Abstract
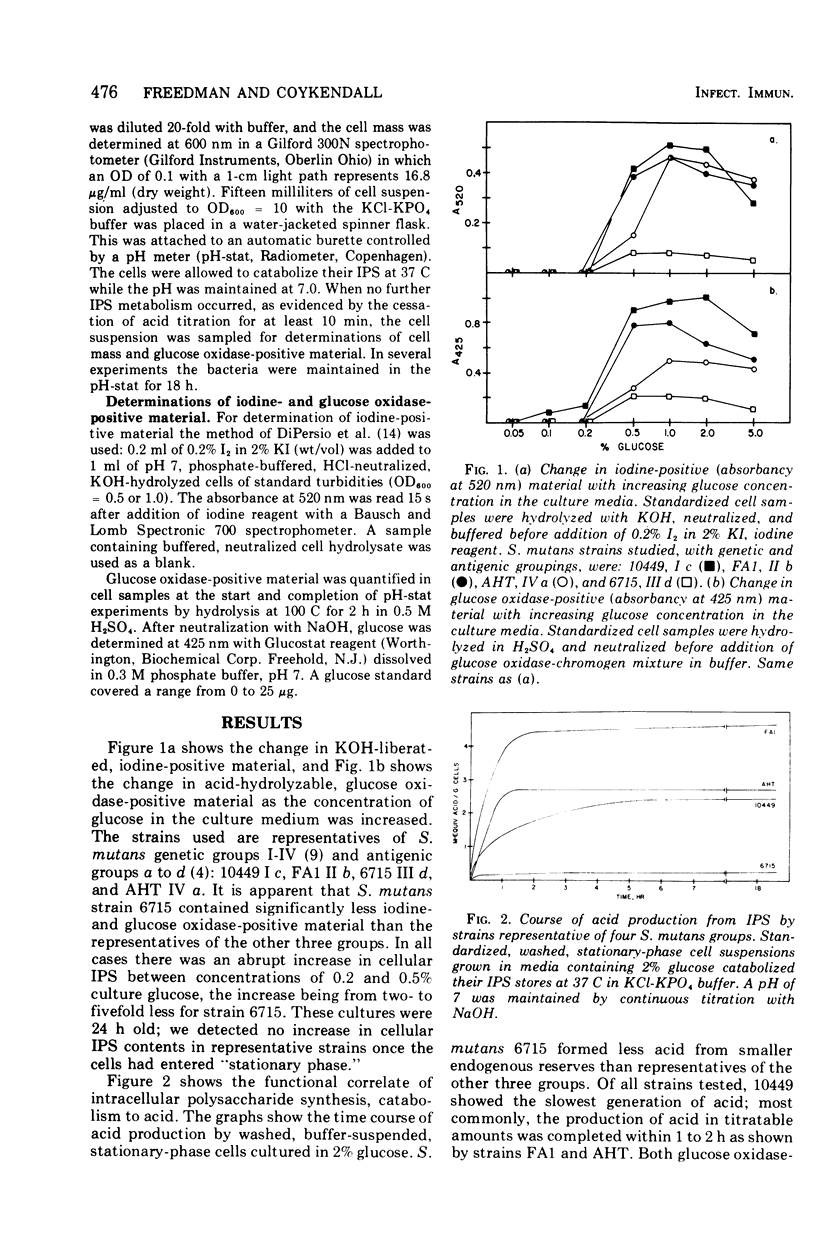
Five strains, representative of Streptococcus mutans genetic group III antigenic group d, synthesized and degraded less intracellular polysaccharide (IPS) then 17 strains representative of other S. mutans groups. The strains that synthesize IPS degraded it rapidly. The production of acid in titratable amounts from endogenous IPS was usually complete within 1 h. IPS synthesis in S. mutans increased abruptly at culture glucose concentrations between 0.2 and 0.5% and was quantitated as both iodine-and glucose oxidase-positive material in cell hyrolysates. IPS degradation was measured by acid production in a pH-stat maintained at 7. The existence within group III d of a strain recently shown to be cariogenic in experimental animals suggest that IPS may not be a prerequisite for virulence in these cariogenic bacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman K. S., Gibbons R. J. Iodophilic polysaccharide synthesis by human and rodent oral bacteria. Arch Oral Biol. 1966 May;11(5):533–542. doi: 10.1016/0003-9969(66)90160-9. [DOI] [PubMed] [Google Scholar]
- Berman K. S., Gibbons R. J., Nalbandian J. Localization of intracellular polysaccharide granules in Streptococcus mitis. Arch Oral Biol. 1967 Oct;12(10):1133–1138. doi: 10.1016/0003-9969(67)90061-1. [DOI] [PubMed] [Google Scholar]
- Bowen W. H. The induction of rampant dental caries in monkeys (Macaca irus). Caries Res. 1969;3(3):227–237. doi: 10.1159/000259597. [DOI] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of Streptococcus mutans strains in some selected areas of the world. Odontol Revy. 1972;23(4):401–410. [PubMed] [Google Scholar]
- Bratthall D. Immunofluorescent identification of Streptococcus mutans. Odontol Revy. 1972;23(2):181–196. [PubMed] [Google Scholar]
- Brown A. T., Patterson C. E. Heterogeneity of Streptococcus mutans strains based on their mannitol-1-phosphate dehydrogenases: criterion for rapid classification. Infect Immun. 1972 Sep;6(3):422–424. doi: 10.1128/iai.6.3.422-424.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coykendall A. L. Base composition of deoxyribonucleic acid isolated from cariogenic streptococci. Arch Oral Biol. 1970 Apr;15(4):365–368. doi: 10.1016/0003-9969(70)90063-4. [DOI] [PubMed] [Google Scholar]
- Coykendall A. L. Four types of Streptococcus mutans based on their genetic, antigenic and biochemical characteristics. J Gen Microbiol. 1974 Aug;83(2):327–338. doi: 10.1099/00221287-83-2-327. [DOI] [PubMed] [Google Scholar]
- Coykendall A. L. Genetic heterogeneity in Streptococcus mutans. J Bacteriol. 1971 Apr;106(1):192–196. doi: 10.1128/jb.106.1.192-196.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coykendall A. L., Specht P. A., Samol H. H. Streptococcus mutans in a wild, sucrose-eating rat population. Infect Immun. 