Abstract
Study Objectives:
The choice and variety of pediatric masks for continuous positive airway pressure (CPAP) is limited in the US. Therefore, clinicians often prescribe modified adult masks. Until recently a mask for children aged < 7 years was not available. This study evaluated apnea-hypopnea index (AHI) equivalence and acceptability of a new pediatric CPAP mask for children aged 2-7 years (Pixi; ResMed Ltd, Sydney, Australia).
Methods:
Patients aged 2-7 years were enrolled and underwent in-lab baseline polysomnography (PSG) using their previous mask, then used their previous mask and the VPAP III ST-A flow generator for ≥ 10 nights at home. Thereafter, patients switched to the Pixi mask for ≥ 2 nights before returning for a PSG during PAP therapy via the Pixi mask. Patients then used the Pixi mask at home for ≥ 21 nights. Patients and their parents/guardians returned to the clinic for follow-up and provided feedback on the Pixi mask versus their previous mask.
Results:
AHI with the Pixi mask was 1.1 ± 1.5/h vs 2.6 ± 5.4/h with the previous mask (p = 0.3538). Parents rated the Pixi mask positively for: restfulness of the child's sleep, trouble in getting the child to sleep, and trouble in having the child stay asleep. The Pixi mask was also rated highly for leaving fewer or no marks on the upper lip and under the child's ears, and being easy to remove.
Conclusions:
The Pixi mask is suitable for children aged 2-7 years and provides an alternative to other masks available for PAP therapy in this age group.
Citation:
Kushida CA, Halbower AC, Kryger MH, Pelayo R, Assalone V, Cardell CY, Huston S, Willes L, Wimms AJ, Mendoza J. Evaluation of a new pediatric positive airway pressure mask. J Clin Sleep Med 2014;10(9):979-984.
Keywords: children, positive airway pressure, sleep disordered breathing, patient interface
In children, sleep disordered breathing (SDB) is associated with a range of negative behavioral problems, such as hyperactivity and aggression, and significant adverse health outcomes, including developmental delays and cardiovascular risk.1–4 There is a range of treatment options available for SDB, and a proportion of children with this condition require treatment with continuous positive airway pressure (CPAP) therapy5,6 or noninvasive ventilation (NIV).7–9
The paucity of US Food and Drug Administration (FDA)-approved pediatric masks is a significant issue when treating children with CPAP and noninvasive ventilation therapy in the USA.5 As a result, many pediatric sleep specialists have to modify available masks designed for adults to use with their pediatric patients.4 Another issue is that many of the pediatric masks available have not been specifically designed for the unique needs of children and are instead simply scaled down versions of adult masks.4,9,10 In addition to the lack of pediatric masks, many children requiring positive airway pressure (PAP) therapy have craniofacial anomalies and asymmetric faces, which make it extremely difficult to fit PAP masks comfortably without air leaks.9–11 Ill-fitting masks cause a number of adverse effects, including eye irritation and conjunctivitis (from air leaking into the eye), skin irritation, blisters and, in severe cases, necrosis.5
A pediatric-specific mask system (Figure 1) has been developed to address the needs of children with SDB (Pixi; ResMed Ltd, Sydney, Australia) and was approved by the US FDA in December 2010. The headgear of this mask has three points of adjustment. The quick-release buckle is designed to retain the headgear and to prevent children from inadvertently removing the mask, but features a predetermined tension that allows removal by pulling the strap in an emergency. The cushion is designed to conform to each child's unique facial structure and the flexible stabilizers employ stability points on the cheeks away from the forehead. The cushion provides a light sealing force sitting further down the nose to spread mask pressure across the face and to avoid the upper nose bridge. The side-mounted hose can be switched to either side of the cushion to accommodate different sleeping positions without removing the mask. Fitting and removal mechanisms are located away from the child's face for quick access by the caregiver. The headgear and side-mounted hose are designed to sit away from the child's eyes, ears, and mouth. The purpose of this study was to evaluate the usability and efficacy of the Pixi pediatric mask in children with SDB receiving PAP therapy.
Figure 1. Pixi mask and headgear.

BRIEF SUMMARY
Current Knowledge/Study Rationale:There are limited choices for children with sleep related breathing disorders who require use of continuous positive airway pressure (CPAP). The current study tests a new CPAP mask specifically designed and constructed for children.
