Abstract
Objectives:
Cross-species conservation of sleep-like behaviors predicts the presence of conserved molecular mechanisms underlying sleep. However, limited experimental evidence of conservation exists. Here, this prediction is tested directly.
Measurements and Results:
During lethargus, Caenorhabditis elegans spontaneously sleep in short bouts that are interspersed with bouts of spontaneous locomotion. We identified 26 genes required for Drosophila melanogaster sleep. Twenty orthologous C. elegans genes were selected based on similarity. Their effect on C. elegans sleep and arousal during the last larval lethargus was assessed. The 20 most similar genes altered both the quantity of sleep and arousal thresholds. In 18 cases, the direction of change was concordant with Drosophila studies published previously. Additionally, we delineated a conserved genetic pathway by which dopamine regulates sleep and arousal. In C. elegans neurons, G-alpha S, adenylyl cyclase, and protein kinase A act downstream of D1 dopamine receptors to regulate these behaviors. Finally, a quantitative analysis of genes examined herein revealed that C. elegans arousal thresholds were directly correlated with amount of sleep during lethargus. However, bout duration varies little and was not correlated with arousal thresholds.
Conclusions:
The comprehensive analysis presented here suggests that conserved genes and pathways are required for sleep in invertebrates and, likely, across the entire animal kingdom. The genetic pathway delineated in this study implicates G-alpha S and previously known genes downstream of dopamine signaling in sleep. Quantitative analysis of various components of quiescence suggests that interdependent or identical cellular and molecular mechanisms are likely to regulate both arousal and sleep entry.
Citation:
Singh K, Ju JY, Walsh MB, Dilorio MA, Hart AC. Deep conservation of genes required for both Drosophila melanogaster and Caenorhabditis elegans sleep includes a role for dopaminergic signaling. SLEEP 2014;37(9):1439-1451.
Keywords: sleep, C. elegans, Drosophila, dopamine
INTRODUCTION
Sleep is observed in all animal species, but the genetic underpinnings of mammalian sleep remain largely unknown.1 However, the recent identification of several genes and DNA polymorphisms affecting human sleep strongly suggests that this behavior is genetically regulated.2–4 Sleep in model organisms, including zebrafish, fruit flies, and nematodes, has been defined based on behavioral changes shared with human sleep. These changes include sleep-specific posture, spontaneous cessation of movement/feeding, increased arousal thresholds, rapid reversibility, and homeostatic response to sleep deprivation.4–6 Although genetic studies have identified many genes required for sleep in these species,5,7–11 generally the cross-species relevance of these genes has not been examined. Given the shared characteristics of sleep and the conservation of this behavior across the animal kingdom, it seemed likely that the genes and pathways required for sleep are conserved. Studies in mice, fruit flies, and nematodes have established a role for the epidermal growth factor (EGF) signaling pathway, protein kinase G (PKG), cyclic adenosine monophosphate (cAMP)/ protein kinase A (PKA)/cAMP response element binding (CREB), dopamine, neuropeptides, and the Notch signaling pathway in sleep in two or more of these model systems.6,12–26 Here, we more comprehensively address the conservation of genes required for sleep using two invertebrate model organisms.
Caenorhabditis elegans sleep is called quiescence,6 whereas Drosophila melanogaster sleep is called rest.27 In Drosophila, rest is temporally coordinated with the solar light-dark cycle via circadian proteins such as Period, whose abundance oscillates rhythmically on a 24-h cycle.28 Under standard culture conditions, C. elegans quiescence occurs during a period called lethargus and is temporally coordinated with molting of the larval cuticle, which occurs at the end of each larval stage.6 Interestingly, the C. elegans Period ortholog, lin-42, regulates both cuticle molting and quiescence.29 Although the roles of circadian genes in sleep regulation do vary between these invertebrate species, the role of other genes required for entry, maintenance, and exit from sleep may be more directly conserved. However, little experimental evidence exists to support this hypothesis.
To test this putative conservation, we focused on two well-known invertebrate model systems, Drosophila and C. elegans. Published Drosophila literature was reviewed to shortlist genes required for rest. The effect of orthologous C. elegans genes on quiescence and arousal was assessed. We found that C. elegans orthologs of Drosophila genes required for rest also altered both the amount of C. elegans quiescence and arousal during quiescence. Correlation analysis for the C. elegans genes tested herein revealed a simple and direct correlation between arousal thresholds and quiescence bout entry. Finally, the comprehensive analysis presented here allowed us to delineate a conserved genetic pathway acting downstream of the DOP-1 D1 receptors that regulates C. elegans quiescence.
MATERIALS AND METHODS
C. elegans strains
Strains and alleles examined are listed in Table S1 (supplemental material). Animals were reared on standard nematode growth media (NGM) media seeded with OP50 Escherichia coli at 25°C and assayed at 24°C. Animals from the RNA interference (RNAi) sensitized strain, HA2518, were transferred to RNAi feeding plates as L4 larvae and their progeny were assayed for quiescence and arousal defects. In some cases, the effect of RNAi was not seen until the F2 generation. To control for potential off-target effect of the RNAi knockdown, RNAi clones for nonoverlapping regions of the gene of interest were tested. The only potential inso-1(RNAi) off-target gene is csn-1, encoding a component of a COP9 signalosome complex. RNAi studies used NGM plates with 1 mM ampicillin and 1mM isopropyl β-D-1-thiogalactopyranoside (IPTG).30
Plasmids and transgenic strains
Transgenic animals were generated by microinjection of plasmids using standard methods.31 Co-injection markers were transcriptional fusions pGH#832 rab-3p::mCherry (25ng/μl) or pPD48.3333 myo-2::gfp (5 ng/μL) (gfp = green fluorescent protein). Primers are listed in Table S2 (supplemental material). Results for at least two independent transgenic lines were determined and combined for each transgenic genotype reported.
dop-1p::gfp pHA#651, 4.3 kb of sequence upstream the dop-1 start codon was polymerase chain reaction (PCR) amplified and cloned into the pPD49.2634 XmaI site. GFP was cloned behind the dop-1 promoter using KpnI and EcoRI sites.
dop-1p::kin-2(RNAi) pHA#652, 2.5 kb of kin-2 genomic sequence containing exons was PCR amplified and cloned in reverse orientation behind the dop-1 promoter of pHA#581, using KpnI and SacI sites.
dop-1p::crh-1 pHA#653, the crh-1 complementary DNA (cDNA) sequence of 945 bp was PCR amplified and cloned behind the dop-1 promoter of pHA#581, using NheI and KpnI sites.
Total quiescence: RNAi feeding clones described below:
crh-1(RNAi) feeding clone pHA#665 469 bp of the crh-1 cDNA sequence was cloned into pL444030 vector using NheI and NcoI sites.
egl-4(RNAi) feeding clone pHA#666 2.1 kbp of the egl-4 genomic sequence containing exons was PCR-amplified and cloned into pL4440 vector using the SphI site.
cul-3(RNAi) feeding clones pHA#657 and pHA#661 each contain a nonoverlapping, PCR-amplified cul-3 cDNA sequence cloned into XmaI site of pL4440.
lgc-38(RNAi) feeding clones pHA#654 and pHA#664 contain a nonoverlapping PCR-amplified lgc-38 genomic and cDNA sequence, respectively cloned into NheI and SacI sites of pL4440.
cya-1(RNAi) feeding clones pHA#662 and pHA#663 contain a nonoverlapping, PCR-amplified cya-1 cDNA sequence cloned into NheI and XmaI sites of pL4440 vector. inso-1(RNAi) feeding clones pHA#659 and pHA#660 each contain a nonoverlapping, PCR-amplified inso-1 cDNA sequence cloned into the SpeI site of pL4440 vector.
jnk-1(RNAi) feeding clone pHA#667 1.8 kbp of the jnk-1 genomic sequence containing exons was PCR-amplified and cloned into pL4440 vector using NheI and SacI sites.
Arousal threshold: RNAi feeding clones described below. The effect on quiescence of these clones was virtually identical to the effect of clones above that altered quiescence (Figure 2A).
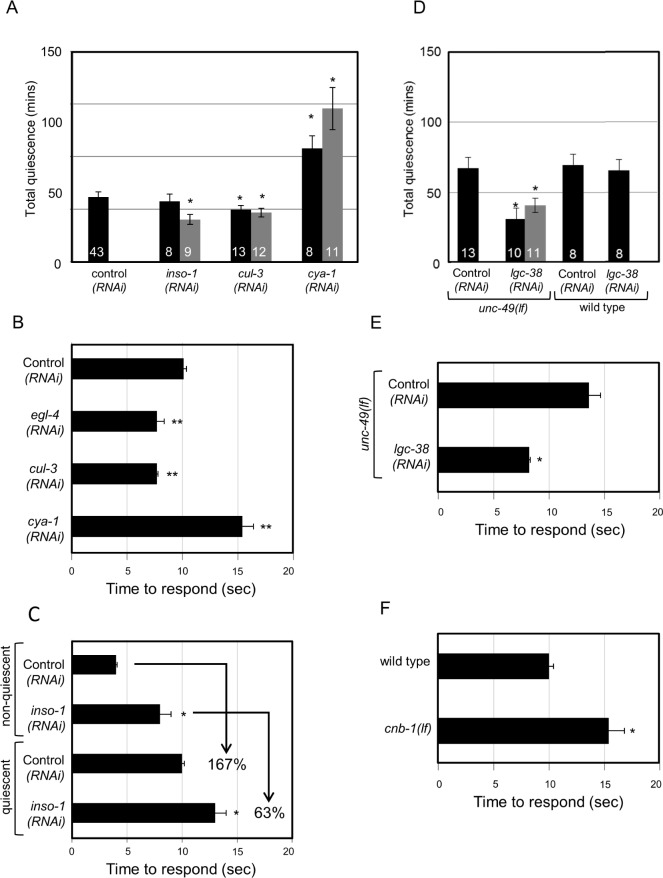
Figure 2.
