Abstract
Study Objectives:
Patients with irritable bowel syndrome (IBS) often report sleep disturbances. Previously, we have shown that self-reported sleep difficulties predicted exacerbations of next-day IBS symptoms, mood disturbance, and fatigue. The purpose of this study was to explore whether objectively measured sleep using actigraphy, as well as self-report, predicts next-day symptoms in women with IBS and to explore whether or not symptoms also predict self-report and objective sleep.
Methods:
Women aged 18-45 years with IBS were community-recruited (n = 24, mean age = 32 ± 8 years). Participants completed sleep and IBS symptom diaries for one menstrual cycle and wore Actiwatch-64 actigraphs for 7 days at home. Statistical analyses used generalized estimating equation (GEE) models.
Results:
Poorer self-reported sleep quality significantly (p < 0.05) predicted higher next-day abdominal pain, anxiety, and fatigue, but was not significant for gastrointestinal (GI) symptoms or depressed mood. Actigraphic sleep efficiency (SEF) significantly predicted worsening next-day anxiety and fatigue, but not abdominal pain, GI symptoms, or depressed mood. On temporally reversed analyses, none of the symptoms significantly predicted subsequent sleep, except that GI symptoms significantly predicted higher actigraphic sleep efficiency.
Conclusion:
This small exploratory study supports previous findings that self-reported sleep disturbance predicted exacerbation of next-day symptoms in women with IBS and extends this relationship using an objective sleep measure. The study adds further evidence that sleep quality predicts subsequent IBS symptoms, but not the converse. The findings from this small study support the importance of additional longitudinal research to further understand the relationships between sleep and IBS.
Citation:
Buchanan DT, Cain K, Heitkemper M, Burr R, Vitiello MV, Zia J, Jarrett M. Sleep measures predict next-day symptoms in women with irritable bowel syndrome. J Clin Sleep Med 2014;10(9):1003-1009.
Keywords: sleep disturbance, sleep quality, psychological, gastrointestinal, actigraphy, polysomnography, symptoms, women, irritable bowel syndrome
Irritable bowel syndrome (IBS) is a common functional gastrointestinal (GI) disorder characterized by intermittent abdominal pain or discomfort associated with bowel pattern alterations (constipation, diarrhea, or mixed-pattern).1 Cross-sectional studies of persons with IBS have shown associations between sleep disturbance and IBS symptoms such as abdominal pain, bloating, diarrhea, and constipation.2–5 Persons with IBS also experience non-GI symptoms including mood disturbances (particularly anxiety and depression) and fatigue.6–8 Although evidence suggests bi-directional interactions of IBS with depression, anxiety, and insomnia,9,10 it is not known how these factors interact day-to-day. Female sex may also be a factor in the interaction of IBS and related symptoms, given that IBS and sleep disturbance are both more common in women than in men.11,12
To better characterize symptom patterns in women with IBS, studies of longitudinal data are needed for exploring the temporal relationships between sleep and symptoms. In particular, it is important to understand whether sleep disturbance and symptoms co-vary over time and whether this relationship is directional. Such information would be useful for understanding potential mechanisms underlying exacerbations of both sleep disturbance and IBS and possibly for targeting treatments (e.g., could treatment of sleep disturbance reduce GI symptoms?).
BRIEF SUMMARY
Current Knowledge/Study Rationale: Self-reported sleep quality predicts next-day symptoms in IBS, but it is not known whether the same is true of objectively measured sleep. Furthermore, studies show that GI symptoms or pain do not necessarily predict sleep disturbance, but little evidence on this relationship is available in persons with IBS.
Study Impact: This study shows that objective as well as subjective sleep outcomes predict next-day IBS symptoms, but that daytime IBS symptoms do not predict subsequent nighttime sleep quality. Clarifying the relationship between sleep quality and IBS symptoms is important for understanding underlying mechanisms of IBS and identifying potential treatment targets in persons with IBS.
When examining symptoms over time, it is recognized that a different picture may emerge from averaged subject responses over the time period versus day-to-day trends within each subject. Examining mean values between subjects (also called the across-subjects effect) reflects the association between two variables in the entire sample without regard to within-person fluctuations over time; i.e., do people who rate one symptom high overall tend to rate the other symptom high or low overall. However, examining covariation of the variables within individuals (the within-subjects effect) reveals information about the day-to-day relationships between symptoms (if individual participants' ratings on one symptom increase, does the other symptom also increase or decrease?). Whereas a trait-like bias toward rating all symptoms high or low may confound across-subjects effects, within-subjects effects could still reflect whether daily changes in one symptom accompany changes in the other. For this reason, research examining across-subjects effects is not sufficient to indicate whether or not symptoms covary day-to-day, and within-subject effects must be examined.
