Abstract
Vitamin C is an essential nutrient in humans and must be obtained through the diet. The aim of this study was to determine vitamin C uptake in healthy volunteers after consuming kiwifruit (Actinidia chinensis var. Hort. 16A), and to determine the amount of fruit required to raise plasma vitamin C to ‘healthy’ (i.e. >50 µmol/l) and ‘optimal’ or saturating levels (i.e. >70 µmol/l). Leucocyte and urinary vitamin C levels were also determined. A total of fifteen male university students with below average levels of plasma vitamin C were selected for the study. Weekly fasting blood samples were obtained for a 4-week lead-in period and following supplementation with, sequentially, half, one, two and three Gold kiwifruit per d for 4–6 weeks each, followed by a final 4-week washout period. The results showed that addition of as little as half a kiwifruit per d resulted in a significant increase in plasma vitamin C. However, one kiwifruit per d was required to reach what is considered healthy levels. Increasing the dose of kiwifruit to two per d resulted in further increases in plasma vitamin C levels as well as increased urinary output of the vitamin, indicating that plasma levels were saturating at this dosage. Dividing the participants into high and low vitamin C groups based on their baseline plasma and leucocyte vitamin C levels demonstrated that it is critical to obtain a study population with low initial levels of the vitamin in order to ascertain a consistent effect of supplementation.
Key words: Plasma vitamin C, Human saturation levels, Kiwifruit supplementation, Leucocytes
Abbreviations: DTPA, diethylene triamine pentaacetic acid; RDI, recommended dietary intake
Introduction
Vitamin C (ascorbate) is an essential nutrient in humans( 1 ), and insufficient dietary intake leads to the potentially fatal deficiency disease, scurvy( 2 ). Vitamin C has a number of important functions in vivo. It is an essential cofactor for a variety of dioxygenase enzymes that hydroxylate amino acids in the synthesis of pro-collagen, carnitine, hormones and neurotransmitters( 3 ) and is also a highly effective water-soluble antioxidant, scavenging both one-electron and two-electron oxidants( 4 ). However, the significance of the antioxidant activity in vivo remains to be determined. Recent research has uncovered vital novel functions for members of the dioxygenase family, including roles in gene regulation and signalling pathways( 5 , 6 ). Vitamin C is now known to be a cofactor for the hydroxylases responsible for the regulation of the transcription factor hypoxia-inducible factor 1( 7 – 10 ), a metabolic sensor that has been implicated in a number of conditions such as cancer, ischaemic cardiovascular disorders and inflammation( 9 , 11 ). As hypoxia-inducible factor 1 is ubiquitously expressed throughout the body, there is a requirement for adequate levels of vitamin C in all tissues.
Numerous epidemiological studies have shown that high dietary intakes and/or high plasma levels of vitamin C are associated with a decreased risk of CVD and cerebrovascular disease and cancer (reviewed in Carr & Frei( 12 )). It is possible that vitamin C may simply be a marker for high fruit and vegetable intake( 13 ) and other plant-derived components may be responsible for the observed health effects. However, based on vitamin C's newly discovered involvement in gene regulation and signalling, it is likely that the vitamin has an essential role in maintaining human health and preventing disease.
The current Australasian recommended dietary intake (RDI) of vitamin C is 45 mg/d for non-smoking men and women( 14 ). Pharmacokinetic studies carried out by Levine et al.( 15 , 16 ) indicate that this intake of vitamin C provides a plasma level which is considered ‘marginal’ or ‘inadequate’, i.e. <23 µmol/l( 17 – 19 ). Thus, the optimal intake of vitamin C required to maintain general health and wellbeing may be higher than the current RDI and is probably closer to a dose at which plasma reaches saturation, i.e. about 70 µmol/l. Along these lines, we and others( 12 , 15 , 16 ) have recommended a vitamin C intake of 120–200 mg/d, which can be obtained from a diet containing the recommended five plus daily servings of fruit and vegetables. It is known that there is a significant proportion of the population that does not achieve these intakes and whose plasma vitamin C levels are well below the recommended optimum( 20 , 21 ).
Although vitamin C is one of the most frequently used dietary supplements( 22 ), a food source of the vitamin may be preferred due to the presence of other potentially beneficial or synergistic constituents. Zespri® Gold kiwifruit (Actinidia chinensis var. Hort 16A) are an outstanding commonly available source of vitamin C, one serving providing twice the current Australasian RDI of the vitamin( 23 ). We recently completed an animal study, using a genetically vitamin C-deficient mouse model (the Gulo mouse), investigating the uptake of vitamin C from kiwifruit gel compared with vitamin C-supplemented water( 24 ). Interestingly, we found that kiwifruit provided significantly higher serum and tissue levels of vitamin C than did supplemented water. This suggests some type of synergistic activity of the whole fruit in this mouse model.
The primary aim of this study was to determine the uptake of vitamin C from kiwifruit in young men with inadequate vitamin C intake, in order to determine the amount of fruit required to raise plasma vitamin C to ‘healthy’ (i.e. >50 µmol/l) and ‘optimal’ or saturating levels (i.e. >70 µmol/l)( 17 ). Several studies have measured plasma uptake of vitamin C from kiwifruit in healthy volunteers( 25 – 27 ), in women with low Fe stores( 28 ), in hyperlipidaemic individuals( 29 ) and in smokers( 30 ). However, in these studies the participants already had high or saturating levels of plasma vitamin C at baseline (i.e. about 50–70 µmol/l), thus abrogating an obvious or consistent effect of supplementation. As such, a major objective of this study was to select individuals with low or below average plasma vitamin C in order to be able to observe a clear effect of supplementation. We monitored the dietary intake, plasma uptake and urinary excretion of the vitamin following supplementation with, sequentially, half, one, two and three Gold kiwifruit per d for 4–6 weeks each, and also measured leucocyte vitamin C levels as an indicator of tissue levels.
Experimental methods
Participants
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Upper South Regional Ethics Committee (consent no. URB/10/06/020). Written informed consent was obtained from all participants. Non-smoking males aged 18–30 years were recruited from local universities for the screening phase of the study.
A total of sixty students underwent a screening interview to ascertain their eligibility for the study. Anthropometric measurements were carried out to determine their BMI and a fasting venous blood sample was drawn to determine their plasma vitamin C levels. Exclusion criteria included: recent smoker (within the previous year), allergy/intolerance to kiwifruit, taking vitamin C-containing supplements (within the past 3 months), taking prescription medication (within the past 3 months), excessive alcohol consumption (more than twenty-one standard drinks per week), high fruit and vegetable consumption (more than five servings per d), current vegetarian or vegan, diabetes mellitus, bleeding disorders, obese (BMI > 35 kg/m2) and fainting due to fear of needles.
