Abstract
Alzheimer’s disease (AD) is associated with insulin resistance and specific regional declines in cerebral metabolism. The effects of a novel mTOT modulating insulin sensitizer (MSDC-0160) were explored in non-diabetic patients with mild AD to determine whether treatment would impact glucose metabolism measured by FDG-PET in regions that decline in AD. MSDC-0160 (150 mg once daily; N=16) compared to placebo (N=13) for 12 weeks did not result in a significant difference in glucose metabolism in pre-defined regions when referenced to the pons or whole brain. However, glucose metabolism referenced to cerebellum was maintained in MSDC-0160 treated participants while it significantly declined for placebo patients in anterior and posterior cingulate, and parietal, lateral temporal, medial temporal cortices. Voxel-based analyses showed additional differences in FDG-PET related to MSDC-0160 treatment. These exploratory results suggest central effects of MSDC-0160 and provide a basis for further investigation of mTOT modulating insulin sensitizers in AD patients.
Keywords: Alzheimer’s disease, FDG-PET, insulin resistance, insulin sensitizer, mTOT modulator.
INTRODUCTION
It is estimated that 5.2 million persons in the United States have dementia due to Alzheimer’s Disease (AD) [1]. By 2050, the number of individuals living with dementia due to AD worldwide is estimated to increase from 36 million to 115 million people with two-thirds of persons affected living in developing countries. Given the worldwide public health impact of AD, increased efforts are needed to develop novel and effective AD interventions that are easy to deploy, not resource-intensive, and which have the potential to be used early in the course of the disease when intervention may have most impact.
AD is a neurodegenerative condition associated with progressive loss of cognitive and functional ability. While the pathogenesis of AD involves neuronal loss associated with the extracellular amyloid and intraneuronal phosphorylated tau [2], other mechanisms also may play a role. With 100 billion neurons interacting in the healthy brain, brain cells require two times more energy than other cells in the body and most of the energy is supplied through glucose metabolism [3]. Alterations in brain glucose metabolism by neurons have been associated with AD pathology [4,5]. Brain imaging studies with 18-F-deoxyglucose positron emission tomography (FDG-PET) have shown a decline in regional cerebral metabolic rate of glucose in persons with AD on the order of 25% as compared to controls [6]. In particular, brain glucose metabolism declines in the medial temporal lobe which incorporates the hippocampus, theposterior cingulate, and temporoparietal cortices [7-9]. It is unknown whether this decline is a specific metabolic effect or represents a general loss of cell function that might be associated with factors such as the insulin resistance that is known to exist in AD [10]. The reduced glucose metabolism associated with AD also has been tied with a progressive loss of mitochondrial function [11,12] and altering mitochondrial dysfunction has been postulated as a target for the treatment of AD together with treatment of insulin resistance [13-15].
As impaired mitochondrial activity has been linked to insulin resistance [14] and insulin sensitizers have been shown to alter glucose metabolism in non-neuronal cells [15], insulin sensitizers originally developed for the treatment of diabetes mellitus have been examined to see if they can be repurposed for the treatment of AD. Studies examining the role of insulin sensitizers on brain glucose metabolism in animal models of AD and in patients with AD have been limited and the results have been mixed [16-20]. Thus far, only PPARγ-activating insulin sensitizers have been tested in AD. The most extensively tested PPARγ agonist rosiglitazone, has not proven effective in phase 3 clinical trials for AD [21].
Recently a mitochondrial target of insulin sensitizers (mTOT) has been identified, which contains key proteins that comprise the mitochondrial pyruvate carrier [22, 23]. MSDC-0160 is a novel mTOT modulating insulin sensitizer that spares activation of PPARγ, and has been shown to be effective as a treatment of diabetes mellitus but with a reduction in the side effects associated with PPARγ activators [24].
In this study, we investigated whether12-week treatment with an mTOT insulin sensitizer could alter brain glucose metabolism in persons with AD and without diabetes. The overall goal of this randomized, placebo-controlled, double-masked, parallel-group, single-site study was to determine whether 3-month treatment with MSDC-0160 would impact glucose metabolism assessed with FDG-PET imaging, a measure of glucose uptake, in key brain regions associated with cognitive decline in dementia due to AD.
MATERIALS AND METHODS
Standard Protocol Approvals, Registrations, and Patient Consents
The clinical trial was approved by the Rush University Medical Center Institutional Review Board (IRB). The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization guidelines for Good Clinical Practice. The trial was registered prior to initiation of the research (NCT01374438, http://www.clinicaltrials.gov). Written informed consent was obtained from all study participants prior to conducting study procedures.
Patients
Clinic-based recruitment efforts primarily were utilized to identify potential study participants. Patients voluntarily consenting to study participation underwent a screening evaluation to confirm study eligibility. Inclusion criteria were age between 55 and 85 years (inclusive), diagnosis of probable AD according to the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association [25]; and a MMSE [26] score greater than or equal to 20. The main exclusionary criteria were a diagnosis of diabetes mellitus including the use of anti-diabetic medications, a fasting plasma glucose greater than 125 mg/dL, or a hemoglobin A1c greater than 6.0%; an inability to participate in FDG-PET imaging; simultaneous enrollment in another clinical trial; and, common medical conditions that could affect the risk profile for study participation including, but not limited to, congestive heart failure or cardiac event in the last six months, cancer in the last five years, renal insufficiency, liver enzyme elevation, and a QTcB greater than 450 seconds on a 12-lead electrocardiogram. The large number of subjects with either diabetes or high levels of hemoglobin A1c were a major factor in the length of time taken to recruit the study.
Participants meeting eligibility criteria were randomized in a 1:1 fashion to active study drug (MSDC-0160 150mg) or a matching placebo after all baseline study procedures were performed. Study drug was provided as encapsulated tablets in bottles and instructions were provided to take one tablet daily in the morning before breakfast for 12 weeks. Allocation to study products was performed using a computer-generated, permuted random length block study treatment randomization code. Using the randomization code, study drug bottles were packaged and shipped to sites by personnel not involved in the study and each bottle had a consecutive number assigned to it. Bottles were dispensed in sequential order to participants that completed baseline procedures. Participants and study staff were masked to study group assignment during the trial. The randomization code was not broken until initial statistical modeling of the primary outcome was complete.
Procedures
Within four weeks of the screening visit, participants meeting study eligibility underwent a baseline visit that included FDG-PET imaging, cognitive testing, and collection of blood samples for peripheral inflammatory markers and Apolipoprotein E ε4 genotyping followed by study drug randomization. Safety assessments and study drug compliance visits were conducted 4 and 8 weeks after the baseline visit. The 12-week visit involved repeating the same procedures as the baseline visit and collecting all unused study drug. A final safety visit was conducted 4 weeks after study drug discontinuation.
