Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is commonly associated with cognitive and functional deficits, some of which are resolved after continuous positive airway pressure (CPAP) treatment. The investigation of brain structural changes before and after treatment could provide deep insights into the pathogenesis and the reversibility of this disorder. We hypothesized that severe OSA patients would have altered white matter (WM) integrity and cognition and that treatment would improve both the structural damage and the cognitive impairment.
Design:
Prospective clinical study.
Setting:
The Sleep Disorders Center and the Center of Excellence in High-Field Magnetic Resonance Imaging at Vita-Salute San Raffaele University, Milan, Italy.
Participants:
Seventeen never-treated consecutive OSA patients were evaluated before and after treatment (after 3 and 12 months) and compared to 15 matched healthy controls.
Intervention:
CPAP.
Measurements:
WM integrity measured by diffusion tensor imaging (DTI) and cognitive performance (measured with neuropsychological testing) before and after 3 and 12 months of CPAP.
Results:
Results in pre-treatment OSA patients showed impairments in most cognitive areas, mood and sleepiness that were associated with diffuse reduction of WM fiber integrity reflected by diminished fractional anisotropy (FA) and mean diffusivity (MD) in multiple brain areas. After 3 months of CPAP, only limited changes of WM were found. However, over the course of 12 months CPAP treatment, an almost complete reversal of WM abnormalities in all the affected regions was observed in patients who were compliant with treatment. Significant improvements involving memory, attention, and executive-functioning paralleled WM changes after treatment.
Conclusions:
Changes of WM DTI “signatures” of brain pathology in OSA patients are appreciable over the course of 12-month treatment with CPAP in most of the regions involved. Recovery of cognitive deficits after treatment is consistent with the presence of a reversible structural neural injury in OSA in patients who were compliant with treatment.
Citation:
Castronovo V, Scifo P, Castellano A, Aloia MS, Iadanza A, Marelli S, Cappa SF, Strambi LF, Falini A. White matter integrity in obstructive sleep apnea before and after treatment. SLEEP 2014;37(9):1465-1475.
Keywords: obstructive sleep apnea, brain white matter, cognition, treatment
INTRODUCTION
OSA is a common clinical sleep disorder that affects at least 2% to 4% of middle-aged women and men.1 OSA is associated with significant medical, cognitive, and psychological sequelae.2–4 Patients with OSA often report mild cognitive deficits that may cause difficulties in work efficiency and performing tasks, such as driving. These cognitive deficits can be due to reduced attention or to defective encoding of information.5 Another possible explanation is that OSA patients may experience difficulty with processing information to form memories during sleep.6 It has been recently demonstrated that hypoxia rather than sleep fragmentation may mediate the association of OSA with mild cognitive impairment or dementia.7 Animal data showed that intermittent hypoxia may cause oxidative stress in the systemic circulation.8 Lanfranchi and Somers have suggested that OSA-related hypoxemia is associated with an increase in sympathetic vasoconstriction and a co-occurring decrease in vascular protective mechanisms which, in turn, results in changes to the structure and function of the blood vessel.9 Aspects of this model have been incorporated into theories of cognitive dysfunction in individuals with OSA, with particular emphasis on vascular involvement of the WM of the brain.10
Conventional structural neuroimaging studies in OSA patients have reported inconclusive findings,11–13 while imaging studies using voxel-based morphometry reported alterations in several gray matter regions, such as in hippocampus.14–20
Recently, Canessa et al. found that reduced gray matter volume in the left hippocampus, left posterior parietal cortex, and right superior frontal gyrus of OSA patients was associated with cognitive dysfunctions.18 Moreover, CPAP treatment resulted in increases in gray matter in the hippocampus and frontal regions that were correlated with improvements in executive function and short term memory. Furthermore, the reversibility of pathological findings after CPAP treatment in hippocampal gray matter has been confirmed by O'Donoghue et al. using MR spectroscopy.21 In the same study, frontal WM abnormalities persisted after 6 months of CPAP treatment, thus hypothesizing irreversible, or at least slowly reversible, differences in brain metabolism, particularly in the frontal lobe.
As regards the detection of subtle structural WM alterations in OSA patients, Macey et al. used DTI to examine FA, a measure of WM integrity, among 41 OSA patients. Patients showed several areas of abnormal FA when compared to controls, suggesting a bilateral and nearly global WM involvement in OSA.10 Moreover, in a recent study Kumar et al. demonstrated that global brain MD values are significantly reduced in OSA, with certain regional sites especially affected, presumably a consequence of axonal, glial, and other cell changes in those areas.22 The findings likely represent acute pathological processes in newly diagnosed OSA subjects. However, in the studies of Macey and Kumar, there was no follow-up with treatment to determine the possible reversibility of WM abnormalities.
Aims of this study were to evaluate WM integrity and cognition in OSA patients and to investigate if the structural damage could be reversed by effective treatment with CPAP. A positive answer would sustain the hypothesis of a “functional” sufferance of cellular tissue, rather than a permanent tissue loss or cellular death.
METHODS
Participants
Seventeen severe treatment-naïve male OSA patients (age range 30-55 years) and 15 male age-and education-matched healthy controls were studied. Inclusion criteria were no psychiatric and/or medical disorders and, for patients, a diagnosis of severe OSA, apnea-hypopnea index [AHI] ≥ 30) and for controls an AHI < 5. Exclusion criteria were symptoms of cognitive deterioration (Mini-Mental score < 24); sleep disorders other than OSA; hypertension (> 150/90); diabetes; use of psychoactive medications; and brain structural abnormalities.