Lethargus quiescence and altered arousal during sleep requires the function of conserved genes. For RNA interference (RNAi) feeding experiments, HA2158 animals expressing the SID-1 double- stranded RNA channel in neurons were used.77 Total quiescence during L4-to-adult lethargus reported in min. Arousal thresholds were determined by measuring the time to respond to 60% dilute 1-octanol. Response time is reported in sec. Error bars represent standard error of the mean (s.e.m.). Gray and black bars represent independent clones in A and D. Numbers inside the bar indicate sample size. For each gene examined, independent sets of control animals were tested in parallel and these were used to determine significance by Student t test. P < 0.05* < 0.01** and < 0.001*** versus control (RNAi), unc-49(lf), or wild-type. Results were grouped and controls were pooled for concise presentation. (A) RNAi knockdown using two independent clones of insomniac (inso-1) and cullin-3 (cul-3) decreased total quiescence with one exception. See Methods for a possible off-target gene for inso-1(RNAi). RNAi knockdown of cyclin A (cya-1) increased total quiescence. (B) RNAi knockdown of egl-4 and cul-3 in sensitized HA2158 animals lowered arousal thresholds during quiescence in comparison with their respective controls. RNAi knockdown of cya-1 increased arousal thresholds. (C) Nonquiescent inso-1(RNAi) animals were mildly 1-octanol sensing defective. Quiescent wild-type animals' response time to dilute 1-octanol increased by 167 ± 11%. However, inso-1(RNAi) animals increased their response time by 63 ± 13%, suggesting that these animals maintain inappropriately low arousal thresholds during quiescence. (D) RNAi knockdown of the lgc-38 γ-aminobutyric acid (GABA) A receptor decreased quiescence in animals lacking the unc-49 GABA A receptor. (E) RNAi knockdown of the lgc-38 in unc-49(lf) animals also decreased arousal thresholds. (F) Nonquiescent cnb-1(lf) animals were defective in their response to mechanosensory stimuli, but responded normally to dilute 1-octanol. Therefore, dilute 1-octanol response was used to demonstrate heightened arousal thresholds during quiescence.
inso-1(RNAi) feeding clone pHA#656, 624 bp of inso-1 genomic sequence containing exons was PCR-amplified and cloned into pL4440 vector using the SpeI site.
cul-3(RNAi) feeding clone pHA#657 (described above).
cya-1(RNAi) feeding clone pHA#655, 1.47 kbp of cya-1 genomic sequence containing exons was PCR-amplified and cloned into pL4440 vector using NheI and XmaI sites.
lgc-38(RNAi) feeding clone pHA#654 (described above).
crh-1(RNAi) feeding clone pHA#665 (described above).
jnk-1(RNAi) feeding clone pHA#667 (described above).
Quiescence and arousal threshold assays
Quiescence during the L4-to-adult molting lethargus was determined using the previously published microfluidic chamber-based assays.19 Image subtraction was performed using the previously published Matlab analysis program.19 A single pixel change above camera noise was the metric for movement detection (lack of quiescence). Cameras used for image acquisition were AxioCam ICc1 (Zeiss, Oberkochen, Germany) (pixel size: 4.65 μm × 4.65 μm), AxioCam MRc Rev3 (Zeiss) (pixel size: 6.45 μm × 6.45 μm) and Stingray F201c (Allied Vision Technologies, Stadtroda, Germany) (pixel size: 4.4 μm × 4.4 μm). Unless indicated otherwise, early-L4 stage larvae were loaded into 1 × 4 mm microfluidic chambers 4-6 h prior to lethargus and the activity was monitored for 12 h. HA2518 animals were loaded in the microfluidic chambers as black-dot/late-L4 stage larvae, just as lethargus began and activity was recorded for 8 h. Images were acquired at 10-sec interval. Unlike the previous analysis presented in Singh et al.,19 in the current study quiescence onset time was defined as a time point after which the fractional quiescence remains > 0.1 for at least 20 minutes. The quiescence exit time was defined as the time point after which the fractional quiescence had remained < 0.1 for at least 20 minutes. The total quiescence was calculated by counting the number of images where no pixel change was detected (compared to the previous image, above camera noise) between quiescence onset and exit time points. Each image subtraction that did not detect motion represents 10 sec of quiescence. Quiescence onset time was subtracted from quiescence exit time to determine the duration of lethargus quiescence and was reported in hours. The number of quiescent bouts that were 10 sec or longer was determined and total number of bouts occurring between the quiescence onset and exit time was reported. Bout frequency was calculated by dividing total number of bouts by lethargus duration for each animal. The duration of all the quiescent bouts occurring between the quiescence onset and exit time was measured and the average bout duration was calculated and reported in sec. We calculated bout durations for each animal and then averaged the results of all animals in a genotype/treatment. Other bout metrics were calculated similarly. goa-1(lf) and gsa-1(gf) animals had profoundly decreased total quiescence. As a result, the fractional quiescence cutoff of > 0.1 for 20 min was never exceeded. Therefore, these animals have total quiescence = 0 min, bout number = 0, and lethargus duration = 0 h (see Table S3, supplemental material). Because lethargus duration for goa-1(lf) and gsa-1(gf) animals was equal to zero, bout number and bout duration for these genotypes could not be determined and were excluded from the correlation analysis presented in Figure 4. Quiescence and arousal thresholds of pde-4(ok1439) animals were presented as pde-4(ce268) animals exhibited normal quiescence (total quiescence of pde-4(ce268) = 59 ± 6 min, P = 0.27). pde-4(ce268) changes a single amino acid, whereas pde-4(ok1439) deletes 1100 bp and likely results in a complete loss of function. For genotypes with most and least total quiescence, bout duration was reexamined at one frame per sec interval. In this reanalysis bout duration > 1 sec was measured and total quiescence determined. This analysis is described in Table S4 (supplemental material).
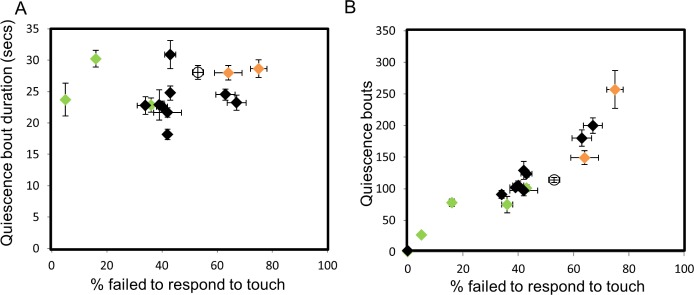
Figure 4.
Assessing correlations between arousal and quiescence. Quiescence bout number and bout duration during lethargus were examined versus arousal thresholds for genotypes tested in Figures 1 and 3. Wild-type is represented as an empty circle. Genes implicated in the dopamine signaling pathway are represented as green and orange rhombi, which represent decreased and increased quiescence, respectively. (A) No correlation was observed between quiescent bout duration and arousal thresholds (r = -0.051, P = 0.580). (B) A direct correlation was detected between quiescent bout numbers and arousal thresholds. Correlation coefficient r = 0.887, P = 1e-08. See Methods for statistical details.
For arousal threshold determination, response to body touch35 was used; exceptions noted later in this paper. A hair was placed on the agar plate evenly seeded with OP50 bacteria and flexed to contact the animal behind the pharynx; locomotion within 1 sec was scored as a response and percentage of animals failing to respond is reported. Arousal thresholds of HA2518 RNAi sensitized animals and cnb-1(lf) animals were performed using 1-octanol diluted to 60% concentration with ethanol. Average response time to 60% octanol of quiescent animals reared on experimental and control RNAi strains is reported in sec. During quiescence, animals responded to touch or dilute 1-octanol by initiating backward locomotion. For touch, any immediate locomotion was scored as response. For dilute octanol, time to initiate locomotion was recorded. Octanol assays stopped at 20 sec. For all genotypes, at least half of the trials were done by an observer blinded as to the genotypes/treatment. Control genotypes were always run in parallel (also blinded). Nonquiescent animals in lethargus were used as controls to determine the basal response to mechanical or chemosensory stimuli. Any immobile, nonfeeding animal was considered quiescent for arousal threshold determination. For gsa-1 and goa-1, rare quiescent animals identified during the appropriate developmental stage were scored for arousal. Arousal threshold results are presented for at least three independent trials with N ≥ 6 per trial for each genotype tested. The following exceptions should be noted: goa-1: N = 1 and kin-2: N = 5 animals (because of scarcity of quiescent animals).
Statistics
Genes examined here are C. elegans orthologs of Drosophila genes that alter the quantity of Drosophila rest. Genes that affect circadian behavior or genes that only alter homeostasis were not included in the analysis. Orthologs were identified and similarity was assessed based on E values from BLAST at the National Center for Biotechnology Information.36 The Expect (E) values for all the C. elegans genes compared to Drosophila genes are listed in Table S5 (supplemental material). The least similar was RCN-1 (E = 3e-27). At least 8 or 20 animals were tested for each quiescence or arousal threshold determination, respectively. For each of the 20 genes examined, independent sets of wild type or control animals were tested in parallel and these were used to determine significance by the Student t test (Table S3 and figure legends). Because multiple comparisons were made, we calculated the false discovery rate (FDR).37,38 The FDR q-value was set at the standard 0.05 to ascribe significance (Table S5). A similar statistical analysis was performed for arousal thresholds (Table S5).
RESULTS
Lethargus quiescence requires the function of conserved genes
The Drosophila rest and C. elegans quiescence share behavioral characteristics of sleep including cessation of movement, altered arousal, and homeostatic regulation.4–6,39 The presence of these common hallmarks suggests that conserved processes regulate sleep in these invertebrate species. For clarity herein, we will refer to sleep in Drosophila as rest and sleep in C. elegans as quiescence.