PET image data was collected at Rush Medical Center using a Phillips PET/CT Gemini TF 16 slice PET/CT scanner and a protocol consistent with ADNI data collection (see Supplemental Materials for further details on the image acquisition protocol). FDG dose was 4.5 – 5.5 mCi, and the scan was acquired from 30 to 60 minutes post-injection, in six 5 minute frames. The CT scan was acquired shortly prior to the emission scan. Images were reconstructed at the imaging site using iterative LOR 3D Ramla (attenuation CTAC-SG and scatter SS-Simul), 128x128 grid, and 256 mm field of view, resulting in a voxel size of 2.0 mm, and slice thickness of 2.0 mm. Smoothing was set to Sharp and all other parameters were set to defaults for the “Brain” protocol, with all corrections set to “On”.
Quality control checks included evaluation of the following: the number of detected coincidence events (for statistical quality), motion assessment across temporal frames, whether the brain was fully in the field of view, scan artifacts such as asymmetry or streaking, and image header checks. The different temporal frames were co-registered using SPM8 (The Wellcome Trust) and averaged to a single static image for each scanning session. Images also were placed into a common spatial orientation and slice thickness for processing and analysis. All scans were spatially normalized to a common anatomical template. The reverse transform was applied to sample the images in their native morphology, without smoothing. A smoothing kernel was then applied to the spatially normalized scans to make them consistent with ADNI reference scans for comparison, and suitable for voxel-based analysis.
Changes in cognitive function by treatment allocation over 12 weeks were measured by the 13-item ADAS-cog scale that is commonly used as the primary cognitive outcome in clinical trials of mild-to-moderate AD. The ADAS-cog assesses memory, language, praxis, attention, and other cognitive abilities. The total ADAS-cog score ranges from 0 (no cognitive deficit) to 70 (severe cognitive deficit), calculated as the numbers of errors a participant made. In order to assess cognition in a complementary manner to the ADAS-cog, two additional cognitive test batteries (a 19-item battery to assess global cognitive function and a 9-item battery to executive function) were administered at baseline and at the 12-week visit. All examiners were trained at the Rush Alzheimer’s Disease Center before conducting the tests of cognitive function. Composite z-scores were determined for global cognitive function and for executive function, respectively. See Supplemental Materials for further details on the cognitive tests and the construction of the z-scores.
The association of treatment with MSDC-0160 as compared to placebo on peripheral blood biomarkers of inflammation was assessed by assaying levels of high molecular weight adiponectin, high-sensitivity C-Reactive Protein (hsCRP), and free-fatty acids (FFA). Venous blood samples were taken, with a maximum of 30 milliliters in total per participant for each of the baseline and end-of-study visits, processed and stored in a -80 degrees Centigrade freezer until batches were shipped on dry ice. High molecular weight adiponectin was measured at Millipore (St. Charles, MO) and all other assays were performed by Medpace Reference Laboratories (Cincinnati, OH). Apolipoprotein Eε4 genotyping on blood samples collected at baseline was performed by Athena Diagnostics (Worcester, MA).
Safety assessments included the recording of adverse events. A study principal investigator and a sponsor-assigned medical monitor reviewed study adverse events. Serious adverse events were reviewed by the Rush University Medical Center Institutional Review Board. Study drug tablet counts were used to measure compliance with treatment.
Choice of Dose
MSDC-0160 or matching placebo capsule was given at 150 mg (QD) based on a recently completed phase 2b study in patients with type 2 diabetes showing this dose produces significant insulin sensitizing effects in a three month treatment window [24].
Sample Size
The objective of this pilot study was to provide preliminary data, without which sample size calculations can be heuristic at best. The sample size of 40 participants was selected in the original protocol to be comparable with other, albeit limited, studies of brain glucose metabolism for insulin sensitizing agents that cross the blood brain barrier.
Statistical Analysis
Baseline characteristics were summarized by treatment group. The Intent-to-Treat cohort included all randomized subjects. Baseline characteristics were compared using 2-sample t-tests or chi-square tests, as appropriate.
Paired-t tests were applied to the Region of Interest values, across treatment and placebo groups and within group. In the protocol, the pons was used as the primary normalization reference region with the cerebellum and whole brain as two other normalization reference regions used to examine result consistency. Treatment and placebo groups also were compared at baseline, with and without correction for age, to determine whether there was a significant difference in initial cerebral metabolic rates of glucose levels that may impact the slope of longitudinal change as a confound to treatment effect.
Voxel-based analyses were performed to identify non-a priori clusters that differed within-group between baseline and 12 weeks, and between placebo and treatment groups at baseline and with respect to longitudinal change. Statistical Parametric Mapping was applied to the data set using SPM (Version 5 or later; http://www.fil.ion.ucl.ac.uk/spm/). The following contrasts were evaluated using SPM: placebo group at baseline vs. 12 weeks; treatment group at baseline vs. 12 weeks; placebo vs. treatment group at baseline; placebo vs. treatment group at 12 weeks; longitudinal differences between placebo and treatment groups using subtraction images; and mixed model to evaluate interactions and treatment effect. Significant clusters were identified at thresholds of 0.001, 0.005, and 0.01, and their associated anatomical locations defined. Prominent clusters that were not previously measured using Region of Interest analysis were sampled on an individual Region of Interest basis, using the anatomical coordinates identified through SPM to guide the ROI definition.
Secondary analyses included the examination of efficacy, safety and tolerability. Efficacy outcomes included the three cognitive function measures. Blood inflammatory markers also were examined at baseline, week 12, and as a change from baseline to week 12. Safety was defined by an overall adverse event rate in the active treatment phase being equivalent to the overall adverse event rate pre- and post-active treatment. Tolerability was defined as a pill compliance rate during the active treatment phase being equivalent by study treatment group. Comparisons between groups were performed using t-tests.
All statistical analyses were performed using SAS software, Version 9.3, of the SAS® system for Linux (SAS Institute, Inc., Cary, NC). All statistical tests were two-tailed at the 0.05 level of significance.
RESULTS
Participant Flow and Baseline Characteristics
The trial was conducted between August 2011 and March 2013. Due to challenges in patient recruitment, particularly because of the large number of subjects with elevated hemoglobin A1c levels, a blinded interim analysis was performed in January 2013 and a decision was made by the sponsor to stop the study with the number of subjects already enrolled at that time. It was clear from the blinded analysis of the image data that changes in the PET imaging patterns were falling into two groups. Also the HbA1c limitation had slowed recruitment at the site so that it would have taken much longer to complete the originally planned number of subjects. Thus, a decision was made to complete the study with 29 subjects in order to utilize the findings from this proof-of-concept trial to design the next trials in the clinical development program. Of the 52 patients who consented to study participation, 23 did not proceed to randomization because they did not meet eligibility criteria. Elevated hemoglobin A1c levels, electrocardiogram QTcB intervals greater than 450 milliseconds, and unstable medications were the most frequent reasons for screening failure (Supplementary Fig. S1 (98.2KB, pdf) ). Of the 29 participants randomized to study drug, 16 were randomized to treatment with MSDC-0160 150 mg and 13 were randomized to placebo. All participants completed the full study duration and were included in the Intent-to-Treat analyses.