Study Impact: This study provides a suitable choice for children with sleep related breathing disorders compared to other CPAP masks.
METHODS
This study had two aims: (1) to assess the efficacy of PAP treatment for SDB in pediatric patients using the Pixi nasal mask as shown by equivalence in the apnea/hypopnea index (AHI) compared with the previously used mask as documented by comprehensive polysomnography (PSG), and (2) to assess participant-parent/legal guardian satisfaction. The study was conducted at 3 clinical sites: The Children's Hospital (Denver, CO, USA), Gaylord Sleep Medicine (Wallingford, CT, USA), and Stanford Center for Human Sleep Research (Redwood City, CA, USA). The protocol was reviewed and approved by institutional review boards at each study site. Written informed consent was obtained from the parents or legal guardians of each child prior to enrollment into the study. The study was performed within the guidelines of Good Clinical Practice and in accordance with the Declaration of Helsinki.
Patients
Our goal by investigator consensus was to have 33 participants recruited for 20 consecutively enrolled participants. Established users of PAP therapy (CPAP or BPAP [bilevel pressure]) aged 2-7 years were eligible for inclusion, irrespective of the type of mask used previously. Established PAP use was defined as having used CPAP or BPAP for ≥ 1 month. Supplemental oxygen use was allowed. All patients who successfully met the inclusion criteria for the study and whose parents or legal guardians signed an informed consent form were consecutively enrolled.
Study Design
The primary endpoint was equivalence of AHI values during PAP therapy using the Pixi mask compared with the patient's previous mask. Secondary endpoints were the extent of unintentional leak, patient/legal guardian satisfaction with the mask, and treatment adherence. Patients participated in a total of 3 clinic visits and 2 follow-up phone calls. PAP usage was assessed using data downloads from flow generators used prior to study entry and from the VPAP III ST-A flow generators during the study; mask leak data were collected from the VPAP III ST-A. Baseline measurements were obtained at the first visit, which included current PAP prescription, average monthly PAP usage (from flow generator download), medical history, and results from the most recent PSG study. Participants who completed a PSG study > 6 months before the baseline visit underwent a new PSG study to provide current baseline data. Baseline PSGs were performed during PAP using the patient's existing pressure settings; titrations were not allowed. Baseline PAP comfort scores (measured using a 100-mm visual analogue scale [VAS]) were rated by parents or legal guardians.
After baseline assessments were obtained, subjects were set-up with a Pixi mask and a VPAP III ST-A flow generator (ResMed Ltd, Sydney, Australia) and were instructed to use their current mask and the VPAP III ST-A for a minimum of 10 nights at home to acclimate to the study flow generator. Thereafter, patients switched to the Pixi mask for a minimum of 2 nights prior to returning to the study site for the second clinic visit. During the acclimation period and switch to the Pixi mask, study staff telephoned the patients' parents/legal guardians to identify or address any issues with the VPAP III ST-A and Pixi mask.
At the second visit, PSG study was undertaken while patients were receiving PAP therapy via the Pixi mask. All PSGs were completed and scored according to standard practice.12 PAP pressure data were collected prior to participants going home with the VPAP III ST-A and the Pixi mask. Patients then used the Pixi mask at home for ≥ 21 nights. A second follow-up telephone call was made shortly after the PSG study to identify and address any issues with PAP treatment. Participants returned to the clinical study site for the final visit, during which total PAP usage and mask leak data were downloaded from the VPAP III ST-A flow generators, and parents/legal guardians completed the final PAP comfort VAS questionnaire and an assessment of the Pixi mask. Ease of use, mask seal, comfort, headgear stability, facial masks, and other features of the Pixi mask were evaluated using a Likert scale from 1 to 10, with 10 being the most positive experience. A median Likert scale rating ≥ 6 was considered “acceptable.”