To determine if orthologs of Drosophila genes required for rest also play a conserved role in C. elegans quiescence, we first identified genes that altered the quantity of Drosophila rest. Genes involved in circadian regulation or solely in homeostasis were excluded from this analysis. C. elegans orthologs of Drosophila genes were identified based on protein sequence similarity using BLAST (Table S5), and the closest C. elegans ortholog was examined in this study. No unambiguous orthologs could be found for six Drosophila genes: Hyperkinetic,40 Fragile X mental retardation,41 homer,42 Relish,43 sleepless,44 and Regulator of cyclin A.45 Publicly available deletion, missense, or nonsense alleles were obtained for the remaining C. elegans genes, whenever possible (Figure 1, Table S1). If no alleles were available, then RNAi by feeding was used to test the effect of genes on C. elegans quiescence (see Figure 2). C. elegans L4-to-adult lethargus quiescence was assessed using a microfluidic chamber-based assay. Total time spent in quiescence in minutes, which will be referred to as total quiescence, was reported19 (Figure 1, see Methods). All genes tested altered C. elegans quiescence. For 18 genes, the change in C. elegans total quiescence was as would be predicted from D. melanogaster literature. When disruption of Drosophila gene function increased rest, disruption of the C. elegans ortholog increased total quiescence, and vice versa. Exceptions were the C. elegans ortholog of Drosophila basket and cyclin A (described in the following paragraphs). Results are summarized in Tables S3 and S6 (supplemental material), presented graphically in Figures 1 and 2, and detailed in the following paragraphs.
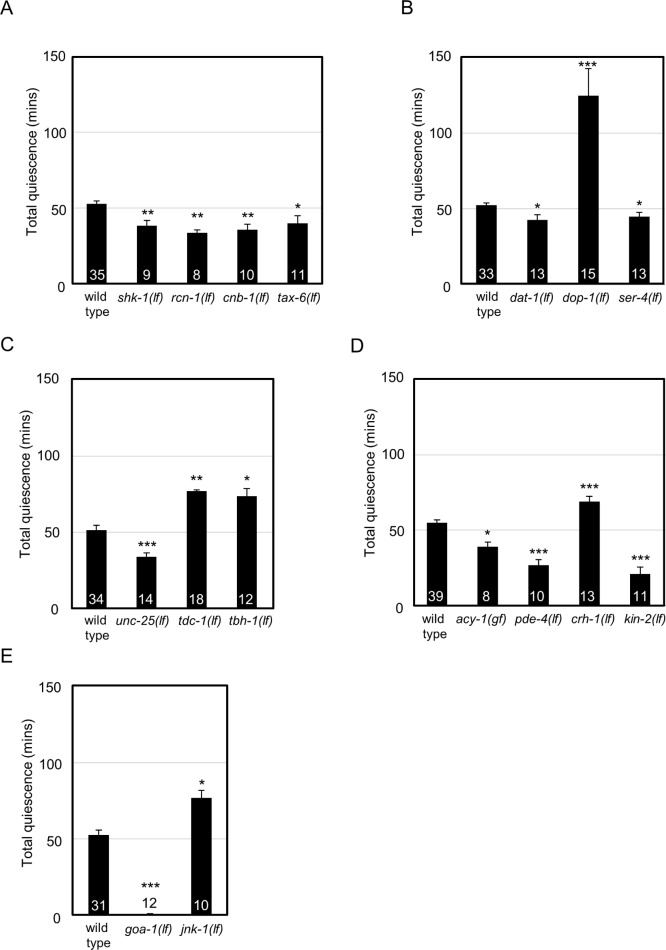
Figure 1.
Lethargus quiescence requires the function of conserved genes. L4-to-adult lethargus quiescence was determined. Total quiescence was reported in min. Error bars represent standard error of the mean (s.e.m.). Numbers inside or above the bar indicate sample size. For each gene examined, independent sets of wild-type control animals were tested in parallel and these were used to determine significance by Student t test. P < 0.05* < 0.01** and < 0.001*** versus wild-type. Results were grouped by pathway/function and controls were pooled for concise presentation. Control is dop-1 promoter driving gfp (dop-1p::gfp). (A) Loss of calcineurin signaling components (rcn-1, cnb-1, tax-6) or Shaker potassium channel (shk-1) function decreased total quiescence. (B) Loss of dopamine transporter (dat-1) or serotonin receptor (ser-4) function decreased total quiescence. Loss of D1 dopamine receptor (dop-1) function increased total quiescence. (C) Loss of γ-aminobutyric acid synthesis (unc-25) decreased total quiescence. Loss of genes required for octopamine biosynthesis increased total quiescence: tyrosine decarboxylase (tdc-1) and dopamine beta hydroxylase (tbh-1). (D) Adenylyl cyclase (acy-1) gain of function or loss of phosphodiesterase (pde-4) decreased total quiescence. Loss of CREB (crh-1) function increased total quiescence. Loss of protein kinase A regulatory subunit (kin-2) function decreased total quiescence. (E) Loss of Go (goa-1) decreased total quiescence. Loss of C-Jun N-terminal kinase (jnk-1) function increased total quiescence.
Shaker and the calcineurin pathway (shk-1, tax-6, cnb-1, rcn-1)
Drosophila Shaker encodes a voltage-gated potassium channel, which regulates membrane repolarization and synaptic signaling.46 Disruption of Drosophila Shaker function reduces rest.47 Consistent with this, decreased total quiescence was observed in animals carrying a deletion allele of the C. elegans Shaker ortholog, shk-1 (Figure 1A). The calcineurin signaling pathway has also been implicated in Drosophila rest.48,49 Calcineurin is a Ca2+/calmodulin dependent protein phosphatase complex containing both a catalytic subunit and a regulatory subunit. Disruption of either catalytic subunit, Pp2B-14D or CanA-14F, or either regulatory subunit, CanB or CanB2, results in reduced Drosophila rest. Additionally, loss of the calcineurin regulator sarah reduces Drosophila rest.48 In C. elegans, the catalytic and regulatory subunits of calcineurin are encoded by tax-6 and cnb-1, respectively50,51; the calcineurin regulator sarah is encoded by rcn-1.52 We found that loss of rcn-1, cnb-1, or tax-6 function decreased C. elegans quiescence. Combined, these results suggest that Shaker potassium channels and calcineurin play conserved roles in sleep across species (Figure 1A).
Neurotransmitter systems (dop-1, dat-1, ser-4, unc-25, unc-49, lgc-38, tbh-1, tdc-1)
In Drosophila, loss of dopamine signaling increases rest; therefore dopamine is said to have a wake-promoting effect.53,54 For example, increasing synaptic dopamine by disrupting the dopamine transporter fumin significantly decreases Drosophila rest.12 Conversely, loss of the D1 dopamine receptor DopR increases Drosophila rest.54 The C. elegans orthologs of fumin and DopR genes are dat-1 and dop-1, respectively.55,56 Consistent with the Drosophila results, dat-1 loss of function decreased total quiescence, and as expected, the dop-1 loss of function increased total quiescence (Figure 1B).
In Drosophila, loss of serotonin signaling decreases rest; serotonin is said to have a sleep-promoting effect.57 Loss of the Drosophila serotonin receptor 5-HT1A decreases rest.57 In C. elegans, ser-458 encodes the protein most similar to Drosophila 5-HT1A. Consistent with Drosophila results, ser-4 loss of function decreased total quiescence in C. elegans (Figure 1B).
γ-Aminobutyric acid (GABA) signaling also affects Drosophila rest. Overexpression of the only Drosophila GABAA receptor, Resistant to dieldrin (Rdl), in LNvs (large ventral lateral neurons) results in increased rest.24 In addition, inhibiting GABAergic neurons via ectopic Shaw channel expression decreases Drosophila rest.59 To test the effect of GABA signaling on C. elegans quiescence, the role of GABA receptor function in quiescence was determined. Similarity searching suggested that in C. elegans both unc-49 and lgc-38 are orthologs of Drosophila Rdl.60 Neither unc-49(lf) nor lgc-38(RNAi) alone affected quiescence (total quiescence = 60 ± 3 min, P = 0.66 or total quiescence = 66 ± 5 min, P = 0.26, respectively and Figure 2D), possibly because of redundant function of the unc-49 and lgc-38. Because no alleles were available for lgc-38, the gene was knocked down using two nonoverlapping RNAi clones in unc-49(lf) animals and quiescence determined. unc-49(lf) animals reared on lgc-38(RNAi) feeding clones had significantly decreased total quiescence compared to those reared on empty vector control (Figure 2C). Complete loss of GABA biosynthesis does not cause lethality in C. elegans. Therefore, we tested animals completely lacking the function of unc-25, which encodes glutamic acid decarboxylase.61 These animals have decreased total quiescence, which is consistent with the effect of GABA loss on Drosophila rest (Figure 1C).
Octopamine, a biogenic amine, also regulates Drosophila sleep.62 Loss of octopamine biosynthesis pathway genes, tyrosine decarboxylase (Tdc) and tyramine beta hydroxylase (TβH), increases rest.62 tdc-1 and tbh-1 are the C. elegans orthologs of Drosophila Tdc and TβH genes.63 Consistent with the Drosophila sleep defects observed in Tdc and TβH flies, tdc-1(lf) and tbh-1(lf) C. elegans had increased total quiescence (Figure 1C).
cAMP signaling (acy-1, pde-4, kin-2, crh-1)
The duration of Drosophila sleep is inversely proportional to cAMP signaling, PKA activity, and CREB activity.64 The Drosophila rutabaga and dunce genes encode adenylyl cyclase and phosphodiesterase, respectively, and they antagonistically regulate cAMP levels. Loss of rutabaga increases rest, whereas loss of dunce decreases rest.64 C. elegans cAMP levels are regulated by two orthologous genes: acy-1 and pde-4.65 Complete loss of acy-1 causes lethality. Therefore, a previously described acy-1 gain of function allele was tested.66 Either acy-1 gain of function or pde-4 loss of function likely increases cAMP levels.67 We found that acy-1(gf) or pde-4(lf) animals exhibited decreased total quiescence, consistent with results in Drosophila (Figure 1D).