Randomized participants had a mean age of 71.7 years (SD=8.4), a mean education level 14.5(SD=3.0) years. A total of 55% of the cohort were women and 14% were African-American. The baseline characteristics of the study participants by treatment allocation are shown in (Table 1). Participants on MSDC-0160 or placebo were matched evenly for most baseline parameters. The drug-treated group, however, had lower Mini-Mental State Examination score (MMSE) at screening (mean=22.8, SD=2.4) as compared to placebo (mean=25.2, SD=2.1). Most of the patients in each group were taking 10 mg donepezil and/or 10 mg memantine. These medications were not adjusted during the trial. There were two patients in each group who were not on AD medications. At least one Apolipoprotein E ε4 allele was present in 63% of the MSDC-0160 group and 69% of the placebo group.
Table 1.
Baseline participant characteristics by treatment group.
| Characteristic | MSCD-0160 (n=16) |
Placebo (n=13) |
|---|---|---|
| Age, mean (SD), years | 72.3 (8.8) | 71.0 (8.0) |
| Female, number (%) | 8 (50) | 8 (62) |
| Education, mean (SD), years | 14.3 (3.4) | 14.9 (2.4) |
| White/Non-Hipsanic, number (%) | 14 (88) | 11 (85) |
| Screening Mini-Mental State Examination Score, mean (SD), out of 30 | 22.8 (2.4) | 25.2 (2.1)* |
| Presence of an Apolipoprotein E e4 allele, number (%) | 10 (63) | 9 (69) |
| Systolic blood pressure, mean (SD), mmHG | 129 (16) | 126 (9) |
| Diastolic blood pressure, mean (SD) mmHG | 79 (7) | 80 (11) |
| Pulse, mean (SD), beats per minute | 64 (10) | 65 (9) |
| HbAlc, mean (SD), percent | 5.63 (0.29) | 5.58 (0.27) |
p = .01
The mean and (standard deviation) is shown for baseline characteristics of the 29 patients (16 in the MSDC-0160-treated group and 13 in the placebo group) who participated in this trial.
Primary Outcome Measure
As shown in (Table 2), daily oral intake of MSDC-0160 150 mg as compared to placebo for 12 weeks did not result in a significant difference across groups in FDG-PET imaging-derived cerebral metabolic rate of glucose when the five pre-specified regions associated with AD regions of interest (ROIs) were referenced to the pons or whole brain (Table 2, left and center panels). However, when the metabolic rate of glucose of the cerebellum was used as the reference, 12-week change from baseline levels in cerebral metabolic rate of glucose for MSDC-0160 treated participants was maintained while it significantly declined for placebo for the posterior cingulate, parietal cortex angular gyrus, lateral temporal cortex, medial temporal cortex, and anterior cingulate-medial frontal cortex (right panel in Table 2).
Table 2.
Cerebral metabolic rate of glucose effects in five pre-specified regions of interest by reference group for daily MSDC-0160 or Placebo over 12 weeks.
| Cerebral Metabolic Rate of Glucose as a Function of Metabolic Rate of Glucose in Reference Group | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reference Group: | Pons | Whole Brain | Cerebellum | |||||
| Bilateral Regions of Interest | MSDC-0160 mean ± SD |
Placebo mean ± SD |
MSDC-0160 mean ± SD |
Placebo mean ± SD |
MSDC-0160 mean ± SD |
Placebo mean ± SD |
p-value | |
| Posterior cingulate | Baseline | 1.15 ± 0.13 | 1.27 ± 0.16 | 1.07 ± 0.09 | 1.13 ± 0.07 | 0.91 ± 0.09 | 0.98 ± 0.11 | |
| Week 12 | 1.11 ± 0.12 | 1.25 ± 0.19 | 1.07 ± 0.08 | 1 13 ± 0.07 | 0.91 ± 0.09 | 0.95 ± 0.12 | ||
| ∆ Week 12 - BL | -0.04 ± 0.04 | -0.02 ± 0.05 | 0.00 ± 0.02 | 0.00 ± 0.02 | 0.01 ± 0.03 | -0.02 ± 0.03 | 0.01 | |
| Parietal cortex (angular gyrus) | Baseline | 1.11 ± 0.17 | 1.20 ± 0.17 | 1.03 ± 0.09 | 1.07 ± 0.08 | 0.87 ± 0.11 | 0.92 ± 0.11 | |
| Week 12 | 1.08 ± 0.17 | 1.18 ± 0.19 | 1.03 ± 0.09 | 1.06 ± 0.08 | 0.88 ± 0.12 | 0.90 ± 0.13 | ||
| ∆ Week 12 - BL | -0.03 ± 0.04 | -0.03 ± 0.05 | 0.00 ± 0.02 | -0.01 ± 0.02 | 0.01 ± 0.02 | -0.03 ± 0.03 | <0.001 | |
| Lateral temporal cortex | Baseline | 1.03 ± 0.13 | 1.08 ± 0.09 | 0.96 ± 0.07 | 0.96 ± 0.03 | 0.81 ± 0.10 | 0.83 ± 0.07 | |
| Week 12 | 1.00 ± 0.12 | 1.06 ± 0.11 | 0.96 ± 0.02 | 0.96 ± 0.04 | 0.82 ± 0.09 | 0.81 ± 0.08 | ||
| ∆ Week 12 - BL | -0.03 ± 0.04 | -0.02 ± 0.04 | 0.00 ± 0.02 | 0.00 ± 0.01 | 0.00 ± 0.02 | -0.02 ± 0.03 | <0.001 | |
| Medial temporal cortex | Baseline | 0.88 ± 0.11 | 0.95 ± 0.09 | 0.82 ± 0.09 | 0.85 ± 0.08 | 0.69 ± 0.10 | 0.73 ± 0.09 | |
| Week 12 | 0.85 ± 0.11 | 0.93 ± 0.11 | 0.82 ± 0.09 | 0.85 ± 0.09 | 0.70 ± 0.10 | 0.72 ± 0.10 | ||
| ∆ Week 12 - BL | -0.02 ± 0.03 | -0.01 ± 0.03 | 0.00 ± 0.03 | 0.00 ± 0.02 | 0.01 ± 0.02 | -0.01 ± 0.02 | 0.02 | |
| Anterior cingulate - medial frontal cortex | Baseline | 1.21 ± 0.13 | 1.23 ± 0.17 | 1.13 ± 0.09 | 1.09 ± 0.11 | 0.95 ± 0.06 | 0.94 ± 0.09 | |
| Week 12 | 1.16 ± 0.12 | 1.20 ± 0.20 | 1.12 ± 0.09 | 1.08 ± 0.11 | 0.95 ± 0.06 | 0.91 ± 0.11 | ||
| ∆ Week 12 - BL | -0.05 ± 0.04 | -0.03 ± 0.04 | -0.01 ± 0.01 | 0.01 ± 0.02 | 0.00 ± 0.02 | -0.03 ± 0.03 | 0.01 | |
Placebo and MSDC-0160 groups CMRglc in pre-specified ROIs in the five listed pre-specified regions was measured as described in the text. Data are show as referenced to the pons (left panel), whole brain (central panel), or cerebellum (right panel). The data are mean and standard deviation (SD) and are presented for baseline, week 12 endpoint, and the individual changes from baseline at endpoint. The differences between the change from baseline at endpoint in the MSDC-0160 as compared to placebo were only significant when using the cerebellum as the reference and the p values for these comparisons are shown in the far right column.