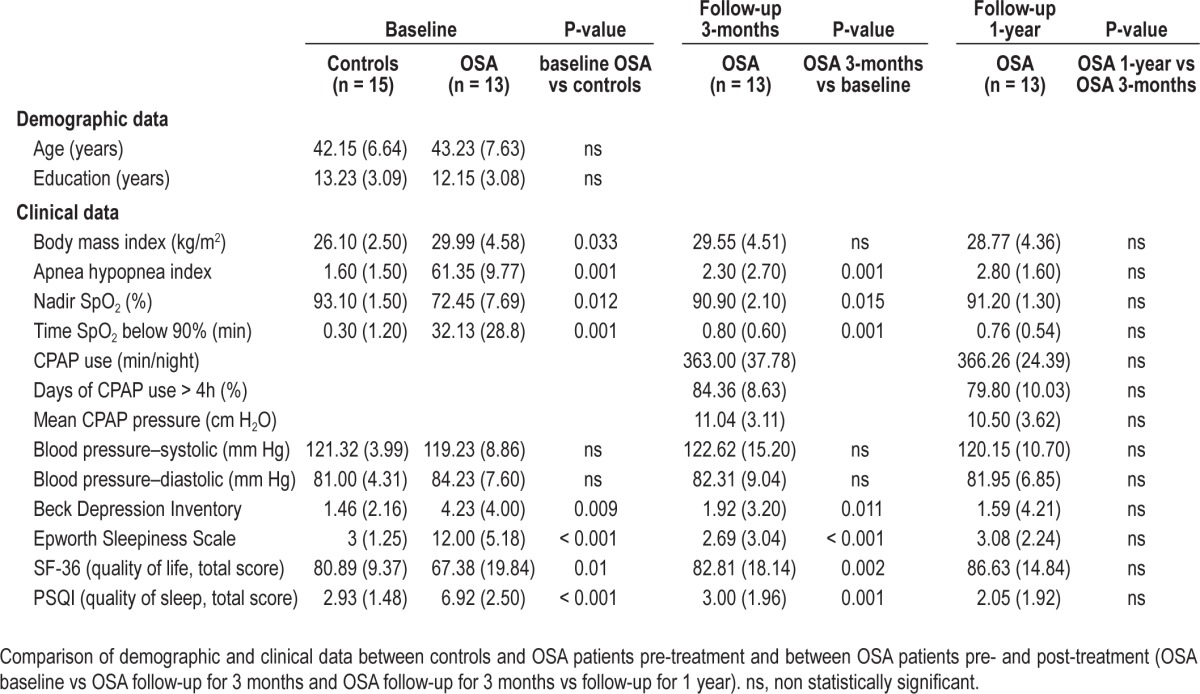
There was no significant demographic difference between patients and controls (Table 1), except for BMI that was significantly lower in controls. All participants provided written informed consent to the experimental procedure, which was approved by the Ethical Committee of Vita-Salute San Raffaele University, Milan, Italy. All patients were evaluated at baseline, after 3 months, and after 12 months of treatment with CPAP (with C-flex, M-series; Philips Respironics, Murrysville, PA). All participants including controls reported regular sleep-wake schedules based on daily sleep diaries with an average total sleep time of 6.9 ± 1.1 h in the 4 days prior the study. Full nocturnal polysomnography (PSG), DTI, neurocognitive functioning (attention, memory and executive function), sleepiness, mood, quality of sleep, and quality of life were assessed at each time point. Healthy controls were evaluated only at baseline. PSG was performed the night before MRI scanning. Standard electroencephalogram, electrooculogram, chin electromyogram, electrocardiogram, airflow, thoracic and abdominal excursions, oximetry, and tibialis electromyogram to screen for periodic leg movements were recorded. Apnea was defined as ≥ 80% drop of respiratory amplitude, lasting ≥ 10 seconds. Hypopnea was defined as a 50% drop of respiratory amplitude, lasting ≥ 10 sec, associated with repeated respiratory effort and arousals or oxygen saturation drops ≥ 3%. The apnea-hypopnea index (AHI) was defined as an index of the number of apnea and hypopnea events per hour of sleep. The lowest nocturnal oxygen saturation (SpO2) value and the time of SpO2 below 90% during total sleep were also recorded.
Table 1.
Demographic and clinical data
All participants underwent magnetic resonance imaging 2 to 3 h after waking the morning after the sleep study.
Three OSA patients dropped out because of low adherence to CPAP (one after 3 months and 2 after 12 months of treatment). In the final analysis, we did not include one patient because of low quality MRI images. The total sample on which we present global results is 13 patients.
Neurocognitive Assessment
All participants underwent a neuropsychological evaluation including Mini Mental State Evaluation (general cognitive function), Digit-span forward (verbal short-term memory) and backward (working-memory), Corsi (visuo-spatial short-term memory), Rey list (learning, recall, and recognition; verbal long-term memory), Trail making test (divided attention), Stroop test (executive functions, inhibition, selective attention), Paced Auditory Serial Addition Test (PASAT; vigilance and executive functions), and Raven progressive matrices (abstract reasoning). An alternative equivalent version of the Rey list learning test was employed at the 2 follow-ups to avoid practice effects. Administration of the neuropsychological test battery lasted approximately 30 minutes. Tests were administered in Italian and scored according to the published procedures.
Additionally, participants completed the self-report Epworth Sleepiness Scale to evaluate daytime somnolence, the Beck Depression Inventory to evaluate mood, the Pittsburgh Sleep Quality Index to evaluate quality of sleep, and SF-36 to assess overall quality of life.
Group differences were investigated using nonparametric two-sample (Mann-Whitney U test) and paired (Wilcoxon signed rank test) t-tests.
Positive Airway Pressure (PAP) Treatment
All patients were fully adherent to manual titration night of PAP. They were all sent home with fixed PAP with C-Flex (Philips Respironics, M series) and reevaluated after 3 months and after 1 year of treatment. Adherence at home was objectively reported by Encore software, and patients with compliance < 4 h/night and < 80% of days of usage were excluded (n = 3, one patient dropped out at 3-month follow-up and 2 at 1-year follow-up).
Magnetic Resonance Imaging Data Acquisition
All subjects underwent a DTI examination using a 3 Tesla Philips Intera scanner (Best, The Netherlands) and an 8-channel SENSE head coil. DTI data were collected with the following acquisition parameters: TR = 6936 msec; TE = 70 msec, b-value = 1000 s/mm2, voxel size = 1.95 × 1.95 × 2.3 mm3, diffusion gradients along 35 non-collinear directions, 2 b-values (0-1000 s/mm2), Sense reduction factor = 2. Total scan time was approximately 11 minutes.