Two conserved downstream components of cAMP signaling pathway are PKA and cAMP response element binding protein (CREB).68 In Drosophila, overexpression of PKA panneuronally or with a heat shock promoter decreases rest.64,69 Conversely, blocking Drosophila CREB activity increases rest.64 C. elegans kin-2 encodes the regulatory subunit of PKA and kin-2 loss of function increases PKA catalytic activity.66 As predicted from the Drosophila study,64 kin-2 loss of function decreased C. elegans total quiescence (Figure 1D). crh-1 encodes the C. elegans CREB ortholog.70 crh-1(lf) animals had increased total quiescence, which is consistent with CREB function in Drosophila rest (Figure 1D). Combined, these results suggest that the cAMP signaling cascade plays a conserved role in Drosophila and C. elegans sleep.
Other signaling (goa-1, jnk-1)
The Drosophila Go protein is encoded by the Goα47a gene and has been implicated in rest. Pan-neuronal expression of Go increases rest, whereas RNAi knockdown of Go decreases rest.71 In C. elegans, Go is encoded by goa-1.72 Complete loss of goa-1 function decreased C. elegans quiescence supporting a conserved role for Go protein in invertebrate sleep (Figure 1E).
The Drosophila gene basket encodes a C-Jun N-terminal kinase that is required for rest.73 RNAi knockdown of basket decreases rest.73 In C. elegans, the closest ortholog of Drosophila basket is encoded by jnk-1.74 C. elegans jnk-1 loss of function caused increased total quiescence (Figure 1E). This gene is one of only two C. elegans orthologs that had discordant results between species, which is discussed in the next paragraphs.
BTB, cullin, and other signaling (inso-1, cul-3, cya-1)
RNAi knockdown of either Drosophila insomniac or Cullin-3 decreases rest. Consistent with this result, insomniac loss of function alleles also decreases rest.75 In C. elegans, cul-3 is the predicted ortholog of Cullin-3.76 The C. elegans gene most similar to insomniac is C52B11.2, which is named inso-1 here. These C. elegans genes have not been previously characterized. A cul-3 mutant allele is not available. A previously uncharacterized inso-1(gk344) deletion allele exists, which removes the predicted first exon of inso-1, but this mutation did not alter quiescence (total quiescence = 50 ± 3 min, P = 0.09). However, examination of inso-1 cDNA clones suggested that translation in inso-1(gk344) animals might initiate at methionine (22) in exon 2 and might not dramatically alter gene function. Therefore, we used RNAi knockdown to test the effect of these two genes on C. elegans quiescence. Expression of double- stranded RNA channels in neurons increases C. elegans sensitivity to RNAi by feeding.77 We confirmed that egl-4(RNAi) in this sensitized background resulted in decreased total quiescence (total quiescence = 42 ± 6 min, P = 0.047), which is consistent with previous work using egl-4(lf) mutant animals.6 The impact of cul-3, the C. elegans ortholog of Cullin-3, on quiescence was tested using two independent RNAi clones. Knockdown of cul-3 by RNAi decreased total quiescence (Figure 2A). Two independent inso-1 RNAi clones were tested. One decreased quiescence compared with that of the control RNAi animals (Figure 2A).75 Although decreased quiescence is consistent with the effect of the Drosophila insomniac on rest, potential off-target effects of inso-1 RNAi cannot be ruled out. See Methods.
Neuronal depletion of Drosophila CycA, which encodes cyclin A, decreases rest.45 The C. elegans ortholog of cyclin A is cya-1.78 Because no deletion alleles were available for cya-1, RNAi knockdown in the sensitized background was used to test the effect on quiescence. RNAi knockdown of cya-1 using two independent clones increased C. elegans total quiescence compared with that in animals raised on control bacteria containing the empty vector (Figure 2A). C-Jun N-terminal kinase and cyclin A are the only two genes for which the change in sleep was inconsistent between the two species.
In summary, all 20 C. elegans genes tested here affected quiescence. For 18 genes, the effect on quiescence was consistent with predictions based on the Drosophila literature. Taken together, these results suggest deep conservation of genes required for invertebrate sleep.
Conserved genes also regulate arousal during C. elegans quiescence
Altered arousal is an essential component of sleep. C. elegans and Drosophila also exhibit increased arousal thresholds during sleep as their responsiveness to sensory stimuli is reduced.4,5,77 In Drosophila, disruption of fumin, Goα47A, or basket gene function perturbs arousal thresholds during rest.12,71 To independently assess the effect of these genes on arousal during C. elegans quiescence, we examined response using chemo-sensory and/or mechanosensory stimuli.19,35 Failure to respond to touch with a hair during quiescence is presented for all C. elegans alleles and is reported as percent failed to respond to touch (Figure 3). Response to 1-octanol during quiescence is reported for RNAi studies (Figures 2B, 2C, and 2E) as the requisite genetic background and/or bacterial strain causes hyper-sensitivity to mechanosensory stimuli. Response to 1-octanol during quiescence is reported for cnb-1(lf) animals (Figure 2F) because these animals exhibited defective response to mechanosensory stimuli. In RNAi studies, nonquiescent animals responded to dilute 1-octanol as quickly as control animals with one exception. Nonquiescent inso-1(RNAi) animals were defective in their response to dilute 1-octanol (8 ± 1 sec) compared with nonquiescent wild type animals (4 ± 0.1 sec), complicating analysis. Quiescent wild type animals increased their response time by 167 ± 11% but inso-1(RNAi) animals only increased their response time by 63 ± 13%, suggesting that inso-1 loss decreases arousal thresholds during quiescence (Figure 2C).
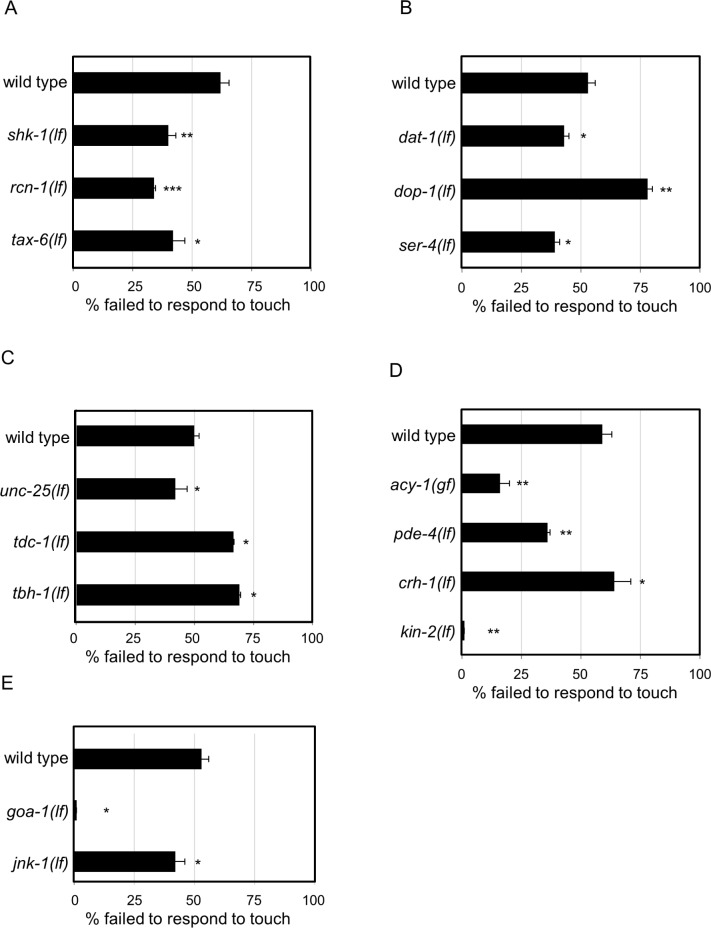
Figure 3.
Conserved genes regulate arousal. Arousal thresholds were determined by counting the number of quiescent animals responding to body touch. Percent of animals failing to respond to body touch is reported in all the figure panels. Error bars represent standard error of the mean (s.e.m.). Non-quiescent animals rarely failed to respond to body touch with a failure rate of 0-10% for all genotypes. Because of their profound quiescence defects, very few quiescent goa-1(lf) (n = 2) and kin-2(lf) (n = 5) animals were found, even when more than 100 animals were sampled. For each gene examined, independent sets of wild-type control animals were tested in parallel and these were used to determine significance by Student t test. P < 0.05* < 0.01** and < 0.001*** versus wild-type. Results were grouped by pathway/function and controls were pooled for concise presentation. (A) Loss of either C. elegans calcineurin signaling genes (rcn-1 and tax-6) or loss of C. elegans Shaker (shk-1) decreased arousal thresholds. (B) Loss of dopamine transporter (dat-1) or serotonin receptor (ser-4) function decreased arousal thresholds. Loss of D1 dopamine receptor (dop-1) function increased arousal thresholds. (C) Loss of γ-aminobutyric acid (GABA) synthesis gene (unc-25) decreased arousal thresholds. Loss of octopamine biosynthesis genes tyrosine decarboxylase (tdc-1) and dopamine beta hydroxylase (tbh-1) increased arousal thresholds. (D) Loss of phosphodiesterase (pde-4) or protein kinase A regulatory subunit (kin-2) function decreased arousal thresholds. Gain of adenylyl cyclase (acy-1) function decreased arousal thresholds. Loss of CREB increased arousal thresholds (crh-1(lf) = 63 ± 0.6 versus parallel wild type control = 51 ± 0.4, P = 0.005). (E) Loss of Go (goa-1) or C-Jun N-terminal kinase (jnk-1) function decreased arousal thresholds.
All 20 genes that affected C. elegans quiescence also altered arousal thresholds (Figures 2B, 2C, 2E, 2F, 3, and Tables S3 and S6). We found decreased arousal thresholds in dat-1 and goa-1 loss of function animals, which is consistent with the decreased arousal thresholds of Drosophila fumin and Goα47A animals.12,71 The only other comparison possible across species was for C-Jun N-terminal kinase orthologs. C. elegans jnk-1 loss of function decreased arousal thresholds, whereas Drosophila basket RNAi is reported to have no effect on arousal during rest.73 For the 17 other orthologous genes, additional studies in Drosophila will be required to test if their function in arousal across species is conserved.