Secondary Outcomes Measures
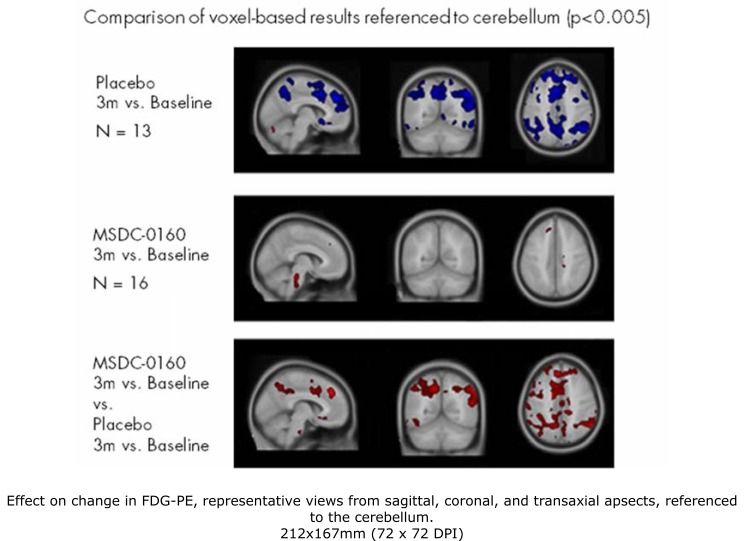
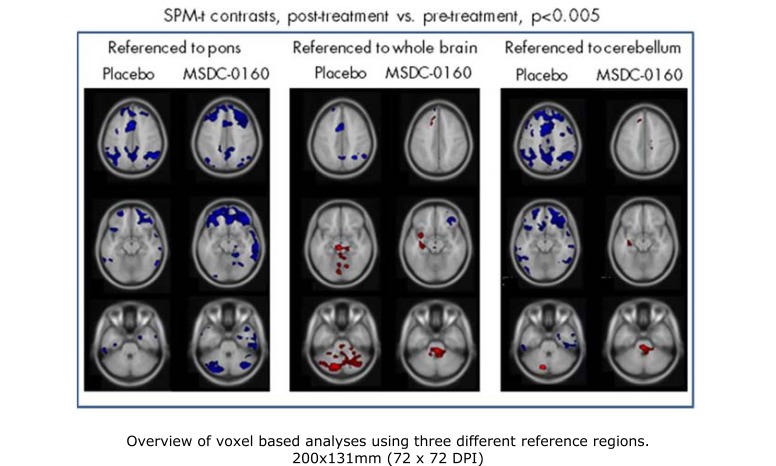
In addition to the primary outcome measure of the regions of interest (ROIs) known to decline in time with AD, we used a voxel based analysis generated by SPM-t test which allowed us to confirm changes inferred from the ratio of the regions of interest shown as well as to identify other regions that might differ between the groups. The pattern of decline in the placebo group was similar, allowing for differences in relative effect using different reference regions. The differences between the treatment group and the placebo group were most evident when using the cerebellum as the reference region. Fig. (1) is a representation of the images showing this comparison in sagittal, coronal, and transverse view for the average decline in the placebo group (upper panel), the lack of change in the MSDC-0160 group (middle panel) and the relative subtraction between the groups (bottom panel). Representative 12 week changes from baseline in both the placebo and MSDC-0160 groups using all three different reference regions are shown in Fig. (2). When referenced to the cerebellum, the placebo group showed an AD-like pattern of decline whereas the MSDC-0160 group showed only a relative increase in the pons and in other small clusters (right panel). However, when referenced to the pons, the placebo group continued to show an AD pattern of decline, whereas the MSDC-0160 group showed a more prominent decrease in the frontal region with additional decreases in the posterior cingulate and cerebellum (central panel). When the whole brain was used as a reference (left panel), the placebo group showed relative increases in the cerebellum and pons while the MSDC-0160 group showed relative increases in the pons, insula, superior temporal gyrus, and parahippocampal gyrus and decreases in frontal subregions.
Fig. (1).
Effect on change in FDG-PET, representative views from sagittal, coronal, and transaxial aspects, referenced to the cerebellum. Significant clusters from with-in group and subtraction analyses relative to cerebellar gray reference region are superimposed on selected transaxial MRI template slices. Blue indicates a decrease in glucose metabolism over the treatment period relative to the reference region, and red a relative increase (or preservation). The placebo group showed an AD-like pattern of decline that was absent in the MSDC-0160 group. The lower panel is a subtraction to show the relative effect of the MSDC-0160 treatment.
Fig. (2).
Overview of voxel based analyses using three reference regions. Significant clusters fromwithin-group analyses were superimposed on selected transaxial MRI template slices.Blue indicates a decrease in glucose metabolism over the treatment period relative to the reference region, and red a relative increase (or preservation). While the Placebo group exhibited an AD-like pattern of decline (and relative preservation), the MSDC-0160 group showed relative increases in regions including parahippocampus when referenced to whole brain and cerebellum, and decreases most evident in frontal and cerebellar gray regions when referenced to pons.
Although we did not expect to see changes in cognition in this short trial, three measures of cognitive function, all of which can measure loss of function in AD, were made at pre-treatment, after 12 weeks of treatment and at a 4 week follow up visit. Table 3 shows that changes in performance on the ADAS-Cog total score, the 19-item cognitive test battery global cognitive summary z-score, and the 7-item Executive Function battery summary z-score at 12 weeks of treatment as compared to baseline. There was no significant difference between the groups over this short time frame.