Power calculations indicated that at 95 % power with a 5 % significance level, a sample size of nine was adequate for detecting a minimum difference of 30 (sd 25) µmol/l as determined from the data presented in Levine et al.( 15 ). However, to allow for potential withdrawal due to the length of the study, fifteen non-smoking participants with less than average baseline plasma vitamin C levels were enrolled. One participant withdrew after completion of the lead-in phase and was not included in subsequent baseline calculations.
Study design
The study comprised a 4-week lead-in phase, an intervention phase of 20 weeks and a 4-week washout phase (Fig. 1). During the lead-in phase, weekly fasting blood samples were drawn to monitor variability in plasma vitamin C levels. At week 4 of the lead-in phase, baseline levels were measured in plasma and total leucocytes as described later and a 24 h urine collection was carried out to determine urinary vitamin C excretion. Participants also completed daily food and drink diaries to monitor their dietary vitamin C intake as indicated in Fig. 1.
Fig. 1.
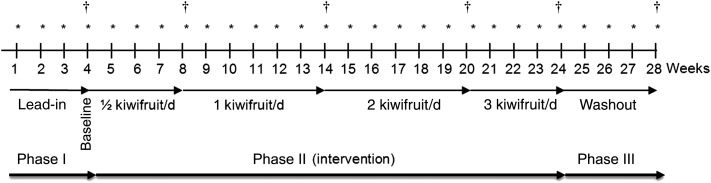
Study design which consisted of a lead-in phase of 4 weeks, an intervention phase of 20 weeks and a washout phase of 4 weeks. * Fasting blood samples taken. † 24 h urine collection and total leucocyte preparations carried out and when food and beverage diaries completed.
At the beginning of the intervention phase, participants were asked to consume half a kiwifruit per d for 4 weeks, followed by one kiwifruit per d for 6 weeks, two kiwifruit per d for 6 weeks and finally three kiwifruit per d for 4 weeks (Fig. 1). A longer time period was chosen for the one and two kiwifruit per d doses to allow the two kiwifruit per d dose to span the 2-week Christmas and New Year break, i.e. participants did not come into the clinic for blood tests during this time, but were provided with additional kiwifruit to allow them to remain on the appropriate dose for the duration of this holiday period. Fasting blood samples were drawn weekly for vitamin C analysis and additional analyses were carried out on 24 h urine samples and total leucocyte preparations at the end of each dose of kiwifruit. For each dose of kiwifruit, participants completed a daily food diary for 1 week.
During the final washout phase of the study the participants returned to their normal diet for 4 weeks (Fig. 1). Blood samples were drawn weekly and a 24 h urine collection and a total leucocyte preparation were carried out at the end of the washout. A 1-week daily food and drink record was completed to monitor dietary vitamin C intake at the end of the washout phase.
Intervention
Zespri® Gold kiwifruit (A. chinensis var. Hort. 16A) were stored at ≤4°C. The vitamin C content was monitored by extracting a sample of kiwifruit in 0·54 m-perchloric acid containing the metal chelator diethylene triamine pentaacetic acid (DTPA) (100 µmol/l) followed by centrifugation( 24 ). The supernatant was analysed by HPLC with electrochemical detection as described later. There was 89 (sd 8) mg vitamin C/100 g fruit (n 4), which is equivalent to about 80 mg vitamin C per kiwifruit.
Sample collection and processing
Plasma
Peripheral blood was collected into 5 ml K3-EDTA vacutainer tubes to stabilise the vitamin C( 31 ) and immediately placed on melting ice. Samples were centrifuged at 4°C, plasma removed and added to an equal volume of ice-cold 0·54 m-HPLC-grade perchloric acid/DTPA solution to precipitate the protein and stabilise the vitamin C. The perchloric acid/DTPA extracts were centrifuged and the deproteinated supernatants were stored at −80°C until HPLC analysis.
Urine
Urine was collected over 24 h into bottles containing K2-EDTA (final concentration 100 µmol/l) to stabilise the vitamin C( 32 ). Samples were diluted twofold with an ice-cold 0·54 m-HPLC-grade perchloric acid/DTPA solution and centrifuged before storage of the supernatants as described earlier.
Leucocytes
Total leucocytes were purified from 20 ml whole blood by dextran sedimentation and hypotonic lysis to remove erythrocytes as described previously( 33 ). The isolated cells were counted using a haemocytometer and vitamin C extracts of pelleted cells were prepared with an ice-cold 0·54 m-HPLC-grade perchloric acid/DTPA solution. The protein content was removed by centrifugation and the supernatants were stored as described earlier.
Analysis of vitamin C by HPLC
The vitamin C content of the kiwifruit, plasma, urine and leucocytes was analysed using reverse-phase HPLC with electrochemical detection using a modified method of Lee et al.( 34 ). Samples were analysed weekly and were separated on a Synergi 4 µ Hydro-RP 80A column 150 × 4·6 mm (Phenomenex NZ Ltd) using a Waters 600 solvent delivery system with a Hitachi L-2200 refrigerated autosampler and an ESA Coulochem II detector (+200 mV electrode potential and 20 µA sensitivity). The mobile phase comprised 80 mm-sodium acetate buffer, pH 4·8, containing DTPA (0·54 mmol/l) and a freshly added paired-ion reagent n-octylamine (1 µmol/l), delivered at a flow rate of 1·2 ml/min. Freshly prepared milli-q water was used for the preparation of all reagents and the mobile phase was sparged with helium. Each sample was analysed in duplicate or triplicate and the vitamin C content was determined to be stable for the duration of the analyses. The stability of the samples was determined under all storage conditions and no loss of ascorbate was observed. A standard curve of sodium-l-ascorbate, standardised spectrophotometrically at 245 nm (ɛ = 9860), was freshly prepared for each HPLC run in 77 mmol/l HPLC-grade perchloric acid containing DTPA (100 µmol/l). Plasma vitamin C content is expressed as μmol/l, urinary vitamin C content is expressed as mg/24 h and total leucocyte vitamin C content is expressed as nmol/108 cells.
Analysis of food and beverage records
The participants recorded their daily food and beverage intake for 1 week for each phase of the study (i.e. at baseline, half a kiwifruit per d, one kiwifruit per d, two kiwifruit per d, three kiwifruit per d and washout). They provided a detailed description of each item, including components of mixed dishes, brand of item, preparation or cooking process, and quantified the items using standard household measures, metric weights or volumes, size of food item or standard serves( 35 ). Food diary entries were followed up with the participants to confirm the accuracy of reporting. The number of servings of fruit and vegetables consumed by each participant was estimated from their food and beverage records using New Zealand Ministry of Health guidelines( 36 ). The vitamin C content of the fruit- and vegetable-containing foods and beverages was estimated using Diet Cruncher software (version 1.6, Way Down South Software) and the New Zealand FOODfiles Food Composition Database (2006).
