Abstract
Observations on the role of ovarian hormones in breast cancer growth, as well as interest in contraception, stimulated research into the biology of estrogens. The identification of the classical receptors ERα and ERβ and the transmembrane receptor GPER and the resolution of the structure of the ligand bound to its receptor established the principal molecular mechanisms of estrogen action. The presence of estrogen-like compounds in many plants used in traditional medicine or ingested as food ingredients, phytoestrogens, as well as the estrogenic activities of many industrial pollutants and pesticides, xenoestrogens, have prompted investigations into their role in human health. Phyto- and xenoestrogens bind to the estrogen receptors with a lower affinity than the endogenous estrogens and can compete or substitute the hormone. Xenoestrogens, which accumulate in the body throughout life, are believed to increase breast cancer risk, especially in cases of prenatal and prepuberal exposure whereas the role of phytoestrogens is still a matter of debate. At present, the application of phytoestrogens appears to be limited to the treatment of post-menopausal symptoms in women where the production of endogenous estrogens has ceased. In this review we discuss chemistry, structure and classification, estrogen signaling and the consequences of the interactions of estrogens, phytoestrogens and xenoestrogens with their receptors, the complex interactions of endogenous and exogenous ligands, the evaluation of the health risks related to xenoestrogens, and the perspectives toward the synthesis of potent third generation selective estrogen receptor modulators (SERMs).
Keywords: Breast cancer, estrogen receptor-alpha, estrogen receptor-beta, estrogen signaling, phytoestrogens, xenoestrogens, selective estrogen receptor modulators
INTRODUCTION
Estrogens (or oestrogens) from the ancient greek word οΐστρος oístrŏs (sting, passion), also denominated follicular hormones, are the most important female sexual hormones and belong to the class of steroid hormones. The three major naturally occurring estrogens in women are estrone (E1), estradiol (E2), and estriol (E3). Endogenous estrogens are produced prevalently by the granulosa cells of the ovarian follicles and corpora lutea, and to a very minor extent by the adrenal cortex. During pregnancy, estrogens are also produced by the placenta. In men, the production of low amounts of the hormone is observed in the testicles. In addition, estrogens are produced by the conversion of testosterone by aromatases present in adipose tissues, which are the main source of estrogens in women after menopause. The cyclic production of estrogens drives the menstrual cycle and the estrogenic burst in puberty drives the development of the mammary glands.
The relation between estrogen production and breast cancer growth is known since 1896 when George Beatson reported that removal of the ovaries from premenopausal women with advanced breast cancer produced a dramatic decrease in tumor size [1], and the development of contraceptive drugs further stimulated research on the estrogen system. The detection of the estrogen receptor by Elwood Jensen and colleagues [2] and the cloning of the ESR1 and ESR2 cDNA that encode the receptors α and β by the groups of Pierre Chambon and Jan Ake Gustafsson [3, 4], have built the base for our current molecular understanding of estrogen signaling.
The identification of a large group of compounds that share some structural features with endogenous estrogens in many plants and among pesticides and industrial pollutants has led to the concept of phytoestrogens and xenoestrogens, respectively. A growing body of evidence, that is reviewed here, shows that phyto- and xenoestrogens can affect the normal function of the endocrine system with both positive and negative effects that rely on the ability to compete with endogenous estrogens for binding with the receptors.
Scope of the present review is to highlight recent research the field of estrogen-like compounds with particular attention to the molecular mechanisms of action. We searched for articles containing “phytoestrogen*” (1310 Pubmed entries) or “xenoestrogens*” (275 Pubmed entries) in the title. It is impossible to cite all the articles identified and we therefore performed an arbitrary selection of articles that appeared to provide particularly relevant information. We apologize for any omission.
PHYTOESTROGENS
Phytoestrogens and Plants
Phytoestrogens are a group of chemical compounds derived from plants that share the ability to bind to the estrogen receptors (ERs) and to trigger estrogen dependent transcription [5]. These compounds are synthesized in plants starting from phenylpropanoids and simple phenols [6]. Crystallographic studies show that the 4-hydroxyl on the B ring of isoflavones mediates binding to ERs [7]. Phytoestrogens are only weakly estrogenic, their activity is 100/10000 lower than that of 17-β-estradiol (E2).
The interest in phytoestrogens derives from reports claiming that the assumption of phytoestrogens correlates with a lower insurgence of mammary carcinoma [8, 9]. Some of these substances (e.g. resveratrol) also act as natural antioxidants, an activity that has been linked to protective effects on the cardiovascular system [10]. Human exposure to phytoestrogens is almost exclusively through diet: estrogenic plant compounds are widespread in grains, vegetables, fruits and drinks (for example coffee). Once ingested, these compounds are either directly excreted or absorbed and eventually broken down into other compounds with estrogenic activity [11]. The levels of exposure to these substances are quite variable essentially depending on the type of diet: elevated levels are observed in infants fed with a soy based nutriments in their first months of life [12-14].
Eastern and southeastern Asian countries, such as Japan, show a reduced incidence of breast cancer as compared to the western countries [15-17]. This has been linked to differences in the diet with a different intake of phytoestrogens, mainly in the form of soy products [18, 19]. Similarly, a lower incidence of prostate cancer in Asia has been correlated with high consumption of soy isoflavones [20]. However, epidemiological observations indicate a correlation and cannot establish a causal relationship since the incidence of many other potential risk factors, including dietary ones, differs between Western and Asian countries. The protective effects against breast cancer of dietary phytoestrogen intake appear to be limited to pre-menopausal women and to affect mainly women who are overweight [21, 22]. Phytoestrogen consumption during adolescence correlates with a decreased risk of breast cancer onset many years later, suggesting that diet is an important factor to be considered starting in youth [23]. A similar correlation between the intake of phytoestrogens and a reduced colorectal cancer risk has also been shown [24].
A. Brief History
The first studies on phytoestrogens date back to 1926 but no effect in human or animal metabolism was observed [25]. In 1940, it was reported that red clover pastures affected the fecundity of grazing sheep [26]. More recently, interest has focused on the potential of phytoestrogens for human health, especially for the treatment of menopausal symptoms and the prevention of osteoporosis, cardiovascular diseases as well as dementia. Phytoestrogenic plants have been used since many centuries in traditional medicine for the treatment of menstrual and menopausal problems [27]. The plants most frequently used were Pueraria mirifica (Kwao Krua), Pueraria lobata (Kudzu), Chinese angelica (Angelica sinensis), Foeniculum vulgare (fennel), but most of all Trifolium pretense (red clover) [28]. The Pueraria species belonging to the fabaceae family are climbing plants native to southern Japan, China, India and Pakistan. An estrogenic phenol was isolated from P. mirifica in 1960. Angelica belongs to the large family of Apiaceae and is present almost everywhere. Angelica sylvestris grows even in the subarctic climate of the Northern Hemisphere.
There is a long list of common foods containing phytoestrogens among which soybeans, tempeh, linseed (flax), sesame seeds, wheatberries, fenugreek, oats, barley, beans, lentils, yams, rice, alfalfa, mung beans, apples, carrots, pomegranates, wheat germ, rice bran, lupin, kudzu, coffee, licorice root, mint, ginseng, hops, bourbon, beer, fennel and anise.
Chemistry and Classification
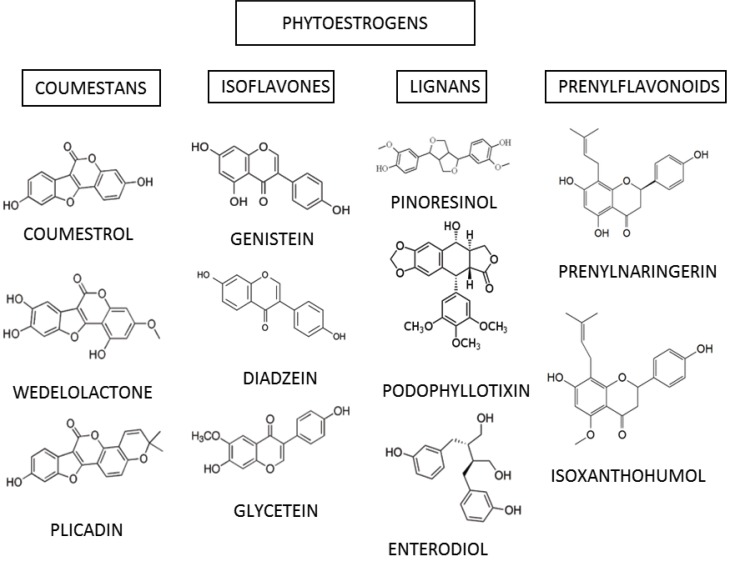
Currently the group of phytoestrogens includes more than 100 molecules, classified according to their chemical structure into flavonoids and non-flavonoids. Flavonoids can be further subdivided into isoflavones, coumestans and prenyl flavonoids. A class of non-flavonoids is constituted by lignans. All compounds are polyphenols with a structural similarity to E2. The ability to bind the ERs is due to the fact that these compounds have a ring similar to that of E2 and possess two hydroxyl groups whose function is to facilitate binding to ERs [29]. A scheme of classification is represented in (Table 1).
Table 1.
Source and Classification of the Most Common Phytoestrogens
| Phytoestrogens | ||
|---|---|---|
| Class | substances | Plants |
| COUMESTANS | COUMESTROL WEDELOLACTONE PLICADIN | CLOVER, ALFA ALFA, SPLIT PEAS, PINTO BEANS |
| ISOFLAVONES | GENISTEIN DIADZEIN GLYCETEIN FORMONONETIN | BEANS, SOYA, RED CLOVER |
| LIGNANS | PINORESINOL PODOPHYLLOTIXIN ENTERODIOL | FLAX SEED, SESAME, SOYBEANS, CROUCIFEROUS VEGETABLES, APRICOT, STRAWBERRIES |
| PRENYLFLAVONOIDS | PRENYLNARINGERIN ISOXANTHOHUMOL | HOPS, BEER |
Isoflavones include compounds like genistein, daidzein, biochanin A and formonetin. Sources of isoflavones are textured soybeans, pulses, red clover and beans. Coumestans are compounds derived from coumarin; food sources high in coumestans include split peas, pinto beans, lima beans, and especially alfalfa and clover sprouts. Prenylated isoflavones include compounds like erysenegalensein E, euchrenone b10, isoerysenegalensein E, 6,8-diprenylorobol, furowanin A and B, millewanins-F, G and H, warangalone, and auriculasin.
Lignans are one of the major classes of phytoestrogens. Plant lignans are polyphenolic substances derived from phenylalanine via dimerization of substituted cinnamic alcohols (see cinnamic acid), known as monolignols, to a dibenzylbutane skeleton. Pinoresinol, podophyllotixin and stegnacin belong to the class of lignans. They are also present in flax seed and sesame. Secoisolariciresinol diglucoside is the principal lignan precursor found in flaxseed. A large abundance of lignans is also present in soybeans, cruciferous vegetables such as broccoli and cabbage, and some fruits, in particular apricots and strawberries. Chemical structures of the most common phytoestrogens found in plants are represented in (Fig. 1).
Fig. (1).
Chemical structures of some of the most common phytoestrogens. The four principal classes and representative members of each class are shown.
Several authors addressed the effects of phytoestrogens on gene expression in breast and prostate cancer cell lines.
For prostate cancer, effects of isoflavones on miRNA expression [30] and the regulation of genes involved in the processes of cell cycle regulation, metastasis and angiogenesis [20] have been described. Genistein has been reported to alter the expression pattern of genes involved in invasion and metastasis in prostate cancer [31]. The ERα positive cell line MCF7 has been frequently used for the characterization of the effects of phytoestrogens on gene expression in breast cancer [32-36]. In most studies high concentrations of the compounds are used in order to obtain measurable effects. We have recently shown that the phytochemicals curcumin, enterolactone and quercetin have estrogenic effects on gene transcription even at low, physiological concentrations that become evident by the correlation analysis of estradiol and phytoestrogen induced gene expression profiles [37].
XENOESTROGENS
Occurrence
Xenoestrogens are chemical compounds derived from chemical and industrial processes that interfere with the endocrine system by mimicking the action of estrogenic hormones.
Over the past 40 years, many epidemiological, experimental and chemical studies (reviewed in [38]) have shown the accumulation of these compounds in the organism and their influence on the endocrine hormonal balance. The U.S. Environmental Protection Agency (EPA) has defined these as endocrine-disrupting compounds (EDCs), i.e. exogenous agents that interfere with the synthesis, secretion, transport, metabolism, binding, action or elimination of blood-borne estrogens naturally present in the body and responsible for homeostasis, reproduction and development processes [39]. The EDCs and their impact on the health of humans, animals and the environment in general have become one of the most active areas of research in toxicology [40, 41].
There are about 70,000 chemical compounds classified as potentially toxic and carcinogenic xenoestrogens (including herbicides, pesticides, fungicides, plastics, polystyrenes and others) some of which are used in the food industry for the production and packing of foods. They are found in soil, water, air, food and detergents. The number of compounds that may have deregulatory endocrine function capacity are over 10,000 and still growing [42].