A previous study by our research group partially addressed this issue by using longitudinal data from a daily diary to examine the temporal associations between sleep and symptoms in women with IBS.3 Separating the across-subject and the within-subject associations revealed a within-subject association; self-reported sleep disturbance predicted individuals' next-day IBS symptoms. This study also showed a significant positive across-subject correlation, indicating that, overall, higher mean sleep disturbance predicted higher mean IBS symptoms. Beyond the approach used in this prior study, potential effects of within-subject self-report bias may be explored by comparing self-report to an objective measure, which should not be subject to bias. Actigraphy offers a low-cost, low-burden approach for obtaining objective longitudinal sleep data; and actigraphy has been shown useful for measuring within-subject fluctuations in sleep over time.13 Thus, actigraphy offers a feasible objective measure for examining covariance of sleep and symptoms in persons with IBS, as well as other populations.
Another growing field of knowledge that pertains to the present research is examination of the directionality of relationships between sleep and symptoms. Much of this evidence has been produced within studies of pain and sleep. Evidence emerging over the past several years demonstrates that nightly sleep disturbance tends to strongly predict next-day pain, but the relationship between daily pain and that night's sleep tends to be weak.14–16 Our previous study was consistent with those results: self-reported sleep disturbances predicted next-day IBS symptoms, but the reverse was not observed—day-of IBS symptoms did not predict that night's self-reported sleep disturbance.3 However, the directionality of the relationship between symptoms and sleep in IBS has not been examined using objective sleep data.
The purpose of this study was to explore whether objectively measured sleep using actigraphy, as well as self-report, predicts next-day IBS symptoms and to explore whether or not IBS symptoms predict self-report and objective sleep. For this study, IBS symptoms included abdominal pain and functional GI symptoms (bloating, diarrhea, and constipation). We also measured mood (depressed mood and anxiety), and fatigue. The specific aims of the study were (1) to examine whether or not self-reported sleep quality ratings from the prior night predict next-day abdominal pain, GI symptoms, mood, and fatigue; (2) to examine whether objective sleep quality measured by actigraphic sleep efficiency from the prior night predicts next-day abdominal pain, GI symptoms, mood, and fatigue; and (3) to explore whether or not day-of symptoms also predict self-reported and/or objective sleep quality that night. We hypothesized that poorer subjective and objective sleep would predict worse ratings on all symptoms, but that the relationships would be weaker for objective sleep (actigraphic sleep efficiency). Consistent with prior sleep research, we hypothesized that sleep would predict symptoms, but not the converse.
METHODS
Subjects
Prior to recruitment, approval was obtained from the University of Washington Human Subjects Board. Women with IBS, 18 to 45 years of age, were recruited through community advertisements (n = 24). To be enrolled, the women in the IBS group had to have a medical diagnosis of IBS and currently be experiencing symptoms compatible with the Rome-II criteria for IBS.17 Women were excluded if they had (1) significant comorbidities (e.g., history or current comorbid abdominal conditions other than IBS, current cardiac dysrhythmia, sleep disorders, pain disorders, psychiatric disorders); (2) medications that could interfere with sleep or affect IBS symptoms (e.g., β-blockers, antihistamines, benzodiazepines, or antidepressants, prokinetic, 5-HT3 antagonist, 5-HT4 agonist drugs, or hormonal contraceptives); or (3) other characteristics that could affect sleep (i.e., body mass index [BMI, kg/m2] > 35, late evening and night work, > 300 mg caffeine consumption in the afternoon-evening, or ≥ 3 servings of alcohol daily). Forty-three women with IBS were enrolled. Nineteen were excluded for insufficient data for various reasons (e.g., withdrew due to time commitment, unwilling to complete sleep laboratory protocol).
Procedures
The purpose of the parent study was to examine polysomnographic and self-reported sleep in women with IBS, previously described elsewhere.18 Informed consent was obtained from participants prior to data collection. Next, the women completed baseline questionnaires, underwent a targeted physical assessment, and were instructed on how to complete the daily sleep and symptom diary. At home, each participant completed the diary each evening over one menstrual cycle, starting with the first day of her next menses. All women underwent PSG monitoring in the sleep laboratory. PSG and actigraphy were collected starting during mid-luteal menstrual phase (based on testing for the luteinizing hormone surge) to control for hormone-related symptom fluctuation.18 At this time, the actigraph was placed on the participant's non-dominant wrist and worn continuously for 10 days (some subjects wore the actigraph a few days longer even though they were instructed to remove it). The first 3 nights were spent in the sleep laboratory and are not included in these analyses as unlikely representative of their regular sleep pattern. Only the nights of concurrent actigraphy and daily diary data collected at home are included.