Statistical analysis
Data are represented as either mean (sd) or mean (sem), as indicated in the text, and P ≤ 0·05 was considered significant. One-way repeated-measures ANOVA with the Fisher least significant deviation pairwise multiple comparison procedure was carried out using Sigma Stat software (version 11, Systat Software Inc.). The differences between paired data were determined by the two-tailed paired t test. The numbers of participants at each data point are indicated in the figures and tables.
Results
Screening
A total of sixty non-smoking male university students were screened for this study (Table 1). Their mean fasting plasma vitamin C level was 49 (sd 16) µmol/l, with a range of 6·8–77 µmol/l. Of those screened, fifteen young men with less than average plasma vitamin C levels, who also satisfied the other inclusion/exclusion criteria, were enrolled in the study. The mean fasting plasma vitamin C level of the enrolled group was 31 (sd 11) µmol/l, with a range of 8·8–44 µmol/l. Other than plasma vitamin C, there were no differences in the characteristics between the screened and the enrolled individuals (Table 1). During the course of the study, four participants withdrew before the end of the intervention phase and three did not complete the washout phase.
Table 1.
Characteristics of individuals screened and enrolled in the study
(Mean values, standard deviations and ranges)
| Screened (n 60) | Enrolled (n 15) | |||||
|---|---|---|---|---|---|---|
| Mean | sd | Range | Mean | sd | Range | |
| Age (years) | 21 | 2·5 | 18–28 | 21 | 2·2 | 18–26 |
| Weight (kg) | 80 | 12 | 59–116 | 80 | 16 | 60–116 |
| Height (cm) | 179 | 7 | 161–200 | 176 | 7 | 161–186 |
| BMI (kg/m2) | 25 | 3·5 | 19–35 | 26 | 4·4 | 20–35 |
| Vitamin C (μmol/l) | 49 | 16 | 6·8–77 | 31 | 11 | 8·8–44 |
Dietary intake of vitamin C
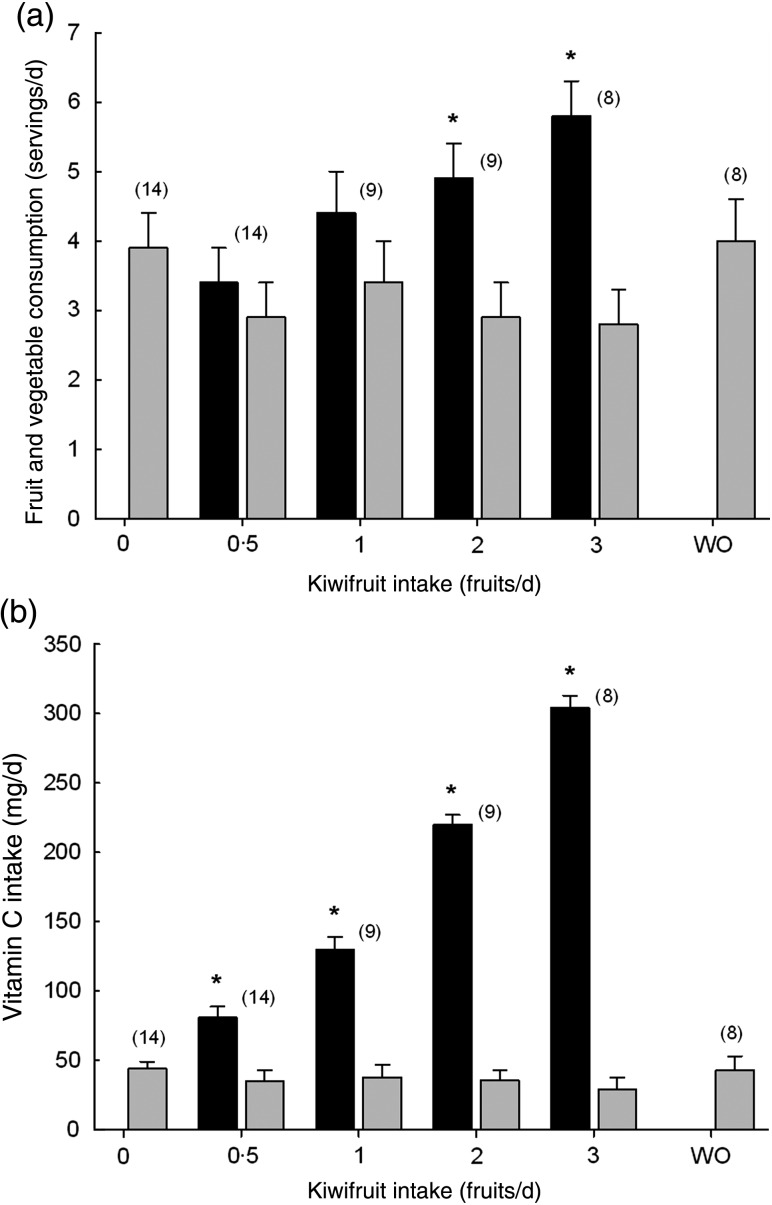
Fresh fruit and vegetables are the major dietary source of vitamin C. The number of fruit and vegetable servings consumed per d by the study participants, as determined from their food and beverage records, indicated a mean consumption of 3·9 (sem 0·5) servings of fruit and vegetables per d by the end of the lead-in period. Addition of kiwifruit to the diet resulted in an increase in fruit and vegetable consumption during the two and three kiwifruit per d supplementation phases of the study, with the average intake approaching six serves per d at the three kiwifruit dosage (Fig. 2(a)). The non-kiwifruit component of the diet was about three serves for the duration of the supplementation period. Consumption of fruit and vegetables returned to baseline levels during the washout period.
Fig. 2.
(a) Daily fruit and vegetable consumption and (b) vitamin C intake by the study
participants. (■), Total fruit and vegetable intake or vitamin C intake;
( ), total minus kiwifruit intervention.
Data are means, with standard errors represented by vertical bars. The numbers of
participants are indicated in parentheses. * Mean value was significantly different
from that at baseline (P < 0·05; one-way repeated-measures
ANOVA with the Fisher least significant deviation pairwise multiple comparison
procedure). WO, washout.
), total minus kiwifruit intervention.
Data are means, with standard errors represented by vertical bars. The numbers of
participants are indicated in parentheses. * Mean value was significantly different
from that at baseline (P < 0·05; one-way repeated-measures
ANOVA with the Fisher least significant deviation pairwise multiple comparison
procedure). WO, washout.