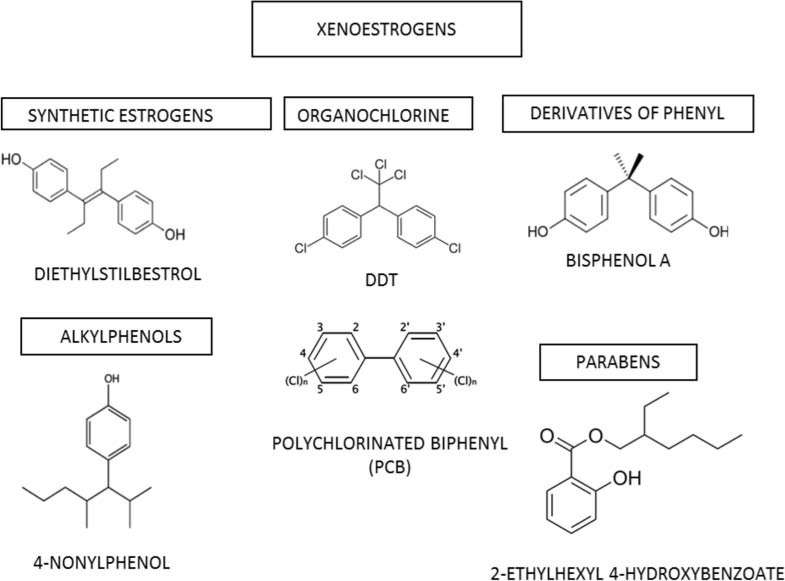
There are various sources of xenoestrogens. A scheme of classification is represented in (Table 2). Organic chlorines, a major source of xenoestrogens, are present in pesticides, products for dry cleaning and feminine hygiene, as well as among the byproducts of the production of plastics. Bisphenol-A, a breakdown of polycarbonate, is present in plastic bottles, in the lining of cans and containers used for foods and juices. A common preservative in processed foods is the xenoestrogen butylated hydroxyanisole. Most of skin lotions, creams, soaps, shampoos, cosmetics contain parabens, stearalkonium chloride and phenoxyethanol as preservatives. Due to their lipophilic nature these chemicals are easily and completely absorbed by the body. Phthalates are commonly found in baby lotions and powders. Many perfumes, air fresheners contain phthalates as artificial fragrances [43].
Table 2.
Source and Classification of the Most Common Xenoestrogens
| Xenoestrogens | ||
|---|---|---|
| Class | substances | Occurrence |
| SYNTHETIC ESTROGENS | DIETHYLSTILBESTROL DIENESTROL MESTRANOL | CATTLE FEED, PHARMACEUTICALS |
| ORGANOCHLORINE | DDT PCB | PETROLIUM DISTILLATES, EMULSIFIABLE CONCENTRATES, WATER WETTABLE POWDERS, GRANULES, AEROSOLS, CANDLE SMOKE, REFILLSFOR VAPORISERS AND LOTIONS |
| DERIVATIVES OF PHENYL | BISPHENOL A | BABY AND WATER BOTTLES, SPORT EQUIPMENT, CDs AND DVDs |
| ALKYLPHENOLS | NONYLPHENOL | STREAM WATER, DETERGENTS, PESTICIDES |
| PARABENS | 2-ETHYLHEXYL-4-HYDROXYBENZOATE | SHAMPOOS, PHARMACEUTICAL, MAKE UP, TOOTHPASTE, FOOD ADDITIVES |
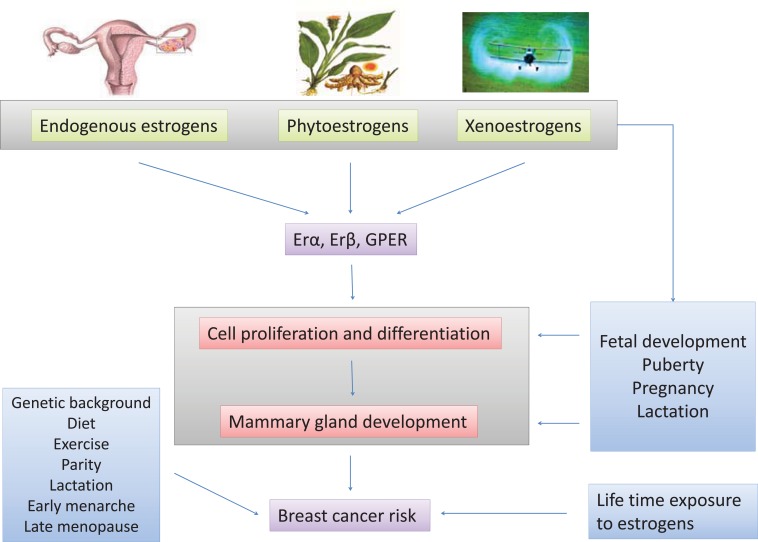
EDCs can affect reproductive behavior and sexual dimorphism mimicking, antagonizing or altering the action of steroid hormones. Exposure to EDCs during critical phases of development (e.g. perinatal and peripuberty) [44-46] can cause significant effects on neurodevelopment and/or reproductive processes [47]. Exposure to EDCs in adulthood may alter physiological processes, including the production of steroids, and the mediated (or independent) actions of classical steroid receptors (Fig. 3). In addition, EDCs may have effects on other substrates, such as on the arylhydrocarbon receptor (AhR) [48], the peroxisome proliferator-activated receptors (PPARs) [48-53], and nuclear receptors including retinoid acid receptors [51].
Fig. (3).
A schematic representation of some sources and mechanisms of action of endocrine disrupting chemicals (EDCs).
A. Brief History
From 1991 to date, there are more than 800 studies cited in Pubmed on the effects of chemicals released into the environment. The history of xenoestrogens began much earlier in 1938, when Dodds and his colleagues synthesized the Diethylstilbestrol (DES) [54, 55], a synthetic molecule with estrogen-like activity (its structure, although not steroid, mimics that of estrogen). This compound has been used from the forties to the seventies in the United States to prevent abortion, as it stimulates the synthesis of estrogen and progesterone in the placenta [56]. As a result of its use the occurrence of clear cell adenocarcinoma of the vagina in women born to mothers who had used the substance during pregnancy, a high incidence of epididymal cysts, testicular atrophy and hardening associated with capsular volume reduction and quantity and quality of ejaculated semen in male offspring have been observed [39, 57]. Many female reproductive disorders of the ovary (aneuploidy, polycystic ovary syndrome, and altered cyclicity) and the uterus (endometriosis, uterine fibroids, fetal growth restriction, and pregnancy loss) are due to exposure to EDCs [58]. The consequences of the use of DES administered until 1971 to millions of women are still observed today [59]. Breast milk exposes the infants to xenoestrogens. The World Health Organization strongly supports breastfeeding for its undisputed advantages for development, yet recognizes the potential risks to health arising from the presence of environmental toxins in breast milk including compounds with estrogenic activity. This contamination is due to persistent pollution. The exposure through breastfeeding can affect the health of children in the early stages of growth [13, 14, 60].
In 1968 Bitman discovered that some pesticides, in particular p-DDT (dichlorodiphenyltrichloroethane), induced estrogen-like responses in reproductive tissues of rats and avian species [61]. Phthalates, including DEHP (Di (2-ethylhexyl) phthalate, the most commonly used), were synthesized for the first time in the twenties and have been extensively used for several decades until the introduction of PVC. Phthalates occur in cosmetics as well as in toys and baby products [62]. Various studies showed the contamination of food through the migration of phthalates from the plastic film coated and plastic food containers [63-72]. In 1996, xenoestrogens were listed as priority risk factors by the European Commission. Since 2001, The Environment Directorate-General of the European Commission has classified DEHP, DBP and BBP as "reproductive-toxic." DEHP and DBP are also anticipated to be human carcinogens. The use of DINP, DIDP and DNOP (Di-n-octylphthalat) was prohibited for the production of clothing, food and toys for infants. In 1992 the World Health Organization (WHO) published a study showing the absorption of DEHP by the soil, its high solubility in the blood and its persistence in plants and animals [73, 74]. The main source of DEHP contamination is air pollution, especially in industrial areas. Bisphenol A (2,2-bis (4-hydroxyphenyl) propane), which was discovered by the Russian AP Dianin in 1891, commonly abbreviated as BPA, is an organic compound with two phenol groups essential in the synthesis of plastics and plastic additives. Although it is considered harmful to humans since the thirties, it was recognized as toxic only in 2008. Several studies have shown that this substance affects male sexual development in the fetus and decreases fertility in adult humans and in mice [75-80].
Chemistry and Classification
The xenoestrogens are part of multiple classes of chemical compounds with different structural characteristics. The main classes are:
synthetic estrogens (for example diethylstilbestrol)
alkylphenols (for example 4-nonylphenol)
derivatives of phenyl (for example bisphenol A)
organochlorines (for example DDT, PCBs)
parabens (for example 2-ethylhexyl 4-hydroxybenzoate) [81]
Estrogen mimetics were known for their effects on wildlife since 1960 when naturalists like Rachel Carson drew the attention on the endocrine disrupting effects of several pesticides, especially DDT [82]. The xenoestrogens are structurally vastly different, but have all in common lipophilic phenolic rings and other hydrophobic components, a characteristic they share with steroid hormones and their nuclear receptor-activating compounds (Fig. 2). They have very weak effects as compared to estrogens, but many studies have shown that low doses of xenoestrogens are sufficient to produce adverse effects on ecology and human health as they rapidly accumulate in adipose tissue [83-85]. The apparent "promiscuity" of estrogen receptors in binding several different ligands has been interpreted as an evolutionary residual of their original function as ligand activated regulatory proteins with a broad affinity spectrum [86]. Some byproducts of industrial production (pesticides, herbicides, plastics processing byproducts, fungicides, additives, cosmetics and pharmaceuticals) act improperly as estrogenic ligands [40].
Fig. (2).
Chemical structures of some of the most common xenoestrogens. Principal chemical groups and representative members are shown.
EFFECTS OF PHYTO- AND XENOESTROGENS ON HUMAN BREAST TISSUE
Proliferation and Apoptosis
It is generally accepted that estrogens and estrogen-like substances exert a proliferative effect in vitro. Hence, historically estrogenic activity was established by the MCF7 proliferation assay [87, 88]. The proliferation of this ERα positive cell line depends on the presence of E2 in the culture medium and the estrogenicity of a compound was assessed by its ability to induce MCF7 proliferation. This effect is mediated through ERα since it disappears in human breast cancer cell lines lacking both α- and β-ERs and in cells like MDA-MB 231 that express only ERβ. With a better understanding of hormone signaling the scenario has become more complex: the proliferative effect mediated by the interaction of ERα with the promoters of target genes is also influenced by membrane bound and cytosolic proteins that participate in triggering extra-nuclear signaling pathways. In addition, the proliferative signal could also be mediated by a member of the G-Protein Coupled Receptor (GPCR) superfamily, GPER [89-94] (see below). The large variety of estrogenic compounds corresponds to diverse affinities for the receptors. Therefore, it is not possible to find a common mechanism of action. Depending on their structure, the compounds can selectively alter the interaction between the ERs and their transcriptional cofactors, thus activating diverse signaling pathways acting as ER agonists or antagonists. One example of this double face action was reported and clearly explained by Marino and colleagues who showed how the flavonoids naringenine and quercetin could reveal in vitro an effect that is opposite to the food contaminant bisphenol A, even if these substances interact all with the same ERα and β: Bisphenol A promotes proliferation of MCF7 breast cancer cells while, under the same culture conditions, the flavonoids naringenine and quercetin inhibit MCF7 proliferation and promote apoptosis [95].
After the advent of genomic technologies, nearly a decade ago, the capability of a molecule to produce estrogenic effects in vitro is usually evaluated by gene expression profiling [37, 96-99]. This analysis, based on the comparison between the gene expression changes induced by E2 and by the substance to be tested, has shown that E2 regulates the expression of hundreds of genes [99, 100]. This technique is much more reliable and sensible than the proliferation assay and recently, by using gene expression profiling, we were able to demonstrate that nonylphenol exerts an estrogenic activity even at very low concentrations on MCF7 cells, thus confirming nonylphenol as an environmental hazard [101].
Embryonic Development and Stem Cells
Estrogen-like molecules could interfere with the physiologic function of endogenous hormones causing, among others, significant effects on the reproductive system both in females and males [102, 103]. This adverse effect is only in part understood, and the wide variability of the results found in the literature depends on the experimental approach, the concentrations tested, the time of exposure and the effects of confounding variables [104]. However, some evidence shows that Bisphenol A could act directly on the nucleus and interfere with the duplication process in embryonic cells leading to aneuploidy or meiotic arrest [105, 106]. Recently, to clarify the role of E2 and E2-like substances during development and differentiation, Jung and co-workers studied the effects of nonylphenol and octylphenol on embryonic stem cells, revealing that these molecules play a role in the differentiation of mouse ES cells by altering the expression of surface markers and maintaining ES cells in an undifferentiated state [107]. Consequently, next to the effect on the reproductive system, estrogenic activity could interfere also with non-reproductive tissues expressing ERs, such as skeletal muscle [108].
Mammary Gland Development and Maturation
Among the reproductive tissues, the mammary gland is one of the major targets for estrogens and estrogen-like molecules. E2 as well as other sex steroid hormones are at least partially responsible for its normal growth, development, and maturation. Most of the studies, performed on rodent mammary glands, show that the influence of estrogen-like compounds depends on the dose, the time of exposure and on the developmental stage at the moment of treatment [109]. The three major critical phases involving gland development: in utero, during puberty and during pregnancy, are under steroid hormone control. It has become evident that any interference by environmental factors, such as EDCs, could alter the normal maturation process, thus leading to serious impairment of mammary gland functions or eventually to cancer [110]. In humans, ductal development of mammary gland starts between the 12th and the 14th week of gestation [111] and consequently the exposure to EDCs during pregnancy is potentially very risky for the fetus (Fig. 3).
Breast Cancer Risk
The link between estrogens and breast cancer is based on the observation that an increased exposure to E2 in women during lifetime (early menarche, delayed menopause, null parity) was associated with an increased risk of developing breast cancer and ovariectomy was used to treat severe forms of the tumor [1]. Today, this association is well established and the observation that the block of ERs by anti-estrogens reduces the risk to develop breast cancer is the most direct evidence of the influence of E2, estrone and estriol on this pathology [112]. Other indirect evidence comes from the observation that the incidence of breast cancer is diminished after the decrease in the use of hormone replacement therapy (HRT) [113]. Estrogens exert their carcinogenic effects by the activation of ER dependent cell growth as well as through an ER independent mechanism such as estrogen-DNA adduct formation [114]. Recently, Tsubura and coworkers [113, 115] showed that early short-term administration of both E2 and progesterone in younger rats induces protection against mammary carcinogenesis, while in older rats it accelerates the development of carcinogen-induced breast cancer. The authors concluded that the protective effect of E2 and PR is true only for short-term treatments while long-term treatments increase the susceptibility to mammary carcinogenesis. This study again emphasizes how age, amount of estrogens and time of exposure are critical factors in favoring or preventing cancer risk and explains how the prolonged exposure to environmental xenoestrogens during the lifespan can be dangerous for human health. The observation that breast cancer incidence is higher in industrialized rather than in under-developed countries, and in urban areas rather than in rural areas [116], accompanied by the observation that most of the increase in breast cancer incidence is due to ER+ tumors [117], also suggests a link between breast cancer and xenoestrogens, with particular regard to environmental EDCs. This link has been experimentally demonstrated for several xenoestrogens [13, 84, 115, 118-121] even though the properties of various chemicals in vitro and in vivo vary greatly in terms of their contribution to breast cancer risk and prevention despite their structural similarities. For example, diethylstilbestrol (DES) and tetrachlorodi-benzo-p-dioxin (TCDD), structurally very dissimilar, both increase cancer susceptibility to breast cancer by retarding normal mammary gland development and maturation. In contrast, Bisphenol A (BPA), structurally similar to DES, has been shown to increase cell proliferation, to induce oxidative stress and to alter cell signaling pathways involved in carcinogenesis and glucose homeostasis in breast cancer cell lines [122]. On the other hand, genistein and resveratrol, despite structural similarities to each other and to E2, show properties of cancer prevention in vitro and in vivo. When given early in postnatal life they accelerate maturation of the mammary gland and protect against mammary carcinogenesis [122].
