Abstract
Study Objectives:
To investigate the comparative efficacy of cognitive behavioral therapy (CBT), Tai Chi Chih (TCC), and sleep seminar education control (SS) on the primary outcome of insomnia diagnosis, and secondary outcomes of sleep quality, fatigue, depressive symptoms, and inflammation in older adults with insomnia.
Design:
Randomized controlled, comparative efficacy trial.
Setting:
Los Angeles community.
Patients:
123 older adults with chronic and primary insomnia.
Interventions:
Random assignment to CBT, TCC, or SS for 2-hour group sessions weekly over 4 months with follow-up at 7 and 16 months.
Measurements:
Insomnia diagnosis, patient-reported outcomes, polysomnography (PSG), and high-sensitivity C-reactive protein (CRP) levels.
Results:
CBT performed better than TCC and SS in remission of clinical insomnia as ascertained by a clinician (P < 0.01), and also showed greater and more sustained improvement in sleep quality, sleep parameters, fatigue, and depressive symptoms than TCC and SS (all P values < 0.01). As compared to SS, CBT was associated with a reduced risk of high CRP levels (> 3.0 mg/L) at 16 months (odds ratio [OR], 0.26 [95% CI, 0.07–0.97] P < 0.05). Remission of insomnia was associated with lower levels of CRP (P < 0.05) at 16 months. TCC was associated with improvements in sleep quality, fatigue, and depressive symptoms as compared to SS (all P's < 0.05), but not insomnia remission. PSG measures did not change.
Conclusions:
Treatment of late-life insomnia is better achieved and sustained by cognitive behavioral therapies. Insomnia treatment and remission reduces a marker of inflammatory risk, which has implications for cardiovascular morbidity and diabetes observed with sleep disturbance in epidemiologic surveys.
Clinical Trial Registration:
ClinicalTrials.gov, NCT00280020
Citation:
Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Breen EC, Witarama T, Yokomizo M, Lavretsky H, Carroll JE, Motivala SJ, Bootzin R, Nicassio P. Cognitive behavioral therapy vs. Tai Chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial. SLEEP 2014;37(9):1543-1552.
Keywords: insomnia, inflammation, late life, behavioral treatment, randomized controlled trial
INTRODUCTION
Insomnia is diagnosed by difficulty in initiating sleep or frequent awakenings and inability to return to sleep, which is associated with distress and daytime impairments due to fatigue and mood symptoms, for example.1 In adults older than 55 years, the prevalence of insomnia disorder exceeds 15%, which is nearly twice that found in adults who are 30 to 50 years old.2 In addition to functional impairments, sleep disturbance increases the risk for chronic disease and mortality in older adults,3,4 possibly related to the association between sleep disturbance and increases in inflammation, including markers such as high sensitivity C-reactive protein (CRP). Indeed, insomnia complaints including difficulties initiating and maintaining sleep, as well as nonrestorative sleep, have been associated with increases in CRP and other markers of inflammation in epidemiologic,5–7 naturalistic observational,8–11 and clinical studies of patients with insomnia disorder.12–14 Epide miologic studies indicate that CRP levels in excess of 3 mg/L predict cardiovascular events,15,16 hypertension,17 weight gain in older adults,18 and type 2 diabetes.19,20
In adults, cognitive behavioral therapy (CBT), a multi-component behavioral intervention that provides sleep education, stimulus control (strengthening associations between bed and sleep), and therapy for anxiety-provoking beliefs about sleep, is an effective treatment for insomnia, with an efficacy that is better sustained than pharmacotherapy.21,22 However, there are only two trials in the elderly with long-term follow-up,23,24 and neither assessed remission of diagnostic insomnia nor daytime impairments such as fatigue. Moreover, it is not known whether such insomnia treatment is associated with decreases in inflammation, even though high levels of CRP are implicated in chronic disease risk in older adults with sleep disturbance.19
In contrast to CBT, which requires a trained clinician, Tai Chi Chih (TCC) is widely available, accessible to patients, and deliverable in a community setting. Controlled trial data have found that this movement meditation can improve sleep quality,25–27 reduce fatigue, depressive symptoms, and markers of inflammation in older adults28–30; yet the efficacy of TCC in the treatment of clinical insomnia is not known. It is thought that TCC targets arousal mechanisms that contribute to insomnia.31–33 The National Center for Complementary and Alternative Medicine (NCCAM) currently designates mind-body therapies as a top research priority.34
The goal of this randomized controlled trial in older adults was to evaluate the comparative benefit of CBT vs. TCC, relative to a sleep, hygiene education control (i.e., Sleep Seminar, SS) on the primary outcome of remission of insomnia diagnosis. Secondary sleep outcomes of sleep diary, patient-reported sleep outcomes, and polysomnographic (PSG) measures were also obtained. Additional secondary outcomes included patient-reported fatigue and sleepiness and clinician-rated depressive symptoms. Inflammation was measured by proportion of those with high CRP (> 3 mg/L), given the clinical significance of this threshold. We hypothesized that CBT would be superior to TCC in the remission of insomnia and related symptoms, with effects on CRP in the long-term (i.e., one year after intervention administration). Secondly, we hypothesized that both CBT and TCC would perform better than SS on these outcomes.
METHODS
Trial Design
After recruitment, telephone screening, interview assessment, laboratory blood tests, and PSG evaluation for sleep apnea, eligible participants were randomly assigned to CBT, TCC, or SS. Each participated in 120 minutes of group class time weekly for 4 months, with assessments at baseline (pre-intervention), 2 and/or 3 months (mid-intervention), 4 months (post-intervention), and 7- and 16 months (follow-up). Because TCC required 4 months for participants to learn, CBT was longer than prior trials,23,24 so that the duration of CBT and SS was comparable to TCC.21 There were no changes to the methods after trial commencement.
Study Participants
This randomized controlled trial was conducted from April 2006 to August 2011 following UCLA institutional review board approval. Study participants, recruited by means of advertisements, were community-dwelling adults older than 55 years of age who fulfilled criteria for primary insomnia in Diagnostic and Statistical Manual, Fourth Edition, Text Revision (DSM-IV-TR)35 and for general insomnia in the International Classification of Sleep Disorders, Second Edition.36 These criteria specify difficulty in initiating or maintaining sleep or non-restorative sleep for at least one month, along with significant distress and daytime impairment.37 DSM-V revised the duration criteria from 1 to 3 months1; we note that all participants also reported the presence of sleep difficulties ≥ 3 times per week for > 3 months.
DSM-IV-TR exclusion criteria of medical and psychiatric disorders were applied. Additional exclusion criteria were: (1) presence of another sleep disorder such as sleep apnea (apneahypopnea index > 15), restless legs, or periodic limb movements (movement index with arousal > 15/h) as determined by one night of PSG; (2) shift work or irregular sleep pattern; (3) regular (≥ 2 times/week) use of hypnotic medications or alcohol for sleep (patients using prescribed or over-the-counter sleep medications < 3 times/week were enrolled after they withdrew from medications); (4) current diagnosis of major depression, unless treated and in remission; (5) cognitive impairment with score < 23 on Mini-Mental Status Examination38; (6) abnormal screening laboratory tests (i.e., complete blood count, liver function tests, thyroid function); (7) tobacco smoking; (8) body mass index > 35 kg/m2; (9) debilitating condition that would impede full participation in the study; or (10) unavailability during the study period.