Analysis of the participants' daily vitamin C intake suggested a mean baseline intake of 44 (sem 5) mg/d of vitamin C. Consuming kiwifruit had a dose-dependent effect on daily vitamin C intake, with a significant increase from a dosage of half a kiwifruit per d onwards (Fig. 2(b)). When participants were given three kiwifruit per d, the average daily intake of vitamin C reached about 300 mg/d. During the 4-week washout period the participants' daily vitamin C intake returned to baseline levels.
Plasma vitamin C
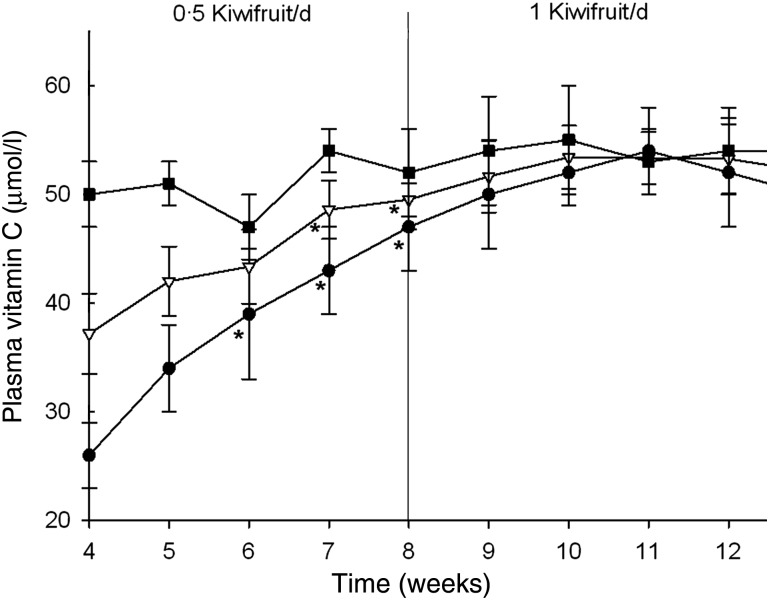
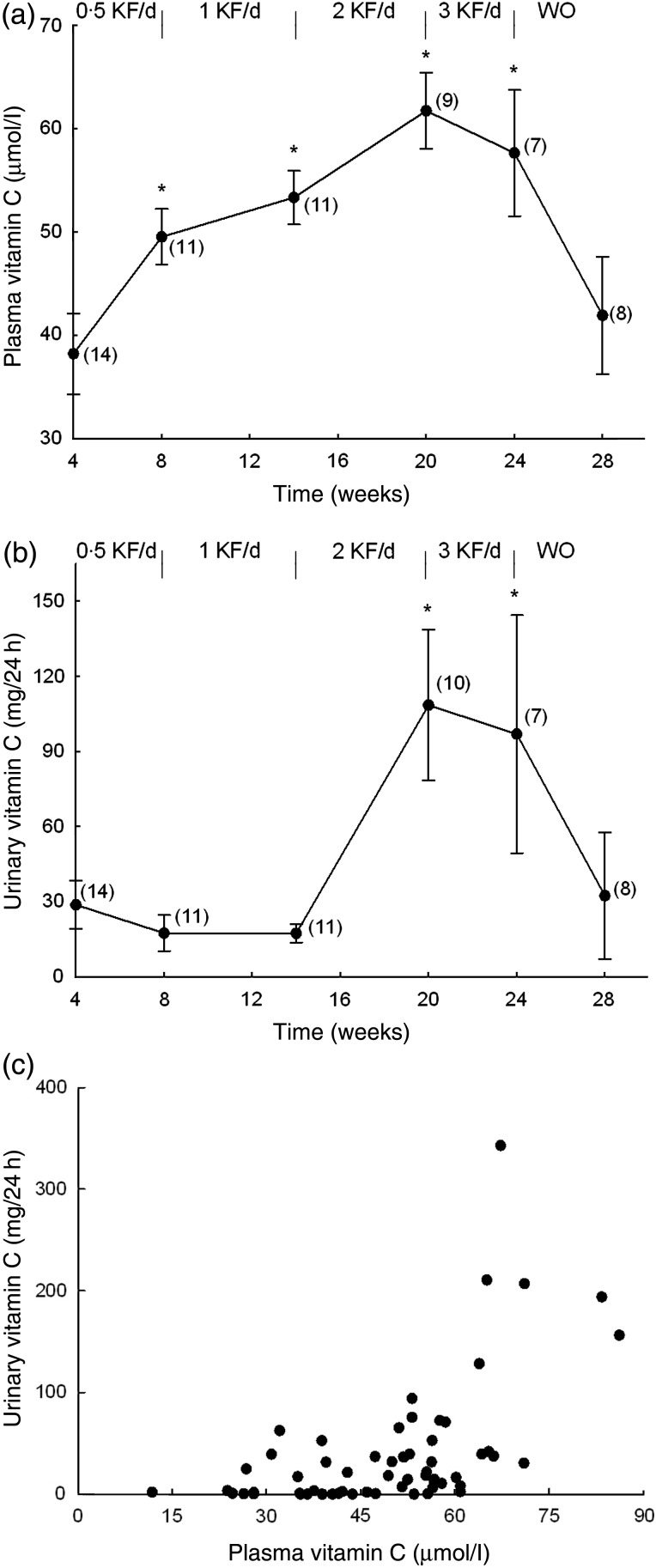
The participants' mean baseline fasting plasma vitamin C concentration was 38 (sem 4) µmol/l (Fig. 3). After 3 weeks of supplementation with half a kiwifruit per d there was a significant increase in plasma vitamin C level to 49 µmol/l (P = 0·010), with a maximum level of 50 µmol/l observed by the fourth week (Figs. 3 and 4(a)). Increasing the dose to one kiwifruit per d increased the average plasma vitamin C to 53 µmol/l after 6 weeks, and two kiwifruit per d for the next 6 weeks resulted in a further increase to 62 µmol/l (Fig. 4(a)). There was a significant difference between the one and two kiwifruit per d doses (P = 0·030). The final dose of three kiwifruit per d initially resulted in a maximal average plasma vitamin C of 68 µmol/l, an increase of 32 µmol/l above baseline, although after 4 weeks the average vitamin C level had dropped back to 58 µmol/l (Fig. 4(a)), with a high degree of variability in plasma vitamin C levels at this dosage. Following a 4-week washout the participants' plasma vitamin C levels had dropped back to 42 µmol/l, close to their starting level of 38 µmol/l.
Fig. 3.