Phytoestrogens and Menopausal Symptoms
Phytoestrogens are largely employed to treat post-menopausal women, as an alternative to E2 for Hormone Replacement Therapy (HRT) with the belief that they possess similar estrogenic properties in reducing menopausal symptoms avoiding, however, the proliferative effects of estrogen itself [123, 124]. Phytoestrogens and xenoestrogens bind to the same receptors and in theory could therefore show the same adverse health effects on female health. Indeed many phytoestrogens are also considered EDCs and the question of whether phytoestrogens can be detrimental to human health has no definitive answer to date [8, 125, 126]. The difference of the effects achieved by phytoestrogens in comparison to E2 is mainly due to their lower ERα binding affinity. Phytoestrogens generally have a higher relative binding affinity for ERβ than ERα and a very weak binding affinity for ERα in comparison to E2 [5, 127-129]. The distinct ER binding capacities affect gene transcription and ER signaling. The effects are further influenced by age, health status and, in case of dietary assumption, by the quality of the gut microflora that contributes to the metabolic conversion of phytoestrogens [130]. The assumption of phytoestrogens during infancy and puberty could be dangerous [14, 23, 131, 132] whereas their use for the treatment of post-menopausal women is thought to provide benefits without serious adverse effects [126, 133]. The antioxidant properties of many phytoestrogens may contribute to prevent the formation of estrogen-DNA adducts, thus preventing ER independent carcinogenic activities [114, 134]. Lignans and isoflavones, orally assumed for treatment of post-menopausal symptoms, possess different pharmacokinetic properties. Isoflavones are absorbed and removed faster than lignans. Consistent with this, a large randomized study has shown that the combination of the different molecules is most efficacious for the control of postmenopausal symptoms during a 24 hour period [135].
MOLECULAR MECHANISMS
Structural Characterization of Classical ERs
Estrogens are involved in different physiological processes, including growth, development and homeostasis of a number of tissues, through binding to and activating the cognate receptors ERα and ERβ. These two “classical” Estrogen Receptors (ERs) belong to the nuclear receptor (NR) superfamily, a class of proteins that includes steroid and other hormones receptors as well as a number of orphan receptors [136]. ERα cDNA was first isolated in the 80s [3]. This protein has long been considered the principal mediator through which estrogen signals are exerted at the transcriptional level. In 1996, a second ER isoform, ERβ, was first cloned as a 477 aminoacid polypeptide from a prostate cDNA library [4, 137]. Two years later ERβ was definitively identified as a 530 residue protein [138]. ERα and ERβ are the products of two genes belonging to different chromosomes (6q25.1 and 14q23-24.1 respectively) [139, 140]. Although only two ERs have been found in mammals, a third ER (ERγ) has been identified in fish species [141]. Another class of nuclear receptors similar to ERs, named ER-related receptors (ERR) has also been identified [142]. Differently from classical ERs, 17β-estradiol (E2) does not bind to ERRs although it still displays affinity for the Estrogen Responsive Element consensus sequence (ERE). From a structural point of view, both ERs are modular proteins composed of six different functional domains (Regions A-F) interacting with each other. The N-terminal “A/B domain” is not well conserved among the protein family members. The A/B domain contains the activating function 1 (AF-1) and different phosphorylation and sumoylation sites [143, 144]. The dimension of this domain is variable and to date, no structural data for this region are available. The third domain of ERs, or C region, is the DNA Binding Domain (DBD). Besides being responsible for the binding to specific DNA sequences, this region plays a role in protein dimerization. The DBD is highly conserved among all nuclear receptors. The three dimensional structure of the DBD has been determined for ERα alone and complexed with DNA [145-148]. The DBDs are composed by globular regions that contain eight cysteine residues and two almost perpendicular α-helices; this region of ERs can be considered as being composed of two interdependent subdomains. The two cysteine clusters allow the tetrahedral coordination of two zinc ions, the first subdomain contains a P-box (proximal box) involved in DNA recognition, while the second, structurally different from the first, contains a D-box (distal box) responsible for DNA dependent DBD dimerization [146, 147]. The P-box amino acid sequence is identical in ERα and ERβ, as a consequence ERα and ERβ bind to EREs with similar specificity and affinity [149]. The D region of ERs is the hinge domain. Little structural information is available, although within this region there are the nuclear localization signal and different sites for sumoylation and acetylation. The C-terminal domain (or E/F region) of ERs is constituted by a large portion of the protein comprising the activating function-2 (AF-2), the dimerization and the ligand binding domain (LBD). In the absence of ligands, ERs bind to the heat-shock proteins (Hsp)70 and Hsp90 through the E/F region and this arrangement allows opening of the steroid binding cleft [150].
The Ligand Binding Domain (LBD)
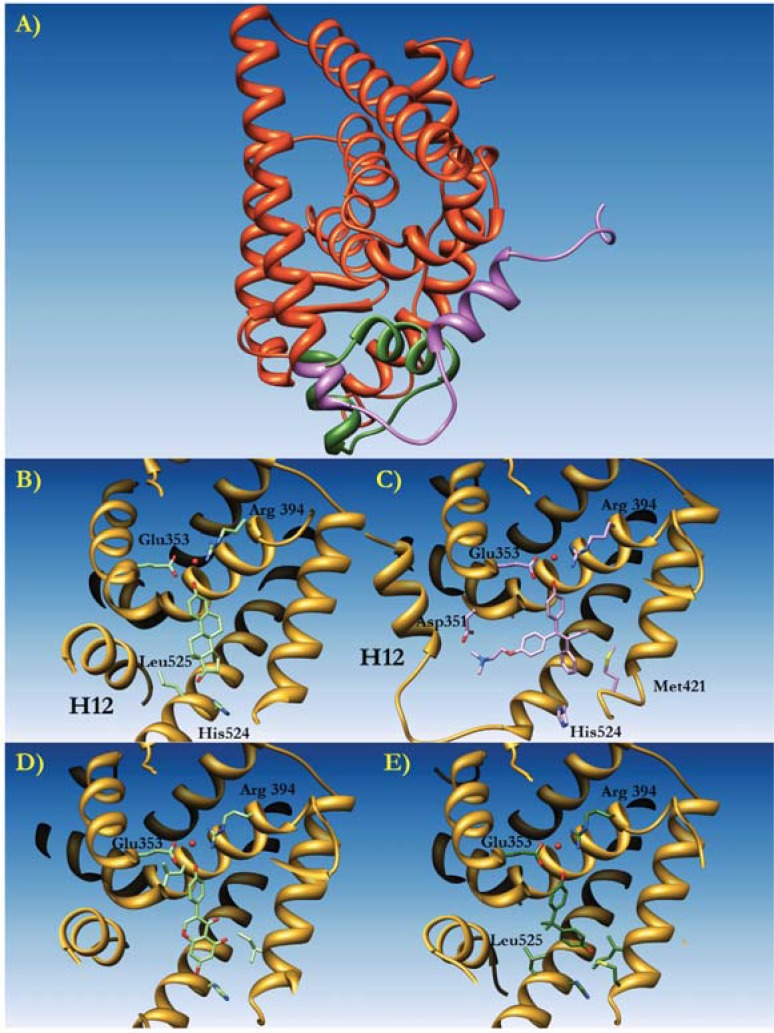
The three-dimensional structure of ER LBDs is very similar to the one of LBDs belonging to different members of the NR superfamily [143]. The overall structure of ERα has been first described in a complex with the natural ligand 17β-estradiol (E2) and with the synthetic antagonist raloxifene [7]. The ERα LBD is composed of 11 α-helices folded into an arrangement of three antiparallel layers (Fig. 4). A core layer composed of helices H4, H5, H6, H8 and H9, is sandwiched by the two external layers, made up by helices H1 and H3 on one side and by H7, H10 and H11 on the other side. The last helix, H12, and two antiparallel β-strands are positioned close to the ligand binding site of the protein. This molecular fold is universal among the LBDs of the NR superfamily. The volume of the ER binding site is about 450Å3, much larger than that of the natural ligand E2, which is about 250Å3. The active site cavity is formed by residues belonging to helices H3, H6, H8 and H11. This surrounded by the antiparallel β-sheet and the C-terminal helix H12 that is involved in the AF-2 transactivation function [7, 143, 144]. The binding of ligands to ERα induces the formation of an AF-2 hydrophobic pocket that regulates the recruitment of cofactors. The natural ligand E2 binds to the cavity by a combination of hydrogen bonds and hydrophobic interactions. The hydroxyl group on the E2 A-ring makes hydrogen bonding directly to the carboxyl group of Glu 353, to the guanidinium group of Arg 394 and to a structural water molecule. On the opposite side, the hydroxyl moiety of the D-ring of E2 is hydrogen bound to His 524. Several non-polar residues are involved in the hydrophobic contact, including Ala 350, Leu 387, Phe 404, Ile 424 and Leu 525. In protein:ligand complexes, helix H12 acts as a “lid” for the active site cavity I, where it is positioned over the ligand binding pocket in contact with helices H3, H5, H6 and H11. H12 is superficially charged: three negatively charged residues Asp 538, Glu542 and Asp 545 are positioned in a plane forming an almost 90° angle with respect to the dimerization interface. This construction is required for transcriptional activation, generating a competent AF-2 able to interact with a number of coactivators. The LBD of ERs can easily form homodimers, however the existence of heterodimers ERα:ERβ has also been demonstrated both in vivo and in vitro [151]. The interface formed by the two ERs, comprising residues belonging to helices H8, H10 and H11 and from the loop between helices H9 and H10, has been demonstrated by crystallographic studies. Considering that proteins crystallized so far omitted the F region (residues 553-595), it is possible that the arrangement suggested could be an artifact of the crystal packing. Indeed, it is possible to argue that the extension after helix H12 could strongly perturb dimeric assemblies. As previously stated, the transcription process requires coactivators that are recognized by ERs through the L-X-X-L-L motif. Furthermore, the AF-2 domain is also able to recognize the motifs L-X-X-Y-L and L-X-X-M-L [152]. Residues from helices H3, H4, and H5 form the co-activator binding site together with helix H12, this latter being in a closed conformation. Helix H12 may eventually not be in contact with the natural ligand E2. The binding mode of partial agonists (such as raloxifene) or antagonists (i.e. ICI 164,384) is similar to that of natural or synthetic agonists (i.e. E2); the only difference is that helix H12 is not aligned over the binding site cavity due to the longer side chains of these moieties. Displacement of H12 prevents the correct assembly of an AF-2 conformation competent for transcription, thus impairing recruitment of co-activators [7, 152].
Fig. (4).
Molecular Modeling of the estrogen receptor ERα complexed to diverse ligands. A) The three dimensional structure of human ERa represented as orange ribbons. Helix XII is colored in green when positioned in the active conformation, and in purple when in an inactivated (i.e. protein bound to an antagonist such as Tamoxifen) conformation. B) Detail of the conformation of Helix XII (H12) with the natural ligand 17-b-estradiol (E2, light green) bound to the active site. Residues involved in ligand binding are drawn as sticks. The active water molecule is represented by a red sphere. C) The conformation of Helix XII (H12) when binding hydroxytamoxifen. D) The conformation of Helix XII (H12) when binding Lasofoxifene. E) The conformation of Helix XII (H12) when binding Bisphenol A.
Many ERα variants have been described [153]. These variants are produced by alternative splicing of the primary ERα transcript. ESR1, the gene encoding ERα on chromosome 6q25.1, spans more than 300,000bp due to very long intronic sequences. The removal of these intronic sequences can lead to splicing variants missing one or more exons [154]. The most abundant splice variant in MCF7 cells and in many tumors lacks exon 4, which encodes for a protein devoid of the hinge region that joins the DNA binding domain to the ligand binding domain and also contains the nuclear location signal [155]. This variant also lacks the short sequence of the ligand binding domain encoded by exon 4. As a consequence, this variant could show altered ligand binding that would differentially affect binding of various ligands. However, translation into stable and functional protein of this variant has not been conclusively shown.