Assessing correlations between arousal and quiescence
Arousal thresholds likely reflect depth and/or quality of sleep. We considered the possibility that arousal might correlate with other metrics of C. elegans sleep, which include total time spent in quiescence, lethargus duration, and/or quiescence bout characteristics (number and duration). Total quiescence, lethargus duration, and bout number are intrinsically correlated based on the methods used to detect and calculate C. elegans quiescence. Therefore, we only considered possible correlations of arousal with bout duration and bout number. The effect of C. elegans genes previously tested on these parameters is reported in Table S3. For lgc-38(RNAi), cul-3(RNAi), inso-1(RNAi), cya-1(RNAi), and cnb-1(lf) animals, arousal thresholds were determined by chemosensory response (see Figures 2B, 2C, 2E, and 2F), which complicated comparison to the other 15 genes, whose arousal thresholds were determined by mechanosensory response. Therefore, we excluded these five genes from the correlation analysis described in the next paragraphs.
We noted that genotypes with increased arousal thresholds usually had both increased total quiescence, and vice versa (Table S3). Total quiescence is a function of quiescent bout duration and bout number. One possibility is that arousal threshold changes might affect bout duration. We tested this by looking for correlation between these two parameters and found that bout duration did not directly correlate with arousal thresholds (Figure 4A). This is not surprising because there is remarkably little variation in bout duration across genotype. Another possibility was that arousal threshold changes might affect bout number, as altered arousal might influence entry into quiescence. We found that bout number directly correlated with arousal thresholds (Figure 4B). In summary, we conclude that mechanisms governing bout duration are likely independent from the mechanisms regulating arousal thresholds. Increased bout number reflects increased propensity to enter a quiescent state. Together, these results make it likely that common genetic pathways likely regulate arousal thresholds and quiescence bout entry.
Genetic pathway for dopamine regulation of C. elegans lethargus quiescence
Combined with the previously published Drosophila and murine studies, our cross-species comparison of genes required for sleep (Figure 1B) confirms that decreased dopamine signaling increases sleep12,13,79 (Figure 1B). Past studies have determined the site of action of D1 receptor as well as the neuronal circuitry of dopamine signaling in Drosophila sleep.80,81 However, little is known about the signaling cascade acting downstream of the D1 receptor in sleep. We undertook genetic and phenotypic rescue experiments to assemble a signaling pathway downstream of dopamine regulating quiescence in C. elegans.
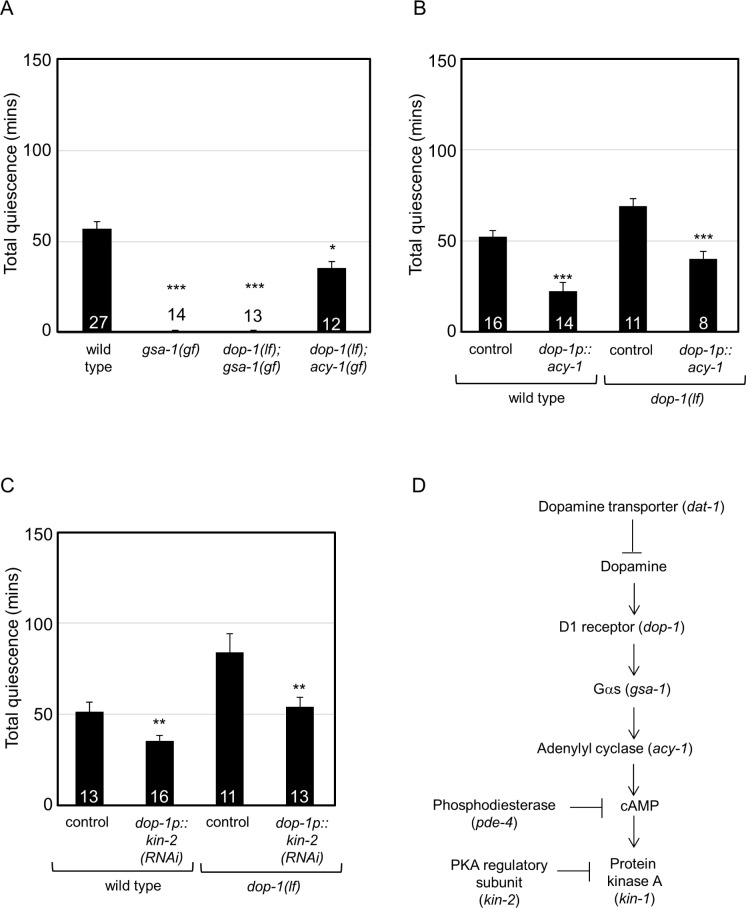
In humans, D1 dopamine receptors couple with various Gα proteins, including Gs and Go.82 Loss of the DOP-1 D1 receptor increased quiescence (Figure 1B, dop-1(lf)). The C. elegans Go ortholog goa-1 is unlikely to act downstream of the DOP-1 D1 receptor as loss of goa-1 decreased total quiescence (Figure 1E, goa-1(lf)). Therefore, the role of C. elegans Gs ortholog GSA-1 was assessed. Loss of gsa-1 function causes lethality; therefore, a previously characterized gsa-1(gf) gain of function allele was tested.65 gsa-1 gain of function decreased total quiescence (Figure 5A, gsa-1(gf)), which was consistent with increased signaling downstream of the DOP-1 D1 receptor. If gsa-1 functions downstream of dop-1, then gsa-1(gf) should suppress the increased quiescence of dop-1(lf) animals. We found that gsa-1(gf);dop-1(lf) double mutant animals had decreased total quiescence compared to dop-1(lf) animals (Figure 5A). Combined, these results suggest that gsa-1 functions downstream of dopamine and D1 dopamine receptors in C. elegans quiescence.
Figure 5.
Genetic pathway for dopamine regulation of C. elegans lethargus quiescence. Genetic epistasis and rescue experiments delineate a dopamine signaling pathway functioning in the regulation of C. elegans quiescence. Total quiescence was reported in min. Error bars represent standard error of the mean (s.e.m.). Numbers inside or above the bar indicate sample size. Results were grouped and controls were pooled for concise presentation. Control is dop-1 promoter driving gfp (dop-1p::gfp). (A) Gain of Gs (gsa-1) function or dop-1;gsa-1 double mutant animals had decreased total quiescence. dop-1;acy-1 double mutant animals had decreased total quiescence. (B) Expression of acy-1 complementary DNA (cDNA) in DOP-1 D1 receptor expressing neurons decreased total quiescence in wild-type animals. Expression of acy-1 cDNA in DOP-1 D1 receptor expressing neurons of dop-1(lf) animals suppressed the quiescence defect. (C) RNA interference (RNAi) knockdown of kin-2 in DOP-1 D1 receptor expressing neurons decreased total quiescence in wild-type animals. In dop-1(lf) animals, RNAi knockdown of kin-2 in DOP-1 D1 receptor expressing neurons suppressed the quiescence defect. *P < 0.05 **P < 0.01 ***P < 0.005 versus wild-type in A; versus transgenic control for each genotype in B and C. (D) A model for dopamine signaling mediated regulation of quiescence. Dopamine binding to D1 dopamine receptor DOP-1 activates downstream signaling via Gs (gsa-1). This results in the activation of adenylyl cyclase (acy-1) that produces cyclic adenosine monophosphate (cAMP). cAMP activates protein kinase A (PKA) to promote wakefulness. Consistently, knockdown of the PKA regulatory subunit (kin-2) in D1 receptor expressing neurons decreased quiescence. It is possible that phosphodiesterase 4 (pde-4 in C. elegans) regulates cAMP levels in this pathway.
The C. elegans adenylyl cyclase acy-1 is known to act downstream of gsa-1 in synaptic signaling and locomotion.66 Similar to the gsa-1(gf) effect on quiescence, acy-1(gf) decreased total quiescence (Figure 1D, acy-1(gf)). If acy-1 functions downstream of dop-1 and gsa-1, then acy-1 gain of function should suppress the increased quiescence of dop-1(lf) animals. Indeed, acy-1(gf); dop-1(lf) double mutant animals have decreased quiescence compared to the dop-1(lf) animals (Figure 5A). Together, these results suggest that both gsa-1 and acy-1 function downstream of or in parallel to dop-1 D1 receptor in quiescence.
Next, we determined if the components of the cAMP signaling pathway, adenylyl cyclase, PKA, and CREB, function in the DOP-1 D1 receptor expressing neurons. The effect of adenylyl cyclase activity in DOP-1 D1 receptor expressing neurons was assessed first. Overexpression of adenylyl cyclase in DOP-1 D1 receptor expressing neurons of wild-type animals was sufficient to decrease quiescence (Figure 5B). Additionally, overexpressing adenylyl cyclase in the D1 dopamine receptor expressing neurons of dop-1(lf) animals suppressed their quiescence defects (Figure 5B). Increased PKA activity in kin-2(lf) animals resulted in decreased total quiescence (Figure 1D, kin-2(lf)), which was concordant with the effect of acy-1(gf) on quiescence (Figure 1D, acy-1(gf)). To determine if increased PKA activity in DOP-1 D1 receptor expressing neurons was sufficient to regulate quiescence, we knocked down kin-2 in these neurons. As predicted, RNAi knockdown of kin-2 in DOP-1 D1 receptor expressing neurons partially recapitulated the quiescence defects of kin-2(lf) animals (Figure 5C). Additionally, RNAi knockdown of kin-2 in DOP-1 D1 receptor expressing neurons of dop-1(lf) animals suppressed their quiescence defects (Figure 5C). These results suggest that manipulating adenylyl cyclase and PKA activity in these neurons was sufficient to regulate quiescence.