Magnetic Resonance Imaging Data Analysis
Voxelwise Diffusion Tensor Imaging (DTI)
Only the patients whose images were acquired and of good quality were included in the group analysis. The final analysis included 13 OSA patients and 15 normal controls. The diffusion weighted images were corrected for the geometric distortions due to eddy currents, and diffusion tensor was estimated on each voxel. Diffusion tensor maps (fractional anisotropy [FA] and mean diffusivity [MD]) were calculated for each subject and each session using Brainvisa software [http://brain-visa.info].
After spatial normalization to the MNI template, the smoothed WM images (FA and MD maps were smoothed with a 8 × 8 × 8 mm kernel) were then statistically tested with SPM5 looking for differences between patients (at each time point) and controls using a 2 independent sample t-test design. Statistical threshold was set at P < 0.005, uncorrected for multiple comparisons, and the minimum cluster size was set at 5 contiguous voxels.
Correlation Analyses between Fractional Anisotropy and Neurocognitive Data
SPM5 was also used for voxel-based correlation analyses across all the participants. Specifically, single correlation analyses between FA global maps, sleepiness (Epworth Sleepiness Scale), quality of sleep (Pittsburgh Sleep Quality Index), neuropsychological tests of attention and executive functions (TEA, Stroop, PASAT, Trial Making A and B, Digit span backward), short-term memory (Digit span forward and Corsi) and long-term memory (Rey lists), all as independent variables, were performed. The dependent variable was FA. We report findings using a threshold of P < 0.005 uncorrected for multiple comparisons, and the minimum cluster size was set at 5 contiguous voxels.
RESULTS
Neurocognitive Assessment
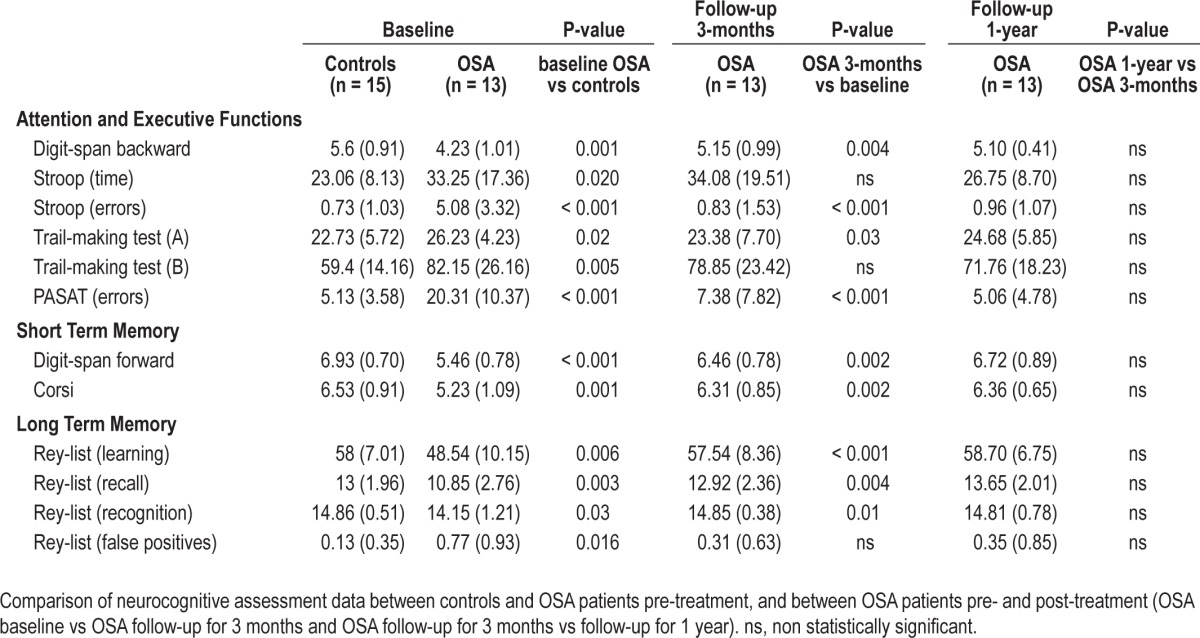
Before treatment, patients and controls were similar on demographic measures, whereas they differed significantly in body mass index (OSA higher than controls) (Table 1) and on all neurocognitive measures (OSA patients poorer than controls) (Table 2; baseline controls vs baseline OSA). After treatment patients showed a significant improvement in sleepiness and in all cognitive tests, except for total time on Stroop test and trial Making test B (executive function) and false-positive Rey list recognition (verbal long-term memory). Also mood (even if always in normal ranges) and quality of life significantly improved after treatment (Table 2; baseline OSA vs follow-up at 3 months). After 1 year, there was no further significant improvement (Table 2; follow-up 3 months vs follow-up 1 year).
Table 2.