Estrogen-like Compounds and ERα
The first three dimensional structure of ERα was solved by X-ray Crystallography and reported in 1997 [7]. After this first structure describing the complex of ERα with E2 and with the selective antagonist raloxifene, an important body of data has been produced. To date, the atomic coordinates of more than 90 complexes with different ligands are deposited within the Protein Data Bank (PDB). Furthermore, different modeling studies have contributed to the identification of novel natural and/or synthetic ligands for ERα [149, 156, 157]. In broad terms, the ERα binding site is composed of a predominantly hydrophobic pocket where the ligand binds, as described previously for the ERα:E2 complex. Natural and synthetic ligands such as genistein [158] and diethylstilbestrol (DES) [159] share similar binding modes. All the ligands are completely encased in the large cleft of the LBD of ERα, with their OH groups superposed to the positions that are occupied by the hydroxyl groups of the A- and D-rings of E2. The three dimensional structure of ERα in complex with the isoflavone genistein (GEN) (PDB code 1X7R) shows how this phytoestrogen occupies the ERα binding site in an identical manner to that of E2 [158]. The hydroxyl group of A-ring makes hydrogen bonds with Glu353 and Arg394, the OH on the D-ring is hydrogen bound to His524 and the A-, C- and D- rings are perfectly overlapped. Our previous modeling studies analyzed the binding modes of resveratrol and its derivatives to ERα, through docking simulations [156]. In this case the hydroxyl groups of resveratrol are also engaged into hydrogen bonds with residues Glu 353, Arg 394 and His 524. Hydrophobic contacts with Leu384, Met387, Leu391, Phe404, Leu525 contribute to stabilize the complex. A similar behavior is also valid for the synthetic estrogen in binding to ERα. In the case of DES, for example, in addition to the interactions described for phytoestrogens, further contacts are made between the LBD and the ligand that may justify the higher receptor affinity for DES (Table 2). These non-polar contacts involve Ala350, Leu384, Phe404 and Leu 428. Particularly interesting is the three-dimensional structure of ERα in complex with raloxifene (RAL) [7] because it represents the first explanation of how an antagonist works at a molecular level. Superposing the three-dimensional structure of the ERα:E2 complex (PDB code 1ERE) with the ERα:raloxifene complex (PDB code 1ERR), a large movement of helix H12 immediately comes to light. A similar movement is found also in the structure of ERα in complex with other antagonists such as 4-Hydroxytamoxifene (4OHT, PDB code 3ERT) [159]. With respect to the configuration adopted in the case of ERα:E2 complex, H12 is rotated on about 130° and translated of about 10Å towards the N-terminal portion of the receptor [7], thus repositioning in a gorge created by helices H3 and H5 C-terminal fraction. 4OHT and RAL A-ring hydroxyl groups are bound to Glu 353 and Arg 394. The RAL second hydroxyl group (on the D-ring, not present in 4OHT) is hydrogen bound to His 524 but in a stereochemistry different from that adopted by E2. The imidazole ring in RAL is rotated to balance the change of position of the oxygen atom in the RAL D-ring with respect to E2. Analogously to 4OHT, a long hydrophobic chain in RAL allows the displacement of H12.
The “third generation“ of selective estrogen receptor modulators (SERMs) adopts a similar binding mode to that described for 4OHT and RAL. The three-dimensional structure of ERα in complex with lasofoxifene (LAS) [160] showed that the hydrogen bonds with Glu353 and Arg394 are maintained in a similar way as in the case of E2, RAL, 4OHT and other ligands. The hydrophobic contacts by the phenyl side chains of LAS are overlaid to the positions occupied by the C- and D-rings of E2. The LAS side chain, protruding from the core of the active site, does not allow the correct positioning of H12, similar to what happens in the case of 4OHT. Eventually, a tertiary amine of the LAS side chain is involved in a weak hydrogen bond with Asp351 (this bond is notably absent in the structure of 4OHT bound to ERα). This interaction neutralizes the charge of Asp351, therefore it may allow the interaction of ERα with a corepressor protein, determining a possible repositioning of AF-2 on the hydrophobic surface of helix 3. It has been shown that this movement of AF-2 is critical for corepressor recruitment in the case of PPARα bound to an antagonist [161].
Estrogen-like Compounds and ERβ
The expression of the two ER subtypes (ERα and ERβ) varies among organs and cell types, a fact that explains most of the differences in the cell type specific responses to phyto- and xenoestrogens [162]. The overall ERβ 3D structure is very similar to the one of ERα described above. The LBD is composed by the canonical sandwich motif where a central layer composed of helices H5, H6, H9 and H10 is flanked on the two sides by H7, H8 and H11 and by H2, H3 and H4 respectively [163]. Despite very similar ligand binding domains, the two receptor subtypes have different binding affinities for several phytoestrogens. Coumestrol can bind ERβ with a 7-fold higher affinity as compared to ERα, while the abortive mycotoxin zearalenone can bind both receptors with no significant difference in affinity. Solid phase binding assays show a higher binding affinity (20- to 30-fold more) of ERβ for genistein, apigenin and kaempferol. This different affinity is probably linked to the position and number of the hydroxyl substituents on the flavone or isoflavone molecule [5]. Genistein binds to ERβ in a cavity between H3 and H11, adopting a similar binding mode to the one of E2. Particularly, the phenolic ring of genistein is superposed to the A ring of E2, and its hydroxyl group is hydrogen bound to Glu305, Arg346 and a functional water molecule. On the other side of the molecule, the flavone part of genistein is superposable to the C- and D- rings of E2 with a hydroxyl group interacting with His 475. Hydrophobic contacts with residues Met295, Leu298, Leu301, Met 336, Leu339, Met340, Ile 373, Ile 376, Phe 377, Leu476 and Val487 contribute to stabilize the complex. The ERβ binding cleft is smaller than the one of ERα (390Å3 versus 490Å3), difference due to the replacement of a Leucine (Leu384 in ERα) with a bulky methionine (position 336 in ERβ). The relatively large size of the ERs binding pockets, compared to other NRs such as the thyroid receptor (TR), suggests that selectivity for these receptors can be generated by a number of different interactions. The very similar architecture of the two binding sites explains why most of the ligands bind to both ERs with similar affinity. However two conservative mutations in the binding clefts may play an important role on the ligand-binding preference for ERβ, by reducing the overall volume of this cavity. Genistein binds ERβ with a 30-fold higher affinity with respect to ERα. The main reason for this increased affinity is the Leu384/Met336 substitution mentioned above [163]. The high binding affinity of genistein for ERβ is also due to the presence of two hydroxyl groups on the flavone rings. Indeed, by eliminating one hydroxyl group (daidzein, biochanin A) or two hydroxyl groups (formononetin) it is possible to observe a great loss in ERβ ligand binding affinity. The flavone apigenin has moderate affinity for both ER subtypes and addition of hydroxyl groups (kaempferol, quercetin) does not increase but decreases the binding affinities [5].
Proteomics and transcriptomics indicate a crucial role of ERβ in the downstream signaling of phytoestrogens, in particular of genistein. In T47D-ERβ cells expressing ERβ and exposed to genistein down-regulation of genes and proteins involved in cell growth and induction of cell cycle arrest and apoptosis have been observed [164]. The binding preference for ERβ has also been reported in other studies [165, 166]. Mak et al. showed that apigenin-induced cancer cell death is mediated by ERβ and not by ERα or androgen receptor. This study examined the growth inhibitory action of apigenin in two ERβ expressing cell lines (DU145 and MDAMB-231) in the presence or in the absence of siRNA-mediated down-regulation of the receptor, showing that ERβ mediated apoptotic effect of apigenin but not of genistein [162]. Genistein analogs have been shown to reduce ERα but not ERβ expression, thereby obtaining anti-proliferative effects similar to genistein at much lower concentrations that are apparently mediated by ERβ [167].
Preferential signaling via ERβ might at least in part be explained by the different effect of phyto- and xenoestrogen binding on co-activator recruitment by the two receptor subtypes that has been observed for E2, genistein, diethylstilbestrol, 4-tert-octylphenol, 2',3',4', 5'-tetrachlorobiphenyl-ol, and bisphenol A [168]. Phyto- and xenoestrogens apparently also influence the expression levels of ERα and ERβ [167, 169]. Pennie et al. showed that the activation of the vitellogenin and luteinizing hormone beta promoters differs for the two receptor subtypes and also depends on the specific ligand [170]. The cellular response to estrogen-like compounds therefore depends on the ER subtype expression pattern as well as on the specific ligand.
Non Classical Estrogen Signaling
In contrast to the classical genomic action that takes place in the time-lapse of some hours after stimulation with E2, it has been demonstrated that physiological doses of E2 imply an increase in cAMP levels within seconds in ovariectomized rats [171]. This rapid action was inappropriately named as “non-genomic”. Typically, the rapid action of E2 is not influenced by transcriptional inhibitors (e.g actinomycin D). The non-genomic estrogen signaling cascade involves the generation of second messengers such as Ca2+, cAMP, and NO, and the activation of receptor tyrosine kinases, including EGFR, IGF-1R, PI 3-kinase, Akt, MAPKs, PKA/PKC and Src [172-177]. It has long been thought that the estrogen-responsive receptor in the case of rapid action could be the classical ER itself, or a modified form of the same protein [178, 179]. Indeed, complexes between the classical ERs and G proteins have been described [180]. Furthermore, it has been demonstrated that ER associations with plasma membrane G proteins can mediate NO production [181] and cAMP inhibition [180]. In summary, estrogen can mediate a plethora of rapid cellular activation events. Nevertheless, not all those actions can be attributed to the classical ERs. The ability of E2 to activate G proteins highlighted the role of the orphan G-protein coupled receptor (GPCR) 30 [90, 92, 93], recently renamed GPER (G-Protein coupled Estrogen Receptor).
GPER
Initially cloned in the late 90s and classified as orphan GPCR [91, 182], GPER (initially known as GPR30) is expressed in different tissues throughout the body (lung, liver, prostate, ovary, placenta). Several years later the possible function for GPER was identified by demonstrating protein kinases Erk1 and Erk2 activation induced by E2, as well as by the ER antagonists ICI 182,780 and tamoxifen, in breast cancer cell lines expressing GPER but not in cell lines lacking GPER [89]. Furthermore the up-regulation of c-fos by estrogen and phytoestrogens has also been shown in breast cancer cells [92, 93]. GPER, as the other 906 members of the GPCRs superfamily, is a 7 transmembrane protein (7TM) and its cellular localization is still a matter of debate. It has been described as expressed in the endoplasmatic reticulum [183] as well as in the plasma membrane [184, 185]. There is also the possibility that, under appropriate conditions, GPER could translocate from the endoplasmatic reticulum to the membrane and vice versa. Several studies have been conducted to identify the possible GPER ligands. Initially, binding with E2 was indicated with high specificity and an affinity constant Ki of about 6nM was measured by competition binding essays [94]. Later on, ER antagonists such as tamoxifen and ICI 182,780 were also shown to bind GPER [185], but in contrast to ER mediated effects, these molecules showed agonistic activities for GPER [89]. More recently, different highly selective, non-steroidal GPER antagonists have been reported [186-188], together with the first molecule acting as full antagonist against both classical ERs and GPER [186]. Future studies of GPER function should be greatly facilitated by these novel moieties [189].
GPER Structure
GPCRs are divided into 6 different structural classes in accordance with their homology and functional similarity: Class A (Rhodopsin-like receptors), Class B (Secretin receptors), Class C (Metabotropic glutamate/pheromone receptors), Class D (Fungal mating pheromone receptors), Class E (Cyclic AMP receptors), Class F (Frizzled/Smoothened receptors). Despite the low sequence similarity with other GPCRs, it is possible to classify GPER as belonging to the Class A subfamily. GPER shares a sequence identity of about 24.6% (calculated over 297 residues) with bovine Rhodopsin, which is the first GPCR atomic structure solved (PDB code 1F88) [190]. This protein had been the only GPCR with its three-dimensional structure solved for quite some time, until 2007 when the human β2-adrenergic receptor structure was determined [191]. Therefore, in our efforts to rationally design “in silico” novel GPER ligands, it has been necessary to use a GPER molecular model built by homology using bovine Rhodopsin as an X-ray template. The low degree of homology between these two GPCRs allowed us to build a trustable model only of the seven helices of the GPER transmembrane region. The remaining N-terminal and C-terminal portions of the protein and the cytosolic loops have been modeled “ab-initio” using the programs Robetta [192] and Modeller [193]. The initial GPER model has been validated by different “in vitro” tests [156, 186, 194-196]. The molecular model of GPER includes 375 amino acids folded in seven transmembrane helices forming a helical bundle common to all GPCRs, and a disulphide bond between the Cys130 and Cys207 residues. A N-terminal region (Met1- Phe60) and a C-terminal cytosolic domain (Leu328 - Val375) complete the structure. Helices TM I, TM V, TM VI and TM-VII display kinks induced by proline residues. These kinks are well conserved among GPCRs, and they are necessary to enable the structural rearrangements that are requested for the activation of the G protein effectors [197]. The molecular model shows that the C-terminal region seems to be structured with two more helices: helix VIII (Thr330 - Lys342) and helix IX (Leu345 - Ile360). While helix VIII is present in all Rhodopsin-like GPCRs, helix IX is unexpectedly predicted by the computational secondary structure analysis.
GPER Signaling
Estrogens can start multiple intracellular signaling processes. Although classical ERs have been demonstrated to be the mediators of many of these processes, we are beginning to understand the role played by GPER in mediating the action of diverse estrogenic compounds [198-200]. The activation of different pathways by estrogens has been ascribed to GPER: the MAP kinase Erk1/2 via EGFR transactivation [89], adenylyl cyclase activation [90, 185], and PI3K activation via EGFR transactivation in ER-negative breast cancer cells such as SKBr7 [201]. In particular, GPER couples to a trimeric G-protein, stimulating the cAMP pathway through a Gα [185] and Src kinase through Gβã [90]. subsequently, Src promotes the shedding of heparin-binding EGF-like growth factor and therefore EGFR activation [89], which in turn activates several signaling cascades, such as the ERK, PI3 kinase and phospholipase C pathways [200, 202]. Several studies have demonstrated that E2 triggers various biological effects through GPER [89, 90, 92, 93, 198], whereas it has been shown that estriol (E3) exerts an antagonist effect on this receptor [196].
A series of external signals are translated into changes in cellular functions through multiple intracellular signal transduction pathways. The integration and the cross talk between different metabolic routes are playmakers in determining biological outcomes such as cell proliferation, migration and differentiation. Recent studies show how GPER acts as an important player in this context. The binding to GPER of molecules acting as agonists results in the transactivation of epidermal growth factor receptor (EGFR) and in the activation of the ERK signaling in different cellular scenarios [200]. Moreover, EGFR signaling can up-regulate GPER expression thus establishing a regulatory loop which uses E2 to increase the growth effects observed in ER-negative breast cancer cells [203]. Eventually, it has been shown that the functional cross-talk of GPER with EGFR is extended to ERα in those cell containing these receptors, triggering a complex stimulatory signaling network in hormone-sensitive tumors [204].