Measures
Descriptive Measures
Demographic data included age, occupation, and education. Baseline sleep was assessed using the Pittsburgh Sleep Quality Index (PSQI), which assesses sleep quality and disturbances over the prior month.19 A global PSQI score > 5 yielded a diagnostic sensitivity of 90% and specificity of 87% (κ = 0.75, p < 0.001) in distinguishing good from poor sleepers.20
Self-Reported Sleep
Participants completed their symptom diary each evening by rating the highest level on each item experienced over the past 24 hours. Sleep-related items included difficulty falling asleep, daytime sleepiness, waking up too early, and waking up during the night, rated as 0 (not present), 1 (minimal), 2 (mild), 3 (moderate), or 4 (extreme), and sleep quality and how rested they felt upon awakening rated as 0 (very poor), 1 (poor), 2 (fair), 3 (good), or 4 (very good).
Severity of Daily IBS Symptoms
Participants rated an additional 33 symptoms on the daily diaries. These symptoms were summarized into subscales.3 The GI scales included abdominal pain (mean of 2 items: abdominal pain and stomach pain) and GI symptoms scores (mean of 4 items: diarrhea, constipation, bloating, intestinal gas). Mood was represented by anxiety (mean of 2 items: anxiety and worrying), depressed mood, and fatigue. Each item was rated as 0 (not present), 1 (minimal), 2 (mild), 3 (moderate), or 4 (extreme).
Objective Indicators of Sleep
Sleep was measured objectively using 7 nights of data collected from actigraphy (Actiwatch-64; Philips Respironics, Andover, MA). These devices are piezoelectric accelerometers, detecting movement on all planes. Movement data were sampled at a rate of 32 Hz, and activity counts were recorded in 30-s epochs. The data were analyzed using Actiware 5.57 software (Philips Respironics, Andover, MA). To analyze actigraphy data, the rest interval (i.e., time in bed) is manually entered into Actiware. The rest interval onset and offset were determined and entered by an experienced research assistant according to the standard criteria used by the University of Washington Center for Research on Management of Sleep Disturbances. All scoring was visually inspected by an investigator with expertise in actigraphy (DTB). The primary sleep outcome was sleep efficiency (SEF = total sleep time / time in bed). Other sleep outcomes that are reported descriptively include time in bed (TIB), total sleep time (TST), sleep onset latency (SOL = minutes between bedtime and initial sleep onset), and wake after sleep onset (WASO = wake time between initial sleep onset and final sleep offset). Sleep onset and offset were defined as the first and last periods of 10 min with ≤ 1 epoch of movement/wake.
Data Analysis
The study data were analyzed using SPSS 17. Only subjects with matched actigraphy and diary data for at least 4 nights were included; fewer nights were considered unlikely to reliably represent the relationships between sleep and symptoms over time. Subjects had between 4 and 7 nights of concurrent home data (median = 7 nights). The relationships between sleep and symptoms were explored in a bi-directional manner. In analyses examining whether sleep predicted next-day IBS symptoms, separate analyses were conducted using each of two predictor variables: self-reported sleep quality ratings (representing subjective sleep) and actigraphic SEF (representing objective sleep). There is no objective outcome that is an ideal equivalent for comparison to self-rated sleep quality, but actigraphic SEF likewise is interpreted as reflecting general sleep quality. One would expect these variables to be modestly, but not perfectly, correlated, which was examined using Pearson correlation. The dependent variables were all aforementioned IBS symptoms. In analyses examining whether IBS symptoms predicted sleep, each IBS symptom was used as a predictor variable and the outcomes were self-reported sleep quality ratings and actigraphic SEF.
All analyses of the relationships between sleep and IBS symptoms were conducted using generalized estimating equations (GEE) models. This approach accounts for the time-series correlation of observations within each subject.3 When analyzing clustered symptom data such as these, it is important to separate the within-subject effect from the across-subject effect.21,22 The across-subject effect addresses the question of whether subjects with poor sleep on average also have high IBS symptom severity on average. The within-subject effect addresses the question of whether, within a person, next-day IBS symptom severity is worse than average after nights when sleep is worse than average for that person. The across-subjects term was calculated as the within-subject mean value of the predictor (SEFmean). The within-subject term was calculated as the deviation of the daily value of the predictor from the within-subject mean (SEFday-SEFmean). Therefore, the coefficient of SEFmean would be interpreted as an estimate of across-subject effect and the coefficient of SEFday would be interpreted as an estimate of within-subject effect. Using the GEE approach, separate linear regression models were applied to each pair of variables (predictor and dependent variable) with both across-subjects and within-subjects effects of the predictor. Similar to a simple regression, GEE produces an unstandardized β value that indicates the correspondence of the 2 methods. To facilitate comparison of the β coefficients, the z scores of the predictor and outcome variables were used in the GEE analyses.
