Abstract
Study Objective:
The Psychomotor Vigilance Task (PVT) is one of the leading assays of sustained vigilant attention in sleep research and highly sensitive to the effects of sleep loss. Even though PVT is widely used in sleep deprivation studies, little is known about PVT performance in patients suffering from sleep-wake disorders. We aimed to quantify the impact of sleep-wake disorders on PVT outcome measures and examine whether PVT can distinguish between healthy controls and patients with sleep-wake disorders and whether PVT can distinguish between three different disorders that express excessive daytime sleepiness.
Methods:
We compared PVT data of 143 patients and 67 age- and gender-matched healthy controls. Patients were diagnosed with one of the following sleep-wake disorders: narcolepsy with cataplexy (n = 20), insufficient sleep syndrome (ISS, n = 67) and hypersomnia (HS, n = 56). Several PVT outcomes were analyzed: reciprocal mean reaction time, response variability, number of lapses, number of false reaction time, slowest and fastest 10% of reaction time, and duration of lapses.
Results:
PVT performance was generally better in healthy controls than in patients with any of the sleep-wake disorders analyzed. Patients with narcolepsy and HS performed worse on PVT than subjects with ISS. In controls, but not in patients, older subjects had slower reactions times and higher response variability in PVT.
Conclusions:
PVT performance shows different patterns in patients with different sleep-wake disorders and control subjects and may add useful information to the diagnostic work-up of sleep-wake disorders.
Citation:
Thomann J, Baumann CR, Landolt HP, Werth E. Psychomotor Vigilance Task Demonstrates Impaired Vigilance in Disorders with Excessive Daytime Sleepiness. J Clin Sleep Med 2014;10(9):1019-1024.
Keywords: psychomotor vigilance task, sleepiness, vigilance, sleep-wake disorders, multiple sleep latency test, maintenance of wakefulness test
In current practice and in most clinical studies, diagnostic tests to assess daytime alertness and wakefulness focus almost exclusively on the quantification of excessive daytime sleepiness. Hence, an equally important complaint of patients suffering from sleep-wake disorders has been much less explored, i.e., impaired sustained vigilant performance.
In sleep research, Psychomotor Vigilance Task (PVT) has emerged as one of the most widely used tools to assess vigilant attention.1 Vigilance can be defined as sustained attention and tonic alertness.2 In sleep deprived individuals, vigilance is the component of cognition that is most consistently and dramatically affected.3 The PVT is a reaction-time test that allows the collection of a large amount of data in a relatively short period of time. These characteristics increase the sensitivity of the test to detect even small changes in vigilant attention, which can wax and wane within seconds.4 Increased sleep drive raises performance variability because of competing sleep-initiating and wake-promoting factors.5–7 Thus, the PVT is highly sensitive to sleep deprivation.3,8,9 Its reliability and validity have been amply demonstrated,3 and the test shows virtually no learning curve and is independent of aptitude.10
Research describing PVT performance in sleep-wake disorders is sparse. Some data are available on PVT performance in patients with obstructive sleep apnea (OSA).11–13 In the Wisconsin Sleep Cohort study, the authors found a significant relationship between apnea severity and number of lapses on PVT in older people.11 Previous studies, however, did not compare data from healthy control subjects with those from patients suffering from sleep-wake disorders.
BRIEF SUMMARY
Current Knowledge/Study Rationale: The Psychomotor Vigilance Task (PVT) is one of the leading assays of vigilant attention in sleep research and highly sensitive to the effects of sleep loss. Even though PVT is widely used in sleep deprivation studies, little is known about PVT performance in patients suffering from sleep-wake disorders.
Study Impact: Performance on the PVT shows different patterns in patients with sleep-wake disorders and in controls. Our study suggests that PVT has a potential to be of clinical relevance because it provides extra information in the diagnostic process to distinguish between healthy controls and patients with sleepiness.
Steer clear test (Steer Clear), a computer-based driving simulation task, and the sustained attention to response task (SART), a go/no-go paradigm, are two additional vigilance tests used in some sleep studies. Patients with OSA and narcolepsy performed more poorly on Steer Clear than did control subjects and investigation of vigilance impairment using SART revealed reduced performance in narcolepsy as well as in other sleep-wake disorders with excessive daytime sleepiness.14,15
Previous studies failed to find correlations between different vigilance and sleepiness tests.16,17 These findings suggest that sleepiness and impaired vigilance are distinct entities, based on different dysfunction of sleep-wake modulatory circuits in the central nervous system.18
Altogether, although PVT has been well implemented in sleep research—mostly to assess vigilance in sleep-deprived healthy subjects—our knowledge on its use in patients with sleep-wake disorders is limited. Thus, with the present study, we aimed at evaluating PVT as a clinical routine diagnostic tool in a sleep laboratory. The main goal of this study was to examine (1) whether PVT can distinguish between healthy controls and patients with sleep-wake disorders, and (2) whether PVT can distinguish between different disorders with excessive daytime sleepiness.
