providing endogenous signals with repetitive and synchronized activity, as for instance suggested by Jenni and colleagues.63 Our data indicate that interindividual differences in sleep spindles are related to inter-individual differences in general cognitive abilities as reflected by intelligence scores and learning ability. Therefore, sleep spindles might be an even stronger sleep related learning trait representing a biological indicator for cognitive and learning abilities during sleep in healthy children.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was funded by the Austrian Science Fund (FWF; T397-B02) and the Fonds Gesundes Oesterreich (1689). Dr. Gruber is an employee of the Siesta Group Schlafanalyse GmbH; he has participated in research projects on automated sleep analyses. Prof. Sadeh served as a consultant for Johnson & Johnson. This current study is not related in any way to the projects he worked on with Johnson & Johnson. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank all of the children who participated in this study and their parents and teachers as well as the principals of the schools and the local education authority (Mag. Dipl. Paed. Birgit Heinrich, Prof. Mag. Josef Thurner) in Salzburg who supported this study.
SUPPLEMENTAL MATERIAL
Three-way interaction between ELECTRODE × NIGHT QUARTER × IQ at all 11 electrodes. Group means ± standard error of means are depicted.
REFERENCES
- 1.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 2.Peigneux P, Laureys S, Delbeuck X, Maquet P. Sleeping brain, learning brain. The role of sleep for memory systems. Neuroreport. 2001;12:A111–24. doi: 10.1097/00001756-200112210-00001. [DOI] [PubMed] [Google Scholar]
- 3.Walker MP, Stickgold R. Sleep, memory, and plasticity. Ann Rev Psychol. 2006;57:139–66. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 4.Wang G, Grone B, Colas D, Appelbaum L, Mourrain P. Synaptic plasticity in sleep: learning, homeostasis and disease. Trends Neurosci. 2011;34:452–63. doi: 10.1016/j.tins.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Dev. 2002;73:405–17. doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- 6.Gais S, Lucas B, Born J. Sleep after learning aids memory recall. Learn Mem. 2006;13:259–62. doi: 10.1101/lm.132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backhaus J, Hoeckesfeld R, Born J, Hohagen F, Junghanns K. Immediate as well as delayed post learning sleep but not wakefulness enhances declarative memory consolidation in children. Neurobiol Learn Mem. 2008;89:76–80. doi: 10.1016/j.nlm.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm I, Diekelmann S, Born J. Sleep in children improves memory performance on declarative but not procedural tasks. Learn Mem. 2008;15:373–7. doi: 10.1101/lm.803708. [DOI] [PubMed] [Google Scholar]
- 9.Fischer S, Wilhelm I, Born J. Developmental differences in sleep's role for implicit off-line learning: comparing chirldren with adults. J Cogn Neurosci. 2007;19:214–27. doi: 10.1162/jocn.2007.19.2.214. [DOI] [PubMed] [Google Scholar]
- 10.Wilhelm I, Metzkow-Meszaros M, Knapp S, Born J. Sleep-dependent consolidation of procedural motor memories in children and adults: the pre-sleep level of performance matters. Dev Sci. 2012;15:506–15. doi: 10.1111/j.1467-7687.2012.01146.x. [DOI] [PubMed] [Google Scholar]
- 11.Prehn-Kristensen A, Goder R, Chirobeja S, Bressmann I, Ferstl R, Baving L. Sleep in children enhances preferentially emotional declarative but not procedural memories. J Exp Child Psychol. 2009;104:132–9. doi: 10.1016/j.jecp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Kopasz M, Loessl B, Hornyak M, et al. Sleep and memory in healthy children and adolescents - a critical review. Sleep Med Rev. 2010;14:167–77. doi: 10.1016/j.smrv.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelm I, Prehn-Kristensen A, Born J. Sleep-dependent memory consolidation - What can be learnt from children? Neurosci Biobehav Rev. 2012;36:718–28. doi: 10.1016/j.neubiorev.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Urbain C, Houyoux E, Albouy G, Peigneux P. Consolidation through the looking-glass: sleep-dependent proactive interference on visuomotor adaptation in children. J Sleep Res. 2014;23:44–52. doi: 10.1111/jsr.12082. [DOI] [PubMed] [Google Scholar]
- 15.Wilhelm I, Rose M, Imhof K, Rasch B, Büchel C, Born J. The sleeping child outplays the adult's capacity to convert implicit into explicit knowledge. Nat Neurosci. 2013;16:391–93. doi: 10.1038/nn.3343. [DOI] [PubMed] [Google Scholar]