We found that global loss of CREB function in crh-1(lf) animals increased quiescence (Figure 1D, crh-1(lf)). We confirmed this by RNAi knockdown of crh-1 (Table S3). However, it is not clear if CRH-1 CREB functions in C. elegans DOP-1 D1 neurons. Simultaneous overexpression of two crh-1 splice isoforms in DOP-1 D1 expressing neurons did not rescue the quiescence defects of dop-1(lf) animals (total quiescence of dop-1(lf);dop-1p::crh-1(cDNA) = 82 ± 7 min, P = 0.88). This might be because of insufficient phosphorylation of CRH-1 in dop-1(lf) animals.68 Expression of these two crh-1 splice isoforms did not ameliorate the total quiescence defects of crh-1(lf) animals (total quiescence of crh-1(lf);dop-1p::crh-1(cDNA) = 85 ± 7 min, P = 0.79). As described in other systems, CRH-1 may function downstream of other receptors or signaling cascade that may increase or decrease quiescence.70,83 Combined, these results suggest that cAMP/adenylyl cyclase and PKA function downstream of or parallel to dopamine and the D1 dopamine receptor in C. elegans neurons to regulate quiescence.
DISCUSSION
Sleep has many shared characteristics and is ubiquitous across the animal kingdom, suggesting that the genes and pathways required for this behavior are conserved. However, experimental evidence to support this hypothesis is limited. Previous work has independently identified roles in sleep for PKG, neuropeptides, EGF, and Notch signaling in both flies and nematodes.6,14,15,17–19,22,23 To test molecular conservation of sleep more broadly, we took advantage of two well-defined invertebrate model systems.4,5 A Drosophila literature survey allowed us to shortlist 26 genes required for rest. C. elegans orthologs of 20 of these genes were systematically tested for roles in quiescence and arousal. Disruption of nearly all of the orthologous C. elegans genes increased or decreased quiescence as would be predicted from Drosophila studies. We found few exceptions to this, which are discussed in the next paragraphs. Although only one allele was tested for most genes in the cross-species comparison, the results presented here demonstrate that conserved genes play critical roles in sleep in two well-defined invertebrate species.
Diminished response to sensory stimuli is an essential feature of sleep. Twenty genes altered C. elegans total quiescence, allowing a comprehensive analysis of their effect on arousal. In every genotype, arousal thresholds during quiescence were significantly different from wild-type C. elegans. Because few Drosophila studies report changes in arousal during rest,12,47,73,84 we can only speculate regarding cross-species roles for these genes in arousal. For genes encoding the dopamine transporter or Go, the effect on arousal thresholds was clearly conserved between these two species. It seems likely that many of the other genes described here will play a conserved role in arousal in Drosophila and other animals.
Only two of the genes tested here, jnk-1 and cya-1, had a discordant effect on C. elegans and Drosophila sleep. RNAi knockdown of Drosophila basket, which encodes C-Jun N-terminal kinase, results in decreased rest with no change in arousal thresholds.73 This is not concordant with either RNAi knockdown or loss of function of the C. elegans basket ortholog jnk-1; both manipulations increased total C. elegans quiescence and decreased arousal thresholds73 (and this study). Also, RNAi knockdown of Drosophila cycA, which encodes cyclin A, decreases rest, but knockdown of C. elegans cya-1 increased quiescence45 (and this study). It is unclear why these two genes had discordant effects on sleep in flies and nematodes. Possibly, their downstream targets differ in these species or these genes regulate different aspects of sleep in C. elegans and Drosophila. Previous work established that PKG and EGF play concordant roles in invertebrate sleep,6,17,18 but the Notch pathway affects different aspects of C. elegans and Drosophila sleep.15,19 We conclude that most genes play conserved roles in sleep across species, but a subset of genes play species-specific roles.
Given the large number of genes characterized here, we were able to examine for the first time possible correlations among quiescence metrics, such as bout number, bout duration, and arousal thresholds. Examination of C. elegans quiescence suggested that genotypes with increased total quiescence also had an increased number of quiescent bouts and had an increased lethargus duration, and vice versa (Table S3). There was little change in bout duration or frequency despite the large number of genotypes tested. In other words, increased quiescence generally occurs when mutant animals have more quiescent bouts and spend more time in lethargus. Therefore, common genetic mechanisms likely affect arousal thresholds and bout entry. However, bout duration may be independently regulated from arousal thresholds and may require other genes that will be identified in future studies.
We did discover a simple and relatively direct correlation between arousal thresholds and total time spent in quiescence for 15 genes. In other words, most of the genetic perturbations that decreased total quiescence also resulted in lowered arousal thresholds. Also, genetic perturbations that increased quiescence were almost always associated with higher arousal thresholds. Based on these observations, we considered two possibilities. Low arousal thresholds might result in an inability either to establish or to maintain quiescence during a bout. Counterintuitively, genotypes with aberrantly high arousal thresholds did not have increased bout duration, but they did have increased number of quiescent bouts late in lethargus and had increased lethargus duration. High arousal thresholds may indicate an inappropriate propensity to establish the quiescent state, resulting in more quiescent bouts. Low arousal thresholds may reflect an inability to enter the quiescent state. Alternatively, genes altering arousal thresholds may regulate lethargus duration. However, it is unclear if quiescence changes are the cause or the result of altered arousal thresholds. Also, it is unclear if quiescence and arousal are independently regulated by these 15 genes. This may be clarified in future studies that directly examine molecular and cellular mechanisms underlying arousal and sleep.
Dopamine plays a conserved role in sleep in flies and mice.12,13,53 Here, we extend this role to C elegans. However, the signaling pathways downstream of dopamine and dopamine receptors relevant to sleep were largely unknown. To establish a coherent model of dopamine signaling in sleep, we assembled a genetic pathway based on phenotype and confirmed action in a subset of C. elegans neurons using a combination of genetic epistasis and rescue experiments. Double mutant analysis suggested that Gs and adenylyl cyclase function in the dopamine signaling pathway, downstream of the DOP-1 D1 receptors. Additionally, activation of PKA or overexpression of adenylyl cyclase, only in neurons expressing DOP-1 D1 receptors, was sufficient to rescue the dop-1 quiescence defects (Figure 5), suggesting that adenylyl cyclase and PKA act in parallel or downstream of D1 receptors. DOP-1 D1 receptors are widely expressed in the C. elegans nervous system85; further studies will be needed to determine precisely which dop-1 expressing neurons are critical for sleep. It is likely that dopamine signaling via Gs, adenylyl cyclase, PKA, and perhaps CREB are required for sleep in all animals.
Results presented here confirm that the genetic underpinnings of sleep are broadly conserved in invertebrates. Some vertebrate orthologs of genes required for invertebrate sleep have been implicated previously in sleep.1,4 However, the connections between these genes and signaling pathways in vertebrate sleep remain mysterious. Invertebrate model systems can be used to rapidly identify and delineate these conserved pathways.
DISCLOSURE STATEMENT
This was not an industry supported study. Supported by National Institutes of Health (NIH) R01 GM078171 and NS055813. There was no off-label or investigational use. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. Huiyan Huang, Altar Sorkac, and other members of the laboratory for helpful discussion and feedback. We are grateful for advice and/or reagents from the C. elegans knockout consortia and numerous members of the C. elegans community, including Dr. Mark Alkema. Some strains were provided by the CGC (Caenorhabditis genetics center), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
SUPPLEMENTAL MATERIAL
Strains and alleles used in this study
Primers used in this study
Compilation of behavioral analysis of sleep and arousal for all genotypes
Analysis of correlation: arousal thresholds and bout duration (≥ 1 sec long)
False discovery rate analysis and cross-species orthology
Effect of Caenorhabditis elegans and Drosophila genes on quantity of sleep and arousal.