Statistical Analysis
Evaluable participants, defined as those who completed the PSG study with their previous mask at baseline and with the Pixi mask, were included in the efficacy analysis. A paired t-test was used to determine if there was a significant difference between total AHI during PAP using the previous mask versus the Pixi mask. Paired t-tests were also used to compare PAP comfort questionnaire scores and adherence data (average daily usage) for the 2 masks. A Wilcoxon rank sum test was used to compare the proportion of evaluations with an “acceptable” rating for each feature in the usability questionnaire. In all cases, p-values were based on a 2-sided test with a type I error of 0.05. All statistical calculations were performed in SAS, Version 9.2 (SAS Institute Inc., Cary NC).
RESULTS
Forty-one consecutive eligible patients were invited to participate in the study. Parents/legal guardians signed informed consent forms for 17 children, 16 of whom were enrolled in the study; 14 were included in the efficacy analysis (Figure 2). The rigors of the protocol, parents having scheduling difficulties for the patient visits, and travel time to and from the laboratories were the most common reasons for the parents deciding not to enroll their children in the study. With the exception of the final PSG, usage and usability questionnaire data were available for 15 patients. Reasons for withdrawal prior to the final study visit were withdrawal of consent for reasons unrelated to the study procedures or Pixi mask (n = 1), inability to tolerate the study mask (n = 1) and nonattendance at the clinical site to complete baseline study procedures (n = 1). Patient characteristics at baseline are shown in Table 1. PAP mode during both previous and Pixi mask use was fixed in two-thirds of patients and bilevel in the remaining third. Eight subjects used a fixed pressure, ranging from 6 to 12 cmH2O (mean 8.8 ± 2.4 cm H2O). Six subjects used bilevel pressure, with inspiratory positive airway pressure (IPAP) of 12-17 (mean 14.5 ± 2.2) cm H2O and expiratory positive airway pressure (EPAP) of 7-13 cm (mean 9.2 ± 2.0) H2O.
Figure 2. Flow chart of study protocol.
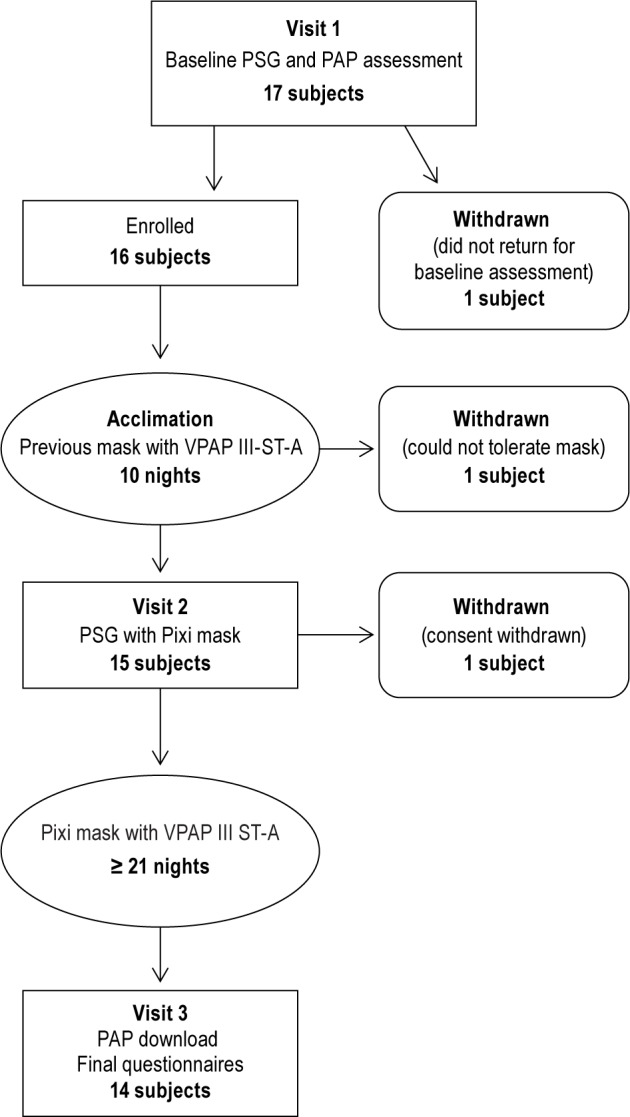
PAP, positive airway pressure; PSG, polysomnography.
Table 1.
Patient characteristics at baseline.