RESULTS
Subjects
The mean age of the sample was 31.9 ± 8.1 years. Most participants were unmarried (67%), college-educated (63%), employed in a technical or service profession (75%), and racially identified as white (75%). On the PSQI, 58% of the sample met or exceeded the threshold indicating poor sleep. Mean values on sleep and symptom outcomes are shown in Table 1.
Table 1.
Mean symptom and sleep outcomes in women with IBS.
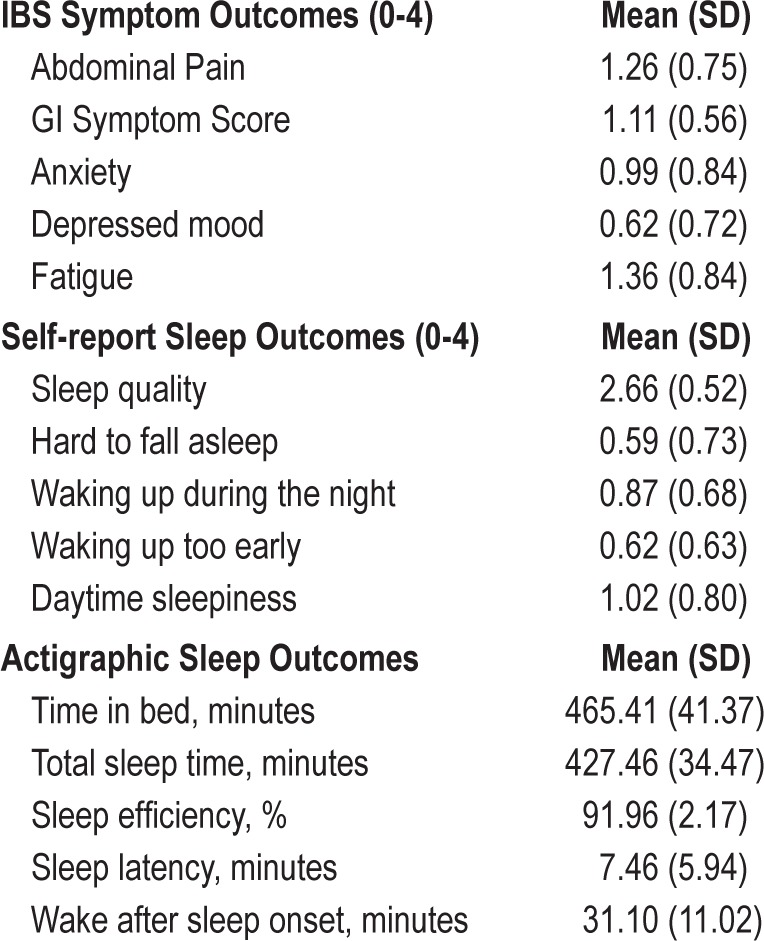
Self-Reported Sleep Quality Ratings and IBS Symptoms
Sleep quality ratings from the subject diaries showed a significant within-subject relationship between sleep quality and IBS symptoms (see Table 2). Reduced self-reported sleep quality significantly predicted next-day symptom exacerbation of abdominal pain, anxiety, and fatigue. Sleep quality did not significantly predict GI symptoms or depressed mood the next day. Across-subject effects were not significant, though the coefficients for anxiety and fatigue were negative and rather large (β = -0.42 and -0.32, respectively, p < 0.20).
Table 2.
Self-reported sleep quality ratings as predictors of symptoms in women with IBS.
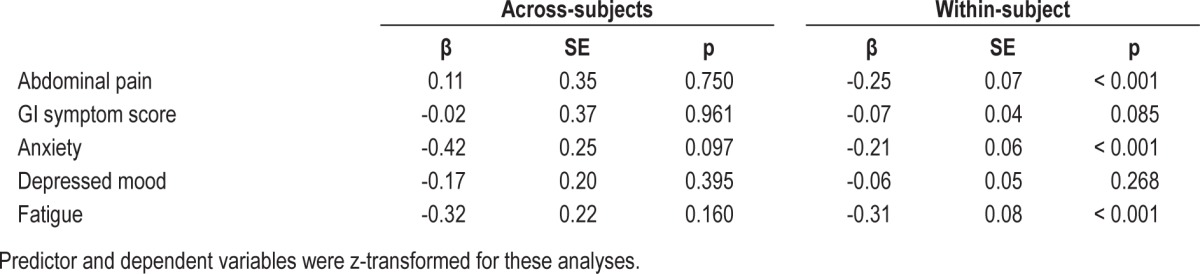
Actigraphic Sleep Efficiency and IBS Symptoms
Scores on self-rated sleep quality and actigraphic SEF were significantly correlated, but the relationship was of low magnitude (r = 0.227, p = 0.001). Reduced actigraphic SEF significantly predicted within-subject next-day anxiety and fatigue but not abdominal pain, GI symptom scores, or depressed mood (see Table 3). Certain across-subject effects were significant, but in the opposite direction than was expected. Persons with increased levels of SEF on actigraphy had significantly higher overall anxiety, depressed mood, and fatigue.