CONCLUSIONS
Given its importance in human physiology and pathophysiology, estrogen signaling has been extensively studied, revealing a highly complex system of cellular signaling and transcription control. Classical ERs and the G-protein coupled receptor GPER bind endogenous estrogens, phyto- and xenoestrogens with varying effects that depend on the conformational changes induced through the interactions of the ligand with specific residues in the binding pouch of the receptors. Since the human organism is exposed to a variety of endogenous and exogenous ER and GPER ligands, it is very difficult to single out the effects of each compound. Endocrine disrupting activities, well established for many industrial pollutants and pesticides, appear to influence the cancer risk depending on the time and duration of exposure and the developmental stage in which it occurs. The contribution of phytoestrogens to human health is still debated and at present their application appears limited to the attenuation of postmenopausal symptoms. Further research needed to unravel the relationship between the different chemical structures and the biological effects elicited will eventually lead to the identification of new, potent and selective estrogen receptor modulators. A better understanding of the mechanisms underlying the action of phytoestrogens might allow for issuing precise dietary cancer prevention and women’s health strategies. Since xenoestrogens contribute to the rising cancer risk their industrial and agricultural use must be strictly limited and tightly controlled. New compounds with some structural similarity to estrogens must be monitored for eventual estrogenic activities on cell proliferation and gene transcription.
ACKNOWLEDGEMENTS
These studies were supported by AIRC (Associazione Italiana per la Ricerca sul Cancro) the Ministero della Salute Progetto Finalizzato and Grande Progetto Strategico, the Ministero dell'Istruzione, dell'Università e della Ricerca PRIN (Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale) 2010NECHBX_003, the Compagnia San Paolo di Torino and the Regione Liguria.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Beatson G. On the treatment of inoperable cases of carcinoma of the mamma suggestions for a new method of treatment with illustrative cases. Lancet. 1896;148:162– 165. [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen EV, Jacobsonk HI. Basic guides to the mechanism of estrogen action. Recent Prog. Horm. Res. 1962;18:387–414. [Google Scholar]
- 3.Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M, Chambon P. Cloning of the human estrogen receptor cDNA. Proc. Natl. Acad. Sci. USA. 1985;82(23):7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 6.Hahlbrock F. lavonoids in The biochemistry of plants a comprehensive treatise. Secondary plant products. 1981;7:425–456. [Google Scholar]
- 7.Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389(6652):753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 8.Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010;31(4):400–419. doi: 10.1016/j.yfrne.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.This P, de Cremoux P, Leclercq G, Jacquot Y. A critical view of the effects of phytoestrogens on hot flashes and breast cancer risk. Maturitas. 2011;70(3):222–226. doi: 10.1016/j.maturitas.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Pilsakova L, Riecansky I, Jagla F. The physiological actions of isoflavone phytoestrogens. Physiol Res. 2010;59(5):651–664. doi: 10.33549/physiolres.931902. [DOI] [PubMed] [Google Scholar]
- 11.Makela S, Santti R, Salo L, McLachlan JA. Phytoestrogens are partial estrogen agonists in the adult male mouse. Environ. Health Perspect. 1995;103(7):123–127. doi: 10.1289/ehp.103-1518873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy based infant formula. Lancet. 1997;350(9070):23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 13.Dorea JG. Maternal xenoestrogen exposure and breast feeding. J. Perinatol. 2005;25(8):558–559. doi: 10.1038/sj.jp.7211338. [DOI] [PubMed] [Google Scholar]
- 14.Massart F, Harrell JC, Federico G, Saggese G. Human breast milk and xenoestrogen exposure a possible impact on human health. J. Perinatol. 2005;25(4):282–288. doi: 10.1038/sj.jp.7211251. [DOI] [PubMed] [Google Scholar]
- 15.Low YL, Taylor JI, Grace PB, Dowsett M, Scollen S, Dunning AM, Mulligan AA, Welch AA, Luben RN, Khaw KT, Day NE, Wareham NJ, Bingham SA. Phytoestrogen exposure correlation with plasma estradiol in postmenopausal women in European Prospective Investigation of Cancer and Nutrition-Norfolk may involve diet-gene interactions. Cancer Epidemiol Biomarkers Prev. 2005;14(1):213–220. [PubMed] [Google Scholar]
- 16.Grace PB, Taylor JI, Low YL, Luben RN, Mulligan AA, Botting NP, Dowsett M, Welch AA, Khaw KT, Wareham NJ, Day NE, Bingham SA. Phytoestrogen concentrations in serum and spot urine as biomarkers for dietary phytoestrogen intake and their relation to breast cancer risk in European prospective investigation of cancer and nutrition-norfolk. Cancer Epidemiol. Biomarkers Prev. 2004;13(5):698–708. [PubMed] [Google Scholar]
- 17.Adlercreutz H, Fotsis T, Bannwart C, Wahala K, Makela T, Brunow G, Hase T. Determination of urinary lignans and phytoestrogen metabolites potential antiestrogens and anticarcinogens in urine of women on various habitual diets. J. Steroid Biochem. 1986;25(5B):791–797. doi: 10.1016/0022-4731(86)90310-9. [DOI] [PubMed] [Google Scholar]
- 18.Rice S, Whitehead SA. Phytoestrogens and breast cancer--promoters or protectors? Endocr. Relat. Cancer. 2006;13(4):995–1015. doi: 10.1677/erc.1.01159. [DOI] [PubMed] [Google Scholar]
- 19.Messina M J, Persky V, Setchell K D, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr. Cancer. 1994;21(2):113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 20.Handayani R, Rice L, Cui Y, Medrano TA, Samedi VG, Baker HV, Szabo NJ, Shiverick KT. Soy isoflavones alter expression of genes associated with cancer progression, including interleukin-8 in androgen-independent PC-3 human prostate cancer cells. J. Nutr. 2006;136(1):75–82. doi: 10.1093/jn/136.1.75. [DOI] [PubMed] [Google Scholar]
- 21.Cotterchio M, Boucher BA, Kreiger N, Mills CA, Thompson LU. Dietary phytoestrogen intake--lignans and isoflavones--and breast cancer risk (Canada) Cancer Causes Control. 2008;19(3):259–272. doi: 10.1007/s10552-007-9089-2. [DOI] [PubMed] [Google Scholar]
- 22.Linseisen J, Piller R, Hermann S, Chang-Claude J. Dietary phytoestrogen intake and premenopausal breast cancer risk in a German case-control study. Int. J Cancer. 2004;110(2):284–290. doi: 10.1002/ijc.20119. [DOI] [PubMed] [Google Scholar]
- 23.Thanos J, Cotterchio M, Boucher BA, Kreiger N, Thompson LU. Adolescent dietary phytoestrogen intake and breast cancer risk (Canada) Cancer Causes Control. 2006;17(10):1253–1261. doi: 10.1007/s10552-006-0062-2. [DOI] [PubMed] [Google Scholar]
- 24.Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey A, Harper P. Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. J Nutr. 2006;136(12):3046–3053. doi: 10.1093/jn/136.12.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartman J, Strom A, Gustafsson JA. Current concepts and significance of estrogen receptor beta in prostate cancer. Steroids. 2012;77(12):1262–1266. doi: 10.1016/j.steroids.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Urpi-Sarda M, Morand C, Besson C, Kraft G, Viala D, Scalbert A, Besle JM, Manach C. Tissue distribution of isoflavones in ewes after consumption of red clover silage. Arch. Biochem. Biophys. 2008;476(2):205–210. doi: 10.1016/j.abb.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Ferrari A. Soy extract phytoestrogens with high dose of isoflavones for menopausal symptoms. J. Obstet. Gynaecol. Res. 2009;35(6):1083–1090. doi: 10.1111/j.1447-0756.2009.01058.x. [DOI] [PubMed] [Google Scholar]
- 28.Geller S E, Shulman L P, van Breemen R B, Banuvar S, Zhou Y, Epstein G, Hedayat S, Nikolic D, Krause E C, Piersen C E, Bolton J L, Pauli G F, Farnsworth N R. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause. 2009;16(6):1156–1166. doi: 10.1097/gme.0b013e3181ace49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zand R S, Jenkins D J, Diamandis EP. Steroid hormone activity of flavonoids and related compounds. Breast Cancer Res. Treat. 2000;62(1):35–49. doi: 10.1023/a:1006422302173. [DOI] [PubMed] [Google Scholar]
- 30.Rabiau N, Trraf H K, Adjakly M, Bosviel R, Guy L, Fontana L, Bignon YJ, Bernard-Gallon DJ. miRNAs differentially expressed in prostate cancer cell lines after soy treatment. In Vivo. 2011;25(6):917–921. [PubMed] [Google Scholar]
- 31.Li Y, Sarkar FH. Down-regulation of invasion and angiogenesis-related genes identified by cDNA microarray analysis of PC3 prostate cancer cells treated with genistein. Cancer Lett. 2002;186(2):157–164. doi: 10.1016/s0304-3835(02)00349-x. [DOI] [PubMed] [Google Scholar]
- 32.Richter D U, Abarzua S, Chrobak M, Scholz C, Kuhn C, Schulze S, Kupka MS, Friese K, Briese V, Piechulla B, Jeschke U. Effects of phytoestrogen extracts isolated from flax on estradiol production and ER/PR expression in MCF7 breast cancer cells. Anticancer Res. 2010;30(5):1695–1699. [PubMed] [Google Scholar]
- 33.Lee YJ, Jin YR, Lim WC, Park WK, Cho JY, Jang S, Lee SK. Ginsenoside-Rb1 acts as a weak phytoestrogen in MCF-7 human breast cancer cells. Arch. Pharm. Res. 2003;26(1):58–63. doi: 10.1007/BF03179933. [DOI] [PubMed] [Google Scholar]
- 34.Gallo D, Ferlini C, Fabrizi M, Prislei S, Scambia G. Lack of stimulatory activity of a phytoestrogen-containing soy extract on the growth of breast cancer tumors in mice. Carcinogenesis. 2006;27(7):1404–1409. doi: 10.1093/carcin/bgi338. [DOI] [PubMed] [Google Scholar]
- 35.Ren L, Marquardt M A, Lech JJ. Estrogenic effects of nonylphenol on pS2, ER and MUC1 gene expression in human breast cancer cells-MCF-7. Chem. Biol. Interact. 1997;104(7):55–64. doi: 10.1016/s0009-2797(97)03767-8. [DOI] [PubMed] [Google Scholar]
- 36.Shiizaki K, Goto K, Ishige A, Komatsu Y. Bioassay of phytoestrogen in herbal medicine used for postmenopausal disorder using transformed MCF-7 cells. Phytother. Res. 1999;13(6):498–503. doi: 10.1002/(sici)1099-1573(199909)13:6<498::aid-ptr495>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 37.Bachmeier B E, Mirisola V, Romeo F, Generoso L, Esposito A, Dell'eva R, Blengio F, Killian PH, Albini A, Pfeffer U. Reference profile correlation reveals estrogen-like trancriptional activity of Curcumin. Cell. Physiol. Biochem. 2010;26(3):471–482. doi: 10.1159/000320570. [DOI] [PubMed] [Google Scholar]
- 38.Sonnenschein C, Soto AM. An updated review of environmental estrogen and androgen mimics and antagonists. J. Steroid. Biochem. Mol. Biol. 1998;65(1-6):143–150. doi: 10.1016/s0960-0760(98)00027-2. [DOI] [PubMed] [Google Scholar]
- 39.Diamanti-Kandarakis E, Bourguignon J P, Giudice L C, Hauser R, Prins G S, Soto A M, Zoeller R T, Gore AC. Endocrine-disrupting chemicals an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson CS, Bulayeva NN, Wozniak AL, Alyea RA. Xenoestrogens are potent activators of nongenomic estrogenic responses. Steroids. 2007;72(2):124–134. doi: 10.1016/j.steroids.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maradonna F, Batti S, Marino M, Mita D G, Carnevali O. Tamoxifen as an emerging endocrine disruptor effects on fish reproduction and detoxification target genes. Ann. N. Y. Acad. Sci. 2009;1163:457–459. doi: 10.1111/j.1749-6632.2008.03653.x. [DOI] [PubMed] [Google Scholar]
- 42.Yu SJ, Keenan SM, Tong W, Welsh WJ. Influence of the structural diversity of data sets on the statistical quality of three-dimensional quantitative structure-activity relationship (3D-QSAR) models: predicting the estrogenic activity of xenoestrogens. Chem. Res. Toxicol. 2002;15(10):1229–1234. doi: 10.1021/tx0255875. [DOI] [PubMed] [Google Scholar]
- 43.Frye C A, Bo E, Calamandrei G, Calza L, Dessi-Fulgheri F, Fernandez M, Fusani L, Kah O, Kajta M, Le Page Y, Patisaul HB, Venerosi A, Wojtowicz AK, Panzica GC. Endocrine disrupters: a review of some sources effects and mechanisms of actions on behaviour and neuroendocrine systems. J. Neuroendocrinol. 2012;24(1):144–159. doi: 10.1111/j.1365-2826.2011.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DiVall SA. The influence of endocrine disruptors on growth and development of children. Curr. Opin. Endocrinol. Diabetes Obes. 2013;20(1):50–55. doi: 10.1097/MED.0b013e32835b7ee6. [DOI] [PubMed] [Google Scholar]
- 45.Yeung BH, Wan HT, Law AY, Wong CK. Endocrine disrupting chemicals Multiple effects on testicular signaling and spermatogenesis. Spermatogenesis. 2011;1(3):231–239. doi: 10.4161/spmg.1.3.18019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fowler PA, Bellingham M, Sinclair KD, Evans NP, Pocar P, Fischer B, Schaedlich K, Schmidt JS, Amezaga MR, Bhattacharya S, Rhind SM, O'Shaughnessy PJ. Impact of endocrine-disrupting compounds (EDCs) on female reproductive health. Mol. Cell. Endocrinol. 2012;355(2):231–239. doi: 10.1016/j.mce.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 47.Guzman C, Zambrano E. [Endocrine disruptor compounds and their role in the developmental programming of the reproductive axis] Rev. Invest Clin. 2007;59(1):73–81. [PubMed] [Google Scholar]