Interventions
CBT as previously described by Morin et al.21 was modified to teach behavioral strategies for management of daytime activity levels and enhancement of mood, because insomnia is often accompanied by a variety of daytime complaints including mood disturbance. Whereas incorporation of a mood module expanded, theoretically and pragmatically, the scope of traditional CBT for insomnia,21 this module was very compatible with the behavioral approach to insomnia treatment. The mood module was designed to promote understanding of the reciprocal relationship between sleep and mood, and how to implement strategies to improve mood either as a consequence of poor sleep or as a determinant of sleep disturbance. Addressing mood throughout the protocol in an integrated manner was believed to augment the efficacy of the intervention and also contribute to the maintenance and generalization of the intervention during follow-up. Additionally, we believed that comparability of CBT and TCC would be further optimized, in comparison with SS, because TCC has been found to improve depressive symptoms28,30 in addition to its effects on sleep quality.25 TCC emphasized control over physical function and arousal-related responsiveness, which is thought to contribute to insomnia,39 through the performance of repetitious, nonstrenuous, slow-paced movement. Sleep seminar (SS) provided educational information related to the physical, medical, and psychosocial factors of aging and their contribution to sleep problems in aging. SS also provided education on sleep hygiene practices. Although sleep hygiene education has been found to produce modest improvements in sleep, this active control has been found to be inferior to other behavioral therapies such as CBT.21 Each intervention was taught by one therapist (CBT: Motivala; TCC: Hollister; SS: Levin) who had at least one year experience in delivery of the treatment modality but no prior experience in sleep medicine, and supervised by another therapist (CBT: Nicassio; TCC: Taggert; SS: Irwin) who had extensive (> 10 years) experience in the treatment modality to maintain therapist fidelity in delivery of the treatments as manualized. The supervising therapist evaluated treatment integrity and attended ≥ 3 sessions with rating of treatment elements; sessions on average contained > 95% of the required elements. Supplement provides further details.
Treatment acceptance, credibility and expectation for change for each of the treatments was rated by participants after the second treatment session using a 9-item scale adapted from the original developed by Borkovic and Nau; items were scored on a 5-point Likert scale, and a score ≥ 3 on each of the 9 items defined treatment acceptability.40
Primary Outcome
The primary outcome was remission of insomnia diagnosis by DSM-IV-TR criteria using a structured interview and checklist, performed by the study psychiatrist (MRI) who was blind to group assignment. Similar to the diagnostic methods to determine subject inclusion, quantitative sleep parameters were not used.
Secondary Outcomes
Secondary outcomes included improvements in patient-reported outcomes of insomnia symptom severity and sleep quality (i.e., Pittsburgh Sleep Quality Index, PSQI; Athens Insomnia Scale, AIS) and daily diaries of sleep parameters for 2 weeks (i.e., Pittsburgh Sleep Diary). PSG was obtained as described for 2 nights after adaptation,41,42 although prior trials have found little effects of CBT on PSG outcomes.23,43
Additional behavioral outcomes included insomnia-related daytime symptoms of fatigue (Multidimensional Fatigue Symptom Inventory [MDFSI]),44 sleepiness (Epworth Sleepiness Scale evaluated daytime sleepiness),45 and clinician-rated depressive symptoms (i.e., Inventory of Depressive Symptom-atology, IDS-C).46
Given the associations between insomnia and inflammation including levels of CRP,5–14 as well as the clinical significance of high CRP in predicting cardiovascular and diabetes outcomes,47 this study focused on assessment of CRP concentration, with categorization of high CRP (> 3.0 mg/L) after the intervention (4 months) and in the long-term (16 months) using methods previously published.48 Blood sampling for CRP, limited to these 2 time points to minimize subject burden, occurred between 08:00 and 10:00. CRP levels are stable over time and show little diurnal variation.49 If a subject reported recent (i.e., last month) infection, illness, or vaccination, the blood sampling was rescheduled. Because body mass index (BMI) and physical activity are related to CRP levels, body weight and height were obtained and physical activity was evaluated using the Yale Physical Activity Survey, as validated for use in older adults, with estimates of metabolic equivalents per week.50
Sample Size
Based on prior meta-analytic findings in older adults and mean treatment effect (0.76),51 28 per treatment group provided the study with a statistical power of 80% (α = 0.05) to detect significant differential in insomnia diagnosis post-treatment (i.e., a sample size of 25 per group would be sufficient to compare the experimental treatments [CBT, TCC] to the control condition [SS]). Given interest in comparing CBT and TCC in the absence of information about the expected effect size, a 2:2:1 randomization schedule was used in which sufficient power (> 80%) was maintained for the primary hypothesis, and increased power for comparisons between CBT and TCC.
Randomization
The randomization sequence was generated via computerized random number generator prior the start of the trial in blocks of 3 conditions (CBT: TCC: SS; 2:2:1). Blocks of 7-10 participants were used to ensure that allocated treatment groups were filled within 1 to 2 months of participant assessment. Once groups of 7-10 participants were accrued to be assigned either to CBT, TCC, or SS, the study coordinator requested the next group randomization. To maintain allocation concealment, none of the research staff who assessed subjects or enrolled participants had access to the randomization list; staff were specifically told that simple randomization was being used such that any of the 3 treatments was possible for each group assignment. In addition, the individual (RO), who managed the randomization list, never interacted with any participants nor viewed any data from the participants prior to their assignment to condition.
Blinding
Outcome assessors were unaware of group assignments.
Statistical Methods
Intervention effects on DSM-IV-TR insomnia diagnosis were tested by χ2 analysis. Intervention effects on the secondary continuous outcome measures were tested on an intention-to-treat basis using a mixed model approach; data from all randomized participants were included with no imputation of missing data. The mixed model approach utilizes all available data and generates unbiased estimates under the assumption that data are missing at random (MAR); or more restrictively, missing completely at random (MCAR). Because it would require information that is, by definition, not available, there are no well-established tests of the MAR assumption; hence, the MCAR assumption was tested.
A priori linear contrasts tested group differences from baseline to 16-month follow-up across all available time points, controlling for multiple comparisons, in which time was indexed as months of follow-up. To reduce the potential for type I error, only primary and key secondary outcomes were tested. For pairwise comparisons, the least significant difference (LSD) method was utilized. For CRP, additional analyses examined intervention effects on the proportion of those with high CRP, including evaluation of the odds ratio of CBT and TCC vs. SS. Secondary analyses evaluated the effects of insomnia remission at 4 months on CRP levels. Data were available on > 95% of the subjects at all time points among those who completed follow-up assessments. Analyses were carried out with IBM SPSS for Windows, version 19.