Table 3.
Cognition effect of daily MSDC-0160 versus Placebo over 12 weeks.
| MSCD-0160 mean ± SD (n) |
Placebo mean ± SD (n) |
|
|---|---|---|
| ADAS-cog | ||
| Baseline | 30.5 ± 9.0 (15) | 25.8 ± 7.0 (12) |
| Week 12 | 30.9 ± 8.4 (16) | 29.5 ± 10.1 (12) |
| ∆ Week 12 - Baseline | 0.7 ± 3.6 (15) | 2.2 ± 2.9 (11) |
| Cognitive Test Battery, z-score | ||
| Baseline | -0.17 ± 0.47 (16) | 0.06 ± 0.41 (13) |
| Week 12 | -0.23 ± 0.48 (16) | 0.09 ± 0.60 (13) |
| ∆ Week 12 - Baseline | -0.06 ± 0.11 (16) | 0.03 ± 0.59 (13) |
| Executive Function Battery, z-score | ||
| Baseline | -0.13 ± 0.38 (15) | 0.01 ± 0.39 (11) |
| Week 12 | -0.21 ± 0.55 (16) | 0.01 ± 0.52 (11) |
| ∆ Week 12 - Baseline | -0.04 ± 0.25 (15) | 0.00 ± 0.45 (11) |
Cognitive tests (ADAS-Cog, Cognitive Test Battery, and Executive Function Battery were conducted in home visits as described in the text. Data are mean and standard deviation at baseline, endpoint, and the individual change from baseline at endpoint. For some of these tests, not all subjucts had valid composite scores and incomplete scores were not included in the average. The number of subjects included in each case is shown in parentheses (n).
As shown in (Table 4), treatment with MSDC-0160 as compared to placebo resulted in a significant increase in high molecular weight adiponectin levels in the blood (p<0.001), suggesting an improvement in insulin sensitivity. There was not a statistically significant change in circulating hs-CRP or FFA, although these values changed in the direction (decreased) that would be expected based upon the pharmacology of the insulin sensitizer [24].
Table 4.
Inflammation effect of daily MSDC-0160 versus Placebo over 12 weeks.
| Inflammatory Marker | MSDC-0160 mean ± SD (n) |
Placebo mean ± SD (n) |
P-value * |
|---|---|---|---|
| HMW Adiponectin, micromol/L | |||
| Baseline | 8684 ± 5356 (16) | 10896 ± 9284 (13) | 0.4 |
| Week 12 | 26506 ± 13684 (16) | 11691 ± 10112 (13) | 0.003 |
| ∆ Week 12 - Baseline | 17822 ± 10940 (16) | 795 ± 4477 (13) | <0.001 |
| hs-CRP, micromol/L | |||
| Baseline | 9.73 ± 30.90 (16) | 1.15 ± 0.98 (13) | 0.3 |
| Week 12 | 0.83 ± 0.58 (16) | 1.82 ± 2.34 (13) | 0.1 |
| ∆ Week 12 - Baseline | -8.91 ± 30.94 (16) | 0.67 ± 1.65(13) | 0.3 |
| FFA, micromol/L | |||
| Baseline | 0.56 ± 0.28 (16) | 0.55 ± 0.26 (13) | 0.9 |
| Week 12 | 0.49 ± 0.22 (16) | 0.60 ± 0.30 (13) | 0.3 |
| ∆ Week 12 - Baseline | -0.07 ± 0.31 (16) | 0.04 ± 0.21 (13) | 0.3 |
| Triglycerides, mg/dL | |||
| Baseline | 83.87 ± 40.99 (16) | 96.38 ± 37.80 (13) | 0.4 |
| Week 12 | 84.47 ± 35.59 (16) | 92.00 ± 43.96 (13) | 0.6 |
| ∆ Week 12 - Baseline | 0.6 ± 16.29 (16) | -4.38 ± 29.32 (13) | 0.8 |
Baseline, endpoint, and individual change from baseline at endpoint are shown for high molecular weight adiponectin, hsCRP, fatty acids, and triglycerides, measured in the respective plasma samples as described in the text. Data are mean and standard deviation with the total number of subjects (n) in parentheses.
There was no treatment differences by ApoE e4 allele presence in any of the parameters measured in this study.
Safety and Tolerability
There was no early study discontinuation by any subject, and all 29 participants randomized completed the study. In total, 13 (44.8%) subjects had at least one treatment-emergent adverse event during the study: five (38.5%) subjects in the placebo group and eight (50.0%) subjects in the MSDC-0160 150 mg group. Overall, the most common system organ classes of treatment-emergent adverse events were infections (17.2%) and gastrointestinal disorders (13.8%) (Supplementary Table S1 (98.2KB, pdf) ). One subject in the MSDC-0160 150 mg group reported two serious adverse events of pneumonia (severe) and urinary tract infection (moderate in severity). Neither event was considered to be related to treatment with MSDC-0160 150 mg.
No significant weight gain or liver enzyme changes were noted with treatment (Supplementary Table S1 (98.2KB, pdf) ). Hemoglobin levels, a measure of hemodilution in g/dL, significantly declined with 12 weeks of treatment with MSDC-0160 (mean=-0.6, SD=0.8) as compared to placebo (mean=0.3, SD=0.7). This change occurred within the first 4 weeks of treatment and did not further change over the remaining 8 weeks (not shown). As expected in these subjects with normal circulating glucose, there was no effect of treatment on peripheral insulin levels.
Mean compliance with study medication based on capsule counts was 99.0% (SD=2.7) for the placebo group (13 subjects) and 99.5% (SD=2.7) for the MSDC-0160 150 mg group (16 subjects). All patients in the treatment group were positive for plasma levels of drug and active metabolite which averaged 2630 ng/ml (parent and metabolite) at trough.
DISCUSSION
This exploratory clinical trial was the first test of a prototype mTOT modulating insulin sensitizer in patients with mild AD without diabetes mellitus. This study was conducted to determine whether MSDC-0160 might alter brain glucose metabolism as assessed by changes in the relative update of FDG as assessed by PET imaging. Changes in the FDG-PET pattern suggest that treatment with this insulin sensitizer has central effects on the pattern of glucose metabolism. It is important to note that all evaluations were made after 12 week of treatment and at trough levels of the drug. Thus, any differences are likely not due to acute changes in glucose metabolism but are reflective of the 12 week treatment, which likely results from changes reflecting alteration in function and/or pathology with time.