Neurocognitive assessment data
Fractional Anisotropy Analyses (Controls vs Patients)
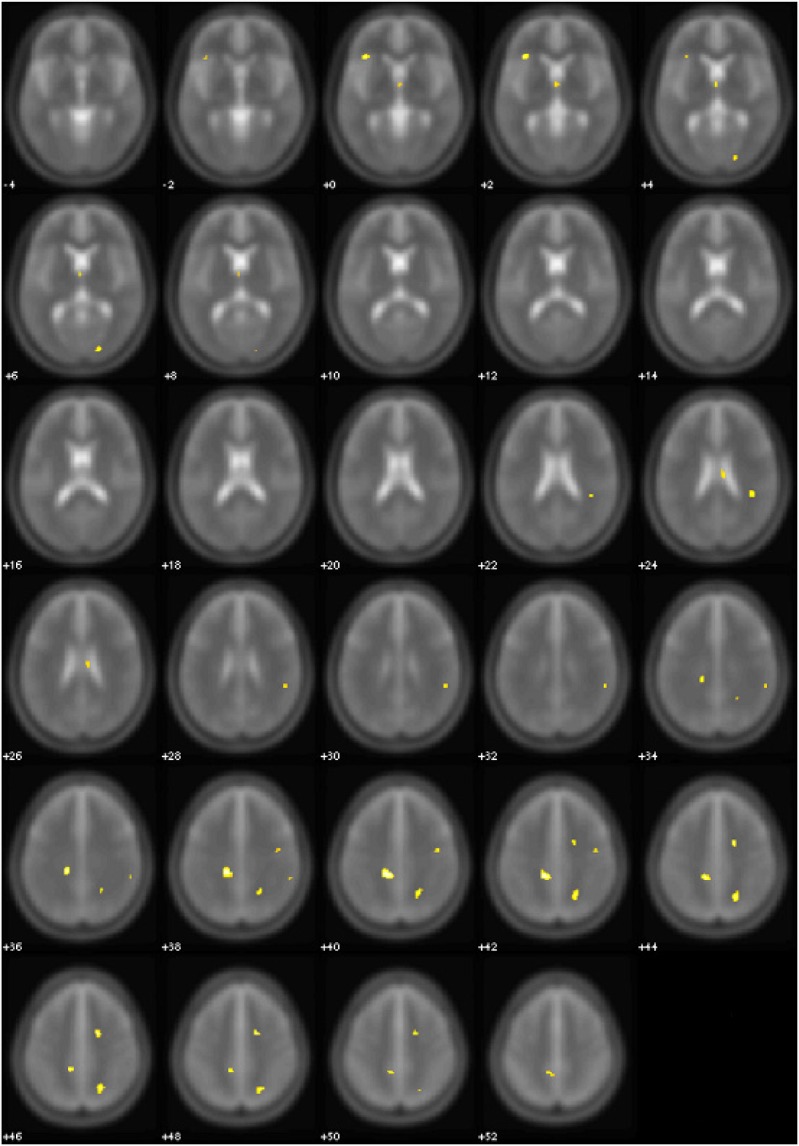
At baseline, OSA patients showed lower FA values in multiple supratentorial brain areas than controls (Table 3A, Figure 1). In the right hemisphere, a significant decrease of FA values was observed at the level of subcortical WM matter of superior and inferior parietal lobe, including fibers of the superior longitudinal fascicle, and at the level of deep frontal WM, corresponding to a portion of the arcuate fascicle. Moreover, another region showed lower FA corresponding to transcallosal connections from medial prefrontal area. In the left hemisphere, a large area of subcortical WM of superior parietal lobe, containing fibers of the superior longitudinal fascicle, showed lower FA in the OSA group, as well as another region in the left inferior frontal gyrus including fibers of the frontal portion of uncinate fascicle.
Table 3A.
Clusters of lower FA regions in OSA patients vs controls before and after treatment
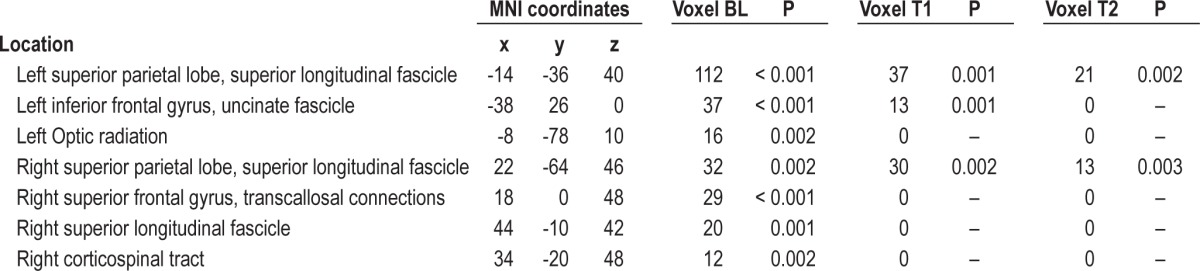
Figure 1.
Areas of significant (yellow, P < 0.005 uncorrected) FA differences between OSA patients at baseline and normal controls. Controls > OSA patients. Numbers indicate the z position in MNI template.
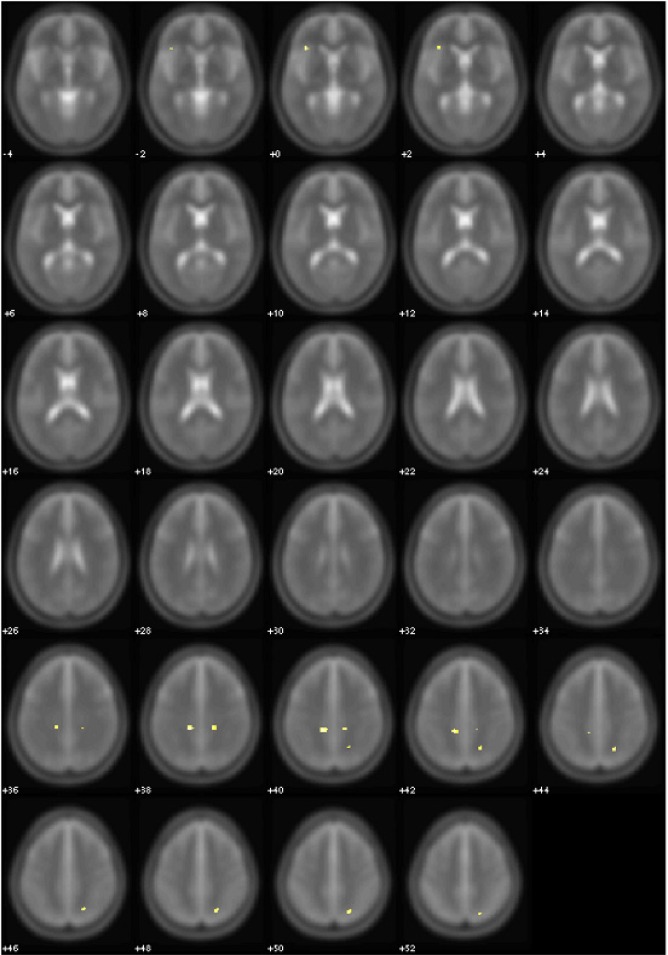
After 3 months of CPAP treatment, some of these areas showed a decrease of extension in OSA patients compared to controls (Table 3A, Figure 2). Clusters of pathological voxels were located in the same areas found at the baseline evaluation that, after therapy, were significantly smaller.
Figure 2.
Areas of significant (yellow, P < 0.005 uncorrected) FA differences between OSA patients after 3 months of treatment and normal controls. Controls > OSA patients. Numbers indicate the z position in MNI template.