Role of the Funding Source
The National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
RESULTS
Baseline Characteristics of the Patients
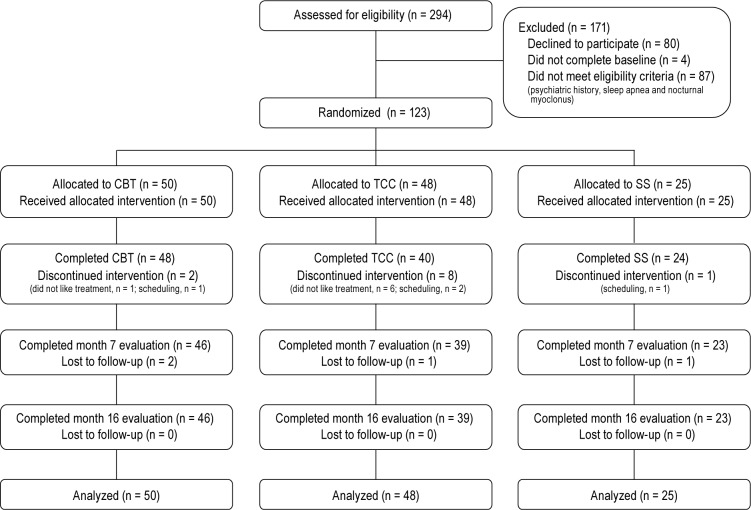
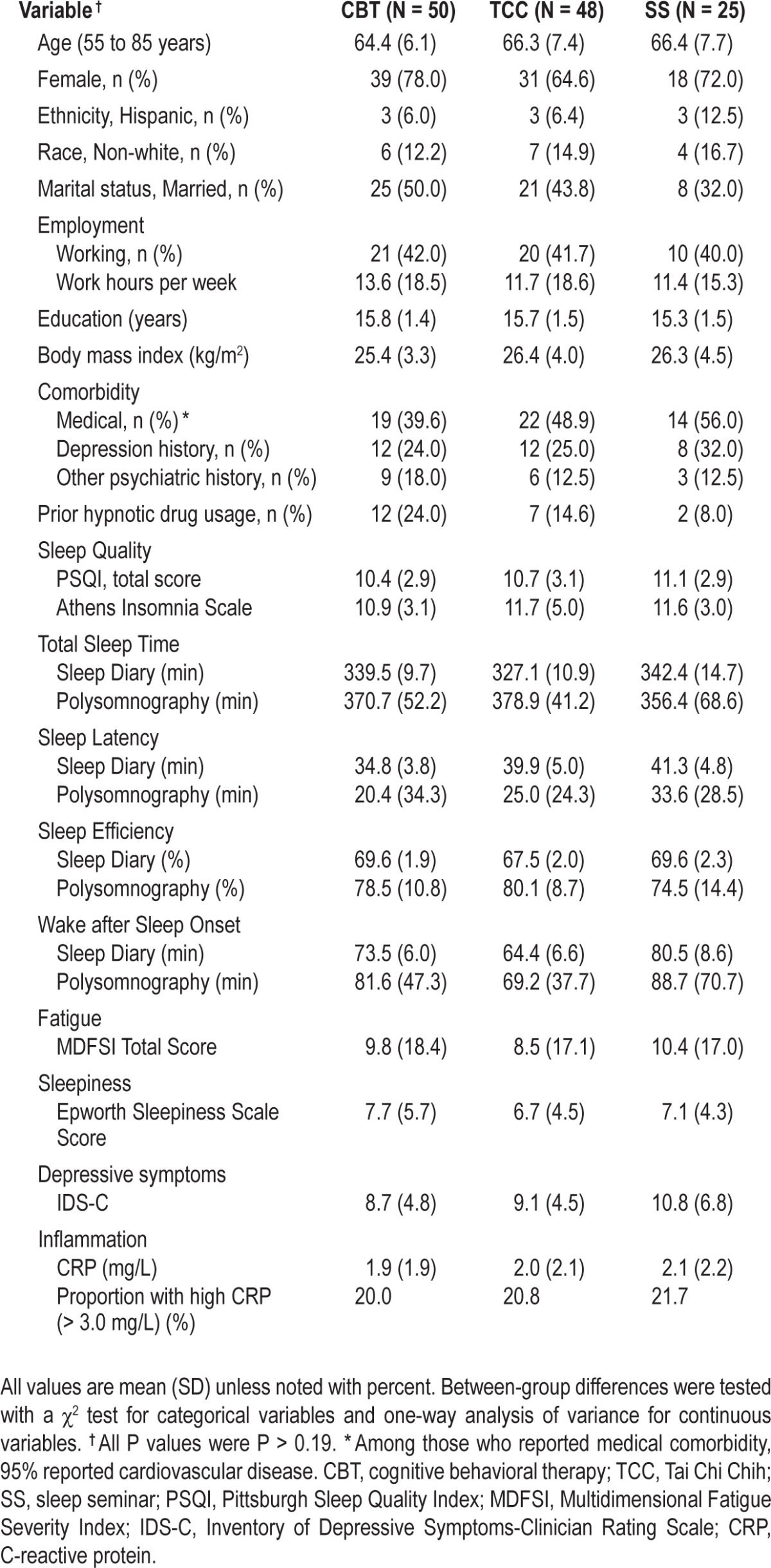
Of a total of 294 who underwent baseline assessment, 87 were identified as not eligible. Of the 207 who were eligible, 123 agreed to participate and also completed the entire baseline assessment including a night of PSG (59%; Figure 1). Treatment groups were comparable with regard to background characteristics (Table 1).
Figure 1.
Screening, randomization, and completion of post-intervention, 7-month, and 16-month evaluations. CBT, cognitive behavioral therapy; TCC, Tai Chi Chih; SS, sleep seminar.
Table 1.
Baseline characteristics of participants, by treatment group.
A total of 112 (92%) participants completed their assigned interventions (4 months), and 108 (89%) completed follow-up (16 months). Those who did not complete the intervention were likely to be younger (t121 = 2.12; P < 0.05) and have higher scores on the PSQI (t121 = 1.72; P = 0.09) and the MDFSI (t121 = 1.78; P = 0.05), whereas those who did not complete the follow-up were more likely to have higher scores on the IDS-C (t110 = 2.82; P = 0.05) and the MDFSI (t110 = 3.23; P < 0.001). Other demographic and outcome variables did not differ between the completers and non-completers at months 4 or 16. At month 4, the drop-out rate tended to be higher in the TCC group as compared to CBT and SS (χ2(2) = 5.77; P = 0.06), but at month 16 the retention rate was similar (χ2(2) = 3.16; P = 0.21). Tests of the continuous data suggested missing values fit the MCAR assumption (χ2(486) = 523.1; P = 0.12) though EM estimates and the results regarding non-completers above suggest a slight bias toward those not completing the trial to have poorer outcomes.
The average rate of session attendance was similar (i.e., 79% in CBT; 81% in TCC and 74% in SS; F2,120 = 0.99; P = 0.38). Additionally, nearly all participants perceived the 3 interventions as acceptable treatments as defined above to improve their insomnia symptoms (98% in CBT; 94% in TCC; 95% in SS; χ2(2) = 0.58; P = 0.74). However, at the conclusion of treatment, SS and TCC participants reported significantly less confidence that their treatment would be effective for others, as compared to those in CBT (F2,91 = 10.3; P < 0.001). Nevertheless, post-treatment perception that treatments were acceptable remained high and not different (100% in CBT, 91% in TCC; 96% in SS; χ2(2) = 3.58; P = 0.17). Among the TCC participants, 90% continued to practice during the follow-up period from months 4 to 16, although average frequency of practice for > 30 min decreased from 3.3 (SD, 2.2) days to 2.3 (SD, 2.0) days (t33 = 3.16; P = 0.004).
There were no significant between-group changes from baseline to post-treatment in occasional sedative-hypnotic medication use (F2,106.4 = 0.36; P = 0.70), body mass index (F2,90.9 = 1.71; P = 0.19), or physical activity (i.e., metabolic equivalents per week as estimated by the Yale Physical Activity Survey50; F2,104.8 = 1.79; P = 0.17). Given that TCC involved a physical activity component not found in CBT or SS, the absence of within group change in physical activity in TCC suggests that participants substituted TCC for other aerobic activity.
Primary Outcome
Diagnostic assessment of insomnia using DSM-IVTR criteria at 4 months showed that CBT resulted in a nearly two-fold greater rate of remission than TCC and SS (χ2 = 9.34, P < 0.01; Figure 2). For CBT vs. SS, the number needed to treat was 3 (95% CI, 1.8–8.5), the absolute risk reduction was 33.3% (95% CI, 11.8%–4.8%), and the relative risk reduction was 72.7% (95% CI, 19.3%–100%). This represents a medium to large effect size (estimated d = 0.65). Results comparing CBT vs. SS were not substantively different if those lost at post-treatment were considered remitters (χ2 = 6.89; P < 0.01) or non-remitters (χ2 = 7.04; P < 0.01). More individuals failed to complete the post-treatment assessment in the TCC group; hence, as compared to effect sizes between CBT and SS, effect size differences between TCC and CBT or SS were much more variable under different assumptions of missing data at follow-up. Assuming those lost at post-treatment were all non-remitters, the effect size of CBT vs TCC was estimated d = 0.71 and TCC vs. SS was estimated d = 0.10. Assuming those lost at post-treatment were all remitters, the effect size of CBT vs. TCC was estimated d = 0.23 and TCC vs SS was estimated d = 0.54. The former option seems more tenable.