Weekly plasma vitamin C levels of the combined group (▼; n 14), low vitamin C participants (•; n 7) and high vitamin C participants (■; n 7). Data are means, with standard errors represented by vertical bars. * Mean value was significantly different from that at baseline (P < 0·05; one-way repeated-measures ANOVA with the Fisher least significant deviation pairwise multiple comparison procedure).
Fig. 4.
(a) Plasma vitamin C levels of study participants and (b) urinary excretion of vitamin C by the study participants as a function of daily kiwifruit (KF) intake. Data are means, with standard errors represented by vertical bars. The numbers of participants are indicated in parentheses. * Mean value was significantly different from that at baseline (P < 0·05; one-way repeated-measures ANOVA with the Fisher least significant deviation pairwise multiple comparison procedure). WO, washout. (c) Correlation of plasma vitamin C with urinary excretion of vitamin C. Data points (n 62) were obtained from participants at each stage of the study (baseline, 0·5 KF/d, 1 KF/d, 2 KF/d, 3 KF/d, WO).
Preliminary statistical analysis indicated that the plasma vitamin C data were bimodal. Therefore, the participants were divided into two groups around their baseline mean at week 4, i.e. 37 µmol/l (Fig. 3). No significant increase in plasma vitamin C was observed for the higher group (with a baseline mean of 50 µmol/l vitamin C). In contrast, the lower group (with a baseline mean of 26 µmol/l vitamin C) showed an earlier response to supplementation as compared with the combined group, with a significant increase observed following the first week on half a kiwifruit per d (Fig. 3). A maximum average plasma vitamin C concentration of 67 µmol/l was reached in the lower group after 6 weeks of supplementation with two kiwifruit per d, an increase of 43 µmol/l above baseline.
Urinary vitamin C
Urinary vitamin C levels were measured in order to monitor excretion of the vitamin. The mean baseline vitamin C level was 29 (sem 10) mg/24 h (Fig. 4(b)) and these levels remained unchanged until the two kiwifruit dosage, when a significant increase in urinary vitamin C level to 109 (sem 30) mg/24 h was observed (Fig. 4(b)). This suggests that plasma saturation is not occurring until this dosage. Urinary vitamin C levels at the three kiwifruit dosage were highly variable. Following the 4-week washout period the urinary vitamin C values dropped back to baseline. Correlation of plasma vitamin C with urinary vitamin C indicated a marked increase in urinary output of the vitamin only at plasma concentrations of >60 µmol/l vitamin C (Fig. 4(c)). This was generally achieved by our participants when they consumed two or three kiwifruit per d and indicates that plasma levels are a more accurate measure of saturation than the amount of fruit consumed.
Leucocyte vitamin C
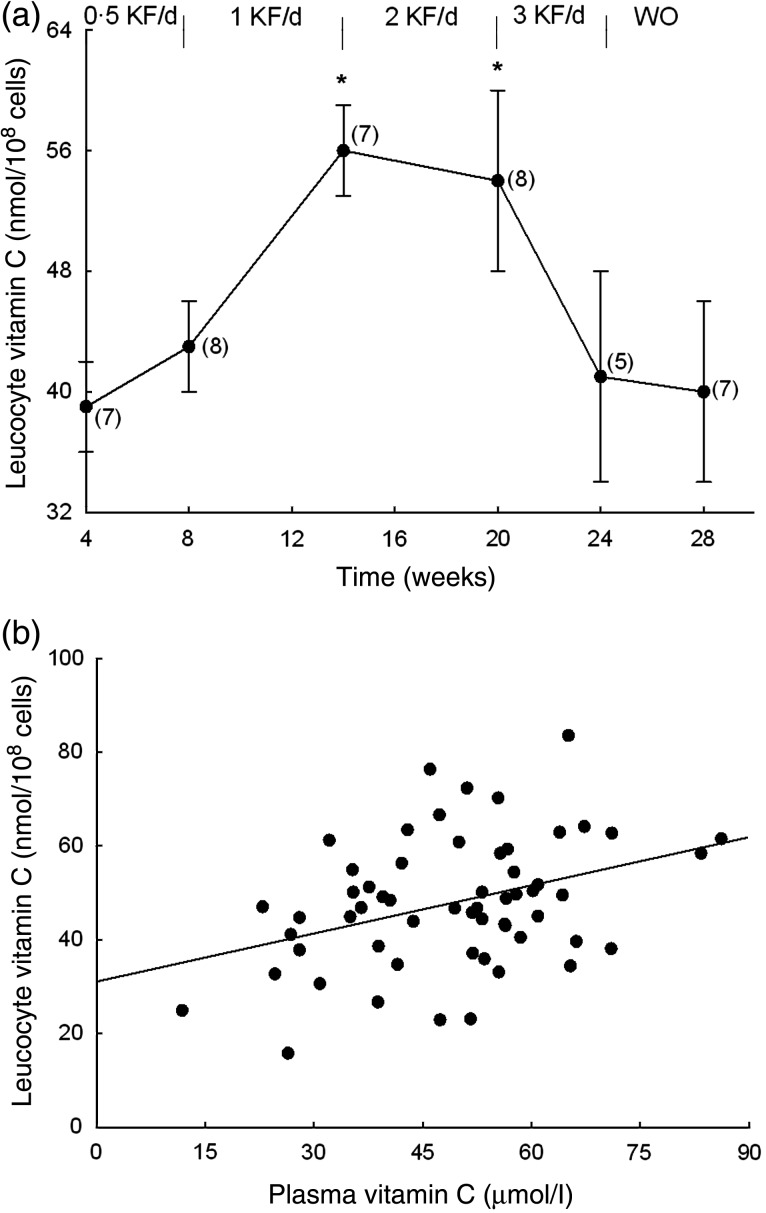
The baseline vitamin C level of the participants' leucocytes was 49 (sem 3) nmol/108 cells. There was a small increase in leucocyte vitamin C levels with intervention, with the two kiwifruit per d dosage reaching 53 (sem 5) nmol/108 cells (P = 0·057). However, when the participants were divided into two groups around their baseline mean at week 4, the low vitamin C group (39 (sem 3) nmol/108 cells) showed a significant increase in vitamin C at both the one and two kiwifruit per d dosages (Fig. 5(a)). Interestingly, there was a significant drop in leucocyte vitamin C at the three kiwifruit per d dosage, which may reflect the drop in plasma levels in this subgroup. A significant correlation of plasma vitamin C with leucocyte vitamin C was observed (R 0·374, P = 0·004) (Fig. 5(b)).
Fig. 5.