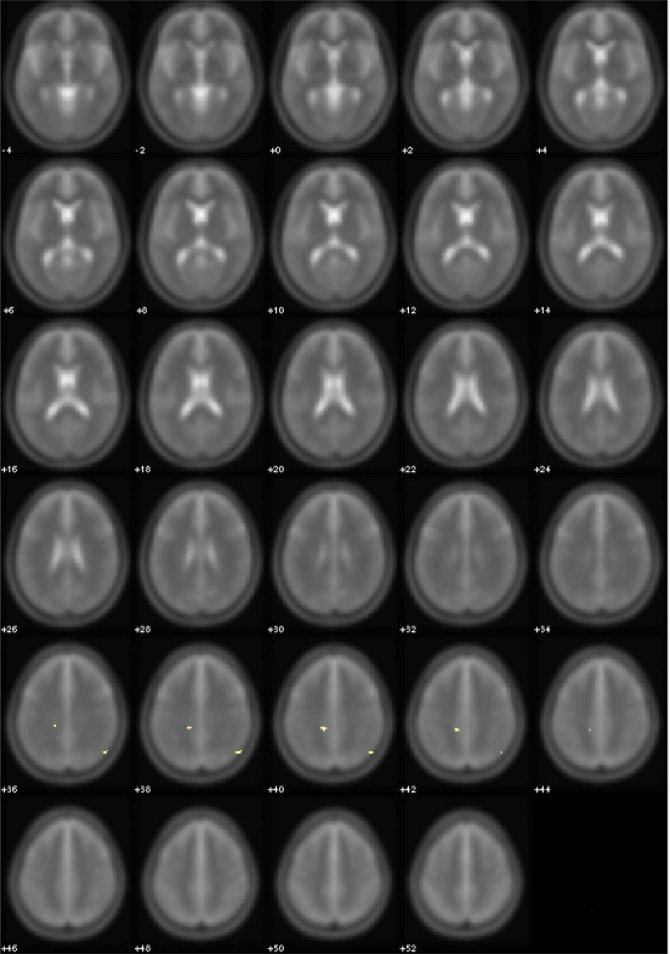
After 12 months of CPAP treatment, all these areas were significantly reduced in extension, both with respect to baseline and to the 3 months follow-up (Table 3A, Figure 3).
Figure 3.
Areas of significant (yellow, P < 0.005 uncorrected) FA differences between OSA patients after 12 months of treatment and normal controls. Controls > OSA patients. Numbers indicate the z position in MNI template.
Mean Diffusivity Analyses (Controls vs Patients)
At baseline, OSA patients showed also lower MD values than controls in some of the areas of lower FA (Table 3B). In the right hemisphere, a significant decrease of MD values was observed at the level of subcortical WM of superior parietal lobe, including fibers of the superior longitudinal fascicle, and at the level of deep frontal WM, corresponding to a portion of the arcuate fascicle. In the left hemisphere, the same large area of subcortical WM of superior parietal lobe, containing fibers of the superior longitudinal fascicle, showed lower MD in the OSA group.
Table 3B.
Clusters of lower MD regions in OSA patients vs controls before and after treatment
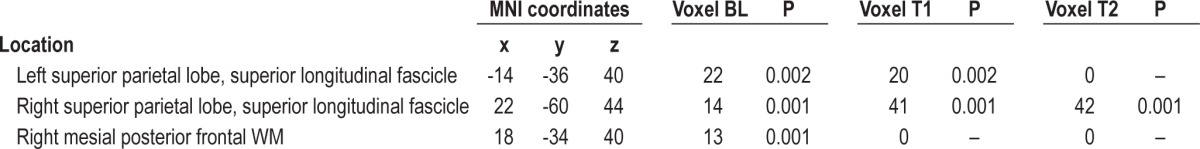
After 3 months of treatment with CPAP, the left area showed a slight decrease in size in OSA patients compared to controls (Table 3B, Figure 4). Clusters of pathological voxels were located in the same areas found at the baseline that, after therapy resulted smaller.
Figure 4.
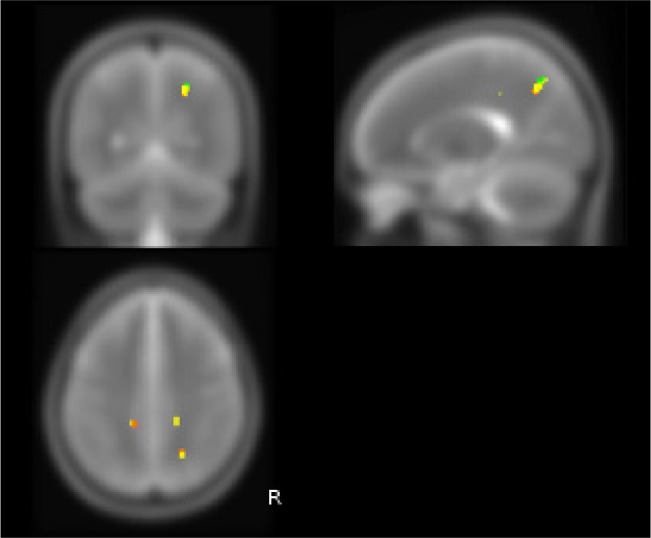
White matter brain MD changes in OSA patients before and after CPAP treatment (P < 0.005 corrected). Red areas show decreased MD in untreated OSA patients at baseline compared to controls; yellow areas show decreased MD in OSA patients after 3 months treatment compared to controls; green areas show decreased MD in OSA patients after 12 months treatment compared to controls. Orange color corresponds to areas of overlap between red and yellow ROIs.
The cluster of voxels in right parietal WM, corresponding to fibers of superior longitudinal fascicle, showed an increase of extension during treatment: the analysis of MD values at the level of this pathological “region of interest” after 3 months of treatment showed a trend toward an increase of MD values. However, despite the near-normalization of MD values in the core of this area, the increase of extension of the same “region of interest” suggested an incomplete reversal of WM abnormalities in this region. After 12 months of CPAP treatment, the trends found in the first follow-up were confirmed.