Figure 2.
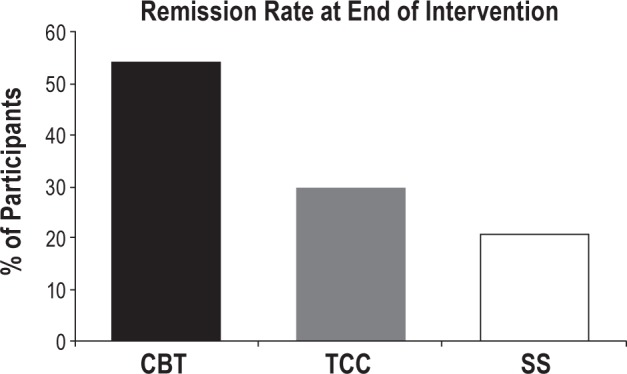
Percentage of participants with remission of DSM-IV-TR insomnia at 4 months (post-intervention) in the CBT, TCC, and SS treatment groups. Insomnia diagnosis was made by clinician interview using DSM-IV-TR criteria. At post-intervention, the rate of remission was 54.2% in CBT vs. 29.7% in TCC (χ2 = 7.25, P < 0.05) and 20.8% in SS (χ2 = 5.1, P < 0.01); the rate of remission was similar in the TCC and SS groups. Conversely, the percentage of participants who continued to have DSM-IV-TR insomnia was lower in the CBT group (45.8%) than in the TCC (70.3%) and SS groups (79.2%). The percentage of participants with insomnia was similar in the TCC and SS groups (χ2 = 0.60, P = 0.44). CBT, cognitive behavioral therapy; TCC, Tai Chi Chih; SS, sleep seminar.
Secondary Outcomes of Sleep
Insomnia symptom severity as indexed by the Pittsburgh Sleep Quality Index (PSQI), showed an overall treatment effect (F2,118.8 = 9.73; P < 0.001), in which CBT resulted in greater improvement in global sleep quality than TCC (t531.3 = 2.64; P < 0.01; estimated d = 0.27) and SS (t522.7 = 2.70; P < 0.01; estimated d = 0.44) from baseline to 16 months (Table S1, Figure 3). Specific time contrasts also showed that TCC resulted in improvements of global sleep quality as compared to SS at months 4 and 7 (P values < 0.05), but not at month 16 (Figure 3). Results using the Athens Insomnia Scale were similar (Table S1). Among the TCC participants, there was no relationship between practice time and improvement in sleep quality at month 4, and no relationship between decrease in practice time from months 4 to 16 and sleep quality at month 16.
Figure 3.
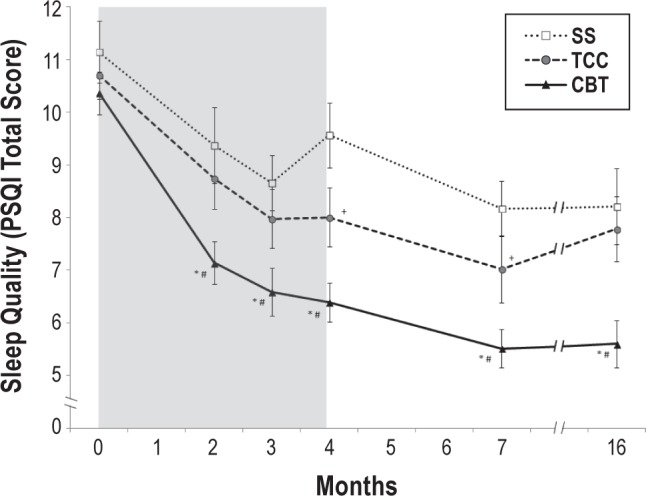
Change in global sleep quality from baseline to month 16 follow-up in the CBT, TCC, and SS treatment groups. Total scores on the Pittsburgh Sleep Quality Index (PSQI) range from 0 to 21, with higher scores indicating worse sleep quality. Values are means; bars indicate standard error of measurement. Measurements were obtained at baseline and months 2 and 3 (mid-intervention), 4 (post-intervention), and 7 and 16 (follow-up). Shaded area indicates period of administration of intervention following baseline assessment. * Significant linear trend contrasts between CBT vs SS (t522.7 = 2.70; P < 0.01). # Significant linear trend contrasts between CBT vs TCC (t531.3 = 2.64; P < 0.05). + Significant pairwise comparisons between TCC vs SS. CBT, cognitive behavioral therapy; TCC, Tai Chi Chih; SS, sleep seminar.
Sleep parameters (total sleep time, TST; sleep latency, SL; sleep efficiency SE; wakefulness after sleep onset, WASO) were indexed using 2 methodologies: sleep diaries and PSG. Sleep diaries showed that CBT resulted in greater improvements in SL, SE, and WASO, but not TST, from baseline to month 16 as compared to TCC and SS (P values < 0.05; Table S1). No differences were found between TCC and SS for any of the sleep diary measures of sleep parameters (all P values > 0.10). PSG measures of TST, SL, SE, and WASO from baseline to month 4 did not differ for the groups (all P values > 0.10; Table S1).
Secondary Outcomes of Fatigue and Depression
For fatigue severity as indexed by the Multidimensional Fatigue Symptom Inventory (MFSI), linear trend contrasts found that the slope of decrease of MFSI scores was greater in the CBT group as compared to TCC and SS (all P values < 0.05; estimated d's: 0.32 and 0.36, respectively; Table S2). Specific contrasts also showed that TCC resulted in lower levels of fatigue than SS at months 2, 4, and 7 (all P values < 0.05). No treatment effects were found for daytime sleepiness. For clinician rated depressive symptom severity as indexed by IDS-C, CBT also resulted in greater decreases in depressive symptom severity than TCC and SS (all P values < 0.05, estimated d's: 0.20 and 0.32, respectively; Figure 4). In addition, specific contrasts showed that TCC resulted in lower levels of depressive symptoms than SS at months 2, 3, and 4 (P's < 0.05; Table S2; Figure 4). Additional analyses with the IDS-C, in which items related to sleep disturbance were excluded, yielded similar results.
Figure 4.
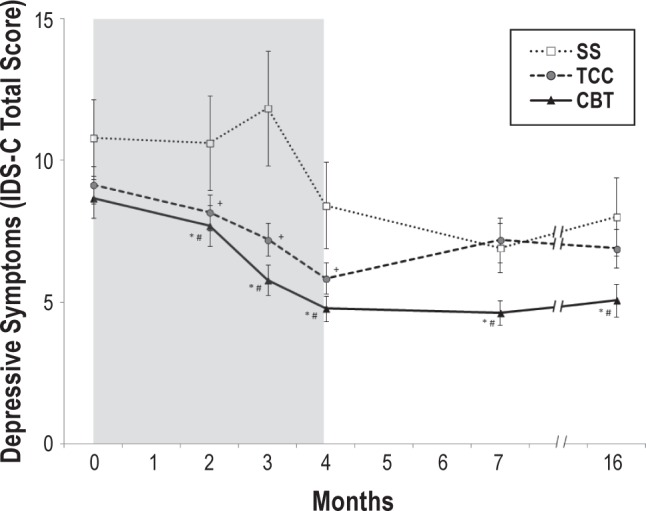
Change in depressive symptoms severity from baseline to month 16 follow-up in the CBT, TCC, and SS treatment groups. Total scores on the Inventory of Depressive Symptomatology-Clinician Rating Scale (IDS-C) range from 0 to 84, with higher scores indicating more severe depressive symptoms. Values are means; bars indicate standard error of measurement. Measurements were obtained at baseline and months 2 and 3 (mid-intervention), 4 (post-intervention), and 7 and 16 (follow-up). Shaded area indicates period of administration of intervention following baseline assessment. * Significant linear trend contrasts between CBT vs SS (t521.1 = 1.98.; P < 0.05). # Significant linear trend contrasts between CBT vs TCC (t523.0 = 1.98; P < 0.05). + Significant pairwise comparisons between TCC vs SS. Similar results were found when the sleep disturbance items of the IDS-C were removed. CBT, cognitive behavioral therapy; TCC, Tai Chi Chih; SS, sleep seminar.