AHI with the Pixi mask was 1.1 ± 1.5/h vs 2.6 ± 5.4/h with the previous mask (p = 0.3538). In subjects with available data (n = 14), 64% had an AHI < 1/h with the previous mask and 71% had an AHI of < 1/h with the Pixi mask.
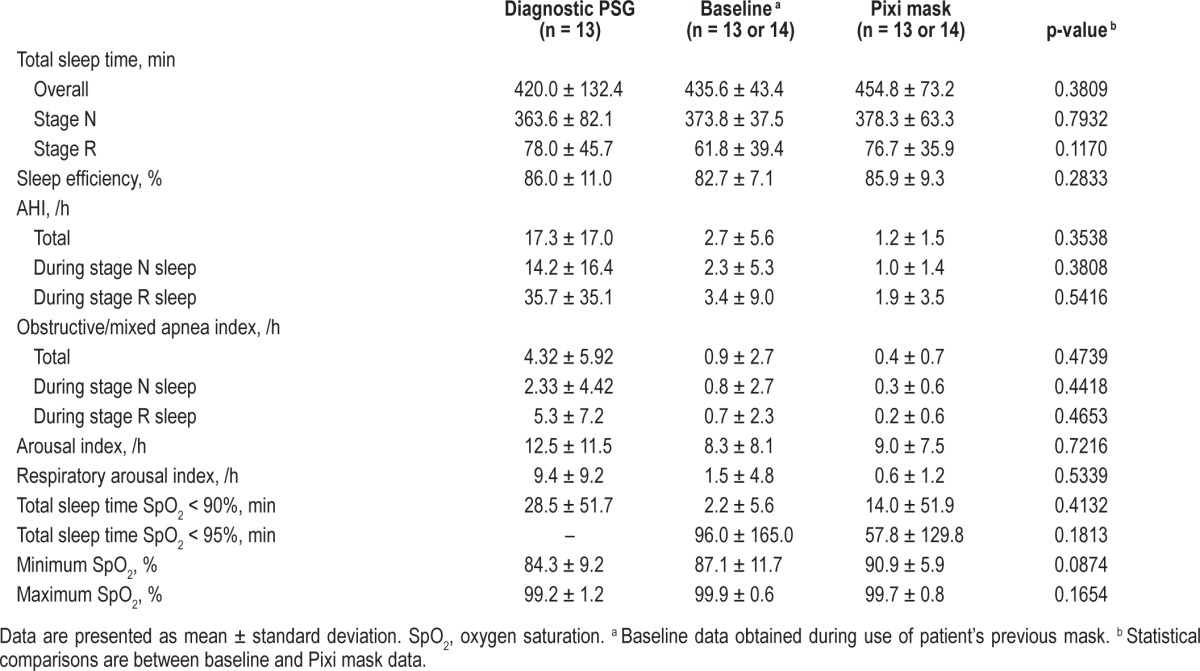
Although numerical differences between masks were observed, favoring the Pixi mask, these did not reach statistical significance, such as a nonsignificant increase in time spent in stage R (REM) sleep during use of the Pixi mask (Table 2). Sleep efficiency, total arousal index and respiratory arousal index were similar during PAP with the previous or Pixi mask (Table 2). Oxygen desaturation during total sleep time (TST SpO2 < 90% and TST SpO2 < 95%) tended to be higher with the Pixi mask (Table 2). One child had a cold during PAP therapy with the Pixi mask and was using nocturnal oxygen during the sleep study; when data from this patient were excluded from the analysis of PSG results, mean TST SpO2 < 90% and TST SpO2 < 95% were 0.1 ± 0.1 min and 29.4 ± 83.2 min, respectively.
Table 2.
Polysomnography findings.
Median PAP usage with the Pixi mask was 27.6 ± 9.9 days. Average daily usage was similar for the previous mask + previous flow generator and the Pixi mask + VPAP III ST-A (7.4 ± 2.6 h and 7.2 ± 2.5 h, respectively; p = 0.9854).
When only study data from the VPAP III ST-A were analyzed, PAP usage was lower on average by 1 h per night with the Pixi mask compared with the previous mask used during the acclimation period (7.2 ± 2.5 h vs 8.4 ± 2.4 h, respectively; p = 0.0035). Similar results were obtained when analysis was limited to the 12 patients who had complete data at baseline and the final visit (Pixi mask usage 7.1 ± 2.5 h/night vs previous mask usage 8.1 ± 2.1 h/night; p = 0.0278). The previous mask was used with the VPAP III ST-A for approximately 10 nights, while the Pixi mask and VPAP III ST-A were used together for ≥ 21 nights.