(a) Leucocyte vitamin C levels of the low vitamin C group as a function of daily kiwifruit (KF) intake. Data are means, with standard errors represented by vertical bars. The numbers of participants are indicated in parentheses. * Mean value was significantly different from that at baseline (P < 0·05; two-tailed paired t test). WO, washout. (b) Correlation of plasma vitamin C with leucocyte vitamin C. Data points (n 58) were obtained from participants at each stage of the study (baseline, 0·5 KF/d, 1 KF/d, 2 KF/d, 3 KF/d, WO). Linear regression analysis provided an R value of 0·374 and a P value of 0·004.
Discussion
A significant downfall of many supplementation studies is the high initial vitamin C status of the participants, which can abrogate a clear effect of supplementation on the plasma vitamin levels( 25 – 30 ). Therefore, a major objective of this study was to recruit individuals with low initial vitamin C status. We screened sixty male university students to determine their vitamin C status and enrolled fifteen of the lowest for the study. Despite thirteen of these fifteen individuals already having what is considered by some to be ‘adequate’ plasma levels of the vitamin (i.e. >23 µmol/l)( 17 – 19 ), supplementation of the participants with as little as half a kiwifruit per d resulted in a significant increase in their plasma vitamin C levels by the third week. Supplementation with one, two and three kiwifruit per d resulted in further increases in plasma vitamin C levels.
The primary aim of this study was to investigate the effect of a high vitamin C-containing fruit such as kiwifruit on plasma vitamin C levels and specifically to determine the dosage required to reach ‘healthy’ and ‘optimal’ levels. Considerable debate exists as to what constitutes ‘healthy’ or ‘optimal’ intake, as evidenced by the differing RDI for the vitamin; in Australasia 45 mg/d is considered adequate for both men and women whereas the USA and Canada recommend 90 mg/d for men and 75 mg/d for women. The current understanding is that a dose which gives a saturated plasma level (about 70 µmol/l) is ‘optimal’, as this is likely to provide additional health benefits of vitamin C beyond preventing scurvy( 17 , 37 ). In our study, enhanced urinary output of vitamin C was not observed until plasma levels were >60 µmol/l, suggesting that plasma saturation occurs at this level. We found that this required the addition of two kiwifruit per d to the diet, with an overall daily intake of around 220 mg vitamin C. Levine et al. also reported plasma saturation at a vitamin C intake of about 200 mg/d( 15 , 16 ).
It has been difficult to define what constitutes a ‘healthy’ plasma level of vitamin C. Clinical guidelines of severe vitamin C deficiency (plasma <11 µmol/l) and marginal vitamin C deficiency (plasma <23 µmol/l) have long been established and relate to the risk of developing scurvy( 17 , 19 ). Lykkesfeldt & Poulsen( 17 ) recently proposed a new category of suboptimal vitamin C status which is based on the supposition that if a plasma concentration of 70 µmol/l and above is ‘optimal,’ then those individuals with plasma concentrations between 23 and 50 µmol/l have plasma concentrations which are suboptimal. The clinical significance of the suboptimal category has not yet been clarified; however, we and others( 38 ) have measured a significant correlation between leucocyte and plasma vitamin C levels, suggesting that tissue levels respond to increased intake and optimal plasma concentrations. In our study, plasma concentrations of 50 µmol/l and above were obtained by supplementation of individuals with a minimum of one kiwifruit per d. This is readily achievable as part of a balanced diet.
When the study participants were divided into two groups around their mean plasma vitamin C level, those participants with the lowest level at baseline (mean of 26 µmol/l vitamin C) showed an earlier and greater response to supplementation, with half to three kiwifruit per d giving increases in plasma vitamin C levels ranging from 22 to 43 µmol/l. This compares with the increases observed for the combined data of 14–32 µmol/l. Collins et al.( 25 ) showed a more modest dose-dependent increase in plasma vitamin C, with increases from 7 to 16 µmol/l, following supplementation with one, two and three kiwifruit per d. However, in that study, participants were not selected on the basis of low vitamin C and had reasonably high levels prior to supplementation. In comparison with the low group, the high baseline vitamin C group (mean plasma levels of 50 µmol/l) showed little effect of supplementation. This lack of a dose-dependent effect with the high group is consistent with the observations of two previous kiwifruit supplementation studies( 26 , 27 ), and probably reflects the high initial vitamin C status of the participants. If plasma levels are approaching saturation it will be difficult to demonstrate an effect, as any excess will be excreted( 15 , 17 ). Our study therefore provides a clear demonstration of the importance of recruiting a suitable population for supplementation studies.
Leucocytes readily accumulate vitamin C and our results support the earlier findings of Levine et al.( 15 , 16 ), who reported saturation of leucocytes at an intake of 100 mg/d. We observed an increase in total blood leucocyte vitamin C levels in the low vitamin C participants with the consumption of one and two kiwifruit per d and an increase in the combined group at a dosage of two kiwifruit per d. Previous studies in which leucocytes were separated into mononuclear cells (monocytes and lymphocytes) and granulocytes (neutrophils) have indicated a more variable dose-dependent effect with mononuclear cell vitamin C levels than with granulocytes( 15 , 16 , 38 ). Since mononuclear cells comprise about 40 % of leucocyte samples and contain two to three times the amount of vitamin C as granulocytes, using a total blood leucocyte preparation may abrogate an obvious dose-dependent effect of supplementation.
The participants' baseline daily vitamin C intake was 44 mg/d, which is close to the Australasian RDI of 45 mg/d, and indicates a vitamin C intake of about 11 mg/serving of fruit and vegetables. Despite the lack of contribution of half a kiwifruit per d to the participants' average number of fruit and vegetable servings, this dose did nevertheless result in a significant increase in their average daily vitamin C intake (to about 80 mg/d) and did significantly increase plasma levels. The one kiwifruit per d dose resulted in an average vitamin C intake of 130 mg/d, which is close to the intake recommended to prevent chronic disease, i.e. a ‘healthy’ intake( 12 ). The dosage of two kiwifruit per d resulted in an average vitamin C intake of 220 mg/d, which is comparable with the dose recommended to reach plasma saturation, i.e. an ‘optimal’ intake( 37 ). We observed that some of the participants incorporated the kiwifruit intervention into their daily diet by reducing their intake of other fruits and vegetables, while other participants consumed the kiwifruit in addition to their normal daily diet. Interestingly, the participants who incorporated the kiwifruit intervention into their baseline diet still exhibited a significant increase in their daily vitamin C intake with intervention. This reflects the significantly higher amount of vitamin C in kiwifruit compared with many other fruits and vegetables and emphasises the effectiveness of consuming a food with high vitamin C content.