Secondary Outcomes of Inflammation
Insomnia treatment was hypothesized to reduce the risk of high CRP (> 3.0 mg/L), in which insomnia remission would be associated with a reduction of CRP. The percentage of participants with high CRP differed between the groups from baseline to month 16, in which high CRP was less likely to be found in the CBT participants as compared to TCC and SS (χ2(1) = 4.0, P = 0.04; Figure 5). As compared to SS, CBT was associated with a reduced risk of having high CRP at 4 months (odds ratio [OR], 0.37 [95% CI, 0.11–1.18]; P = 0.08) and at 16 months (OR, 0.26 [CI, 0.07–0.97]; P < 0.05) (Figure 5, Table S3). As compared to SS, there was a non-significant effect of TCC on lower risk of high CRP at 4 months (OR, 0.39 [CI, 0.11–1.3] P = 0.10), but no difference was found at 16 months (OR, 0.8; [CI, 0.25–2.5] P = 0.47). As compared to SS, the change in proportion CBT participants with high CRP from baseline to month 16 was significantly greater (χ2(1) = 4.0, P = 0.04), with a nearly 50% decrease in the CBT group. Change in proportion of TCC participants with high CRP from baseline to month 16 was similar to SS (P > 0.70). Although CBT and SS showed a divergence in the proportion of high CRP, change in proportion of high CRP from baseline to month 16 in the SS group was not significant (P > 0.30).
Figure 5.
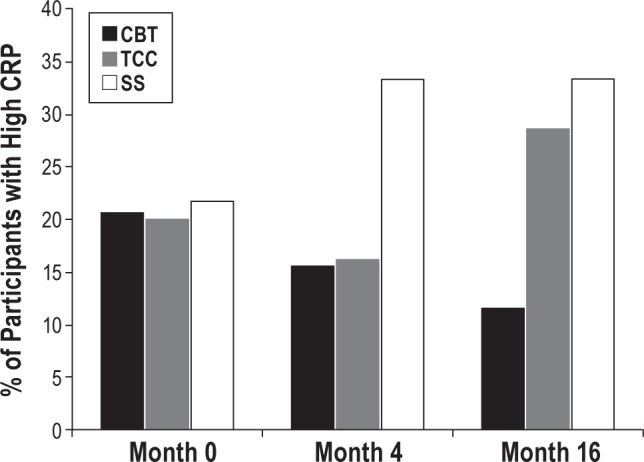
Percentage of participants with high C-reactive protein (CRP) at baseline, 4 months, and 16 months in the CBT, TCC, and SS treatment groups. High CRP was defined by levels in excess of 3.0 mg/L. High CRP was less likely to be found in the CBT participants as compared to SS (χ2(1) = 4.0, P = 0.04). As compared to SS, CBT was associated with a reduced risk of having high CRP at 4 months (odds ratio [OR], 0.37 [95% CI, 0.11–1.18]; P = 0.08) and at 16 months (OR, 0.26 [CI, 0.07–0.97]; P < 0.05). As compared to SS, TCC was not associated with reduced risk of high CRP at 4 months (OR, 0.39 [CI, 0.11–1.3] P = 0.10), or at 16 months (OR, 0.8; [CI, 0.25–2.5] P = 0.47). In SS, change in percentage with high CRP from baseline to month 16 was not significant (P > 0.30). CBT, cognitive behavioral therapy; TCC, Tai Chi Chih; SS, sleep seminar.
To further evaluate the association between insomnia treatment and reduction of CRP levels, the sample was stratified into those who showed a remission of insomnia disorder and those who continued to fulfill criteria for insomnia. As compared to non-remitters, those with remission of insomnia had similar levels of CRP at 4 months (t = 0.18, P = 0.86), but significantly lower levels of CRP at 16 months (t = 1.7, P < 0.05; Figure 6).
Figure 6.
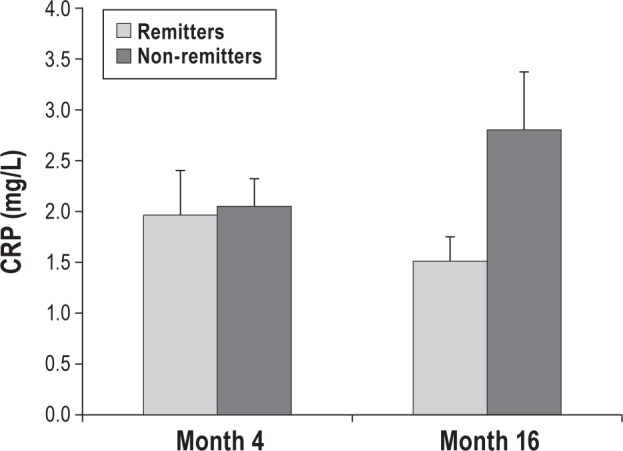
Levels of C-reactive protein (CRP) in participants with and without remission of DSM-IV-TR insomnia. As compared to non-remitters, those with remission of insomnia had similar levels of CRP at 4 months (t = 0.18, P = 0.86), but significantly lower levels of CRP at 16 months (t = 1.7, P < 0.05).
DISCUSSION
This is the first randomized, controlled trial that has evaluated the comparative efficacy of TCC vs. CBT, a standardized behavioral intervention for insomnia. We found that CBT is more effective in the treatment of insomnia in older adults than TCC or the control intervention (SS). CBT produced a significant rate of remission of diagnostic insomnia that was nearly 2-fold greater than either TCC or SS. The additional benefits of CBT included improvements in sleep parameters, sleep quality, and symptoms of daytime fatigue and depression but not daytime sleepiness, which were sustained one year after treatment. Contrary to our hypothesis, and despite the fact that we had the power to detect a clinically significant effect, TCC did not yield improvements in rates of insomnia remission and sleep parameters as compared to SS. However, administration of TCC improved sleep quality, fatigue, and depression as compared to SS, although these gains were less robust than CBT and were not maintained for the duration of the follow-up.
Along with improvements in sleep outcomes, this study provides novel insight that insomnia treatment is related to decreases in a clinically relevant maker of inflammation, CRP. Given that high levels of CRP are prospectively associated with incident cardiovascular disease15,16 and diabetes,52 and that levels of CRP in excess of 3.0 mg/L indicate substantially elevated cardiovascular risk,47 it is noteworthy that CBT treatment was associated with a significant decrease in the risk of high CRP. The proportion of participants with high CRP at baseline decreased by nearly 50% one year after treatment with an odds ratio (0.26) relative to SS, which is comparable to the benefit reported with vigorous physical activity53 or weight loss.54 Moreover, among those who showed remission of insomnia disorder, average levels of CRP were nearly 50% lower one year after treatment than those who continued to fulfill criteria for insomnia disorder. The maintenance of sleep improvements appears to play a critical role in the reduction of CRP; for example in TCC, the decreases of CRP that emerged at post-intervention were no longer present one year after treatment when improvements in sleep outcomes were lost. Decreases in CRP cannot be attributed to changes in body weight or physical activity because neither of these factors changed in any of the groups. Finally, when insomnia is untreated and sleep disturbance persists (i.e., SS participants), it appears that CRP progressively increases similar to the effects of psychological stress on the inflammatory marker interleukin-6 in older adults.55 Together, these findings indicate that it is even more critical to treat insomnia in this population who are already at elevated risk for aging related inflammatory disease.