Although there were no differences in median leak for the Pixi mask (5.2 ± 7.7 L/min) versus the previous mask (4.6 ± 5.8 L/min) (p = 0.8049), maximum leak was less for the Pixi mask (47.5 ± 18.2 L/min vs 61.0 ± 21.7 L/min for the previous mask; p = 0.0021).
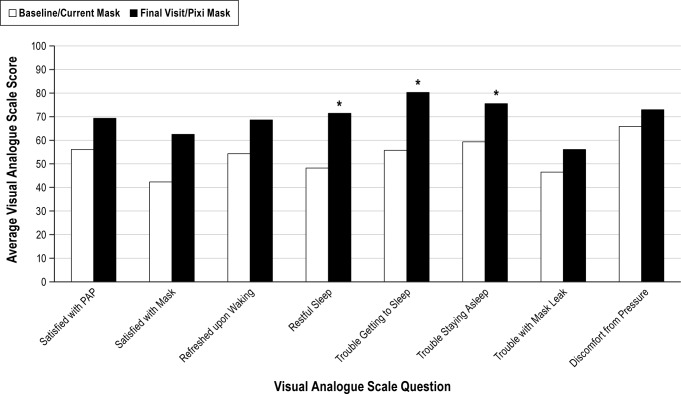
Visual analogue scale scores completed by parents/legal guardians indicated more favorable experiences with the Pixi mask compared with the previous mask; there were statistically significant differences between the 2 masks for 3 of the 8 parameters: restfulness of the child's sleep, ability to get to sleep, and ability to stay asleep (Figure 3).
Figure 3. PAP Comfort Questionnaire (visual analogue scale [VAS]).
* p < 0.05 vs baseline/previous mask.
Parents/caregivers generally rated the Pixi mask as acceptable at the final visit. Two parameters (“mask seal change during use” and “cushion marks on the side of the nose”) had a median rating of 6 (“acceptable”) (Figure 4), and several parameters had a median score of ≥ 9 all of which were significantly better than 6, the “acceptable” rating (p < 0.05; “ease/ difficulty adjusting the headgear,” “marks on the nose bridge, upper lip, and under the ears,” “ease/difficulty removing the mask,” “obtrusiveness of the mask,” and “mask performance”). No safety issues were reported with the Pixi mask.
Figure 4. Pixi mask Likert scale ratings.
A rating of 10 is the most positive experience; a median rating ≥ 6 is considered “acceptable.”
DISCUSSION
Although the number of children being prescribed PAP therapy is growing, there are still relatively few pediatric patients receiving this treatment and there is a general lack of data in this area. Published data on the use of nasal CPAP in children show that it is effective and well tolerated in > 80% of recipients,5,13 and benefits have even been observed in very young patients (age < 1 year).14
The majority of CPAP masks are designed for adults with fully developed facial features and contours. Use of these masks in children does not result in formation of a good seal, and adult-sized headgear does not fit well. The Pixi mask incorporates a number of comfort and safety features, including construction based on anthropometric data from children aged 2 years and older, taking into account bone structure, facial characteristics and skin sensitivity. In addition, the interface comes with unobtrusive, adjustable headgear designed specifically for pediatric patients.
In this study, the Pixi mask consistently rated better than the patients' previous PAP interface across all measures examined in this study, with statistically significant differences for restful sleep, ability to get to sleep, and ability to stay asleep. In addition, median ratings for all 18 usability parameters were “acceptable” or higher. The numerical differences in total sleep time, and time spent in stage N and stage R sleep trended in favor of Pixi over the previous mask, but did not reach statistical significance. Although this study showed that there was no significant difference in AHI during PAP therapy with the Pixi mask compared with previously used interfaces, there was a trend toward a lower AHI with the Pixi mask. The failure to achieve statistical significance may have been the result of the small number of patients in this study, given a pediatric patient population and the rigors of the protocol. Recruiting large numbers of participants into pediatric PAP studies is also difficult because the total number of children receiving PAP is very small; another study of CPAP in children, also conducted at 3 centers, was able to enroll a total of 21 patients.9 One of the trade-offs of increasing the number of participants in pediatric PAP trials is that the heterogeneity of the study population increases.15 Mode of PAP therapy (BPAP or CPAP) has been shown to have no significant effect on adherence to PAP,16 so this was unlikely to be a confounding factor in the current study. Another possible limitation is patient selection bias; in this study it was minimized by the recruitment of consecutive patients seen by sleep specialists and use of broad inclusion criteria with minimal exclusion criteria; however, some self-selection bias may occur since parents of patients can choose not to sign informed consent and thus not participate in the study.