- 16.Gais S, Molle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–4. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith C, Macneill C. Impaired Motor Memory for a Pursuit Rotor Task Following Stage-2 Sleep Loss in College-Students. J Sleep Res. 1994;3:206–13. doi: 10.1111/j.1365-2869.1994.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 18.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–11. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 19.Schabus M, Gruber G, Parapatics S, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27:1479–85. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 20.Morin A, Doyon J, Dostie V, et al. Motor sequence learning increases sleep spindles and fast frequencies in post-training sleep. Sleep. 2008;31:1149–56. [PMC free article] [PubMed] [Google Scholar]
- 21.Bergmann TO, Mölle M, Diedrichs J, Born J, Siebner HR. Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage. 2012;59:2733–42. doi: 10.1016/j.neuroimage.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 22.Eschenko O, Molle M, Born J, Sara SJ. Elevated sleep spindle density after learning or after retrieval in rats. J Neurosci. 2006;26:12914–20. doi: 10.1523/JNEUROSCI.3175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35:1154–65. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Sejnowski TJ, Destexhe A. Why do we sleep? Brain Research. 2000;886:208–23. doi: 10.1016/s0006-8993(00)03007-9. [DOI] [PubMed] [Google Scholar]
- 25.Steriade M. Coherent oscillations and short-term plasticity in corticothalamic networks. Trends Neurosci. 1999;22:337–45. doi: 10.1016/s0166-2236(99)01407-1. [DOI] [PubMed] [Google Scholar]
- 26.Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25:9398–405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buzsaki G. The hippocampo-neocortical dialogue. Cerebral Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- 28.Nader R, Smith C. A role for stage 2 sleep in memory processing. In: Maquet P, Smith C, Stickgold R, editors. Sleep and brain plasticity. New York: Oxford University Press; 2003. pp. 87–98. [Google Scholar]
- 29.Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132:529–35. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Lustenberger C, Maric A, Durr R, Achermann P, Huber R. Triangular relationship between sleep spindle activity, general cognitive ability and the efficiency of declarative learning. PLoS One. 2012;7:e49561. doi: 10.1371/journal.pone.0049561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolas A, Petit D, Rompre S, Montplaisir J. Sleep spindle characteristics in healthy subjects of different age groups. Clin Neurophysiol. 2001;112:521–7. doi: 10.1016/s1388-2457(00)00556-3. [DOI] [PubMed] [Google Scholar]
- 32.Scholle S, Zwacka G, Scholle HC. Sleep spindle evolution from infancy to adolescence. Clin Neurophysiol. 2007;118:1525–31. doi: 10.1016/j.clinph.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Tarokh L, Carskadon MA. Developmental changes in the human sleep EEG during early adolescence. Sleep. 2010;33:801–9. doi: 10.1093/sleep/33.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanguay PE, Ornitz EM, Kaplan A, Bozzo ES. Evolution of sleep spindles in childhood. Electroencephalogr Clin Neurophysiol. 1975;38:175–81. doi: 10.1016/0013-4694(75)90227-8. [DOI] [PubMed] [Google Scholar]
- 35.Ellingson RJ. Development of sleep spindle bursts during the first year of life. Sleep. 1982;5:39–46. [PubMed] [Google Scholar]
- 36.Shibagaki M, Kiyono S, Watanabe K. Spindle evolution in normal and mentally retarded children: a review. Sleep. 1982;5:47–57. doi: 10.1093/sleep/5.1.47. [DOI] [PubMed] [Google Scholar]
- 37.Zeitlhofer J, Gruber G, Anderer P, Asenbaum S, Schimicek P, Saletu B. Topographic distribution of sleep spindles in young healthy subjects. J Sleep Res. 1997;6:149–55. doi: 10.1046/j.1365-2869.1997.00046.x. [DOI] [PubMed] [Google Scholar]
- 38.Ueda K, Nittono H, Hayashi M, Hori T. Spatiotemporal changes of slow wave activities before and after 14 Hz/12 Hz sleep spindles during stage 2 sleep. Psychiatry Clin Neurosci. 2001;55:183–4. doi: 10.1046/j.1440-1819.2001.00817.x. [DOI] [PubMed] [Google Scholar]
- 39.Shinomiya S, Nagata K, Takahashi K, Masumura T. Development of sleep spindles in young children and adolescents. Clin Electroencephalogr. 1999;30:39–43. doi: 10.1177/155005949903000203. [DOI] [PubMed] [Google Scholar]
- 40.Nagata K, Shinomiya S, Takahashi K, Masumura T. [Developmental characteristics of frontal spindle and centro-parietal spindle] No To Hattatsu. 1996;28:409–17. [PubMed] [Google Scholar]
- 41.Busby K, Pivik RT. Sleep patterns in children of superior intelligence. J Child Psychol Psychiatry. 1983;24:587–600. doi: 10.1111/j.1469-7610.1983.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 42.Geiger A, Huber R, Kurth S, Ringli M, Jenni OG, Achermann P. The sleep EEG as a marker of intellectual ability in school age children. Sleep. 2011;34:181–9. doi: 10.1093/sleep/34.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geiger A, Huber R, Kurth S, Ringli M, Achermann P, Jenni OG. Sleep electroencephalography topography and children's intellectual ability. Neuroreport. 2012;23:93–7. doi: 10.1097/WNR.0b013e32834e7e8f. [DOI] [PubMed] [Google Scholar]