The mechanism of action that leads to a reduction in the risk of high CRP following remission of insomnia is not known. However, patients with insomnia disorder show sympathetic hyperactivation, as evidenced by an absent nocturnal autonomic drop,56 resulting in higher levels of sympathetic catecholamines.57 In turn, β-adrenergic signaling can activate nuclear factor (NF)-κB, upregulate transcription of proinflammatory cytokine genes, and lead to increased production of interleukin-6, which drives increases in CRP.19 Hence, treatment of insomnia may reduce sympathetic nervous system activity and contribute to the relative reduction in CRP. In turn, inflammatory cytokines can signal the central nervous system and alter symptoms of fatigue58–60 and depression,19,61,62 which suggests that reduction in proinflammatory activity may have salutary effects on these insomnia related symptoms. Finally, arousal mechanisms fail to decline from waking to sleep states in insomnia patients, which is thought to contribute to daytime fatigue.31 Because nocturnal sympathetic activity is lowest during slow wave sleep and highest during REM sleep,63 further research is needed to evaluate whether insomnia treatment affects nocturnal arousal mechanisms and/or the sleep architecture, with attendant effects on sympathetic activation, inflammatory biology, and fatigue.
This is one of largest controlled trials comparing behavioral therapies for insomnia in older adults. We found six published randomized controlled trials of CBT for insomnia in older adults,23,24,43,64–66 and only two studies evaluated sleep using subjective and objective outcomes with long-term follow-up; one of these used a wait-list control and objectively assessed sleep with actigraphy rather than PSG.23,24 The present study replicates large effect sizes for CBT on sleep outcomes of quality and continuity; consistent with prior trials in older adults,51 CBT did not alter sleep duration. This study is novel in systematic evaluation of insomnia remission, along with daytime impairments, which together comprise the diagnosis of insomnia.
CBT employed a mood enhancement module that is not typically included in other CBT therapies, which might have contributed to the improvement in the daytime depressive symptoms, and to the relative benefit of CBT as compared to TCC. However, TCC was also found to reduce depressive symptoms consistent with prior trials.28,30 Additionally, in contrast to other CBT trials, sleep restriction was not used due to the known effects of short sleep duration on inflammation,41,42 although long sleep has also been found to increase markers of inflammation.67 Nevertheless, the effects of CBT on chronic insomnia are nearly identical to prior results,24,43 in showing clinically significant remission of insomnia diagnosis. CBT has not been consistently found to alter PSG in older adults.23,43 Finally, duration of CBT in his trial was long and comparable to TCC, which requires more time for participants to learn and master the movements; a typical course of CBT for insomnia is considerably shorter, lasting 6 to 8 weeks.
Consistent with prior studies that have solely targeted older adults with sleep complaints, but not clinical insomnia, TCC yields modest improvements in subjective assessments of sleep quality as compared to a control condition.25 Meta-analyses show that tai chi, when regularly practiced, has a role in symptom management,68 but the present findings do not support the use of TCC as a treatment for clinically significant insomnia in older adults nor as a substitute for a behavioral intervention that specifically targets insomnia symptoms.
There are limitations that require consideration. As is the case with behavior-based treatments, participants were aware of their intervention assignment, which may have introduced biases in the results. However, to minimize the influence of preexisting beliefs and expectations, participants were informed that the study was designed to test the effects of three different treatments on sleep; expectations of benefit and post-treatment acceptability were similar in the three groups. Secondly, this study used one set of therapists to deliver TCC intervention, and another set to deliver CBT and SS treatments; several therapists with expertise in all treatment modalities would have been better balanced and it is possible that differences between therapists might have contributed to the treatment effects. Third, it is possible that the scope of the CBT that included a mood enhancement component disadvantaged TCC. However, key differences between the two groups were found principally in the maintenance of benefit in the long-term after the intervention had ended. Fourth, generalizability of these results to a more diverse community sample or a primary care population are limited by recruitment of older adults by advertisement, overrepresentation of women, and exclusion of those with comorbid medical or psychiatric illness and/or an inability to stop regular use of hypnotic medications, which yielded a low participation rate. Fifth, the assessment of daytime functioning was limited to the use of questionnaires to assess fatigue, depressive symptoms, and sleepiness. Finally, there was a lack of effect of any of the treatment on PSG data. However, in clinical practice, patients with insomnia do not receive sleep recordings and physicians base the success of any given treatment on patient reports of improved sleep.
Given its impact on insomnia related symptoms, further research is needed to evaluate whether TCC might be used as a stepped approach, for example, in the management of prodromal sleep disturbance before the onset of clinically severe, chronic insomnia in older adults. However, CBT as a sleep-focused, non-drug behavioral intervention effectively treats chronic insomnia in late life with evidence that these therapeutic gains are well maintained. Of particular importance, CBT along with remission of insomnia contributed to clinically meaningful reductions in CRP. Together our findings demonstrate that CBT for insomnia in older adults is an efficacious treatment for both achieving remission of insomnia and modifying an inflammatory marker of cardiovascular disease and diabetes risk.
DISCLOSURE STATEMENT
This was not an industry supported study. Primary Funding Source was the National Institute of Aging. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank all the study participants for their support and dedication to this research project; the physicians for providing medical clearance (Drs. Lara Kierlin, Hyong Jin Cho, Marissa Caudill); the intervention instructors (Kate Hollister, Jennifer Levin, Roberta Taggert); the sleep research assistants (Jenna Robbins, Nick Dietz), Merrill Collins for providing music during the TCC session (www.SpiralingMusic.com); and the members of the institutional review board at UCLA, and data safety monitoring board members (Drs. Annette Stanton and Julienne Bower).
Footnotes
A commentary on this article appears in this issue on page 1407.
SUPPLEMENTAL MATERIAL
Description of Interventions
The cognitive behavior therapy for sleep quality (CBT) was a multicomponent (i.e., behavioral, cognitive, educational) intervention as previously described by Morin et al.,1 with modification to teach behavioral strategies for management of daytime activity levels and enhancement of mood. CBT was administered in groups of 7-10 subjects by two co-therapists, a licensed clinical psychologist and a Ph.D. level psychologist, each with specialty training in behavioral medicine. Using a manualized approach, each session dictated objectives, patient skills, and treatment activities, in which therapists provided direct role-playing and other skill-development exercises designed to increase patients' self-efficacy in managing their insomnia. CBT included five treatment modules: (1) Biopsychosocial Model and Insomnia provided sleep education and discussion of the role of biological, psychological, social, and behavioral factors that affect sleep, such as stress, cognitive arousal, poor sleep hygiene, and mood disturbance. (2) Cognitive Restructuring and Sleep Disturbance used cognitive restructuring principles to help patients identify maladaptive sleep cognitions, neutralize their effect, and facilitate more adaptive thinking about sleep and its importance, including training in other cognitive coping strategies such as relaxing self-talk, imagery, and distraction methods (e.g., repetition of a calming phrase, thought). (3) Stimulus Control targeted sleep behavior directly by instructing patients to go to bed only when sleepy; use the bed only for sleep and sexual activity and not other behaviors that compete with sleep; leave the bedroom after being unable to fall asleep within 20 minutes; repeat this process as often as necessary either before falling asleep or after awakening from sleep; and establish and adhere to a fixed time of arising each morning. The sleep restriction component was not emphasized, as shortened sleep duration may confound measures of inflammation. For example, Vgontzas et al. found that insomnia with short sleep duration is associated with increases in inflammation.2,3 (4) Mood Enhancement assisted patients in developing behavioral goals in areas where sleep has disrupted their functioning and mood (e.g., work, social, physical activity), with the use of self-rewards (e.g., leisure, resting, relaxation), scheduling of pleasant events and mental exercises to increase awareness of positive emotional states. Whereas incorporation of this mood module expanded, theoretically and pragmatically, the scope of traditional CBT for insomnia,1 this module was very compatible with the behavioral approach to insomnia as it was designed to promote understanding of the reciprocal relationship between sleep and mood, and how to implement strategies to improve mood either as a consequence of poor sleep or as a determinant of sleep disturbance. Additionally, incorporation of this module was done in an integrated manner throughout the throughout the protocol. (5) Skill Consolidation and Adherence was devoted to the development of individual treatment plans for follow-up, including performance of skills, and relapse prevention training methods4 to help patients cope with situations that have contributed to poor sleep disturbance or that have interfered with the implementation of the insomnia management protocol.