REFERENCES
- 1.Schade MA, Reynolds NK, Dollins CM, Miller KG. Mutations that rescue the paralysis of Caenorhabditis elegans ric-8 (synembryn) mutants activate the G alpha(s) pathway and define a third major branch of the synaptic signaling network. Genetics. 2005;169:631–49. doi: 10.1534/genetics.104.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates EA, Victor M, Jones AK, Shi Y, Hart AC. Differential contributions of Caenorhabditis elegans histone deacetylases to huntingtin polyglutamine toxicity. J Neurosci. 2006;26:2830–8. doi: 10.1523/JNEUROSCI.3344-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JI, Dhakal BK, Lee J, et al. The Caenorhabditis elegans homologue of Down syndrome critical region 1, RCN-1, inhibits multiple functions of the phosphatase calcineurin. J Mol Biol. 2003;328:147–56. doi: 10.1016/s0022-2836(03)00237-7. [DOI] [PubMed] [Google Scholar]
- 4.McDonald PW, Hardie SL, Jessen TN, Carvelli L, Matthies DS, Blakely RD. Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J Neurosci. 2007;27:14216–27. doi: 10.1523/JNEUROSCI.2992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chase DL, Pepper JS, Koelle MR. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci. 2004;7:1096–103. doi: 10.1038/nn1316. [DOI] [PubMed] [Google Scholar]
- 6.Segalat L, Elkes DA, Kaplan JM. Modulation of serotonin-controlled behaviors by Go in Caenorhabditis elegans. Science. 1995;267:1648–51. doi: 10.1126/science.7886454. [DOI] [PubMed] [Google Scholar]
- 7.Villanueva A, Lozano J, Morales A, et al. jkk-1 and mek-1 regulate body movement coordination and response to heavy metals through jnk-1 in Caenorhabditis elegans. EMBO J. 2001;20:5114–28. doi: 10.1093/emboj/20.18.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carre-Pierrat M, Baillie D, Johnsen R, et al. Characterization of the Caenorhabditis elegans G protein-coupled serotonin receptors. Invert Neurosci. 2006;6:189–205. doi: 10.1007/s10158-006-0033-z. [DOI] [PubMed] [Google Scholar]
- 10.Dusenbery DB, Sheridan RE, Russell RL. Chemotaxis-defective mutants of the nematode Caenorhabditis elegans. Genetics. 1975;80:297–309. doi: 10.1093/genetics/80.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine Functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–60. doi: 10.1016/j.neuron.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Jin Y, Jorgensen E, Hartwieg E, Horvitz HR. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J Neurosci. 1999;19:539–48. doi: 10.1523/JNEUROSCI.19-02-00539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bamber BA, Beg AA, Twyman RE, Jorgensen EM. The Caenorhabditis elegans unc-49 locus encodes multiple subunits of a heteromultimeric GABA receptor. J Neurosci. 1999;19:5348–59. doi: 10.1523/JNEUROSCI.19-13-05348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cirelli C, Bushey D, Hill S, et al. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–92. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- 15.Nakai Y, Horiuchi J, Tsuda M, et al. Calcineurin and its regulator sra/DSCR1 are essential for sleep in Drosophila. J Neurosci. 2011;31:12759–66. doi: 10.1523/JNEUROSCI.1337-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25:7377–84. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebestky T, Chang JS, Dankert H, et al. Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron. 2009;64:522–36. doi: 10.1016/j.neuron.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–62. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 19.Hendricks JC, Williams JA, Panckeri K, et al. A non-circadian role for cAMP signaling and CREB activity in Drosophila rest homeostasis. Nat Neurosci. 2001;4:1108–15. doi: 10.1038/nn743. [DOI] [PubMed] [Google Scholar]
- 20.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–60. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 21.Agosto J, Choi JC, Parisky KM, Stilwell G, Rosbash M, Griffith LC. Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat Neurosci. 2008;11:354–9. doi: 10.1038/nn2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parisky KM, Agosto J, Pulver SR, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–82. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo F, Yi W, Zhou M, Guo A. Go signaling in mushroom bodies regulates sleep in Drosophila. Sleep. 2011;34:273–81. doi: 10.1093/sleep/34.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahama K, Tomita J, Ueno T, Yamazaki M, Kume S, Kume K. Panneuronal knockdown of the c-Jun N-terminal Kinase (JNK) results in a reduction in sleep and longevity in Drosophila. Biochem Biophys Res Commun. 2012;417:807–11. doi: 10.1016/j.bbrc.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 25.Rogulja D, Young MW. Control of sleep by cyclin A and its regulator. Science. 2012;335:1617–21. doi: 10.1126/science.1212476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stavropoulos N, Young MW. insomniac and Cullin-3 regulate sleep and wakefulness in Drosophila. Neuron. 2011;72:964–76. doi: 10.1016/j.neuron.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crocker A, Sehgal A. Octopamine regulates sleep in Drosophila through protein kinase A-dependent mechanisms. J Neurosci. 2008;28:9377–85. doi: 10.1523/JNEUROSCI.3072-08a.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
REFERENCES
- 1.Caylak E. The genetics of sleep disorders in humans: narcolepsy, restless legs syndrome, and obstructive sleep apnea syndrome. Am J Med Genet A. 2009;149A:2612–26. doi: 10.1002/ajmg.a.33087. [DOI] [PubMed] [Google Scholar]
- 2.Retey JV, Adam M, Honegger E, et al. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc Natl Acad Sci U S A. 2005;102:15676–81. doi: 10.1073/pnas.0505414102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotronoulas G, Stamatakis A, Stylianopoulou F. Hormones, hormonal agents, and neuropeptides involved in the neuroendocrine regulation of sleep in humans. Hormones (Athens) 2009;8:232–48. doi: 10.14310/horm.2002.1239. [DOI] [PubMed] [Google Scholar]
- 4.Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011;146:194–207. doi: 10.1016/j.cell.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson MD, Raizen DM. A sleep state during C. elegans development. Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raizen DM, Zimmerman JE, Maycock MH, et al. Lethargus is a Caenorhabditis elegans sleep-like state. Nature. 2008;451:569–72. doi: 10.1038/nature06535. [DOI] [PubMed] [Google Scholar]
- 7.Yokogawa T, Marin W, Faraco J, et al. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol. 2007;5:e277. doi: 10.1371/journal.pbio.0050277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allada R, Siegel JM. Unearthing the phylogenetic roots of sleep. Curr Biol. 2008;18:R670–R9. doi: 10.1016/j.cub.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw P, Ocorr K, Bodmer R, Oldham S. Drosophila aging 2006/2007. Exp Gerontol. 2008;43:5–10. doi: 10.1016/j.exger.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crocker A, Sehgal A. Genetic analysis of sleep. Genes Dev. 2010;24:1220–35. doi: 10.1101/gad.1913110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bushey D, Cirelli C. From genetics to structure to function: exploring sleep in Drosophila. Int Rev Neurobiol. 2011;99:213–44. doi: 10.1016/B978-0-12-387003-2.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25:7377–84. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dzirasa K, Ribeiro S, Costa R, et al. Dopaminergic control of sleep-wake states. J Neurosci. 2006;26:10577–89. doi: 10.1523/JNEUROSCI.1767-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langmesser S, Franken P, Feil S, Emmenegger Y, Albrecht U, Feil R. cGMP-dependent protein kinase type I is implicated in the regulation of the timing and quality of sleep and wakefulness. PLoS One. 2009;4:e4238. doi: 10.1371/journal.pone.0004238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seugnet L, Suzuki Y, Merlin G, Gottschalk L, Duntley SP, Shaw PJ. Notch signaling modulates sleep homeostasis and learning after sleep deprivation in Drosophila. Curr Biol. 2011;21:835–40. doi: 10.1016/j.cub.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donlea J, Leahy A, Thimgan MS, et al. Foraging alters resilience/vulnerability to sleep disruption and starvation in Drosophila. Proc Natl Acad Sci U S A. 2012;109:2613–8. doi: 10.1073/pnas.1112623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Buskirk C, Sternberg PW. Epidermal growth factor signaling induces behavioral quiescence in Caenorhabditis elegans. Nat Neurosci. 2007;10:1300–7. doi: 10.1038/nn1981. [DOI] [PubMed] [Google Scholar]
- 18.Foltenyi K, Greenspan RJ, Newport JW. Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nat Neurosci. 2007;10:1160–7. doi: 10.1038/nn1957. [DOI] [PubMed] [Google Scholar]
- 19.Singh K, Chao MY, Somers GA, et al. C. elegans Notch signaling regulates adult chemosensory response and larval molting quiescence. Curr Biol. 2011;21:825–34. doi: 10.1016/j.cub.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belfer SJ, Chuang HS, Freedman BL, et al. Caenorhabditis-in-drop array for monitoring C. elegans quiescent behavior. Sleep. 2013;36:689–98G. doi: 10.5665/sleep.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graves LA, Hellman K, Veasey S, Blendy JA, Pack AI, Abel T. Genetic evidence for a role of CREB in sustained cortical arousal. J Neurophysiol. 2003;90:1152–9. doi: 10.1152/jn.00882.2002. [DOI] [PubMed] [Google Scholar]
- 22.Turek M, Lewandrowski I, Bringmann H. An AP2 Transcription Factor Is Required for a Sleep-Active Neuron to Induce Sleep-like Quiescence in C. elegans. Curr Biol. 2013;23:2215–23. doi: 10.1016/j.cub.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 23.Choi S, Chatzigeorgiou M, Taylor KP, Schafer WR, Kaplan JM. Analysis of NPR-1 reveals a circuit mechanism for behavioral quiescence in C. elegans. Neuron. 2013;78:869–80. doi: 10.1016/j.neuron.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parisky KM, Agosto J, Pulver SR, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–82. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwanir S, Tramm N, Nagy S, Wright C, Ish D, Biron D. The microarchitecture of C. elegans behavior during lethargus: homeostatic bout dynamics, a typical body posture, and regulation by a central neuron. Sleep. 2013;36:385–95. doi: 10.5665/sleep.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagy S, Wright C, Tramm N, Labello N, Burov S, Biron D. A longitudinal study of Caenorhabditis elegans larvae reveals a novel locomotion switch, regulated by Galphas signaling. Elife. 2013;2:e00782. doi: 10.7554/eLife.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hendricks JC, Finn SM, Panckeri KA, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–38. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 28.Sehgal A, Joiner W, Crocker A, et al. Molecular analysis of sleep: wake cycles in Drosophila. Cold Spring Harb Symp Quant Biol. 2007;72:557–64. doi: 10.1101/sqb.2007.72.018. [DOI] [PubMed] [Google Scholar]
- 29.Monsalve GC, Van Buskirk C, Frand AR. LIN-42/PERIOD controls cyclical and developmental progression of C. elegans molts. Curr Biol. 2011;21:2033–45. doi: 10.1016/j.cub.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 30.Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- 31.Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–70. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frokjaer-Jensen C, Davis MW, Ailion M, Jorgensen EM. Improved Mos1-mediated transgenesis in C. elegans. Nat Methods. 2012;9:117–8. doi: 10.1038/nmeth.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fire A, Harrison SW, Dixon D. A modular set of lacZ fusion vectors for studying gene expression in Caenorhabditis elegans. Gene. 1990;93:189–98. doi: 10.1016/0378-1119(90)90224-f. [DOI] [PubMed] [Google Scholar]