Table 3.
Actigraphic sleep efficiency as a predictor of symptoms in women with IBS.
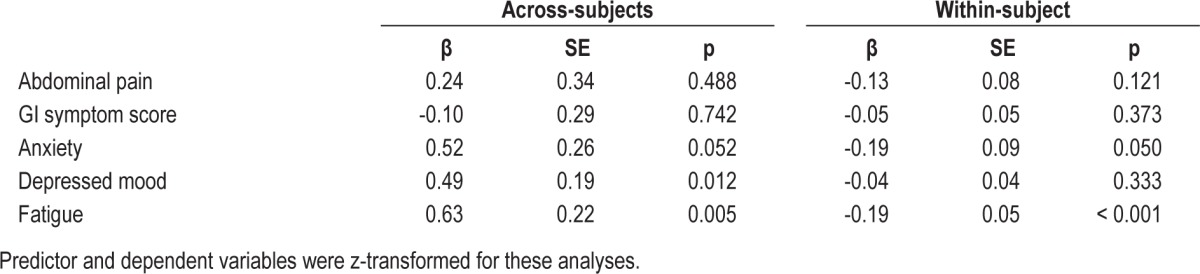
IBS Symptoms as Predictors of Self-reported Sleep Quality Ratings and Actigraphic Sleep Efficiency
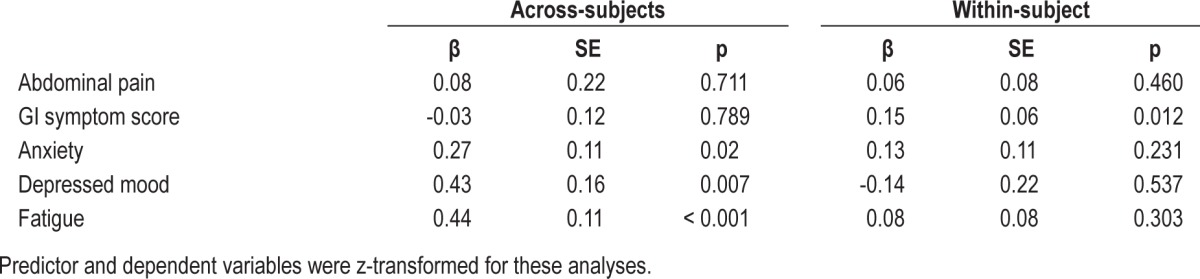
The temporally reversed analysis found no significant within-subject associations of IBS symptoms predicting self-reported sleep quality that night (Table 4). Similar analyses predicting actigraphy SEF found no significant within-subject associations (Table 5) except for GI symptom score, which had a significant (p = 0.012) positive coefficient predicting actigraphy SEF. As expected, the across-subject associations in Tables 4 and 5 agree/correlate with those seen in Tables 2 and 3.
Table 4.
Prediction of self-reported sleep quality ratings by symptoms in women with IBS.
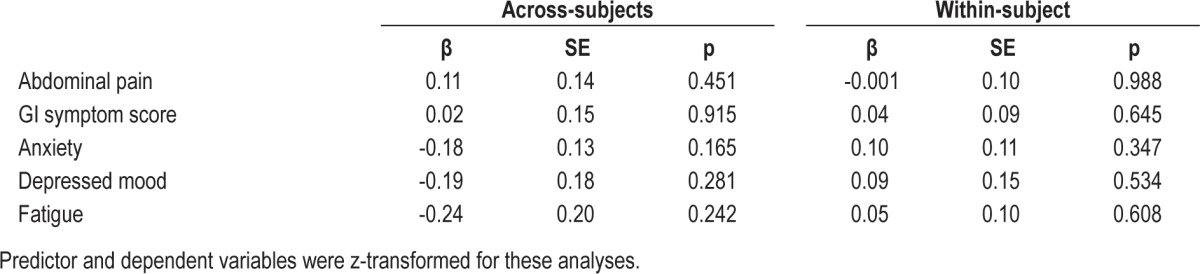
Table 5.