Primary sleep outcomes in each treatment group from baseline to 16 months
Secondary outcomes of fatigue, sleepiness and depressive symptoms in each treatment group from baseline to 16 months
Tai Chi Chih (TCC) is also a multicomponent intervention that integrates physical, psychosocial, emotion, spiritual, and behavioral elements to target arousal mechanisms that are thought to contribute to insomnia.5–7 Because of its mind-body and “meditation through movement” attributes, TCC was well-suited to help older adults cope with fatigue, perceived physical limitations, and negative emotional states, which are commonly associated with insomnia. In contrast with CBT, TCC did not address cognitive activity underlying appraisals of disordered sleep, but instead emphasized control over physical function and arousal-related responsiveness through the performance of repetitious, nonstrenuous, slow-paced movement. TCC was administered in groups of 7-10 subjects master's level instructor who had undergone certification by the national TCC association. Using a manualized approach,8 each session provided objectives and learning activities related to sequentially learning a specific set of 20 exercises with verification of skills attainment weekly. The first 8 weeks emphasized mastery of single forms though multiple repetitions in class and at home; latter weeks focused on class consolidation of daily practice routines with natural breathing integrated into all sessions. Diary assessments were administered to assess frequency and duration of practice between sessions and at follow-up.
Sleep Seminar (SS) was an educational intervention that provided health information related to the physical, medical, and psychosocial factors that contribute to sleep problems in aging with an emphasis on sleep education and sleep hygiene practices. SS delivered some content (i.e., sleep hygiene principles) similar to that of CBT, but in contrast to CBT, SS simply provided educational information without discussion of how these practices might be used to change sleep-wake behaviors. Educational topics included during the 16 weeks were the following: (1) Sleep in late-life including discussion of changes in sleep with aging and misperceptions about sleep duration; (2) What is insomnia, including a review of the definition of insomnia, prevalence, discussion of potential causes of insomnia, and how the sleep diary can be used to evaluate insomnia; (3) Sleep basics including the sleep cycle and stages of sleep including the role of polysomnography in the evaluation of sleep; (4) Review of how sleep changes with age, and how these changes are different than insomnia with consideration of the association between sleep and medical comorbidities; (5) Other sleep disorders and sleep problems that are not insomnia, and how to assess whether they are present including sleep apnea, restless legs syndrome, and parasomnias; (6) Impact of insomnia on health including health functioning, accidents rates, and mood problems (session included discussion of the relationship between insomnia and inflammation and cardiovascular disease risk); (7) Traditional treatment for insomnia with review of medication, non-pharmacologic therapies, and role of sleep hygiene; (8) Sleep hygiene with focus on the use of alcohol, tobacco, certain non-prescription medications, and exercise; (9) Sleep hygiene II including review of age related sleep patterns, bedtime rituals, napping, and behaviors that are not compatible with good sleep such as TV watching in bed; (10) Sleep hygiene III with focus on sleep-wake principles such as the use of regular bedtime, and the impact of the environment such as light, temperature, and noise on sleep; (11) Managing daytime stress with review of certain stress management skills such as progressive muscle relaxation (without instruction), time management, problem solving; (12) Managing daytime stress II with presentation of the impact of the stress on certain disease risk including associations between stress and insomnia, depression and cardiovascular disease; (13) Nutrition and health aging, and relation to sleep with review of the relationships between alcohol, caffeine use, and tobacco smoking and sleep; (14) Exercise and healthy aging with focus on the relationship between aerobic fitness and sleep; and the impact of exercise on sleep patterns as related to sleep hygiene practices; (15) How to get medical help including discussion of how to talk to your physician and access wellness educational resources; (16) Review of major topics discussed with directive question and answer session.
Circulating levels of CRP and proportion with high CRP
SS was administered in groups of 7-10 subjects as didactic presentations by physicians or licensed clinical psychologists as previously described,9 followed by group discussion and self-help quizzes to assess patient learning. SS served as a control for nonspecific treatment elements such as attention, expectation for improvement, and group support that pose rival explanations for the effectiveness of CBT and TCC. As in CBT and TCC, homework was prescribed to include reading of educational materials that expanded on session information.
REFERENCES TO SUPPLEMENT
- 1.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia:update of the recent evidence (1998-2004) Sleep. 2006;29:1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 2.Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 4.Keefe FJ, Caldwell DS. Cognitive behavioral control of arthritis pain. Med Clin North Am. 1997;81:277–90. doi: 10.1016/s0025-7125(05)70515-0. [DOI] [PubMed] [Google Scholar]
- 5.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 6.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–67. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Med Rev. 1997;1:97–108. doi: 10.1016/s1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 8.Stone JF. Tai Chi Chih, Joy Through Movement: Good Karma Publishing, Incorporated. 1996.