- 48.Rogers JA, Metz L, Yong VW. Review Endocrine disrupting chemicals and immune responses a focus on bisphenol-A and its potential mechanisms. Mol Immunol. 2013;53(4):421–430. doi: 10.1016/j.molimm.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Caserta D, Bordi G, Ciardo F, Marci R, La Rocca C, Tait S, Bergamasco B, Stecca L, Mantovani A, Guerranti C, Fanello EL, Perra G, Borghini F, Focardi SE, Moscarini M. The influence of endocrine disruptors in a selected population of infertile women. Gynecol. Endocrinol. 2013;29:444–447. doi: 10.3109/09513590.2012.758702. [DOI] [PubMed] [Google Scholar]
- 50.Choi J S, Oh J H, Park H J, Choi M S, Park S M, Kang S J, Oh M J, Kim S J, Hwang S Y, Yoon S. miRNA regulation of cytotoxic effects in mouse Sertoli cells exposed to nonylphenol. Reprod. Biol. Endocrinol. 2011;9:126. doi: 10.1186/1477-7827-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheshenko K, Pakdel F, Segner H, Kah O, Eggen RI. Interference of endocrine disrupting chemicals with aromatase CYP19 expression or activity, and consequences for reproduction of teleost fish. Gen. Comp. Endocrinol. 2008;155(1):31–62. doi: 10.1016/j.ygcen.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Bainy AC. Nuclear receptors and susceptibility to chemical exposure in aquatic organisms. Environ. Int. 2007;33(4):571–575. doi: 10.1016/j.envint.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Zmuda JM, Modugno F, Weissfeld JL, Cauley JA, Trump DL, Moffett SP, Ferrell RE. Peroxisome proliferator-activated receptor-gamma polymorphism body mass and prostate cancer risk evidence for gene-environment interaction. Oncology. 2006;70(3):185–189. doi: 10.1159/000093805. [DOI] [PubMed] [Google Scholar]
- 54.Sohail Z. DES banned again. Nature. 1973;243(5401):6. [PubMed] [Google Scholar]
- 55.Dodds ECGL, Lawson W, Robinson R. Oestrogenic activity of certain synthetic compounds. Nature. 1938;141:247–248. [Google Scholar]
- 56.Lopez-Espinosa MJ, Silva E, Granada A, Molina-Molina JM, Fernandez MF, Aguilar-Garduno C, Olea-Serrano F, Kortenkamp A, Olea N. Assessment of the total effective xenoestrogen burden in extracts of human placentas. Biomarkers. 2009;14(5):271–277. doi: 10.1080/13547500902893744. [DOI] [PubMed] [Google Scholar]
- 57.Caserta D, Maranghi L, Mantovani A, Marci R, Maranghi F, Moscarini M. Impact of endocrine disruptor chemicals in gynaecology. Hum. Reprod. Update. 2008;14(1):59–72. doi: 10.1093/humupd/dmm025. [DOI] [PubMed] [Google Scholar]
- 58.Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC, Guillette LJ., Jr Female reproductive disorders the roles of endocrine disrupting compounds and developmental timing. Fertil Steril. 2008;90(4):911–940. doi: 10.1016/j.fertnstert.2008.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoover R N, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville A L, Colton T, Hartge P, Hatch EE, Herbst A L, Karlan BY, Kaufman R, Noller KL, Palmer JR, Robboy SJ, Saal RC, Strohsnitter W, Titus-Ernstoff L, Troisi R. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N. Engl. J. Med. 2011;365(14):1304–1314. doi: 10.1056/NEJMoa1013961. [DOI] [PubMed] [Google Scholar]
- 60.Moral R, Wang R, Russo IH, Lamartiniere CA, Pereira J, Russo J. Effect of prenatal exposure to the endocrine disruptor bisphenol A on mammary gland morphology and gene expression signature. J. Endocrinol. 2008;196(1):101–112. doi: 10.1677/JOE-07-0056. [DOI] [PubMed] [Google Scholar]
- 61.Bitman J, Cecil HC, Harris SJ, Fries GF. Estrogenic activity of o,p'-DDT in the mammalian uterus and avian oviduct. Science. 1968;162(3851):371–372. doi: 10.1126/science.162.3851.371. [DOI] [PubMed] [Google Scholar]
- 62.Schettler T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006;29(1):134–139. doi: 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 63.Sharman M, Read W A, Castle L, Gilbert J. Levels of di-(2-ethylhexyl)phthalate and total phthalate esters in milk cream butter and cheese. Food Addit. Contam. 1994;11(3):375–385. doi: 10.1080/02652039409374236. [DOI] [PubMed] [Google Scholar]
- 64.Petersen J H, Jensen L K. Phthalates and food-contact materials enforcing the 2008 European Union plastics legislation Food Addit. . Contam. Part A Chem. Anal. Control Expo Risk Assess. 2010;27(11):1608–1616. doi: 10.1080/19440049.2010.501825. [DOI] [PubMed] [Google Scholar]
- 65.Kappenstein O, Vieth B, Luch A, Pfaff K. Toxicologically relevant phthalates in food. EXS. 2012;101:87–106. doi: 10.1007/978-3-7643-8340-4_4. [DOI] [PubMed] [Google Scholar]
- 66.Xu Q, Yin X, Wang M, Wang H, Zhang N, Shen Y, Xu S, Zhang L, Gu Z. Analysis of Phthalate Migration from Plastic Containers to Packaged Cooking Oil and Mineral Water. J. Agric. Food Chem. 2010 doi: 10.1021/jf102821h. [DOI] [PubMed] [Google Scholar]
- 67.Rothenbacher T, Schwack W. Rapid identification of additives in poly(vinyl chloride) lid gaskets by direct analysis in real time ionisation and single-quadrupole mass spectrometry. Rapid Commun. Mass Spectrom. 2010;24(1):21–29. doi: 10.1002/rcm.4350. [DOI] [PubMed] [Google Scholar]
- 68.Gartner S, Balski M, Koch M, Nehls I. Analysis and migration of phthalates in infant food packed in recycled paperboard. J. Agric. Food Chem. 2009;57(22):10675–10681. doi: 10.1021/jf902683m. [DOI] [PubMed] [Google Scholar]
- 69.Fromme H, Lahrz T, Piloty M, Gebhart H, Oddoy A, Ruden H. Occurrence of phthalates and musk fragrances in indoor air and dust from apartments and kindergartens in Berlin (Germany) Indoor Air. 2004;14(3):188–195. doi: 10.1111/j.1600-0668.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- 70.Tsumura Y, Ishimitsu S, Kaihara A, Yoshii K, Nakamura Y, Tonogai Y. Di(2-ethylhexyl) phthalate contamination of retail packed lunches caused by PVC gloves used in the preparation of foods. Food Addit. Contam. 2001;18(6):569–579. doi: 10.1080/02652030120071. [DOI] [PubMed] [Google Scholar]
- 71.Petersen JH, Breindahl T. Plasticizers in total diet samples, baby food and infant formulae. Food Addit. Contam. 2000;17(2):133–141. doi: 10.1080/026520300283487. [DOI] [PubMed] [Google Scholar]
- 72.Aurela B, Kulmala H, Soderhjelm L. Phthalates in paper and board packaging and their migration into Tenax and sugar. Food Addit. Contam. 1999;16(12):571–577. doi: 10.1080/026520399283713. [DOI] [PubMed] [Google Scholar]
- 73.Olea N, Pazos P, Exposito J. Inadvertent exposure to xenoestrogens. Eur. J. Cancer Prev. 1998;7(1):S17–S23. doi: 10.1097/00008469-199802001-00005. [DOI] [PubMed] [Google Scholar]
- 74.Olea N, Olea-Serrano F, Lardelli-Claret P, Rivas A, Barba-Navarro A. Inadvertent exposure to xenoestrogens in children. Toxicol. Ind. Health. 1999;15(1-2):151–158. doi: 10.1191/074823399678846682. [DOI] [PubMed] [Google Scholar]
- 75.Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, Lanphear BP. Prenatal bisphenol A exposure and early childhood behavior. Environ. Health. Perspect. 2009;117(12):1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dallinga J W, Moonen E J, Dumoulin J C, Evers J L, Geraedts J P, Kleinjans JC. Decreased human semen quality and organochlorine compounds in blood. Hum. Reprod. 2002;17(8):1973–1979. doi: 10.1093/humrep/17.8.1973. [DOI] [PubMed] [Google Scholar]
- 77.Irvine DS. Declining sperm quality: a review of facts and hypotheses. Baillieres Clin. Obstet. Gynaecol. 1997;11(4):655–671. doi: 10.1016/s0950-3552(97)80005-3. [DOI] [PubMed] [Google Scholar]
- 78.Golden R J, Noller K L, Titus-Ernstoff L, Kaufman R H, Mittendorf R, Stillman R, Reese EA. Environmental endocrine modulators and human health an assessment of the biological evidence. Crit. Rev. Toxicol. 1998;28(2):109–227. doi: 10.1080/10408449891344191. [DOI] [PubMed] [Google Scholar]
- 79.Izzotti A, Longobardi M, Cartiglia C, D'Agostini F, Kanitz S, De Flora S. Pharmacological modulation of genome and proteome alterations in mice treated with the endocrine disruptor bisphenol A. Curr. Cancer Drug Targets. 2010;10(2):147–154. doi: 10.2174/156800910791054220. [DOI] [PubMed] [Google Scholar]
- 80.Izzotti A, Kanitz S, D'Agostini F, Camoirano A, De Flora S. Formation of adducts by bisphenol A an endocrine disruptor in DNA in vitro and in liver and mammary tissue of mice. Mutat Res. 2009;679(1-2):28–32. doi: 10.1016/j.mrgentox.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 81.Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, Tong W, Shi L, Perkins R, Sheehan DM. The estrogen receptor relative binding affinities of 188 natural and xenochemicals structural diversity of ligands. Toxicol. Sci. 2000;54(1):138–153. doi: 10.1093/toxsci/54.1.138. [DOI] [PubMed] [Google Scholar]
- 82.Kroll G. The "Silent Springs" of Rachel Carson mass media and the origins of modern environmentalism. Public Underst Sci. 2001;10(4):403–420. doi: 10.1088/0963-6625/10/4/304. [DOI] [PubMed] [Google Scholar]
- 83.Coyle YM. The effect of environment on breast cancer risk. Breast Cancer Res Treat. 2004;84(3):273–288. doi: 10.1023/B:BREA.0000019964.33963.09. [DOI] [PubMed] [Google Scholar]
- 84.Folmar LC, Hemmer M, Denslow ND, Kroll K, Chen J, Cheek A, Richman H, Meredith H, Grau EG. A comparison of the estrogenic potencies of estradiol ethynylestradiol diethylstilbestrol nonylphenol and methoxychlor in vivo and in vitro. Aquat. Toxicol. 2002;60(1-2):101–110. doi: 10.1016/s0166-445x(01)00276-4. [DOI] [PubMed] [Google Scholar]
- 85.Vandenberg LN, Colborn T, Hayes TB, Heindel J J, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012;33(3):378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baker ME. Co-evolution of steroidogenic and steroid-inactivating enzymes and adrenal and sex steroid receptors. Mol. Cell. Endocrinol. 2004;215(1-2):55–62. doi: 10.1016/j.mce.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 87.Soto A M, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ. Health Perspect. 1995;103(7):113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blom A, Ekman E, Johannisson A, Norrgren L, Pesonen M. Effects of xenoestrogenic environmental pollutants on the proliferation of a human breast cancer cell line (MCF-7) Arch. Environ. Contam. Toxicol. 1998;34(3):306–310. doi: 10.1007/s002449900322. [DOI] [PubMed] [Google Scholar]
- 89.Filardo E J, Quinn J A, Bland K I, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog GPR30 and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000;14(10):1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 90.Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30 stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol. Endocrinol. 2002;16(1):70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 91.Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45(3):607–617. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- 92.Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Ando S. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17beta-estradiol and phytoestrogens in breast cancer cells. J. Biol. Chem. 2004;279(26):27008–27016. doi: 10.1074/jbc.M403588200. [DOI] [PubMed] [Google Scholar]
- 93.Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Ando S, Maggiolini M. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol. Endocrinol. 2006;20(3):631–646. doi: 10.1210/me.2005-0280. [DOI] [PubMed] [Google Scholar]
- 94.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 95.Marino M, Pellegrini M, La Rosa P, Acconcia F. Susceptibility of estrogen receptor rapid responses to xenoestrogens Physiological outcomes. Steroids. 2012;77(10):910–917. doi: 10.1016/j.steroids.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 96.Cicatiello L, Scafoglio C, Altucci L, Cancemi M, Natoli G, Facchiano A, Iazzetti G, Calogero R, Biglia N, De Bortoli M, Sfiligoi C, Sismondi P, Bresciani F, Weisz A. A genomic view of estrogen actions in human breast cancer cells by expression profiling of the hormone responsive transcriptome. J Mol Endocrinol. 2004;32(3) doi: 10.1677/jme.0.0320719. [DOI] [PubMed] [Google Scholar]
- 97.Terasaka S, Inoue A, Tanji M, Kiyama R. Expression profiling of estrogen-responsive genes in breast cancer cells treated with alkylphenols chlorinated phenols parabens or bis and benzoylphenols for evaluation of estrogenic activity. Toxicol. Lett. 2006;163(2):130–141. doi: 10.1016/j.toxlet.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 98.Inoue A, Seino Y, Terasaka S, Hayashi S, Yamori T, Tanji M, Kiyama R. Comparative profiling of the gene expression for estrogen responsiveness in cultured human cell lines. Toxicol In Vitro. 2007;21(4):741–752. doi: 10.1016/j.tiv.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 99.Inoue A, Yoshida N, Omoto Y, Oguchi S, Yamori T, Kiyama R, Hayashi S. Development of cDNA microarray for expression profiling of estrogen-responsive genes. J. Mol Endocrinol. 2002;29(2):175–192. doi: 10.1677/jme.0.0290175. [DOI] [PubMed] [Google Scholar]