Prediction of actigraphic sleep efficiency by symptoms in women with IBS.
DISCUSSION
Results of this study confirm prior findings that self-reported sleep disturbances are associated with next-day symptoms in persons with IBS.3 Findings showed that within-subjects poorer self-reported sleep quality predicted higher next-day abdominal pain, anxiety, and fatigue, but not GI symptom scores or depressed mood. This study also provides evidence on the within-subject relationships between sleep and next-day symptoms using an objective (actigraphic) sleep outcome. Reduced actigraphic SEF was significantly associated with next-day exacerbation of anxiety and fatigue. The relationship between actigraphic SEF and abdominal pain trended in the same direction as with self-reported sleep quality, but was not significant. Although no conclusions should be reached from a nonsignificant trend in this small study, further research could be useful to explore whether this trend is underpowered or spurious. No effect or trending was observed between actigraphic SEF and GI symptoms and depressed mood. The observation that the within-subject relationship between sleep and some symptoms were present but weaker on objective versus subjective measures suggests that this relationship may be partly, but not entirely, explained by self-report bias.
The one symptom that was consistently unrelated to subjective or objective sleep was depressed mood. Studies of adults and adolescents with pain have shown that depressive symptoms increased the risk of developing sleep disturbance months to years later,14,15 but such analyses do not suggest whether or not daily fluctuations in depressed mood are related to that night's sleep quality. A recent study of temporal associations between sleep and symptoms found that negative mood (not specifically depression) predicted worse self-reported sleep quality but not lower actigraphic SEF that night (the converse was not examined).16 Consideration of this literature along with the present findings suggests that, although depression appears to be important predictor of sleep disturbance over time, the relationship between daily fluctuations of depressive symptoms and sleep disturbance remains unclear.
Across-subjects effects differed substantially between the analyses of self-reported sleep quality and actigraphic sleep efficiency. Across-subject effects of self-reported sleep quality on symptoms were not significant. The difference between within-subjects and across-subjects effects indicates that, although poorer self-reported sleep quality did not predict higher symptoms within the general sample (across-subjects effects), day-to-day changes in sleep quality did predict symptom fluctuations (abdominal pain, anxiety, and fatigue) within-subjects. Across-subjects associations of mean actigraphic SEF with abdominal pain and GI symptoms were not significant, but associations with mean severity of depressed mood, anxiety, and fatigue showed relationships in an opposite direction than expected. Whereas one would expect persons with higher SEF to have lower levels of these next-day IBS symptoms, the analyses showed that subjects with higher SEF displayed exacerbation of (or perhaps increased attention to) next-day anxiety, depression, and fatigue. Recognizing that actigraphy measures movement (i.e., sleep behavior) as a proxy for neurologically defined sleep, it is possible that persons with these symptoms were awake but lying still, which could be misclassified as “sleep” on actigraphy. The behavioral pattern of quiescent wakefulness is consistent with a pattern commonly observed in persons with insomnia.23 Although actigraphy is known to generally overidentify sleep, as could be the case in these subjects, prior evidence on the validity of actigraphy indicates that it remains useful for quantifying within-subject changes from night to night.13 Therefore, these across-subjects effects are less likely to call into question the findings that within-subjects changes in SEF are associated with symptom levels.
The present study also explored the directionality of the relationships between sleep and IBS symptoms by conducting the temporally-reversed GEE analyses with the IBS symptoms as predictors of that night's self-reported sleep quality and actigraphic SEF. The overall picture presented from the analyses using IBS symptoms as the predictor variables indicates that exacerbation of daily IBS symptoms seems not to predict that night's sleep quality or efficiency. This finding is mostly consistent with our earlier study with a different sample of IBS subjects, which also showed that IBS symptoms did not predict that night's sleep quality.3 The finding of higher GI symptom scores predicting higher actigraphic SEF was unexpected and remains unexplained in the absence of further data on potential causes. Across-subjects effects were the same as the analyses using sleep outcomes as the predictors. This is expected because across-subjects analyses do not address day-to-day variation, but rather summarize whether the sample means of two variables are related. Thus reversing the predictor and outcome variables would not substantially change the relationships between the variables in across-subjects analyses.