1974 Jul;10(1):216–219. doi: 10.1128/iai.10.1.216-219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley P., Wood J. M., Saxton C. A., Leach S. A. The polymerisation of dietary sugars by dental plaque. Caries Res. 1967;1(2):112–129. doi: 10.1159/000259506. [DOI] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer DIRKS O. The effect of carbohydrate restriction on the presence of Streptococcus mutans, Streptococcus sanguis and iodophilic polysaccharide-producing bacteria in human dental plaque. Caries Res. 1970;4(2):114–123. doi: 10.1159/000259633. [DOI] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer Dirks O. The relationship between extracellular polysaccharide-producing streptococci and smooth surface caries in 13-year-old children. Caries Res. 1969;3(2):190–199. doi: 10.1159/000259582. [DOI] [PubMed] [Google Scholar]
- DiPersio J. R., Mattingly S. J., Higgins M. L., Shockman G. D. Measurement of intracellular iodophilic polysaccharide in two cariogenic strains of Streptococcus mutans by cytochemical and chemical methods. Infect Immun. 1974 Sep;10(3):597–604. doi: 10.1128/iai.10.3.597-604.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Hausner T., Clewell D. B. Buoyant densities of DNA from various strains of Streptococcus mutans. Arch Oral Biol. 1972 Jun;17(6):1001–1003. doi: 10.1016/0003-9969(72)90123-9. [DOI] [PubMed] [Google Scholar]
- Edwardsson S. Characteristics of caries-inducing human streptococci resembling Streptococcus mutans. Arch Oral Biol. 1968 Jun;13(6):637–646. doi: 10.1016/0003-9969(68)90142-8. [DOI] [PubMed] [Google Scholar]
- FITZGERALD R. J., JORDAN H. V., STANLEY H. R. Experimental caries and gingival pathologic changes in the gnotobiotic rat. J Dent Res. 1960 Sep-Oct;39:923–935. doi: 10.1177/00220345600390052701. [DOI] [PubMed] [Google Scholar]
- FITZGERALD R. J., KEYES P. H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dent Assoc. 1960 Jul;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- Fitzgerald D. B., Fitzgerald R. J. Induction of dental caries in gerbils. Arch Oral Biol. 1966 Jan;11(1):139–140. doi: 10.1016/0003-9969(66)90126-9. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Tanzer J. M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):189–196. doi: 10.1128/iai.10.1.189-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., KAPSIMALIS B. Synthesis of intracellular iodophilic polysaccharide by Streptococcus mitis. Arch Oral Biol. 1963 May-Jun;8:319–329. doi: 10.1016/0003-9969(63)90024-4. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. J. METABOLISM OF INTRACELLULAR POLYSACCHARIDE BY STREPTOCOCCUS MITIS AND ITS RELATION TO INDUCIBLE ENZYME FORMATION. J Bacteriol. 1964 Jun;87:1512–1520. doi: 10.1128/jb.87.6.1512-1520.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., SOCRANSKY S. S. Intracellular polysaccharide storage by organisms in dental plaques. Its relation to dental caries and microbial ecology of the oral cavity. Arch Oral Biol. 1962 Jan-Feb;7:73–79. doi: 10.1016/0003-9969(62)90050-x. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., König K. G., Mühlemann H. R. Modifications of the oral bacterial flora and their influence on dental caries in the rat. I. The effects of inoculating 4 labelled strains of streptococci. Helv Odontol Acta. 1965 Oct;9(2):121–129. [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta. 1967 Oct;11(2):131–152. [PubMed] [Google Scholar]