Correlation Analyses between Fractional Anisotropy and Neurocognitive and Clinical Data
The clinical relevance of the FA changes is supported by the results of the correlation analysis (Table 4). Reduced FA was correlated with higher scores on the sleepiness scale (Epworth Sleepiness Scale) and quality of sleep (Pittsburgh Sleep Quality Index) and with an increase of the time required to perform executive tasks, combined with more errors (Stroop, PASAT, Trial making A and B). In contrast, higher FA values were associated with better performance on short-term and long-term memory tasks.
Table 4.
Clusters of significant correlation between FA and neurocognitive and clinical data
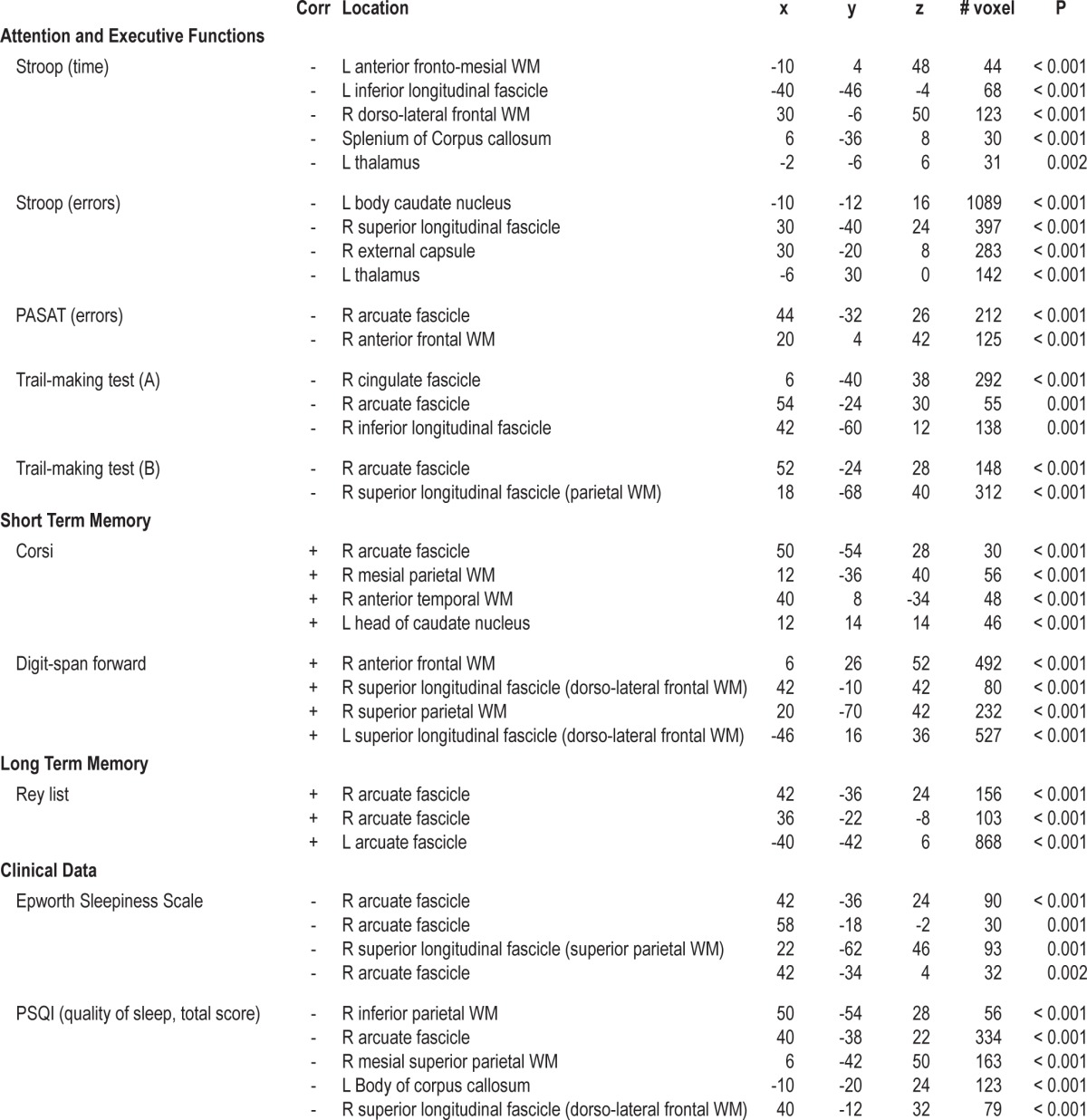
Specifically, the right hemisphere in the superior longitudinal fascicle, mainly at the level of the arcuate fascicle, has been involved in the correlations with sleep indexes (Epworth Sleepiness Scale, Pittsburgh Sleep Quality Index); the left thalamus and caudate nucleus and the splenium of the corpus callosum in the correlations with attention and executive functions indexes (Stroop, PASAT); the right arcuate fascicle and the superior and inferior longitudinal fascicles with Trial making A and B. Finally, the left parietal and frontal caudate nuclei were involved in the correlations with short-term memory indexes (Digit forward and Corsi test), and the arcuate fascicles with the correlations with verbal long-term memory indexes (Rey lists).
DISCUSSION
The goal of this study was to assess the presence of structural changes in the WM of OSA patients and to evaluate their possible reversibility, by demonstrating a normalization of DTI-derived metrics, like FA and MD, over the course of CPAP treatment.
OSA is associated with cognitive and functional deficits, most of which improve after effective treatment with CPAP in those patients who are compliant. Functional alterations of the brain in OSA likely result, at least in part, from injury to neural struc tures also at the level of WM tracts, but the nature of such injury is not fully understood.
DTI measures the flow of water through the tissue. This flow is relatively more focused and directed when tissue is compact, for example in intact WM (axonal) tracts of the brain. Compromised WM tracts can have functional consequences such as slowed information processing, impaired neurocognitive performance, and even aberrant emotional functioning, all of which have been reported in OSA.4,23
This study demonstrated compromised DTI metrics among OSA patients compared to controls across a number of WM tracts. Moreover, the study found only some limited changes in DTI after 3-month treatment, but more notable changes were visible over the course of one-year treatment.
Our study contributes to the existing literature in two primary ways. It is the first study to examine change in DTI with treatment. Two previous studies have demonstrated the involvement of the WM of the brain in OSA.10,22 These studies have employed a similar technique and have confirmed that the WM of the brains of OSA patients appears less organized than the ones of matched healthy controls. No studies to date, however, have attempted to examine changes to DTI after effective treatment.