This analysis showed subtle differences in the pattern of FDG-PET uptake in various brain regions in the MSDC-0160-treated group as compared to the subjects in the placebo group after 12 weeks of treatment. In the 5 regions of interest known to decline during the course of AD (the primary endpoint), a significant difference between the groups was found only when the cerebellum was used as the reference (normalization) region. The preservation of uptake observed in MSDC-0160 treated subjects relative to this reference could be consistent with a preservation of function in these regions as a result of the drug treatment. However, there could be other reasons for these relative changes in the FDG-PET images. Based on limited data prior to the study, the pons was selected a priori as the primary reference region with the cerebellum and whole brain as to additional references regions. Each reference region has inherent advantages and disadvantages. For example, the whole brain while stable is least sensitive to longitudinal change and the pons is susceptible to motion artifact. The cerebellum has been used in some studies as a preferred normalization reference to measure differences between AD subjects and controls due to its relative preservation (slower loss of metabolic activity) during the course of the disease as compared to temporo-parietal, cingulate, and frontal regions [27] and this should likely be selected as a primary comparison going forward although most information can be gained by using multiple reference regions. Additionally, changes in FDG-PET found by the voxel based analysis using all the reference regions suggest additional hypotheses relating to MSDC-0160 treatment that may be tested in future trials.
Voxel-based analyses showed differences between placebo and MSDC0160 subjects for all reference regions applied. As discussed above, within-group placebo results were consistent with the patterns of decline reported in typical AD patients for all three reference regions [7-9]. These involved decreases in cingulate and temporoparietal cortices when referenced to pons and cerebellum, and relative increases in cerebellum, pons, and thalamus (relatively preserved in AD) when referenced to whole brain. In contrast, however, the MSDC-0160 group did not show a pattern typical of AD-decline regardless of the reference region selected. Regions affected were sensitive to reference region selection. When referenced to pons, the MSDC-0160 group showed relative decreases most prominent in frontal, cingulate, and cerebellar regions. Referenced to whole brain, relative increases were observed in parahippocampal gyrus and insula, and relative decreases were seen in cerebellum. Normalized to cerebellum, relative increases or preservation of pons, thalamus, and parahippocampal regions were seen in MSDC-0160 treated subjects. None of these effects are consistent with the longitudinal decline expected in AD patients. Interpretation is limited due to the relative nature of the FDG-PET measurements, the small study size, and the short timeframe for disease related metabolic decline to have occurred. In any event, there were differences in the patterns associated with drug treatment as compared to those in the control group. Given the timing of the PET scans relative to treatment administration, changes are likely reflective of longer term (12 week) effects of the treatment and not acute effects upon glucose transport.
It is uncertain why an insulin sensitizer may be associated with a decrease in brain glucose metabolism in some regions such as the cerebellum and frontal regions as occurred within the group MSDC-0160 when referenced to pons. Pioglitazone, which also has anti-inflammatory activity, has been reported to generally reduce FDG uptake into the mouse brain in regions other than pons [28]. It has been suggested that this might due to an increased utilization of lactate rather than dependence on glucose uptake (as measured by FDG-PET). Thus, contributions to FDG-PET could result from changes in function in addition to changes in the number of functional cells. It also is important to note that if brain cells involved in cognitive function utilize fuel more efficiently, the ancillary regions in the thalamus or cerebellum that can support memory would be less taxed and their glucose uptake might be lowered. In this case, lower glucose uptake in the cerebellum and thalamus may reflect more efficient brain network function. There is precedence, for example, for lower glucose metabolism when healthy adults were administered methylphenidate and given a cognitive task [29]. It also is possible that the decreases seen in the voxel-based analysis of MSDC-0160 patients when referenced to pons may be related to a reduction in inflammation. The distribution of decreased metabolism in the MSDC-0160 group referenced to pons had greater overlap with the typical distribution of amyloid in the brains of Alzheimer’s disease patients [30] than that of AD related metabolic decline. Amyloid burden has been associated with inflammation [31], and peripheral biomarkers confirmed that MSDC-0160 acts as an anti-inflammatory agent, consistent with preclinical findings and results in diabetic patients. These hypotheses could be tested in future studies using measurement of amyloid plaque and inflammation by different PET tracers, and by the study of the effects on FDG-PET uptake during active cognitive tasks together with blood sampling for absolute quantization. It also would likely be instructive to use more precise measurements of regional cerebral glucose metabolism to dissect the nature of the metabolic changes that are occurring [32].
Based on the short time frame of the study, cognitive function was not significantly changed with treatment over 12 weeks as measured using three complementary cognitive outcomes. None-the-less, the placebo group, which started from a less impaired baseline, tended to worsen in the ADAS-Cog score with time, while there was no change in the scores of the MSDC-0160 group. This study showed that this insulin sensitizer has peripheral effects in these patients that are similar to those previously seen in diabetic subjects, particularly with respect to the elevation of HMW adiponectin, a biomarker for improved insulin sensitivity. As peripheral insulin levels did not change, the changes in FDG-PET images likely reflect a combination of metabolic effects and maintenance of cell function that occurs centrally as discussed above. These results now set the framework for the examination of the potential of this pharmacology on longer-term treatment such that the potential effects on cognitive function can be assessed.
Prior work with insulin sensitizers in AD and brain glucose metabolism has been limited. A sub-study of a clinical trial with rosiglitazone as compared to placebo in persons with mild-to-moderate AD and without diabetes showed an initial one-month increase in cerebral rate of glucose (at 4 mg rosiglitazone) but no difference after six months or one year (after the dose was increased to 8 mg rosiglitazone) [20]. Moreover, phase 3 trials with rosiglitazone have not met their clinical endpoints [21]. However, rosiglitazone was given at low doses, so the results may mean that a peripheral effect on glucose utilization might not result in a change in brain function that would reflect sustained differences in glucose metabolism as assessed by FDG-PET over the longer term. A preliminary clinical trial of pioglitazone in persons with AD with diabetes showed a 3-month change in cerebral blood flow to the parietal region using SPECT in persons treated with study drug as compared to placebo [33]. Both rosiglitazone and pioglitazone are PPARγ agonists. This study of MSDC-0160 is the first to test an insulin sensitizer of the mTOT modulator class [34] in persons with AD and without diabetes. This molecule is more specific for the newly identified mitochondrial target of the TZDs which modulates the important nutrient sensors mTOR and AMPK [35], which are known to regulate important mitochondrial functions that are affected in AD [15]. Ongoing rodent studies demonstrate that MSDC-0160 and is principle metabolite enter brain mitochondria after oral dosing and impact the nutrient sensing pathways in the brain. The limitations of this study include the small sample size and heterogeneity of the subjects inherent in an AD population which may have reduced the ability to detect results of significance, the relative nature of the regional cerebral glucose metabolism measurements, and the short term of treatment necessitated by the early stage in the development of this compound. Recruitment of study subjects that met study criteria also was challenging as many older persons with dementia due to AD also have diabetes mellitus and/or pre-diabetes. Studying an insulin sensitizer for broad application in persons with mild AD may require studies that include recruitment of persons with and without diabetes, examining whether the presence of diabetes modifies the association of treatment with brain glucose metabolism and/or cognition. While the Apolipoprotein E ε4 genotype is known to impact the effectiveness of some AD therapeutics [36], there was no obvious difference in the response to MSDC-0160 in carriers and non-carriers in this study. However, given the size of this study and the fact that it was not of long enough duration to determine the effects on cognition, the impact of this genetic risk factor on the effectiveness of this agent will need to be formally addressed in future trials. Strengths of the study included no loss to follow-up, whereby all subjects who were randomized completed baseline and 12 week PET-imaging, and did so with high compliance to study drug. In addition, a peripheral biologic effect was noted on adiponectin levels which suggests both an anti-inflammatory action as well as improved insulin sensitivity. Finally, the safety profile in older persons with AD showed no difference in the report of adverse events by treatment group. The hemodilution effect of treatment with MSDC-0160 stabilized after four weeks of treatment yet should continue to be monitored in future clinical studies. These preliminary imaging, tolerance, and biologic results provide a basis for further investigation of mTOT modulating insulin sensitizers to alter the brain glucose metabolism and to potentially impact the pathology associated with mild AD.