- 44.Chatburn A, Coussens S, Lushington K, Kennedy D, Baumert M, Kohler M. Sleep spindle activity and cognitive performance in healthy children. Sleep. 2013;36:237–43. doi: 10.5665/sleep.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gruber R, Wise MS, Frenette S, et al. The association between sleep spindles and IQ in healthy school-age children. Int J Psychophysiol. 2013;89:229–40. doi: 10.1016/j.ijpsycho.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 46.Werth E, Achermann P, Dijk DJ, Borbely AA. Spindle frequency activity in the sleep EEG: individual differences and topographic distribution. Electroencephalogr Clin Neurophysiol. 1997;1035:535–42. doi: 10.1016/s0013-4694(97)00070-9. [DOI] [PubMed] [Google Scholar]
- 47.De Gennaro L, Ferrara M, Vecchio F, Curcio G, Bertini M. An electroencephalographic fingerprint of human sleep. NeuroImage. 2005;26:114–22. doi: 10.1016/j.neuroimage.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 48.De Gennaro L, Marzano C, Fratello F, et al. The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Ann Neurol. 2008;64:455–60. doi: 10.1002/ana.21434. [DOI] [PubMed] [Google Scholar]
- 49.Schabus M, Hödlmoser K, Gruber G, et al. Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. Eur J Neurosci. 2006;23:1738–46. doi: 10.1111/j.1460-9568.2006.04694.x. [DOI] [PubMed] [Google Scholar]
- 50.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14:190–5. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- 51.Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–51. [PubMed] [Google Scholar]
- 52.Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997;38:581–6. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 53.Petermann F, Petermann U. Hamburger-Wechsler Intelligenztest für Kinder. Bern: Huber; 2007. [Google Scholar]
- 54.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 55.Stickgold R. Dissecting sleep-dependent learning and memory consolidation. Comment on Schabus M et al. Sleep spindles and their significance for declarative memory consolidation. Sleep 2004;27(8):1479-85. Sleep. 2004;27:1443–5. doi: 10.1093/sleep/27.8.1443. [DOI] [PubMed] [Google Scholar]
- 56.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events. [Google Scholar]
- 57.Anderer P, Gruber G, Parapatics S, et al. An e-health solution for automatic sleep Classification according to Rechtschaffen and Kales: validation study of the Somnolyzer 24 x 7 utilizing the Siesta Database. Neuropsychobiology. 2005;51:115–33. doi: 10.1159/000085205. [DOI] [PubMed] [Google Scholar]
- 58.Welch PD. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Transactions on Audio Electroacoustics. 1967;AU-15:70–3. [Google Scholar]
- 59.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 60.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–52. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 61.Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34:215–20. doi: 10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]
- 62.Bodizs R, Kis T, Lazar AS, et al. Prediction of general mental ability based on neural oscillation measures of sleep. J Sleep Res. 2005;14:285–92. doi: 10.1111/j.1365-2869.2005.00472.x. [DOI] [PubMed] [Google Scholar]
- 63.Jenni OG, Borbely AA, Achermann P. Development of the nocturnal sleep electroencephalogram in human infants. Am J Physiol Regul Integr Comp Physiol. 2004;286:R528–38. doi: 10.1152/ajpregu.00503.2003. [DOI] [PubMed] [Google Scholar]
- 64.Bruni O, Ferri R, Novelli L, et al. Sleep spindle activity is correlated with reading abilities in developmental dyslexia. Sleep. 2009;32:1333–40. doi: 10.1093/sleep/32.10.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 66.Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci U S A. 2009;106:5177–80. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–3. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 68.Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–9. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 69.Molle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011;34:1411–21. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurth S, Jenni OG, Riedner BA, Tononi G, Carskadon MA, Huber R. Characteristics of sleep slow waves in children and adolescents. Sleep. 2010;33:475–80. doi: 10.1093/sleep/33.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Three-way interaction between ELECTRODE × NIGHT QUARTER × IQ at all 11 electrodes. Group means ± standard error of means are depicted.