At baseline, we found a reduction of WM fiber integrity in OSA patients, reflected by diminished FA and MD in multiple brain areas. We then quantitatively evaluated the longitudinal changes of DTI indices following patients for one year and evaluating them at 3 and 12 months into treatment. The intention was to examine the effects of CPAP among patients who were adherent to treatment over the short-term and long-term course of therapy. The study was also the first to begin to address the time course for change to the WM tracts of the brain in treated OSA patients. Changes were limited over the first 3 months of treatment as compared to normal control values, but they were clearer after a 1-year period.
A limitation of our study is that healthy subjects were not re-evaluated at 3 months and 1 year, a restriction caused by practical considerations of study cost and participant availability for repeated scanning. In principle, the absence of this control would not allow one to exclude either an effect of learning on cognitive performance or of “spontaneous” brain changes. However, several considerations speak against this interpretation. Our neuropsychological tests were unlikely to be prone to significant learning effects, except for the Rey list learning, for which an alternate equivalent form was used for follow-up evaluations. Moreover no spontaneous improvement in test performance has never been reported with sham-CPAP treatment.24 Moreover for the post-treatment neural changes observed in patients, we do not believe that a time interval of 1 year could create significant WM changes in control subjects in that range of age.25 Although the mechanisms of injury expected to be operating with OSA are largely unclear, as changes identified by DTI are sensitive to different under lying pathologies, our results suggest that some of the structural changes resulted from an (early) reversible insult, rather than atrophy that is the end result of some injurious process. Axonal changes are not necessarily as extreme as the cell death or shrinkage associated with atrophy, since axons may undergo di ameter reduction and lose much of their myelin sheath without axonal death, an effect found in animal models of intermittent hypoxia.8
Moreover, cerebral perfusion is chronically altered in OSA and reduced during apneic events, creating a potential for ischemic damage, particularly at the level of small vessels. Recent studies showed that DTI changes related to small vessel disease largely correspond to axonal chang es, rather than gliosis.
We hypothesize that early axonal damage from mild ischemic injury causes reversible injury, reflected as diminished FA; a more subtle alteration of membrane structure could represent the pathological substrate of a consensual reduction of MD, rather than an increase, found in our patients at baseline and partially recovered after therapy. Specifically, we speculate that some osmotic imbalances induced by hypoxia and ischemia in an early stage can contribute to focal enlargement and constriction, or beading, in axons and dendrites. This can cause change in cell membrane morphology sufficient to significantly hinder water mobility and thereby decrease diffusivity; beading of the neurite membrane is, therefore, a consequence of ischemia-induced swelling.26 In vivo, visualization of dendritic beading using two-photon microscopy shows that beading occurs within minutes of an ischemic event and resolves quickly after reperfusion.27 Similar characteristics are evident in apparent diffusion coefficient in vivo.28 This possible explanation of our findings is consistent with the recent study of Kumar et al.,22 in which the authors speculate that hypoxia/ ischemia-induced injury from apnea events leads to an osmotic imbalance causing cell swelling which, in extreme hypoxia, can result in cytotoxic edema: at an early, acute stage these modifications likely explain the reduction of MD values found in OSA patients.
Another explanation of our results could be related to an inflammatory condition that can directly damage brain tissue by impairing blood vessel integrity, leading to an increase in inflammatory cells in the cerebrospinal fluid and perivascular spaces in the brain.29 However, inflammation is generally accompanied by an increase of water diffusivity, where higher MD values may be interpreted as a reduction in the ordered anisotropic tissue structure. This suggests a loss of cell membrane integrity rather than a subtle morphological alteration, leading to higher net displacement of water molecules.30
Our results regarding WM alterations in OSA and their recovery after therapy should be considered together with our previous findings of gray matter volume changes, which were already evident after 3 months of treatment.18,20 Further gray matter structural changes were not evident after 12 months of treatment in the same patients. These results suggest that the WM of the brain takes longer to respond to treatment than the gray matter does and could explain the apparent different findings on WM changes after treatment reported by O'Donoghue et al.21 In their study, frontal lobes abnormalities in metabolite ratios were not modified after 6-month CPAP treatment, suggesting that axonal or glial pathology in OSA is a slowly reversible process, as clearly confirmed by our study. Changes of WM DTI “signatures” of brain pathology in OSA are appreciable over the course of 1-year CPAP treatment in most of the areas involved, but some regions in the right parietal lobe showed only a partial reversal of DTI abnormalities even after 12 months of treatment.
It is important to note, despite the mechanism underlying our reported brain changes, that both voxel-based morphometry and DTI demonstrate change with treatment. This may be the most important aspect of both studies, regardless of the timing or extent of this change. Simply demonstrating changes with treatment is the first step in showing that at least some of the impairments seen in the brains of patients with OSA are remediable with treatment and are not due to permanent damage, suggesting the ability of OSA patients to recover from brain dysfunction.
Changes to the brain can affect all of these secondary outcomes with time. Our studies now suggest that the timing of the measurement of such changes may provide insight into the brain dysfunction itself or to the mechanisms of this dysfunction. Clinical trials of CPAP should not limit themselves to a single measurement of outcome. If our data are upheld by future research, it is possible that some changes will accompany gray matter changes seen with treatment, while others will linger until changes are seen in the WM tracts of the brain. Future studies will need to first replicate these findings and then attempt to coordinate the differential time courses with the time courses seen for behavioral changes with treatment. One must, however, keep in mind that changes with treatment are highly subject to the sensitivity of the measures employed. The more sensitive measures are likely to demonstrate change prior to those measures that are less sensitive to change.
In conclusion, this study provides the first evidence that the structural brain abnormalities observed in WM regions susceptible to hypoxemia can change with treatment. The recovery of cognitive deficits after effective treatment of OSA is consistent with the presence of a reversible structural neural injury, which would resolve with ventilatory treatment. Therefore, adherence to treatment can lead not only to clinical, but also to brain structural recovery.