CONCLUSION
This new class insulin sensitizer is well tolerated in patients with AD, has effects on the biomarker adiponectin as seen previously in diabetic patients, and alters the pattern of FDG-PET uptake in the brain suggesting that this treatment might have central effects on brain metabolic function. Longer term testing of compounds with this mechanism on brain metabolism and cognition is warranted to further define the pharmacological effects and to determine if this approach has disease modifying potential.
ACKNOWLEDGEMENTS
This research was conducted by Metabolic Solutions Development Company through a grant from the Alzheimer’s Drug Discovery Foundation (New York, NY, USA). Metabolic Solutions Development Company provided input in study design; analysis and interpretation of data; the writing of the report; and the decision to submit the paper for publication. RCS and DCM had full access to the entire dataset. DCM performed an independent analysis for the imaging outcomes while RCS and AWC performed an independent, blinded analysis of the dataset for cognitive, inflammatory marker, and compliance outcomes.
We are indebted to the study participants and their caregivers. We thank Phillip M. Green, MD, at Borgess Geriatric Assessment Center (Kalamazoo, MI) and William H. Baer, MD, PharmD at VARI-ClinXus of Van Andel Institute (Grand Rapids, MI) for sharing information about this research study opportunity with their patients. We thank Lindsay Pluff , Jamie Plenge, MS, Diane Beuving and Angeline Shashlo for study coordination; Zachary Hill and Neelum T. Aggarwal, MD, for performing study visits; John Gibbons, MS, for data management; Greg LaMonica and Amjad Ali, MD, for PET-imaging acquisition at Rush University Medical Center (Chicago, IL) and Randolph Andrews for image processing at Abiant, Inc (Grayslake, IL); W. Fan for blinded statistical programming and analysis; Allison Bovard for central laboratory sample management and analysis at MedPace Laboratories (Cincinnati, OH); Mary Conklin for study monitoring; and Wade J. Adams for the analysis of the pharmacokinetics.
AUTHOR CONTRIBUTIONS
RCS, DCM, and JRC wrote the manuscript. RCS, DCM, DAF, JTVL, and JRC designed the research. RCS and DCM performed the research. RCS, DCM, AC, DAF, and JRC analyzed the data.
CONFLICT OF INTEREST
JRC and JTV are employees and/or part owners of Metabolic Solutions Development Company. RCS serves on the Board of Directors of the Alzheimer’s Association – Greater Illinois Chapter; serves as a member of the Investigator Consultation Network for Merck Research Laboratories; served on a research advisory panel for Accera, Inc, and a clinical advisory panel for Nutricia, Inc.; receives or recently received research support as Site PI or Site Subinvestigator from Ceregene, Inc., Danone Research BV, Eisai, Inc., Elan Pharmaceuticals, Inc., Eli Lilly, Inc., Genentech, Inc., Merck & Co., Inc., Navidea Biopharmaceuticals, Pamlab, L.L.C., and Pfizer, Inc.; and receives research support from the NIH and from the Illinois Department of Public Health Alzheimer’s Disease Assistance Center Grant. AC and DAF reports no financial disclosures relevant to the work.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers web site along with the published article.
REFERENCES
- 1.Alzheimer’s Association.2013 Alzheimer s disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC , et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer Dement. 2013:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pellerin L. Food for thought: the importance of glucose and other energy substrates for sustaining brain function under varying levels of activity. Diabetes Metab. 2010;36:S59–63. doi: 10.1016/S1262-3636(10)70469-9. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Yan SD. Mitochondrial Abeta: a potential cause of metabolic dysfunction in Alzheimer’s disease. IUBMB Life. 2006;58:686–694. doi: 10.1080/15216540601047767. [DOI] [PubMed] [Google Scholar]
- 5.Blass JP. Brain metabolism and brain disease: is metabolic deficiency the proximate cause of Alzheimer dementia?. J Neurosci Res. 2001;66:851–856. doi: 10.1002/jnr.10087. [DOI] [PubMed] [Google Scholar]
- 6.Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S , et al. Brain fuel metabolism. aing.and Alzheimer s disease. . Nutrition . 2011;27:3–20. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Rinne JO, Mosconi L, Pirraglia L, Rusinek H, DeSanti S, Nina Kemppainen N , et al. Regional analysis of FDG and PIB-PET images in normal aging. mild cognitive impairent.and Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2008; 3512 :2169–2181. doi: 10.1007/s00259-008-0833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL , et al. Associations between cognitive. functinal.and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011; 327:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen K, Langbaum JB, Fleisher AS, Ayutyanont N, Reschke C, Lee W , et al. Twelve-month metabolic declines in probable Alzheimer's disease and amnestic mild cognitive impairment assessed using an empirically pre-defined statistical region-of-interest: findings from the Alzheimer's Disease Neuroimaging Initiative. Neuroimage. 2010;51:654–664. doi: 10.1016/j.neuroimage.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talbot K, Wang H-Y, Kazi H, Han L-Y, Bakshi KP, Stucky A , et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance. IRS-1 dysregulaion.and cognitive decline. J Clin Invest. 2012; 1224 :1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parihar M, Brewer GJ. Mitoenergetic failure in Alzheimer disease. Am J Physiol Cell Physiol. 2007;292:C8–C23. doi: 10.1152/ajpcell.00232.2006. [DOI] [PubMed] [Google Scholar]