The present study findings have implications for both understanding and treating IBS symptoms. A growing body of evidence in sleep research has focused on the directionality of relationships between sleep and pain. A recent study by Tang and colleagues16 examined the directionality of relationships between pain and sleep in a sample of patients with varied types of chronic pain. Findings showed that pre-sleep pain did not predict that night's diary-reported sleep quality or actigraphic SEF; however, diary sleep quality and actigraphic SEF both predicted next-day daytime pain. Consistent with the present study, self-reported sleep quality was a stronger predictor than actigraphic SEF. Tang et al. also found that pre-sleep cognitive arousal more strongly predicted self-reported sleep quality and actigraphic SEF than pain. Similarly, another study by our group has shown that women with IBS were more susceptible than controls to sleep disruption when faced with the pre-sleep stressor of having to give a public talk in the morning.24 This evidence suggests that women with IBS may experience a vicious cycle of increased reactivity to pre-sleep events, subsequent poor sleep, and exacerbation of abdominal pain, anxiety, and fatigue the following day. This suggests that a stronger emphasis on pre-sleep events for stress reduction may be therapeutic in the management of IBS symptoms.
Few studies have examined the mechanisms underlying associations between sleep and IBS symptoms. Some evidence suggests that pathological processes in IBS may be related to dysregulation of autonomic nervous system (ANS) control of GI function, although current evidence on the relative contributions of sympathetic and parasympathetic function to IBS symptom subgroups (i.e., diarrhea-predominant versus constipation-predominant) is contradictory.25–27 Sleep research also has suggested ANS involvement in sleep disruption, with evidence supporting an association between insomnia and sympathetic dominance.28,29 It is possible that ANS dysregulation may be a common factor underlying both IBS symptoms and sleep disturbances reported in persons with IBS, or perhaps a certain IBS subgroup. Research examining the temporal associations between sleep disturbance and autonomic activity (e.g., heart rate variability) might clarify pathophysiologic mechanisms of symptom exacerbation in IBS, as well as potential treatment targets.
This exploratory study has several limitations that must be considered. First, the sample was small and was limited to only women aged 18 to 45 years. Research has shown less severe sleep disturbance and more severe IBS symptoms in subjects who were younger than 50 years old and some evidence suggests less severe sleep disturbance scores in women with IBS versus men.2 Therefore, future research should explore whether day-to-day associations between sleep and symptoms are affected by these demographic factors and whether the relationship holds true for the male gender and other age groups. Furthermore, the findings were based on 4-7 nights of data due to the necessity of data from adjacent days/nights (i.e., data on one night's sleep and the next day symptoms, one day's symptoms and that night's sleep). Ideally, the replication of these findings would use 7- to 14-day actigraphic records to provide better characterization of fluctuations of subjective/objective sleep and IBS symptoms.30
Finally, as indicated in Table 2, this community-recruited sample was only mildly to moderately symptomatic for sleep disturbance and the other symptoms assessed. This pattern is to be expected from a community sample, which may not experience symptoms severe enough to warrant treatment as would be seen in a clinic-recruited sample. In this small sample, the lack of active symptomatology may have reduced the strength of associations between sleep and symptoms. Thus, further exploration of the study findings, including non-significant trends, in a sample recruited for greater symptom variability would expand the presently limited information on day-to-day sleep and symptom fluctuation in IBS.
In summary, this study supports prior observations that self-reported sleep quality predicts some next-day symptoms in women with IBS. The study adds to the literature evidence from objective sleep measures suggesting that the relationship between sleep and IBS symptoms cannot be fully explained by self-report bias. However, only anxiety and fatigue were significantly predicted by objectively measured SEF. Further research is needed to better characterize the relationships between sleep and IBS symptoms, as such evidence would clarify understanding of these comorbid problems and potentially identify mechanisms underlying both conditions.
DISCLOSURE STATEMENT
This was not an industry supported study. Financial support was provided by NINR, NIH (NR01094, P30 NR04001). The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Ms. Katina Velloth for the actigraphy analyses, and especially, the women who gave so generously of their time to participate in this study.