- 34.Fire A, Kondo K, Waterston R. Vectors for low copy transformation of C. elegans. Nucleic Acids Res. 1990;18:4269–70. doi: 10.1093/nar/18.14.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S. The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci. 1985;5:956–64. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 37.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–84. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 38.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 39.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–7. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 40.Bushey D, Huber R, Tononi G, Cirelli C. Drosophila Hyperkinetic mutants have reduced sleep and impaired memory. J Neurosci. 2007;27:5384–93. doi: 10.1523/JNEUROSCI.0108-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bushey D, Tononi G, Cirelli C. The Drosophila fragile X mental retardation gene regulates sleep need. J Neurosci. 2009;29:1948–61. doi: 10.1523/JNEUROSCI.4830-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naidoo N, Ferber M, Galante RJ, et al. Role of Homer proteins in the maintenance of sleep-wake states. PLoS One. 2012;7:e35174. doi: 10.1371/journal.pone.0035174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams JA, Sathyanarayanan S, Hendricks JC, Sehgal A. Interaction between sleep and the immune response in Drosophila: a role for the NFkappaB relish. Sleep. 2007;30:389–400. doi: 10.1093/sleep/30.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koh K, Joiner WJ, Wu MN, Yue Z, Smith CJ, Sehgal A. Identification of SLEEPLESS, a sleep-promoting factor. Science. 2008;321:372–6. doi: 10.1126/science.1155942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogulja D, Young MW. Control of sleep by cyclin A and its regulator. Science. 2012;335:1617–21. doi: 10.1126/science.1212476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papazian DM, Schwarz TL, Tempel BL, Jan YN, Jan LY. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science. 1987;237:749–53. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- 47.Cirelli C, Bushey D, Hill S, et al. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–92. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- 48.Nakai Y, Horiuchi J, Tsuda M, et al. Calcineurin and its regulator sra/DSCR1 are essential for sleep in Drosophila. J Neurosci. 2011;31:12759–66. doi: 10.1523/JNEUROSCI.1337-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomita J, Mitsuyoshi M, Ueno T, et al. Pan-neuronal knockdown of calcineurin reduces sleep in the fruit fly, Drosophila melanogaster. J Neurosci. 2011;31:13137–46. doi: 10.1523/JNEUROSCI.5860-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bandyopadhyay J, Lee J, Lee J, et al. Calcineurin, a calcium/calmodulin dependent protein phosphatase, is involved in movement, fertility, egg laying, and growth in Caenorhabditis elegans. Mol Biol Cell. 2002;13:3281–93. doi: 10.1091/mbc.E02-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song HO, Ahnn J. Calcineurin may regulate multiple endocytic processes in C. elegans. BMB Rep. 2011;44:96–101. doi: 10.5483/BMBRep.2011.44.2.96. [DOI] [PubMed] [Google Scholar]
- 52.Lee JI, Dhakal BK, Lee J, et al. The Caenorhabditis elegans homologue of Down syndrome critical region 1, RCN-1, inhibits multiple functions of the phosphatase calcineurin. J Mol Biol. 2003;328:147–56. doi: 10.1016/s0022-2836(03)00237-7. [DOI] [PubMed] [Google Scholar]
- 53.Andretic R, van Swinderen B, Greenspan RJ. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 2005;15:1165–75. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 54.Lebestky T, Chang JS, Dankert H, et al. Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron. 2009;64:522–36. doi: 10.1016/j.neuron.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jayanthi LD, Apparsundaram S, Malone MD, et al. The Caenorhabditis elegans gene T23G5.5 encodes an antidepressant- and cocaine-sensitive dopamine transporter. Mol Pharmacol. 1998;54:601–9. [PubMed] [Google Scholar]
- 56.Suo S, Sasagawa N, Ishiura S. Identification of a dopamine receptor from Caenorhabditis elegans. Neurosci Lett. 2002;319:13–6. doi: 10.1016/s0304-3940(01)02477-6. [DOI] [PubMed] [Google Scholar]
- 57.Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–62. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 58.Carre-Pierrat M, Baillie D, Johnsen R, et al. Characterization of the Caenorhabditis elegans G protein-coupled serotonin receptors. Invert Neurosci. 2006;6:189–205. doi: 10.1007/s10158-006-0033-z. [DOI] [PubMed] [Google Scholar]
- 59.Agosto J, Choi JC, Parisky KM, Stilwell G, Rosbash M, Griffith LC. Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat Neurosci. 2008;11:354–9. doi: 10.1038/nn2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bamber BA, Beg AA, Twyman RE, Jorgensen EM. The Caenorhabditis elegans unc-49 locus encodes multiple subunits of a heteromultimeric GABA receptor. J Neurosci. 1999;19:5348–59. doi: 10.1523/JNEUROSCI.19-13-05348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin Y, Jorgensen E, Hartwieg E, Horvitz HR. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J Neurosci. 1999;19:539–48. doi: 10.1523/JNEUROSCI.19-02-00539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crocker A, Sehgal A. Octopamine regulates sleep in Drosophila through protein kinase A-dependent mechanisms. J Neurosci. 2008;28:9377–85. doi: 10.1523/JNEUROSCI.3072-08a.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine Functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–60. doi: 10.1016/j.neuron.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 64.Hendricks JC, Williams JA, Panckeri K, et al. A non-circadian role for cAMP signaling and CREB activity in Drosophila rest homeostasis. Nat Neurosci. 2001;4:1108–15. doi: 10.1038/nn743. [DOI] [PubMed] [Google Scholar]
- 65.Reynolds NK, Schade MA, Miller KG. Convergent, RIC-8-dependent Galpha signaling pathways in the Caenorhabditis elegans synaptic signaling network. Genetics. 2005;169:651–70. doi: 10.1534/genetics.104.031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schade MA, Reynolds NK, Dollins CM, Miller KG. Mutations that rescue the paralysis of Caenorhabditis elegans ric-8 (synembryn) mutants activate the G alpha(s) pathway and define a third major branch of the synaptic signaling network. Genetics. 2005;169:631–49. doi: 10.1534/genetics.104.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Charlie NK, Thomure AM, Schade MA, Miller KG. The Dunce cAMP phosphodiesterase PDE-4 negatively regulates G alpha(s)-dependent and G alpha(s)-independent cAMP pools in the Caenorhabditis elegans synaptic signaling network. Genetics. 2006;173:111–30. doi: 10.1534/genetics.105.054007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Delghandi MP, Johannessen M, Moens U. The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cell Signal. 2005;17:1343–51. doi: 10.1016/j.cellsig.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 69.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–60. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 70.Kimura Y, Corcoran EE, Eto K, et al. A CaMK cascade activates CRE-mediated transcription in neurons of Caenorhabditis elegans. EMBO Rep. 2002;3:962–6. doi: 10.1093/embo-reports/kvf191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo F, Yi W, Zhou M, Guo A. Go signaling in mushroom bodies regulates sleep in Drosophila. Sleep. 2011;34:273–81. doi: 10.1093/sleep/34.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lochrie MA, Mendel JE, Sternberg PW, Simon MI. Homologous and unique G protein alpha subunits in the nematode Caenorhabditis elegans. Cell Regul. 1991;2:135–54. doi: 10.1091/mbc.2.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takahama K, Tomita J, Ueno T, Yamazaki M, Kume S, Kume K. Panneuronal knockdown of the c-Jun N-terminal Kinase (JNK) results in a reduction in sleep and longevity in Drosophila. Biochem Biophys Res Commun. 2012;417:807–11. doi: 10.1016/j.bbrc.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 74.Kawasaki M, Hisamoto N, Iino Y, Yamamoto M, Ninomiya-Tsuji J, Matsumoto K. A Caenorhabditis elegans JNK signal transduction pathway regulates coordinated movement via type-D GABAergic motor neurons. EMBO J. 1999;18:3604–15. doi: 10.1093/emboj/18.13.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stavropoulos N, Young MW. insomniac and Cullin-3 regulate sleep and wakefulness in Drosophila. Neuron. 2011;72:964–76. doi: 10.1016/j.neuron.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kipreos ET, Lander LE, Wing JP, He WW, Hedgecock EM. cul-1 is required for cell cycle exit in C. elegans and identifies a novel gene family. Cell. 1996;85:829–39. doi: 10.1016/s0092-8674(00)81267-2. [DOI] [PubMed] [Google Scholar]
- 77.Calixto A, Chelur D, Topalidou I, Chen X, Chalfie M. Enhanced neuronal RNAi in C. elegans using SID-1. Nat Methods. 2010;7:554–9. doi: 10.1038/nmeth.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kreutzer MA, Richards JP, De Silva-Udawatta MN, et al. Caenorhabditis elegans cyclin A- and B-type genes: a cyclin A multigene family, an ancestral cyclin B3 and differential germline expression. J Cell Sci. 1995;108(Pt 6):2415–24. doi: 10.1242/jcs.108.6.2415. [DOI] [PubMed] [Google Scholar]
- 79.Liu Q, Liu S, Kodama L, Driscoll MR, Wu MN. Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Curr Biol. 2012;22:2114–23. doi: 10.1016/j.cub.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ueno T, Tomita J, Tanimoto H, et al. Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat Neurosci. 2012;15:1516–23. doi: 10.1038/nn.3238. [DOI] [PubMed] [Google Scholar]
- 81.Seugnet L, Suzuki Y, Vine L, Gottschalk L, Shaw PJ. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr Biol. 2008;18:1110–7. doi: 10.1016/j.cub.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kimura K, White BH, Sidhu A. Coupling of human D-1 dopamine receptors to different guanine nucleotide binding proteins. Evidence that D-1 dopamine receptors can couple to both Gs and G(o) J Biol Chem. 1995;270:14672–8. doi: 10.1074/jbc.270.24.14672. [DOI] [PubMed] [Google Scholar]
- 83.Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cell Signal. 2004;16:1211–27. doi: 10.1016/j.cellsig.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 84.Wu MN, Koh K, Yue Z, Joiner WJ, Sehgal A. A genetic screen for sleep and circadian mutants reveals mechanisms underlying regulation of sleep in Drosophila. Sleep. 2008;31:465–72. doi: 10.1093/sleep/31.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wani KA, Catanese M, Normantowicz R, Herd M, Maher KN, Chase DL. D1 dopamine receptor signaling is modulated by the R7 RGS protein EAT-16 and the R7 binding protein RSBP-1 in Caenorhabditis elegans motor neurons. PLoS One. 2012;7:e37831. doi: 10.1371/journal.pone.0037831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Strains and alleles used in this study
Primers used in this study
Compilation of behavioral analysis of sleep and arousal for all genotypes
Analysis of correlation: arousal thresholds and bout duration (≥ 1 sec long)
False discovery rate analysis and cross-species orthology
Effect of Caenorhabditis elegans and Drosophila genes on quantity of sleep and arousal.