Somewhat surprisingly, there was a drop in plasma and urinary vitamin C levels after 4 weeks on three kiwifruit per d, although these levels did not differ significantly from two kiwifruit per d. The variability in plasma and urinary vitamin C levels at this higher dosage probably reflects a degree of non-compliance by some of the participants. This is likely to be the case, as meta-analysis has indicated that plasma vitamin C reflects dietary intake( 39 ). Another possible reason for the observed decrease is the inhibition of intestinal uptake of vitamin C by kiwifruit-derived flavonoids( 40 ). Similarly, the significant drop in leucocyte vitamin C levels at the three kiwifruit per d dosage probably reflects participant non-compliance at this dosage or possible inhibition of cellular vitamin C uptake by kiwifruit-derived flavonoids( 41 ).
Several studies have suggested that kiwifruit consumption has significant biological effects. In addition to containing high concentrations of vitamin C, kiwifruit are also a good source of vitamins E and K, folate, K, fibre, carotenoids and polyphenols, and these compounds may also confer health benefits. Kiwifruit have been shown to improve digestive health( 42 – 44 ), modulate lipid profiles( 26 , 29 ) and reduce platelet aggregation( 26 , 27 , 45 ). There is some evidence that consumption of one to three kiwifruit per d reduces endogenous levels of oxidised pyrimidines and purines in the DNA of healthy individuals( 25 , 27 ) and affects DNA repair activity( 25 , 27 , 46 ). Bohn et al. demonstrated up-regulation of a number of DNA repair genes in blood cells from male smokers supplemented with three kiwifruit per d for 8 weeks( 47 ). Consumption of Gold kiwifruit has also been shown to improve Fe absorption in women with low-Fe status( 28 ), probably due to the ability of vitamin C to enhance the absorption of non-haem Fe.
Overall, the results of our human study show kiwifruit to be an excellent source of vitamin C in humans. Addition to the daily diet of as little as half a kiwifruit resulted in a significant increase in plasma vitamin C in men who, despite consuming up to four serves of fruit and vegetables per d, still had below average vitamin C status. However, one kiwifruit per d was sufficient to achieve ‘healthy’ plasma levels of vitamin C (i.e. >50 µmol/l) and with two or three per d, ‘optimal’ plasma levels of the vitamin were reached. Our data confirm the pharmacokinetic data of Levine et al.( 15 , 16 ) and indicate that plasma vitamin C levels in humans saturate at an intake of about 200 mg/d (equivalent to approximately two kiwifruit per d), whereas leucocytes saturate at an intake of about 100 mg/d (equivalent to about one kiwifruit per d). This may therefore suggest that vitamin C supplementation is equivalent to consumption of the vitamin in foods. Finally, our study indicated that in order to observe a consistent effect of supplementation, it is critical to obtain a study population that has low initial vitamin C levels.
Acknowledgements
We express our gratitude to the young men who participated in this study, many of whom showed a great deal of dedication and perseverance. We acknowledge Simon Kerridge for Diet Cruncher analysis of the food and beverage diaries, Jo Kepple for allowing us generous access to the Primorus Clinical Trials Unit and Lynley Drummond for consultation on study design.
There are no conflicts of interest. Financial support for this study was provided by the University of Otago, Dunedin, New Zealand and Zespri International Ltd, New Zealand. The contributions of each author are as follows: M. C. M. V. and A. C. C. for study design; A. C. C. for recruitment and co-ordination; S. M. for phlebotomy and sample processing; J. P. and A. C. C. for sample and data analyses; A. C. C., J. P. and M. C. M. V. for manuscript preparation. Each author has seen and approved the contents of the manuscript.
References
- 1.Burri BJ & Jacob RA (1997) Human metabolism and the requirement for vitamin C In Vitamin C in Health and Disease, pp. 341–366 [Packer L and Fuchs J, editors]. New York: Marcel Dekker [Google Scholar]
- 2.Sauberlich HE (1997) A history of scurvy and vitamin C In Vitamin C in Health and Disease, pp. 1–24 [Packer L and Fuchs J, editors]. New York: Marcel Dekker [Google Scholar]
- 3.Tsao CS (1997) An overview of ascorbic acid chemistry and biochemistry In Vitamin C in Health and Disease, pp. 25–58 [Packer L and Fuchs J, editors]. New York: Marcel Dekker [Google Scholar]
- 4.Carr A & Frei B (1999) Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J 13, 1007–1024 [DOI] [PubMed] [Google Scholar]
- 5.Loenarz C & Schofield CJ (2008) Expanding chemical biology of 2-oxoglutarate oxygenases. Nat Chem Biol 4, 152–156 [DOI] [PubMed] [Google Scholar]
- 6.Arrigoni O & De Tullio MC (2002) Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta 1569, 1–9 [DOI] [PubMed] [Google Scholar]
- 7.Vissers MC, Gunningham SP, Morrison MJ, et al. (2007) Modulation of hypoxia-inducible factor-1 alpha in cultured primary cells by intracellular ascorbate. Free Radic Biol Med 42, 765–772 [DOI] [PubMed] [Google Scholar]
- 8.Knowles HJ, Raval RR, Harris AL, et al. (2003) Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res 63, 1764–1768 [PubMed] [Google Scholar]
- 9.Gao P, Zhang H, Dinavahi R, et al. (2007) HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell 12, 230–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirota K, Semenza GL (2005) Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem Biophys Res Commun 338, 610–616 [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL (2000) HIF-1 and human disease: one highly involved factor. Genes Dev 14, 1983–1991 [PubMed] [Google Scholar]
- 12.Carr AC & Frei B (1999) Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr 69, 1086–1107 [DOI] [PubMed] [Google Scholar]
- 13.Block G, Norkus E, Hudes M, et al. (2001) Which plasma antioxidants are most related to fruit and vegetable consumption? Am J Epidemiol 154, 1113–1118 [DOI] [PubMed] [Google Scholar]
- 14.National Health and Medical Research Council (2006) Vitamin C In Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes, pp. 119–125 Canberra: NHMRC Publications [Google Scholar]