- 100.Terasaka S, Aita Y, Inoue A, Hayashi S, Nishigaki M, Aoyagi K, Sasaki H, Wada-Kiyama Y, Sakuma Y, Akaba S, Tanaka J, Sone H, Yonemoto J, Tanji M, Kiyama R. Using a customized DNA microarray for expression profiling of the estrogen-responsive genes to evaluate estrogen activity among natural estrogens and industrial chemicals. Environ. Health Perspect. 2004;112(7):773–781. doi: 10.1289/ehp.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amaro AA, Esposito AI, Mirisola V, Mehilli A, Rosano C, Noonan DM, Albini A, Pfeffer U, Angelini G. Endocrine disruptor agent Nonyl phenol exerts an estrogen-like transcriptional activity on estrogen receptor positive breast cancer cells. Curr. Med. Chem. 2013 doi: 10.2174/09298673113209990169. [DOI] [PubMed] [Google Scholar]
- 102.Lee PC, Lee W. In vivo estrogenic action of nonylphenol in immature female rats. Bull. Environ. Contam. Toxicol. 1996;57(3):341–348. doi: 10.1007/s001289900196. [DOI] [PubMed] [Google Scholar]
- 103.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308(5727):1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hunt PA, Susiarjo M, Rubio C, Hassold TJ. The bisphenol A experience: a primer for the analysis of environmental effects on mammalian reproduction. Biol Reprod. 2009;81(5):807–813. doi: 10.1095/biolreprod.109.077008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.George O, Bryant BK, Chinnasamy R, Corona C, Arterburn JB, Shuster CB. Bisphenol A directly targets tubulin to disrupt spindle organization in embryonic and somatic cells. ACS Chem. Biol. 2008;3(3):167–179. doi: 10.1021/cb700210u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eichenlaub-Ritter U, Vogt E, Cukurcam S, Sun F, Pacchierotti F, Parry J. Exposure of mouse oocytes to bisphenol A causes meiotic arrest but not aneuploidy. Mutat. Res. 2008;651(1-2):82–92. doi: 10.1016/j.mrgentox.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 107.Jung E M, Choi K C, Yu F H, Jeung EB. Effects of 17beta-estradiol and xenoestrogens on mouse embryonic stem cells. Toxicol. In Vitro. 2010;24(6):1538–1545. doi: 10.1016/j.tiv.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 108.Galluzzo P, Rastelli C, Bulzomi P, Acconcia F, Pallottini V, Marino M. 17beta-Estradiol regulates the first steps of skeletal muscle cell differentiation via ER-alpha-mediated signals. Am. J. Physiol Cell Physiol. 2009;297(5):C1249–C1262. doi: 10.1152/ajpcell.00188.2009. [DOI] [PubMed] [Google Scholar]
- 109.Fenton SE. Endocrine-disrupting compounds and mammary gland development early exposure and later life consequences. Endocrinology. 2006;147(6):S18–S24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- 110.Moon HJ, Han SY, Shin J H, Kang I H, Kim T S, Hong J H, Kim S H, Fenton S E. Gestational exposure to nonylphenol causes precocious mammary gland development in female rat offspring. J. Reprod. Dev. 2007;53:333–344. doi: 10.1262/jrd.18055. [DOI] [PubMed] [Google Scholar]
- 111.Russo J, Russo I H. Biological and molecular bases of mammary carcinogenesis. Lab. Invest. 1987;57(2):112–137. [PubMed] [Google Scholar]
- 112.Vogel V G. The NSABP Study of Tamoxifen and Raloxifene (STAR) trial. Expert Rev. Anticancer Ther. 2009;9(1):51–60. doi: 10.1586/14737140.9.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Katalinic A, Rawal R. Decline in breast cancer incidence after decrease in utilisation of hormone replacement therapy. Breast Cancer Res. Treat. 2008;107(3):427–430. doi: 10.1007/s10549-007-9566-z. [DOI] [PubMed] [Google Scholar]
- 114.Hinrichs B, Zahid M, Saeed M, Ali M F, Cavalieri E L, Rogan E G. Formation of diethylstilbestrol-DNA adducts in human breast epithelial cells and inhibition by resveratrol. J. Steroid Biochem. Mol. Biol. 2011;127(3-5):276–281. doi: 10.1016/j.jsbmb.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsubura A, Uehara N, Matsuoka Y, Yoshizawa K, Yuri T. Estrogen and progesterone treatment mimicking pregnancy for protection from breast cancer. In Vivo. 2008;22(2):191–201. [PubMed] [Google Scholar]
- 116.Dey S, Soliman A S, Hablas A, Seifeldin I A, Ismail K, Ramadan M, El-Hamzawy H, Wilson M L, Banerjee M, Boffetta P, Harford J, Merajver S D. Urban-rural differences in breast cancer incidence by hormone receptor status across 6 years in Egypt. Breast Cancer Res. Treat. 2010;120(1):149–160. doi: 10.1007/s10549-009-0427-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dey S, Soliman A S, Merajver S D. Xenoestrogens may be the cause of high and increasing rates of hormone receptor positive breast cancer in the world. Med. Hypotheses. 2009;72(6):652–656. doi: 10.1016/j.mehy.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 118.McPherson K, Steel C M, Dixon J M. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ. 2000;321(7261):624–628. doi: 10.1136/bmj.321.7261.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mitra A K, Faruque F S, Avis A L. Breast cancer and environmental risks: where is the link? J. Environ. Health. 2004;66(7):24–32. [PubMed] [Google Scholar]
- 120.Vivacqua A, Recchia A G, Fasanella G, Gabriele S, Carpino A, Rago V, Di Gioia M L, Leggio A, Bonofiglio D, Liguori A, Maggiolini M. The food contaminants bisphenol A and 4-nonylphenol act as agonists for estrogen receptor alpha in MCF7 breast cancer cells. Endocrine. 2003;22(3):275–284. doi: 10.1385/ENDO:22:3:275. [DOI] [PubMed] [Google Scholar]
- 121.Hess-Wilson J K, Boldison J, Weaver K E, Knudsen K E. Xenoestrogen action in breast cancer: impact on ER-dependent transcription and mitogenesis. Breast Cancer Res. Treat. 2006;96(3):279–292. doi: 10.1007/s10549-005-9082-y. [DOI] [PubMed] [Google Scholar]
- 122.Jenkins S, Betancourt A M, Wang J, Lamartiniere C A. Endocrine-active chemicals in mammary cancer causation and prevention. J. Steroid Biochem. Mol. Biol. 2012;129(3-5):191–200. doi: 10.1016/j.jsbmb.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 123.Davis S R. Phytoestrogen therapy for menopausal symptoms? BMJ. 2001;323(7309):354–355. doi: 10.1136/bmj.323.7309.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tice J A, Ettinger B, Ensrud K, Wallace R, Blackwell T, Cummings S R. Phytoestrogen supplements for the treatment of hot flashes: the Isoflavone Clover Extract (ICE) Study: a randomized controlled trial. JAMA. 2003;290(2):207–214. doi: 10.1001/jama.290.2.207. [DOI] [PubMed] [Google Scholar]
- 125.Bar-El D S, Reifen R. Soy as an endocrine disruptor: cause for caution? J. Pediatr. Endocrinol. Metab. 2010;23(9):855–861. doi: 10.1515/jpem.2010.138. [DOI] [PubMed] [Google Scholar]
- 126.Bedell S, Nachtigall M, Naftolin F. The pros and cons of plant estrogens for menopause. J. Steroid Biochem. Mol. Biol. 2012 doi: 10.1016/j.jsbmb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 127.Martin P M, Horwitz K B, Ryan D S, McGuire W L. Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology. 1978;103(5):1860–1867. doi: 10.1210/endo-103-5-1860. [DOI] [PubMed] [Google Scholar]
- 128.Kuiper G G, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson J A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 129.Sotoca A M, Ratman D, van der Saag P, Strom A, Gustafsson J A, Vervoort J, Rietjens I M, Murk A J. Phytoestrogen-mediated inhibition of proliferation of the human T47D breast cancer cells depends on the ERalpha/ERbeta ratio. J. Steroid Biochem. Mol. Biol. 2008;112(4-5):171–178. doi: 10.1016/j.jsbmb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 130.Setchell K D, Brown N M, Desai P B, Zimmer-Nechimias L, Wolfe B, Jakate A S, Creutzinger V, Heubi J E. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J. Nutr. 2003;133(4):1027–1035. doi: 10.1093/jn/133.4.1027. [DOI] [PubMed] [Google Scholar]
- 131.Rozman K K, Bhatia J, Calafat A M, Chambers C, Culty M, Etzel R A, Flaws J A, Hansen D K, Hoyer P B, Jeffery E H, Kesner J S, Marty S, Thomas J A, Umbach D. NTP-CERHR expert panel report on the reproductive and developmental toxicity of genistein. Birth Defects Res. B Dev. Reprod. Toxicol. 2006;77(6):485–638. doi: 10.1002/bdrb.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Duffy C, Perez K, Partridge A. Implications of phytoestrogen intake for breast cancer. CA Cancer J. Clin. 2007;57(5):260–277. doi: 10.3322/CA.57.5.260. [DOI] [PubMed] [Google Scholar]
- 133.Pruthi S, Qin R, Terstreip S A, Liu H, Loprinzi C L, Shah T R, Tucker K F, Dakhil S R, Bury M J, Carolla R L, Steen P D, Vuky J, Barton D L. A phase III, randomized, placebo-controlled, double-blind trial of flaxseed for the treatment of hot flashes: North Central Cancer Treatment Group N08C7. Menopause. 2012;19(1):48–53. doi: 10.1097/gme.0b013e318223b021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Takemura H, Sakakibara H, Yamazaki S, Shimoi K. Breast cancer and flavonoids - A role in prevention. Curr. Pharm. Des. 2013 doi: 10.2174/1381612811319340006. [DOI] [PubMed] [Google Scholar]
- 135.Sammartino A, Tommaselli G A, Gargano V, di Carlo C, Attianese W, Nappi C. Short-term effects of a combination of isoflavones, lignans and Cimicifuga racemosa on climacteric-related symptoms in postmenopausal women: a double-blind, randomized, placebo-controlled trial. Gynecol. Endocrinol. 2006;22(11):646–650. doi: 10.1080/09513590601010722. [DOI] [PubMed] [Google Scholar]
- 136.Pawlak M, Lefebvre P, Staels B. General molecular biology and architecture of nuclear receptors. Curr. Top. Med. Chem. 2012;12(6):486–504. doi: 10.2174/156802612799436641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392(1):49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 138.Ogawa S, Inoue S, Watanabe T, Hiroi H, Orimo A, Hosoi T, Ouchi Y, Muramatsu M. The complete primary structure of human estrogen receptor beta (hER beta) and its heterodimerization with ER alpha in vivo and in vitro. Biochem. Biophys. Res. Commun. 1998;243(1):122–126. doi: 10.1006/bbrc.1997.7893. [DOI] [PubMed] [Google Scholar]
- 139.Gosden J R, Middleton P G, Rout D. Localization of the human oestrogen receptor gene to chromosome 6q24-q27 by in situ hybridization. Cytogenet. Cell Genet. 1986;43(3-4):218–220. doi: 10.1159/000132325. [DOI] [PubMed] [Google Scholar]
- 140.Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjold M, Gustafsson J A. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J. Clin. Endocrinol. Metab. 1997;82(12):4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- 141.Hawkins M B, Thornton J W, Crews D, Skipper J K, Dotte A, Thomas P. Identification of a third distinct estrogen receptor and reclassification of estrogen receptors in teleosts. Proc. Natl. Acad. Sci. U S A. 2000;97(20):10751–10756. doi: 10.1073/pnas.97.20.10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Giguere V, Yang N, Segui P, Evans RM. Identification of a new class of steroid hormone receptors. Nature. 1988;331(6151):91–94. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- 143.Kumar R, Thompson E B. The structure of the nuclear hormone receptors. Steroids. 1999;64(5):310–319. doi: 10.1016/s0039-128x(99)00014-8. [DOI] [PubMed] [Google Scholar]
- 144.McEwan I J. Sex, drugs and gene expression: signalling by members of the nuclear receptor superfamily. Essays Biochem. 2004;40:1–10. doi: 10.1042/bse0400001. [DOI] [PubMed] [Google Scholar]
- 145.Schwabe J W, Neuhaus D, Rhodes D. Solution structure of the DNA-binding domain of the oestrogen receptor. Nature. 1990;348(6300):458–461. doi: 10.1038/348458a0. [DOI] [PubMed] [Google Scholar]
- 146.Schwabe J W, Chapman L, Finch J T, Rhodes D. The crystal structure of the estrogen receptor DNA-binding domain bound to DNA: how receptors discriminate between their response elements. Cell. 1993;75(3):567–578. doi: 10.1016/0092-8674(93)90390-c. [DOI] [PubMed] [Google Scholar]
- 147.Schwabe J W, Chapman L, Finch J T, Rhodes D, Neuhaus D. DNA recognition by the oestrogen receptor: from solution to the crystal. Structure. 1993;1(3):187–204. doi: 10.1016/0969-2126(93)90020-h. [DOI] [PubMed] [Google Scholar]
- 148.Schwabe J W, Chapman L, Rhodes D. The oestrogen receptor recognizes an imperfectly palindromic response element through an alternative side-chain conformation. Structure. 1995;3(2):201–213. doi: 10.1016/s0969-2126(01)00150-2. [DOI] [PubMed] [Google Scholar]
- 149.Rosano C, Stec-Martyna E, Lappano R, Maggiolini M. Structure-based approach for the discovery of novel selective estrogen receptor modulators. Curr. Med. Chem. 2011;18(8):1188–1194. doi: 10.2174/092986711795029645. [DOI] [PubMed] [Google Scholar]
- 150.Pratt W B, Galigniana M D, Morishima Y, Murphy P J. Role of molecular chaperones in steroid receptor action. Essays Biochem. 2004;40:41–58. doi: 10.1042/bse0400041. [DOI] [PubMed] [Google Scholar]
- 151.Matthews J, Gustafsson J A. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol. Interv. 2003;3 (5):281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 152.Pike A C, Brzozowski A M, Hubbard R E. A structural biologist's view of the oestrogen receptor. J. Steroid Biochem. Mol. Biol. 2000;74(5):261–268. doi: 10.1016/s0960-0760(00)00102-3. [DOI] [PubMed] [Google Scholar]