DISCLOSURE STATEMENT
This study was supported by Respironics Foundation, Pittsburgh, Pennsylvania. The work was performed at the Sleep Disorders Center and the Center of Excellence in High-Field Magnetic Resonance Imaging at Scientific Institute and University Ospedale San Raffaele, Milan, Italy. Dr. Castronovo is consultant to Philips Respironics. Dr. Aloia is an employee of Philips Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge the help of Mr. Daniele Biz zozero, MSc; Mr. Antonio Massimo, MSc; and Miss Cristina Martinelli, MSc (Sleep Disorders Center, Scientific Institute and University Ospedale San Raffaele, Milan, Italy) for polysomnographic recordings.
ABBREVIATIONS
- CPAP
continuous positive airway pressure
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- MD
mean diffusivity
- OSA
obstructive sleep apnea
- WM
white matter
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Brown WD. The psychosocial aspects of obstructive sleep apnea. Semin Respir Crit Care Med. 2005;26:33–43. doi: 10.1055/s-2005-864199. [DOI] [PubMed] [Google Scholar]
- 3.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: A critical review. J Int Neuropsychol Soc. 2004;10:772–85. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 4.Ferini-Strambi L, Baietto C, Di Gioia MR, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP) Brain Res Bull. 2003;61:87–92. doi: 10.1016/s0361-9230(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 5.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: A meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 6.Kloepfer C, Riemann D, Nofzinger EA, et al. Memory before and after sleep in patients with moderate obstructive sleep apnea. J Clin Sleep Med. 2009;5:540–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gozal D, Row BW, Gozal E, et al. Temporal aspects of spatial task performance during intermittent hypoxia in the rat: evidence for neurogenesis. Eur J Neurosci. 2003;18:2335–42. doi: 10.1046/j.1460-9568.2003.02947.x. [DOI] [PubMed] [Google Scholar]
- 9.Lanfranchi P, Somers VK. Obstructive sleep apnea and vascular disease. Respir Res. 2001;2:315–9. doi: 10.1186/rr79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–77. [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmerman ME, Aloia MS. A review of neuroimaging in obstructive sleep apnea. J Clin Sleep Med. 2006;2:461–71. [PubMed] [Google Scholar]
- 12.Ayalon L, Peterson S. Functional central nervous system imaging in the investigation of obstructive sleep apnea. Curr Opin Pulm Med. 2007;13:479–83. doi: 10.1097/MCP.0b013e3282f0e9fb. [DOI] [PubMed] [Google Scholar]
- 13.Davies CW, Crosby JH, Mullins RL, et al. Case control study of cerebrovascular damage defined by magnetic resonance imaging in patients with OSA and normal matched control subjects. Sleep. 2001;24:715–20. doi: 10.1093/sleep/24.6.715. [DOI] [PubMed] [Google Scholar]
- 14.Macey PM, Henderson LA, Macey KE, et al. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:1382–7. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- 15.Morrell MJ, McRobbie DW, Quest RA, Cummin AR, Ghiassi R, Corfield DR. Changes in brain morphology associated with obstructive sleep apnea. Sleep Med. 2003;4:451–4. doi: 10.1016/s1389-9457(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 16.Yaouhi K, Bertran F, Clochon P, et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. J Sleep Res. 2009;18:36–48. doi: 10.1111/j.1365-2869.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 17.Joo EY, Tae WS, Lee MJ, et al. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep. 2010;33:235–41. doi: 10.1093/sleep/33.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: Brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183:1419–26. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 19.Torelli F, Moscufo N, Garreffa G, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage. 2011;54:787–93. doi: 10.1016/j.neuroimage.2010.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Donoghue FJ, Briellmann RS, Rochford PD, et al. Cerebral structural changes in severe obstructive sleep apnea. Am J Respir Crit Care Med. 2005;171:1185–90. doi: 10.1164/rccm.200406-738OC. [DOI] [PubMed] [Google Scholar]
- 21.O'Donoghue FJ, Wellard RM, Rochford PD, et al. Magnetic resonance spectroscopy and neurocognitive dysfunction in obstructive sleep apnea before and after CPAP treatment. Sleep. 2012;35:41–8. doi: 10.5665/sleep.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar R, Chavez AS, Macey PM, Woo MA, Yan-Go FL, Harper RM. Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res. 2012;90:2043–52. doi: 10.1002/jnr.23083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar R, Macey PM, Cross RL, Woo MA, Yan-Go FL, Harper RM. Neural alterations associated with anxiety symptoms in obstructive sleep apnea syndrome. Depress Anxiety. 2009;26:480–91. doi: 10.1002/da.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim W, Bardwell WA, Loredo JS, et al. Neuropsychological effects of 2-week continuous positive airway pressure treatment and supplemental oxygen in patients with obstructive sleep apnea: a randomized placebo-controlled study. J Clin Sleep Med. 2007;3:380–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong WJ, Guo JN, Zhao JN, Xie WB, Chen WJ, Wu W. Changes of axial and radial diffusivities in cerebral white matter led by normal aging. Diagn Interv Imaging. 2012;93:47–52. doi: 10.1016/j.diii.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Budde MD, Frank JA. Neurite beading is sufficient to decrease the apparent diffusion coefficient after ischemic stroke. Proc Natl Acad Sci U S A. 2010;107:14472–77. doi: 10.1073/pnas.1004841107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li P, Murphy TH. Two-photon imaging during prolonged middle cerebral artery occlusion in mice reveals recovery of dendritic structure after reperfusion. J Neurosci. 2008;28:11970–9. doi: 10.1523/JNEUROSCI.3724-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li F, Liu KF, Silva MD, et al. Acute postischemic renormalization of the apparent diffusion coefficient of water is not associated with reversal of astrocytic swelling and neuronal shrinkage in rats. AJNR Am J Neuroradiol. 2002;23:180–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Man S, Ubogu EE, Ransohoff RM. Inflammatory cell migration into the central nervous system: A few new twists on an old tale. Brain Pathol. 2007;17:243–50. doi: 10.1111/j.1750-3639.2007.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sotak CH. Nuclear magnetic resonance (NMR) measurement of the apparent diffusion coefficient (ADC) of tissue water and its relationship to cell volume changes in pathological states. Neurochem Int. 2004;45:569–82. doi: 10.1016/j.neuint.2003.11.010. [DOI] [PubMed] [Google Scholar]