One of the major issues in pediatric PAP therapy is adherence, which has consistently been reported to be suboptimal.15–17 Along with education and support for the patient and their care-givers and removing financial burdens, use of a comfortable and well-fitting mask has been described as an integral factor for the successful use of CPAP in children17 and in general.18 In a multivariate analysis, the type of interface was independently associated with a higher risk of CPAP non-adherence. In addition, adherence to CPAP with nasal masks was higher than adherence to therapy with an oronasal mask.18 In pediatric patients, a comfortable and well-fitting mask minimizes the development of complications such as pressure sores and mid-facial hypoplasia.19 Regular reassessment (every 6 to 12 months) of the CPAP interface in children is recommended because of their growing facial structures.5,17,20
In this study, we observed an hour difference in PAP usage between the Pixi mask and the children's usual mask. The discordance between the usage and the subjective improvement with the Pixi mask is difficult to explain, but there are a few possibilities: (1) the new mask benefitted the participants to a degree that subjective improvement was achieved despite a shorter usage time; (2) the children may have fallen asleep faster with the new mask; (3) the small sample size; and/or (4) the heightened favorable impressions of a novel vs. usual mask by participants' parents/guardians.
In conclusion, the Pixi mask was at least equivalent to previous interfaces in children receiving PAP therapy. It was easy to use and was well accepted by patients and their caregivers. Given the shortage of child-specific options for delivery of PAP therapy, the Pixi mask offers a useful alternative for delivery of treatment for SDB in pediatric patients.
DISCLOSURE STATEMENT
This study was funded by ResMed Ltd., Sydney, NSW, Australia. Dr. Kryger has received research funding from ResMed and Respironics, and consulting fees from Inspire, Medtronic, and Merck. Dr. Kushida was the Principal Investigator on studies supported by Pacific Medico Co., Resmed, Apnex Medical, Impax Laboratories, Inc., and Cephalon. He has consulting relationships with Apnex, Seven Dreamers Laboratories, Noven Pharmaceuticals, UCB, and Zephyr and has received royalties from Philips Respironics. Dr. Halbower is an advisor to AVISA device company, unrelated to this manuscript, and advised ResMed during the development of the Pixi mask outlined in this manuscript. Chia-Yu Cardell has received research support from ResMed Ltd. Ms. Willes is an independent, statistical consultant hired to analyze the results for this study and has no other financial relationship with the study sponsor other than as a fee-based consultant for statistical services provided. Ms. Wimms and Ms. Mendoza are full time employee of ResMed Ltd. The other authors have indicated no financial conflicts of interest. Medical writing assistance was provided by Nicola Ryan, independent medical writer, on behalf of ResMed. The work was performed at the Children's Hospital, Denver, CO, Gaylord Sleep Medicine, Wallingford, CT, and Stanford Center for Human Sleep Research, Redwood City, CA.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BPAP
bilevel positive airway pressure
- CPAP
continuous positive airway pressure
- NIV
noninvasive ventilation
- PAP
positive airway pressure
- PSG
polysomnography
- SDB
sleep disordered breathing
- SpO2
oxygen saturation
- TST
total sleep time
REFERENCES