- 15.Levine M, Conry-Cantilena C, Wang Y, et al. (1996) Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A 93, 3704–3709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine M, Wang Y, Padayatty SJ, et al. (2001) A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci U S A 98, 9842–9846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lykkesfeldt J & Poulsen HE (2010) Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br J Nutr 103, 1251–1259 [DOI] [PubMed] [Google Scholar]
- 18.Hampl JS, Taylor CA & Johnston CS (2004) Vitamin C deficiency and depletion in the United States: the Third National Health and Nutrition Examination Survey, 1988 to 1994. Am J Public Health 94, 870–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob RA (1990) Assessment of human vitamin C status. J Nutr 120, Suppl. 11, 1480–1485 [DOI] [PubMed] [Google Scholar]
- 20.Schleicher RL, Carroll MD, Ford ES, et al. (2009) Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr 90, 1252–1263 [DOI] [PubMed] [Google Scholar]
- 21.Mosdol A, Erens B & Brunner EJ (2008) Estimated prevalence and predictors of vitamin C deficiency within UK's low-income population. J Public Health (Oxf) 30, 456–460 [DOI] [PubMed] [Google Scholar]
- 22.Schutz HG, Read M, Bendel R, et al. (1982) Food supplement usage in seven Western states. Am J Clin Nutr 36, 897–901 [DOI] [PubMed] [Google Scholar]
- 23.Nishiyama I, Yamashita Y, Yamanaka M, et al. (2004) Varietal difference in vitamin C content in the fruit of kiwifruit and other actinidia species. J Agric Food Chem 52, 5472–5475 [DOI] [PubMed] [Google Scholar]
- 24.Vissers MCM, Bozonet SM, Pearson JF, et al. (2011) Dietary ascorbate affects steady state tissue levels in vitamin C-deficient mice: tissue deficiency after sub-optimal intake and superior bioavailability from a food source (kiwifruit). Am J Clin Nutr 93, 292–301 [DOI] [PubMed] [Google Scholar]
- 25.Collins AR, Harrington V, Drew J, et al. (2003) Nutritional modulation of DNA repair in a human intervention study. Carcinogenesis 24, 511–515 [DOI] [PubMed] [Google Scholar]
- 26.Duttaroy AK & Jorgensen A (2004) Effects of kiwi fruit consumption on platelet aggregation and plasma lipids in healthy human volunteers. Platelets 15, 287–292 [DOI] [PubMed] [Google Scholar]
- 27.Brevik A, Gaivao I, Medin T, et al. (2011) Supplementation of a western diet with golden kiwifruits (Actinidia chinensis var. ‘Hort 16A'): effects on biomarkers of oxidation damage and antioxidant protection. Nutr J 10, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beck K, Conlon CA, Kruger R, et al. (2011) Gold kiwifruit consumed with an iron-fortified breakfast cereal meal improves iron status in women with low iron stores: a 16-week randomised controlled trial. Br J Nutr 105, 101–109 [DOI] [PubMed] [Google Scholar]
- 29.Chang WH & Liu JF (2009) Effects of kiwifruit consumption on serum lipid profiles and antioxidative status in hyperlipidemic subjects. Int J Food Sci Nutr 60, 709–716 [DOI] [PubMed] [Google Scholar]
- 30.Karlsen A, Svendsen M, Seljeflot I, et al. (2011) Compliance, tolerability and safety of two antioxidant-rich diets: a randomised controlled trial in male smokers. Br J Nutr 106, 557–571 [DOI] [PubMed] [Google Scholar]
- 31.Lykkesfeldt J (2012) Ascorbate and dehydroascorbic acid as biomarkers of oxidative stress: validity of clinical data depends on vacutainer system used. Nutr Res 32, 66–69 [DOI] [PubMed] [Google Scholar]
- 32.Chalmers AH, Cowley DM & McWhinney BC (1985) Stability of ascorbate in urine: relevance to analyses for ascorbate and oxalate. Clin Chem 31, 1703–1705 [PubMed] [Google Scholar]
- 33.Boyum A (1968) Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl 97, 77–89 [PubMed] [Google Scholar]
- 34.Lee W, Hamernyik P, Hutchinson M, et al. (1982) Ascorbic acid in lymphocytes: cell preparation and liquid-chromatographic assay. Clin Chem 28, 2165–2169 [PubMed] [Google Scholar]
- 35.Gibson R (2002) Dietary assessment In Essentials of Human Nutrition, 2nd ed., pp. 449–466 [Mann J and Truswell AS, editors]. Oxford: Oxford University Press [Google Scholar]
- 36.Ministry of Health (2003) Food and Nutrition Guidelines for Healthy Adults - a Background Paper, p. 148. Wellington: Ministry of Health [Google Scholar]
- 37.Levine M, Padayatty SJ & Espey MG (2011) Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr 2, 78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans RM, Currie L & Campbell A (1982) The distribution of ascorbic acid between various cellular components of blood, in normal individuals, and its relation to the plasma concentration. Br J Nutr 47, 473–482 [DOI] [PubMed] [Google Scholar]
- 39.Dehghan M, Akhtar-Danesh N, McMillan CR, et al. (2007) Is plasma vitamin C an appropriate biomarker of vitamin C intake? A systematic review and meta-analysis. Nutr J 6, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song J, Kwon O, Chen S, et al. (2002) Flavonoid inhibition of sodium-dependent vitamin C transporter 1 (SVCT1) and glucose transporter isoform 2 (GLUT2), intestinal transporters for vitamin C and Glucose. J Biol Chem 277, 15252–15260 [DOI] [PubMed] [Google Scholar]
- 41.Park JB & Levine M (2000) Intracellular accumulation of ascorbic acid is inhibited by flavonoids via blocking of dehydroascorbic acid and ascorbic acid uptakes in HL-60, U937 and Jurkat cells. J Nutr 130, 1297–1302 [DOI] [PubMed] [Google Scholar]
- 42.Rush EC, Patel M, Plank LD, et al. (2002) Kiwifruit promotes laxation in the elderly. Asia Pac J Clin Nutr 11, 164–168 [DOI] [PubMed] [Google Scholar]
- 43.Chan AO, Leung G, Tong T, et al. (2007) Increasing dietary fiber intake in terms of kiwifruit improves constipation in Chinese patients. World J Gastroenterol 13, 4771–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang CC, Lin YT, Lu YT, et al. (2010) Kiwifruit improves bowel function in patients with irritable bowel syndrome with constipation. Asia Pac J Clin Nutr 19, 451–457 [PubMed] [Google Scholar]
- 45.Karlsen A, Svendsen M, Seljeflot I, et al. (2012) Kiwifruit decreases blood pressure and whole-blood platelet aggregation in male smokers. J Hum Hypertens (epublication ahead of print version 19 January 2012). [DOI] [PubMed] [Google Scholar]
- 46.Brevik A, Karlsen A, Azqueta A, et al. (2011) Both base excision repair and nucleotide excision repair in humans are influenced by nutritional factors. Cell Biochem Funct 29, 36–42 [DOI] [PubMed] [Google Scholar]
- 47.Bohn SK, Myhrstad MC, Thoresen M, et al. (2010) Blood cell gene expression associated with cellular stress defense is modulated by antioxidant-rich food in a randomised controlled clinical trial of male smokers. BMC Med 8, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]