- 12.Swerdlow RH. Brain aging. Alzheimer s disase.and mitochondria. Biochim Biophys Acta. 2011; 1812:1630–1639. doi: 10.1016/j.bbadis.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva DF, Selfridge JE, Lu J, Lezi E, Cardoso SM, Swerdlow RH. Mitochondrial abnormalities in Alzheimer’s disease: possible targets for therapeutic intervention. Adv Pharmacol. 2012;64:83–126. doi: 10.1016/B978-0-12-394816-8.00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. CurrAlz. 2012;Res 9:35–66. doi: 10.2174/156720512799015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colca JR, Feinstein DL. Altering mitochondrial dysfunction as an approach to treating Alzheimer's disease. Adv Pharmacol. 2012;64:155–176. doi: 10.1016/B978-0-12-394816-8.00005-2. [DOI] [PubMed] [Google Scholar]
- 16.Hanyu H, Sato T, Kiuchi A, Sakurai H, Iwamoto T. Pioglitazone improved cognition in a pilot study on patients with Alzheimer’s disease and mile cognitive impairment with diabetes mellitus. J Am Geriatr Soc. 2009;571:177–179. doi: 10.1111/j.1532-5415.2009.02067.x. [DOI] [PubMed] [Google Scholar]
- 17.Hanyu H, Sato T, Sakuri H, Iwamoto T. The role of tumor necrosis factor alpha in the cognitive improvement after peroxisome proliferator activator receptor gamma agonist pioglitazone treatment in Alzheimer’s disease. J Am Geriatr Soc. 2010;585:1000–1001. doi: 10.1111/j.1532-5415.2010.02841.x. [DOI] [PubMed] [Google Scholar]
- 18.Miller BW, Willett KC, Desilets AR. Rosiglitazone and pioglitazone for the treatment of Alzheimer's disease. Ann Pharmacother. 2011;45:1416–1424. doi: 10.1345/aph.1Q238. [DOI] [PubMed] [Google Scholar]
- 19.Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-? agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging. 2011;32:1626–1633. doi: 10.1016/j.neurobiolaging.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Tzimopoulou S, Cunningham VJ, Nichols TE, Searle G, Bird NP, Mistry P , et al. A multi-center randomized proof-of-concept clinical trial applying [18F]FDG-PET for evaluation of metabolic therapy with rosiglitazone XR in mild to moderate Alzheimer’s disease. J. Alzheimers Dis. 2010;22:1241–1256. doi: 10.3233/JAD-2010-100939. [DOI] [PubMed] [Google Scholar]
- 21.Harrington C, Sawchak S, Chiang C, Davies J, Donovan C, Saunders AM , et al. Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild-to-moderate Alzheimer's disease: two phase 3 studies. curr Alzheimer Res. 2011;85:592–606. doi: 10.2174/156720511796391935. [DOI] [PubMed] [Google Scholar]
- 22.Colca JR, McDonald WG, Cavey GS, Cole SL, Holewa DD, Brightwell-Conrad AS , et al. Identification of a mitochondrial target of thiazolidinedione insulin sensitizers mTOT)-relationship to newly identified mitochondrial pyruvate carrier proteins. PLOS One. 2013;8 e61551:1–10. doi: 10.1371/journal.pone.0061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Divakaruni AS, Wiley SE, Rogers GW, Andreyev AY, Petrosyan S, Loviscach M , et al. Thiazolidinediones are acute. specific inhibitors of the mitochondrial pyruvate carrier. PNAS. 2013;110:5422–5427. doi: 10.1073/pnas.1303360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colca JR, VanderLugt JT, Adams WJ, Shashlo A, McDonald WG, Liang J , et al. Clinical proof of concept with MSDC-0160. a prototype mTOT modulating insulin sensitizer. Clin Pharm Ther. 2013;93:352–359. doi: 10.1038/clpt.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Bauer CM, Cabral HJ, Greve DN, Killiany RJ. Differentiating between normal aging. Mild Cognitive Impairent.and Alzheimer s disease with FDG-PET Effects of normalization region and partial volume correcting method. . J Alzheimers Dis Parkinsonism. 2013;31 [Google Scholar]
- 28.Galea E, Feinstein DL, Lacombe P. Pioglitazone does not increase cerebral glucose utilization in a murine model of Alzheimer’s disease and decreases it in wild-type mice. Diabetologia. 2006;49:2153–2161. doi: 10.1007/s00125-006-0326-0. [DOI] [PubMed] [Google Scholar]
- 29.Volkow NDFowler JS, Wang GJ, Telang F, Logan J, Wong C , et al. Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLOS One. 2008;3:e2017. doi: 10.1371/journal.pone.0002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edison P, Archer HA, Hinz R, Hammers A, Pavese N, Tai YF , et al. Amyloid. hypometaboism.and cognition in Alzheimer disease An [11C]PIB and [18F]FDG PET study. Neurology . 2007;8: 501–508. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- 31.Okello A, Edison P, Archer HA, Turkheimer FE, Kennedy J, Bullock R , et al. Microglial activation and amyloid deposition in mild cognitive impairment: A PET study. Neurology. 2009;72:56–62. doi: 10.1212/01.wnl.0000338622.27876.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosconi L, Tsui WH, Rusinek H, De Santi S, Li Y, Wang GJ , et al. Quantitation. regional vulnerabiity.and kinetic modeling of brain glucose metabolism in mild Alzheimer s disease. Eur J Nucl Med Mol Imaging . 2007; 34:1467–1479. doi: 10.1007/s00259-007-0406-5. [DOI] [PubMed] [Google Scholar]
- 33.Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR agonist pioglitazone in mild Alzheimer’s disease. Neurobiol Aging. 2011;32:16261633. doi: 10.1016/j.neurobiolaging.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Colca JR, Tanis SP, McDonald WG, Kletzein RF. Insulin sensitizers in 2013 New insights for the development of novel therapeutic agents to treat metabolic disease. Exp Opin Invest Drugs. 2013;23:1–7. doi: 10.1517/13543784.2013.839659. [DOI] [PubMed] [Google Scholar]
- 35.Colca JR, McDonald WG, Kletzien RF. The Mitochondrial Target of Thiazolidinediones Diabetes. Obesity and Metabolism in press. 2014 doi: 10.1111/dom.12308. [DOI] [PubMed] [Google Scholar]
- 36.Schneider LS, Lahiri DK. The perils of Alzheimer's drug development. Curr Alzheimer Res. 2009;61:77–8. doi: 10.2174/156720509787313871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publishers web site along with the published article.