- 1.Chang SJ, Chae KY. Obstructive sleep apnea syndrome in children: Epidemiology, pathophysiology, diagnosis and sequelae. Korean J Pediatr. 2010;53:863–71. doi: 10.3345/kjp.2010.53.10.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chervin RD, Dillon JE, Bassetti C, Ganoczy DA, Pituch KJ. Symptoms of sleep disorders, inattention, and hyperactivity in children. Sleep. 1997;20:1185–92. doi: 10.1093/sleep/20.12.1185. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb DJ, Vezina RM, Chase C, et al. Symptoms of sleep-disordered breathing in 5-year-old children are associated with sleepiness and problem behaviors. Pediatrics. 2003;112:870–7. doi: 10.1542/peds.112.4.870. [DOI] [PubMed] [Google Scholar]
- 4.Halbower AC, McGinley BM, Smith PL. Treatment alternatives for sleep-disordered breathing in the pediatric population. Curr Opin Pulm Med. 2008;14:551–8. doi: 10.1097/MCP.0b013e3283130f80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcus CL, Ward SL, Mallory GB, et al. Use of nasal continuous positive airway pressure as treatment of childhood obstructive sleep apnea. J Pediatr. 1995;127:88–94. doi: 10.1016/s0022-3476(95)70262-8. [DOI] [PubMed] [Google Scholar]
- 6.Section on Pediatric Pulmonology. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:704–12. doi: 10.1542/peds.109.4.704. [DOI] [PubMed] [Google Scholar]
- 7.Kissoon N, Adderley R. Noninvasive ventilation in infants and children. Minerva Pediatr. 2008;60:211–8. [PubMed] [Google Scholar]
- 8.Liner LH, Marcus CL. Ventilatory management of sleep-disordered breathing in children. Curr Opin Pediatr. 2006;18:272–6. doi: 10.1097/01.mop.0000193301.63259.84. [DOI] [PubMed] [Google Scholar]
- 9.Marcus CL, Rosen G, Ward SL, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006;117:e442–51. doi: 10.1542/peds.2005-1634. [DOI] [PubMed] [Google Scholar]
- 10.Waters K. Interventions in the paediatric sleep laboratory: the use and titration of respiratory support therapies. Paediatr Respir Rev. 2008;9:181–91. doi: 10.1016/j.prrv.2008.01.003. quiz 91-2. [DOI] [PubMed] [Google Scholar]
- 11.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 12.Iber C, Ancoli-Israel S, Chesson A, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specification. [Google Scholar]
- 13.Waters KA, Everett FM, Bruderer JW, Sullivan CE. Obstructive sleep apnea: the use of nasal CPAP in 80 children. Am J Respir Crit Care Med. 1995;152:780–5. doi: 10.1164/ajrccm.152.2.7633742. [DOI] [PubMed] [Google Scholar]
- 14.Massa F, Gonsalez S, Laverty A, Wallis C, Lane R. The use of nasal continuous positive airway pressure to treat obstructive sleep apnoea. Arch Dis Child. 2002;87:438–43. doi: 10.1136/adc.87.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beebe DW, Byars KC. Adolescents with obstructive sleep apnea adhere poorly to positive airway pressure (PAP), but PAP users show improved attention and school performance. PloS One. 2011;6:e16924. doi: 10.1371/journal.pone.0016924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uong EC, Epperson M, Bathon SA, Jeffe DB. Adherence to nasal positive airway pressure therapy among school-aged children and adolescents with obstructive sleep apnea syndrome. Pediatrics. 2007;120:e1203–11. doi: 10.1542/peds.2006-2731. [DOI] [PubMed] [Google Scholar]
- 17.O'Donnell AR, Bjornson CL, Bohn SG, Kirk VG. Compliance rates in children using noninvasive continuous positive airway pressure. Sleep. 2006;29:651–8. [PubMed] [Google Scholar]
- 18.Borel JC, Tamisier R, Dias-Domingos S, et al. Type of mask may impact on continuous positive airway pressure adherence in apneic patients. PloS One. 2013;8:e64382. doi: 10.1371/journal.pone.0064382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li KK, Riley RW, Guilleminault C. An unreported risk in the use of home nasal continuous positive airway pressure and home nasal ventilation in children: mid-face hypoplasia. Chest. 2000;117:916–8. doi: 10.1378/chest.117.3.916. [DOI] [PubMed] [Google Scholar]
- 20.Marcus CL. Advances in management of sleep apnea syndromes in infants and children. Pediatr Pulmonol Suppl. 1999;18:188–9. [PubMed] [Google Scholar]