- 9.Nicassio P, Greenberg MA. The effectiveness of cognitive-behavioral and psychoeducational interventions in the management of arthritis. I. In: Weisman MH, Weinblatt M, Louie J, editors. Treatment of Rheumatic Diseases. Orlando: William Saunders; 2001. pp. 147–61. [Google Scholar]
- 10.Smets E, Garssen B, Bonke B, De Haes JC. The multi-dimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–25. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 11.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 12.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–86. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 3.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 4.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 5.Laugsand LE, Vatten LJ, Bjorngaard JH, Hveem K, Janszky I. Insomnia and high-sensitivity C-reactive protein: the HUNT study, Norway. Psychosom Med. 2012;74:543–53. doi: 10.1097/PSY.0b013e31825904eb. [DOI] [PubMed] [Google Scholar]
- 6.Liukkonen T, Rasanen P, Ruokonen A, et al. C-reactive protein levels and sleep disturbances: observations based on the Northern Finland 1966 Birth Cohort study. Psychosom Med. 2007;69:756–61. doi: 10.1097/PSY.0b013e318157cb96. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Lamers F, Hickie IB, He JP, Feig E, Merikangas KR. Differentiating nonrestorative sleep from nocturnal insomnia symptoms: demographic, clinical, inflammatory, and functional correlates. Sleep. 2013;36:671–9. doi: 10.5665/sleep.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu YL, Chuang YF, Fang KC, et al. Higher systemic inflammation is associated with poorer sleep quality in stable haemodialysis patients. Nephrol Dial Transplant. 2009;24:247–51. doi: 10.1093/ndt/gfn439. [DOI] [PubMed] [Google Scholar]
- 9.Okun ML, Coussons-Read M, Hall M. Disturbed sleep is associated with increased C-reactive protein in young women. Brain Behav Immun. 2009;23:351–4. doi: 10.1016/j.bbi.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bornivelli C, Alivanis P, Giannikouris I, et al. Relation between insomnia mood disorders and clinical and biochemical parameters in patients undergoing chronic hemodialysis. J Nephrol. 2008;21(Suppl 13):S78–83. [PubMed] [Google Scholar]
- 11.Okun ML, Hall M, Coussons-Read ME. Sleep disturbances increase interleukin-6 production during pregnancy: implications for pregnancy complications. Reprod Sci. 2007;14:560–7. doi: 10.1177/1933719107307647. [DOI] [PubMed] [Google Scholar]
- 12.Razeghi E, Sahraian MA, Heidari R, Bagherzadeh M. Association of inflammatory biomarkers with sleep disorders in hemodialysis patients. Acta Neurol Belg. 2012;112:45–9. doi: 10.1007/s13760-012-0003-7. [DOI] [PubMed] [Google Scholar]
- 13.Vgontzas AN, Zoumakis M, Papanicolaou DA, et al. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism. 2002;51:887–92. doi: 10.1053/meta.2002.33357. [DOI] [PubMed] [Google Scholar]
- 14.Burgos I, Richter L, Klein T, et al. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun. 2006;20:246–53. doi: 10.1016/j.bbi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–7. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 17.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–51. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 18.Barzilay JI, Forsberg C, Heckbert SR, Cushman M, Newman AB. The association of markers of inflammation with weight change in older adults: the Cardiovascular Health Study. Int J Obes (Lond) 2006;30:1362–7. doi: 10.1038/sj.ijo.0803306. [DOI] [PubMed] [Google Scholar]
- 19.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–32. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunner EJ, Kivimaki M, Witte DR, et al. Inflammation, insulin resistance, and diabetes--Mendelian randomization using CRP haplotypes points upstream. PLoS Med. 2008;5:e155. doi: 10.1371/journal.pmed.0050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004) Sleep. 2006;29:1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 22.Morin CM, Vallieres A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301:2005–15. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morin C, Cholecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281:991–9. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 24.Epstein DR, Sidani S, Bootzin RR, Belyea MJ. Dismantling multicomponent behavioral treatment for insomnia in older adults: a randomized controlled trial. Sleep. 2012;35:797–805. doi: 10.5665/sleep.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin MR, Olmstead R, Motivala SJ. Improving sleep quality in older adults with moderate sleep complaints: A randomized controlled trial of Tai Chi Chih. Sleep. 2008;31:1001–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Li F, Fisher KJ, Harmer P, Irbe D, Tearse R, Weimer C. Tai Chi and self-rated quality of sleep and daytime sleepiness in older adults: a randomized controlled trial. J Am Geratr Soc. 2004;52:892–900. doi: 10.1111/j.1532-5415.2004.52255.x. [DOI] [PubMed] [Google Scholar]
- 27.Li F, Fisher KJ, Weimer C, Shirai M. The effects of Tai Chi training on self-rated sleep quality in older adults: a randomized controlled trial. Sleep. 2003;26:A423. (Abstract Suppl) [Google Scholar]
- 28.Irwin MR, Olmstead R. Mitigating cellular inflammation in older adults: a randomized controlled trial of Tai Chi Chih. Am J Geriatr Psychiatry. 2012;20:764–72. doi: 10.1097/JGP.0b013e3182330fd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irwin MR, Olmstead R, Oxman MN. Augmenting immune responses to varicella zoster virus in older adults: a randomized, controlled trial of Tai Chi. J Am Geriatr Soc. 2007;55:511–7. doi: 10.1111/j.1532-5415.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- 30.Lavretsky H, Alstein LL, Olmstead RE, et al. Complementary use of Tai Chi Chih augments escitalopram treatment of geriatric depression: a randomized controlled trial. Am J Geriatr Psychiatry. 2011;19:839–50. doi: 10.1097/JGP.0b013e31820ee9ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 32.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–67. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 33.Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Med Rev. 1997;1:97–108. doi: 10.1016/s1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 34.National Center for Complementary and Alternative Medicine. Exploring the Science of Complementary and Alternative Medicine: NCCAM Third Strategic Plan 2011-2015. 2011.
- 35.American Psychiatric Association. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision. [Google Scholar]
- 36.American Academy of Sleep Medicine. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International Classification of Sleep Disorders: Diagnostic and Coding Manual. [Google Scholar]
- 37.Mai E, Buysse DJ. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin. 2008;3:167–74. doi: 10.1016/j.jsmc.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 39.Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav Res Ther. 1985;23:263–71. doi: 10.1016/0005-7967(85)90004-x. [DOI] [PubMed] [Google Scholar]
- 40.Borkovec T, Nau SD. Credibility of analogue therapy rationales. J Behav Ther Exper Psychiatry. 1972;3:257–60. [Google Scholar]
- 41.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 42.Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171:887–95. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smets E, Garssen B, Bonke B, De Haes JC. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–25. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 45.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 46.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–86. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 47.Ridker PM, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham Risk Scores. Circulation. 2004;109:1955–9. doi: 10.1161/01.CIR.0000125690.80303.A8. [DOI] [PubMed] [Google Scholar]
- 48.Fuligni AJ, Telzer EH, Bower J, Irwin MR, Kiang L, Cole SW. Daily family assistance and inflammation among adolescents from Latin American and European backgrounds. Brain Behav Immun. 2009;23:803–9. doi: 10.1016/j.bbi.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meier-Ewert HK, Ridker PM, Rifai N, Price N, Dinges DF, Mullington JM. Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clin Chem. 2001;47:426–30. [PubMed] [Google Scholar]
- 50.Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Med Sci Sports Exerc. 2001;33:962–70. doi: 10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 51.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25:3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 52.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 53.Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiol. 2002;13:561–8. doi: 10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 54.Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 55.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. PNAS. 2003;100:9090–5. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Zambotti M, Covassin N, De Min Tona G, Sarlo M, Stegagno L. Sleep onset and cardiovascular activity in primary insomnia. J Sleep Res. 2011;20:318–25. doi: 10.1111/j.1365-2869.2010.00871.x. [DOI] [PubMed] [Google Scholar]
- 57.Irwin M, Clark C, Kennedy B, Gillin JC, Ziegler M. Nocturnal catehcolamines and immune function in insomniacs, depressed patients, and control subjects. Brain Beh Immun. 2003;17:365–72. doi: 10.1016/s0889-1591(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 58.Bower JE, Ganz PA, Irwin MR, Castellon S, Arevalo J, Cole SW. Cytokine genetic variations and fatigue among patients with breast cancer. J Clin Oncol. 2013;31:1656–61. doi: 10.1200/JCO.2012.46.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517–22. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–66. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 61.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–54. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47:881–90. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrin Metab. 1999;84:1979–85. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- 64.Morin CM, Azrin NH. Behavioral and cognitive treatments of geriatric insomnia. J Consult Clin Psychol. 1988;56:748–53. doi: 10.1037//0022-006x.56.5.748. [DOI] [PubMed] [Google Scholar]
- 65.Morin CM, Kowatch RA, Barry T, Walton E. Cognitive-behavior therapy for late-life insomnia. J Consult Clin Psychol. 1993;61:137–46. doi: 10.1037//0022-006x.61.1.137. [DOI] [PubMed] [Google Scholar]
- 66.Rybarczyk B, Lopez M, Benson R, Alsten C, Stepanski E. Efficacy of two behavioral treatment programs for comorbid geriatric insomnia. Psychol Aging. 2002;17:288–98. [PubMed] [Google Scholar]
- 67.Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang C, Collet JP, Lau J. The effect of Tai Chi on health outcomes in patients with chronic conditions: a systematic review. Arch Intern Med. 2004;164:493–501. doi: 10.1001/archinte.164.5.493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary sleep outcomes in each treatment group from baseline to 16 months
Secondary outcomes of fatigue, sleepiness and depressive symptoms in each treatment group from baseline to 16 months
Circulating levels of CRP and proportion with high CRP