- 153.Pfeffer U, Fecarotta E, Vidali G. Coexpression of multiple estrogen receptor variant messenger RNAs in normal and neoplastic breast tissues and in MCF-7 cells. Cancer Res. 1995;55(10):2158–2165. [PubMed] [Google Scholar]
- 154.Ferro P, Forlani A, Muselli M, Pfeffer U. Alternative splicing of the human estrogen receptor alpha primary transcript: mechanisms of exon skipping. Int. J. Mol. Med. 2003;12(3):355–363. [PubMed] [Google Scholar]
- 155.Pfeffer U, Fecarotta E, Castagnetta L, Vidali G. Estrogen receptor variant messenger RNA lacking exon 4 in estrogen-responsive human breast cancer cell lines. Cancer Res. 1993;53(4):741–743. [PubMed] [Google Scholar]
- 156.Lappano R, Rosano C, Madeo A, Albanito L, Plastina P, Gabriele B, Forti L, Stivala L A, Iacopetta D, Dolce V, Ando S, Pezzi V, Maggiolini M. Structure-activity relationships of resveratrol and derivatives in breast cancer cells. Mol. Nutr. Food Res. 2009;53(7):845–858. doi: 10.1002/mnfr.200800331. [DOI] [PubMed] [Google Scholar]
- 157.Martin-Santamaria S, Rodriguez J J, de Pascual-Teresa S, Gordon S, Bengtsson M, Garrido-Laguna I, Rubio-Viqueira B, Lopez-Casas P P, Hidalgo M, de Pascual-Teresa B, Ramos A. New scaffolds for the design of selective estrogen receptor modulators. Org. Biomol. Chem. 2008;6(19):3486–3496. doi: 10.1039/b806918b. [DOI] [PubMed] [Google Scholar]
- 158.Manas E S, Xu Z B, Unwalla R J, Somers W S. Understanding the selectivity of genistein for human estrogen receptor-beta using X-ray crystallography and computational methods. Structure. 2004;12(12):2197–2207. doi: 10.1016/j.str.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 159.Shiau A K, Barstad D, Loria P M, Cheng L, Kushner P J, Agard D A, Greene G L. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95(7):927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 160.Vajdos F F, Hoth L R, Geoghegan K F, Simons S P, LeMotte P K, Danley D E, Ammirati M J, Pandit J. The 2 A crystal structure of the ERalpha ligand-binding domain complexed with lasofoxifene. Protein Sci. 2007;16(5):897–905. doi: 10.1110/ps.062729207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xu H E, Stanley T B, Montana V G, Lambert M H, Shearer B G, Cobb J E, McKee D D, Galardi C M, Plunket K D, Nolte R T, Parks D J, Moore J T, Kliewer S A, Willson T M, Stimmel J B. Structural basis for antagonist-mediated recruitment of nuclear co-repressors by PPARalpha. Nature. 2002;415(6873):813–817. doi: 10.1038/415813a. [DOI] [PubMed] [Google Scholar]
- 162.Mak P, Leung Y K, Tang W Y, Harwood C, Ho S M. Apigenin suppresses cancer cell growth through ERbeta. Neoplasia. 2006;8(11):896–904. doi: 10.1593/neo.06538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pike A C, Brzozowski A M, Hubbard R E, Bonn T, Thorsell A G, Engstrom O, Ljunggren J, Gustafsson J A, Carlquist M. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J. 1999;18(17):4608–4618. doi: 10.1093/emboj/18.17.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sotoca A M, Gelpke M D, Boeren S, Strom A, Gustafsson J A, Murk A J, Rietjens I M, Vervoort J. Quantitative proteomics and transcriptomics addressing the estrogen receptor subtype-mediated effects in T47D breast cancer cells exposed to the phytoestrogen genistein. Mol. Cell. Proteomics. 2011;10(1):M110 002170–0. doi: 10.1074/mcp.M110.002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ho S M. Estrogens and anti-estrogens: key mediators of prostate carcinogenesis and new therapeutic candidates. J. Cell. Biochem. 2004;91(3):491–503. doi: 10.1002/jcb.10759. [DOI] [PubMed] [Google Scholar]
- 166.Mueller S O. Overview of in vitro tools to assess the estrogenic and antiestrogenic activity of phytoestrogens. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;777(1-2):155–165. doi: 10.1016/s1570-0232(02)00282-9. [DOI] [PubMed] [Google Scholar]
- 167.Marik R, Allu M, Anchoori R, Stearns V, Umbricht C B, Khan S. Potent genistein derivatives as inhibitors of estrogen receptor alpha-positive breast cancer. Cancer Biol. Ther. 2011;11(10):883–892. doi: 10.4161/cbt.11.10.15184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Routledge E J, White R, Parker M G, Sumpter J P. Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) alpha and ERbeta. J. Biol. Chem. 2000;275(46):35986–35993. doi: 10.1074/jbc.M006777200. [DOI] [PubMed] [Google Scholar]
- 169.Cappelletti V, Saturno G, Miodini P, Korner W, Daidone M G. Selective modulation of ER-beta by estradiol and xenoestrogens in human breast cancer cell lines. Cell. Mol. Life Sci. 2003;60(3):567–576. doi: 10.1007/s000180300048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pennie W D, Aldridge T C, Brooks A N. Differential activation by xenoestrogens of ER alpha and ER beta when linked to different response elements. J. Endocrinol. 1998;158(3):R11–14. doi: 10.1677/joe.0.158r011. [DOI] [PubMed] [Google Scholar]
- 171.Szego C M, Davis J S. Adenosine 3',5'-monophosphate in rat uterus: acute elevation by estrogen. Proc. Natl. Acad. Sci. U S A. 1967;58(4):1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ho K J, Liao J K. Non-nuclear actions of estrogen: new targets for prevention and treatment of cardiovascular disease. Mol. Interv. 2002;2(4):219–228. doi: 10.1124/mi.2.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kelly M J, Levin E R. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol. Metab. 2001;12(4):152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- 174.Levin E R. Cell localization, physiology, and nongenomic actions of estrogen receptors. J. Appl. Physiol. 2001;91(4):1860–1867. doi: 10.1152/jappl.2001.91.4.1860. [DOI] [PubMed] [Google Scholar]
- 175.Levin E R. Cellular functions of plasma membrane estrogen receptors. Steroids. 2002;67(6):471–475. doi: 10.1016/s0039-128x(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 176.Razandi M, Pedram A, Park S T, Levin E R. Proximal events in signaling by plasma membrane estrogen receptors. J. Biol. Chem. 2003;278(6):2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- 177.Hall J M, Couse J F, Korach K S. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J. Biol. Chem. 2001;276(40):36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 178.Acconcia F, Ascenzi P, Fabozzi G, Visca P, Marino M. S-palmitoylation modulates human estrogen receptor-alpha functions. Biochem. Biophys. Res. Commun. 2004;316(3):878–883. doi: 10.1016/j.bbrc.2004.02.129. [DOI] [PubMed] [Google Scholar]
- 179.Li K M, Todorovic R, Devanesan P, Higginbotham S, Kofeler H, Ramanathan R, Gross M L, Rogan E G, Cavalieri E L. Metabolism and DNA binding studies of 4-hydroxyestradiol and estradiol-3,4-quinone in vitro and in female ACI rat mammary gland in vivo. Carcinogenesis. 2004;25(2):289–297. doi: 10.1093/carcin/bgg191. [DOI] [PubMed] [Google Scholar]
- 180.Navarro C E, Saeed S A, Murdock C, Martinez-Fuentes A J, Arora K K, Krsmanovic L Z, Catt K J. Regulation of cyclic adenosine 3',5'- monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Mol. Endocrinol. 2003;17(12):1792–1804. [PubMed] [Google Scholar]
- 181.Wyckoff M H, Chambliss K L, Mineo C, Yuhanna I S, Mendelsohn M E, Mumby S M, Shaul P W. Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through Galpha(i) J. Biol. Chem. 2001;276(29):27071–27076. doi: 10.1074/jbc.M100312200. [DOI] [PubMed] [Google Scholar]
- 182.Takada Y, Kato C, Kondo S, Korenaga R, Ando J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem. Biophys. Res. Commun. 1997;240(3):737–741. doi: 10.1006/bbrc.1997.7734. [DOI] [PubMed] [Google Scholar]
- 183.Gobeil F, Fortier A, Zhu T, Bossolasco M, Leduc M, Grandbois M, Heveker N, Bkaily G, Chemtob S, Barbaz D. G-protein-coupled receptors signalling at the cell nucleus: an emerging paradigm. Can. J. Physiol. Pharmacol. 2006;84(3-4):287–297. doi: 10.1139/y05-127. [DOI] [PubMed] [Google Scholar]
- 184.Funakoshi T, Yanai A, Shinoda K, Kawano M M, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem. Biophys. Res. Commun. 2006;346(3):904–910. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- 185.Thomas P, Pang Y, Filardo E J, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146(2):624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 186.Lappano R, Santolla M F, Pupo M, Sinicropi M S, Caruso A, Rosano C, Maggiolini M. MIBE acts as antagonist ligand of both estrogen receptor alpha and GPER in breast cancer cells. Breast Cancer Res. 2012;14(1):R12. doi: 10.1186/bcr3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dennis M K, Burai R, Ramesh C, Petrie W K, Alcon S N, Nayak T K, Bologa C G, Leitao A, Brailoiu E, Deliu E, Dun N J, Sklar L A, Hathaway H J, Arterburn J B, Oprea T I, Prossnitz E R. In vivo effects of a GPR30 antagonist. Nat. Chem. Biol. 2009;5(6):421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dennis M K, Field A S, Burai R, Ramesh C, Petrie W K, Bologa C G, Oprea T I, Yamaguchi Y, Hayashi S, Sklar L A, Hathaway H J, Arterburn J B, Prossnitz E R. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J. Steroid Biochem. Mol. Biol. 2011;127(3-5):358–366. doi: 10.1016/j.jsbmb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rosano C, Lappano R, Santolla M F, Ponassi M, Donadini A, Maggiolini M. Recent advances in the rationale design of GPER ligands. Curr. Med. Chem. 2012;19(36):6199–6206. [PubMed] [Google Scholar]
- 190.Palczewski K, Kumasaka T, Hori T, Behnke C A, Motoshima H, Fox B A, Le Trong I, Teller D C, Okada T, Stenkamp R E, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289(5480):739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 191.Rasmussen S G, Choi H J, Rosenbaum D M, Kobilka T S, Thian F S, Edwards P C, Burghammer M, Ratnala V R, Sanishvili R, Fischetti R F, Schertler G F, Weis W I, Kobilka B K. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450(7168):383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 192.Kim D E, Chivian D, Baker D. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 2004;32:W526–W531. doi: 10.1093/nar/gkh468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sali A, Blundell T L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234(3):779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 194.Pupo M, Pisano A, Lappano R, Santolla M F, De Francesco E M, Abonante S, Rosano C, Maggiolini M. Bisphenol A induces gene expression changes and proliferative effects through GPER in breast cancer cells and cancer-associated fibroblasts. Environ. Health Perspect. 2012;120(8):1177–1182. doi: 10.1289/ehp.1104526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lappano R, Rosano C, Santolla M F, Pupo M, De Francesco E M, De Marco P, Ponassi M, Spallarossa A, Ranise A, Maggiolini M. Two novel GPER agonists induce gene expression changes and growth effects in cancer cells. Curr. Cancer Drug Targets. 2012;12(5):531–542. doi: 10.2174/156800912800673284. [DOI] [PubMed] [Google Scholar]
- 196.Lappano R, Rosano C, De Marco P, De Francesco E M, Pezzi V, Maggiolini M. Estriol acts as a GPR30 antagonist in estrogen receptor-negative breast cancer cells. Mol. Cell. Endocrinol. 2010;320(1-2):162–170. doi: 10.1016/j.mce.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 197.Yohannan S, Faham S, Yang D, Whitelegge J P, Bowie J U. The evolution of transmembrane helix kinks and the structural diversity of G protein-coupled receptors. Proc. Natl. Acad. Sci. USA. 2004;101(4):959–963. doi: 10.1073/pnas.0306077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Prossnitz E R, Arterburn J B, Smith H O, Oprea T I, Sklar L A, Hathaway H J. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu. Rev. Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- 199.Prossnitz E R, Sklar L A, Oprea T I, Arterburn J B. GPR30 a novel therapeutic target in estrogen-related disease. Trends Pharmacol. Sci. 2008;29(3):116–123. doi: 10.1016/j.tips.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 200.Maggiolini M, Picard D. The unfolding stories of GPR30 a new membrane-bound estrogen receptor. J. Endocrinol. 2010;204(2):105–114. doi: 10.1677/JOE-09-0242. [DOI] [PubMed] [Google Scholar]
- 201.Filardo E J. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J. Steroid Biochem. Mol. Biol. 2002;80(2):231–238. doi: 10.1016/s0960-0760(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 202.Prossnitz E R, Maggiolini M. Mechanisms of estrogen signaling and gene expression via GPR30. Mol. Cell. Endocrinol. 2009;308(1-2):32–38. doi: 10.1016/j.mce.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Albanito L, Lappano R, Madeo A, Chimento A, Prossnitz E R, Cappello A R, Dolce V, Abonante S, Pezzi V, Maggiolini M. Gprotein-coupled receptor 30 and estrogen receptor-alpha are involved in the proliferative effects induced by atrazine in ovarian cancer cells. Environ. Health Perspect. 2008;116(12):1648–1655. doi: 10.1289/ehp.11297. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 204.Vivacqua A, Lappano R, De Marco P, Sisci D, Aquila S, De Amicis F, Fuqua S A, Ando S, Maggiolini M. G protein-coupled receptor 30 expression is up-regulated by EGF and TGF alpha in estrogen receptor alpha-positive cancer cells. Mol. Endocrinol. 2009;23(11):1815–1826. doi: 10.1210/me.2009-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]