- Guggenheim B. Streptococci of dental plaques. Caries Res. 1968;2(2):147–163. doi: 10.1159/000259553. [DOI] [PubMed] [Google Scholar]
- Hardie J. M., Bowden G. H. Cell wall and serological studies on Streptococcus mutans. Caries Res. 1974;8(4):301–316. doi: 10.1159/000260120. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., FITZGERALD R. J., BOWLER A. E. Inhibition of experimental caries by sodium metabisulfite and its effect on the growth and metabolism of selected bacteria. J Dent Res. 1960 Jan-Feb;39:116–123. doi: 10.1177/00220345600390010501. [DOI] [PubMed] [Google Scholar]
- Krasse B. Human streptococci and experimental caries in hamsters. Arch Oral Biol. 1966 Apr;11(4):429–436. doi: 10.1016/0003-9969(66)90107-5. [DOI] [PubMed] [Google Scholar]
- Littleton N. W., Kakehashi S., Fitzgerald R. J. Recovery of specific "caries-inducing" streptococci from carious lesions in the teeth of children. Arch Oral Biol. 1970 May;15(5):461–463. doi: 10.1016/0003-9969(70)90073-7. [DOI] [PubMed] [Google Scholar]
- McCabe R. M., Keyes P. H., Howell A., Jr An in vitro method for assessing the plaque forming ability of oral bacteria. Arch Oral Biol. 1967 Dec;12(12):1653–1656. doi: 10.1016/0003-9969(67)90200-2. [DOI] [PubMed] [Google Scholar]
- Shklair I. L., Keene H. J. A biochemical scheme for the separation of the five varieties of Streptococcus mutans. Arch Oral Biol. 1974 Nov;19(11):1079–1081. doi: 10.1016/0003-9969(74)90099-5. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Backer Dirks O., Stoppelaar J. D., Jansen H. M. Iodophilic polysaccharide-producing bacteria and dental caries in children consuming fluoridated and non-fluoridated drinking water. Caries Res. 1969;3(2):178–189. doi: 10.1159/000259581. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Pulkkinen A. J. Adherence as an ecological determinant for streptococci in the human mouth. Arch Oral Biol. 1971 Oct;16(10):1131–1141. doi: 10.1016/0003-9969(71)90042-2. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Jansen H. M. The iodophilic polysaccharide synthesized by Stretococcus salivarius. Caries Res. 1968;2(1):47–56. doi: 10.1159/000259543. [DOI] [PubMed] [Google Scholar]
- ZINNER D. D., JABLON J. M., ARAN A. P., SASLAW M. S. EXPERIMENTAL CARIES INDUCED IN ANIMALS BY STREPTOCOCCI OF HUMAN ORIGIN. Proc Soc Exp Biol Med. 1965 Mar;118:766–770. doi: 10.3181/00379727-118-29964. [DOI] [PubMed] [Google Scholar]
- van Houte J., Jansen H. M. Role of glycogen in survival of Streptococcus mitis. J Bacteriol. 1970 Mar;101(3):1083–1085. doi: 10.1128/jb.101.3.1083-1085.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houte J., Winkler K. C., Jansen H. M. Iodophilic polysaccharide synthesis, acid production and growth in oral streptococci. Arch Oral Biol. 1969 Jan;14(1):45–61. doi: 10.1016/0003-9969(69)90020-x. [DOI] [PubMed] [Google Scholar]
- van Houte J., de Moor C. E., Jansen H. M. Synthesis of iodophilic polysaccharide by human oral streptococci. Arch Oral Biol. 1970 Mar;15(3):263–266. doi: 10.1016/0003-9969(70)90084-1. [DOI] [PubMed] [Google Scholar]