REFERENCES
- 1.Drossman D, Corazziari E, Delvaux M, et al., editors. McLean,VA: Degnon Associates, Inc.; 2006. Rome III: The Functional Gastrointestinal Disorders. [Google Scholar]
- 2.Bellini M, Gemignani A, Gambaccini D, et al. Evaluation of latent links between irritable bowel syndrome and sleep quality. World J Gastroenterol. 2011;17:5089–96. doi: 10.3748/wjg.v17.i46.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarrett M, Heitkemper M, Cain KC, Burr RL, Hertig V. Sleep disturbance influences gastrointestinal symptoms in women with irritable bowel syndrome. Dig Dis Sci. 2000;45:952–9. doi: 10.1023/a:1005581226265. [DOI] [PubMed] [Google Scholar]
- 4.Rotem AY, Sperber AD, Krugliak P, Freidman B, Tal A, Tarasiuk A. Polysomnographic and actigraphic evidence of sleep fragmentation in patients with irritable bowel syndrome. Sleep. 2003;26:747–52. doi: 10.1093/sleep/26.6.747. [DOI] [PubMed] [Google Scholar]
- 5.Vege SS, Locke GR, 3rd, Weaver AL, Farmer SA, Melton LJ, 3rd, Talley NJ. Functional gastrointestinal disorders among people with sleep disturbances: a population-based study. Mayo Clin Proc. 2004;79:1501–6. doi: 10.4065/79.12.1501. [DOI] [PubMed] [Google Scholar]
- 6.Deechakawan W, Heitkemper MM, Cain KC, Burr RL, Jarrett ME. Anxiety, depression, and catecholamine levels after self-management intervention in irritable bowel syndrome. Gastroenterol Nurs. 2014;37:24–32. doi: 10.1097/SGA.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 7.Fond G, Loundou A, Hamdani N, et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2014 Apr 6; doi: 10.1007/s00406-014-0502-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Lackner JM, Gudleski GD, Dimuro J, Keefer L, Brenner DM. Psychosocial predictors of self-reported fatigue in patients with moderate to severe irritable bowel syndrome. Behav Res Ther. 2013;51:323–31. doi: 10.1016/j.brat.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. The brain-gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. 2012;61:1284–90. doi: 10.1136/gutjnl-2011-300474. [DOI] [PubMed] [Google Scholar]
- 10.Lackner JM, Ma CX, Keefer L, et al. Type, rather than number, of mental and physical comorbidities increases the severity of symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11:1147–57. doi: 10.1016/j.cgh.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:991–1000. doi: 10.1038/ajg.2012.131. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 13.Taibi DM, Landis CA, Vitiello MV. Concordance of polysomnographic and actigraphic measurement of sleep and wake in older women with insomnia. J Clin Sleep Med. 2013;9:217–25. doi: 10.5664/jcsm.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell P, Tang N, McBeth J, et al. The role of sleep problems in the development of depression in those with persistent pain: a prospective cohort study. Sleep. 2013;36:1693–8. doi: 10.5665/sleep.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palermo TM, Law E, Churchill SS, Walker A. Longitudinal course and impact of insomnia symptoms in adolescents with and without chronic pain. J Pain. 2012;13:1099–106. doi: 10.1016/j.jpain.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang NK, Goodchild CE, Sanborn AN, Howard J, Salkovskis PM. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: a multilevel daily process study. Sleep. 2012;35:675–87A. doi: 10.5665/sleep.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drossman DA. The functional gastrointestinal disorders and the Rome II process. Gut. 1999;45(Suppl 2):II1–5. doi: 10.1136/gut.45.2008.ii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heitkemper M, Jarrett M, Burr R, et al. Subjective and objective sleep indices in women with irritable bowel syndrome. Neurogastroenterol Motil. 2005;17:523–30. doi: 10.1111/j.1365-2982.2005.00700.x. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 20.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53:737–40. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 21.Mancl LA, Leroux BG, DeRouen TA. Between-subject and within-subject statistical information in dental research. J Dent Res. 2000;79:1778–81. doi: 10.1177/00220345000790100801. [DOI] [PubMed] [Google Scholar]
- 22.Neuhaus JM, Kalbfleisch JD. Between- and within-cluster covariate effects in the analysis of clustered data. Biometrics. 1998;54:638–45. [PubMed] [Google Scholar]
- 23.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29:232–9. [PubMed] [Google Scholar]
- 24.Heitkemper MM, Cain KC, Deechakawan W, et al. Anticipation of public speaking and sleep and the hypothalamic-pituitary-adrenal axis in women with irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:626–31. e270–1. doi: 10.1111/j.1365-2982.2012.01915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burr RL, Jarrett ME, Cain KC, Jun SE, Heitkemper MM. Catecholamine and cortisol levels during sleep in women with irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:1148–e97. doi: 10.1111/j.1365-2982.2009.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orr WC, Chen CL. Sleep and the gastrointestinal tract. Neurol Clin. 2005;23:1007–24. doi: 10.1016/j.ncl.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Robert JJ, Elsenbruch S, Orr WC. Sleep-related autonomic disturbances in symptom subgroups of women with irritable bowel syndrome. Dig Dis Sci. 2006;51:2121–7. doi: 10.1007/s10620-006-9305-z. [DOI] [PubMed] [Google Scholar]
- 28.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Rowe M, McCrae C, Campbell J, et al. Actigraphy in older adults: comparison of means and variability of three different aggregates of measurement. Behav Sleep Med. 2008;6:127–45. doi: 10.1080/15402000801952872. [DOI] [PubMed] [Google Scholar]