METHODS
Subjects
We retrospectively analyzed data from patients referred to the sleep laboratory of the Department of Neurology of the University Hospital Zurich between January 2006 and May 2012. We identified 143 patients who underwent PVT examinations as described below, including 62 women (43%) and 81 men (57%), age 40 ± 15 years (mean ± SD). Sleep-wake disorders in these patients were diagnosed according to the International Classification of Sleep Disorders (ICSD-2)19 and included the following diagnoses: hypocretin-deficient narcolepsy with cataplexy (n = 20, age 37 ± 17), actigraphy-proven insufficient sleep syndrome (ISS, n = 67, age 40 ± 14), and hypersomnia disorders of other origin (HS), characterized by excessive daytime sleepiness (Epworth Sleepiness Scale [ESS > 10]; n = 56, age 43 ± 15). Thus all included patients suffered from sleepiness. As all recordings were done during their diagnostic workup, none of the patients took medication that might influence sleepiness.
In addition, we examined 67 age- and gender-matched healthy control subjects, including 28 women (42%) and 39 men (58%), age 42 ± 17 years. We accepted subjects who reported to be healthy, i.e., not suffering from neurological or psychiatric disorder or any disease potentially affecting sleep and wakefulness and having no sleep-wake problems. Based on structured questionnaires and interviews, the following criteria led to exclusion of healthy subjects: presence of any sleep-wake, neurologic, psychiatric, or medical disorders; ESS scores > 10; and intake of medication that might influence PVT outcome. The data of a subset of healthy control subjects (n = 30) were included in a previous publication.20
Diagnostic Workup: Nocturnal Sleep Studies and Daytime Sleepiness Evaluation in Sleep Disordered Patients
To assess sleep satiation before sleep laboratory tests, all patients were examined by 2-week actigraphy. This is a noninvasive method to monitor activity and rest cycles recorded by a wrist actimeter. Thereafter, every patient underwent diagnostic nocturnal polysomnography. For this purpose, we applied a multi-channel recording system (Embla N7000) as described previously.21
The day following polysomnography, all patients underwent multiple sleep latency tests (MSLT) which were performed according to standard criteria.22 In addition, excessive daytime sleepiness was assessed with the Epworth Sleepiness Scale (ESS).23
On a separate day, 127 patients (87%) were examined by means of maintenance of wakefulness tests (MWT), which included 4 test sessions measuring the patients' ability to stay awake in non-stimulating conditions.22 It was performed, preferably close to the day of MSLT, by the majority of patients in all the different diagnostic groups—19/20 (95%) in patients with narcolepsy, 59/67 (88%) in ISS, and 47/56 (84%) in HS.
In addition, the Steer Clear test was performed.14 This computer-based driving simulation task lasts 30 minutes and presents obstacles (steers) on a two-lane highway. Patients are instructed to avoid hitting the obstacles by pressing the space bar on the keyboard to change the lane. This test was applied in 123 patients (88%) (narcolepsy: 19/20 [95%], ISS: 61/67 [91%], HS: 46/56 [82%]). In 116 patients (94%), Steer Clear was performed on the same day as MWT.
Control subjects did not undergo this full diagnostic work-up but were examined with PVT and questionnaires.
Psychomotor Vigilance Task (PVT)
For PVT (PVT-192, Ambulatory Monitoring Inc.), subjects were instructed to press a button as quickly as possible when a red millisecond-counter appeared on a small screen. Upon pressing the button, the screen displayed the reaction time (RT) for one second, thus giving an instant feedback on the individual's performance. Stimuli appeared in a random pattern with an inter-stimulus interval varying between 2 and 10 seconds. Each test lasted 10 min and consisted of approximately 100 stimulus presentations. In every subject, a single 1-min habituation test was performed before the first trial. We assessed the following outcome measures: (1) Reciprocal RT as a measure of speed (1/RT (lapses included) and 1/RT500 (lapses excluded); (2) number of lapses (defined as RT > 500 ms, i.e. inability to respond in a timely fashion when a stimulus was present (data transformed by (SQR(x)+SQR(x+1)); (3) false RT defined as number of false starts, non-physiological responses divided by the number of valid stimuli in percentage; (4) range between the 10th and 90th percentiles of speed as a measure of variability of responses (1/ RT variability, with and without lapses included); (5) the 10% fastest RT; (6) the 10% slowest RT; (7) average lapses time in milliseconds (defined as RT during lapses minus 500 ms); and (8) cumulated lapses time within the 10-min test session.
In every patient, PVT was performed at least twice a day, once during morning hours, once in the afternoon. Mean scores of each day were calculated since no strong variation according to time of day occurred in the control or patient groups.
Some patients performed PVT on several days; however, only PVT results of the first day of each person were included in the study. In 117 patients (82%) this first PVT was performed on the same day as the MSLT, in 26 patients (18%) on the day of the MWT. As PVT results did not differ between patients who performed PVT on the day of the MSLT (napping during the day) and those who performed it on the day of the MWT, data were pooled.
In 30 controls, PVT was performed several times a day; however, only the 2 tests which corresponded to the PVT timing in patients were selected for this study. In 37 controls, PVT was performed once during a normal day, based on previous data demonstrating that a single performance assessment per day may be reliable.24
Statistical Methods
All analyses were performed with SPSS version 21 (SPSS, Inc., Chicago, IL, USA) software. As not all PVT data in patients and controls were normally distributed, data were transformed to gain valid results when using parametric tests. Square root, logarithmic, or inverse transformations were used for positively skewed data. To convert negatively skewed data to normality reflect square root (SQR((xmax+1)-x)), reflect logarithmic or reflect inverse transformation were applied. When transformation was unsuccessful we applied nonparametric analyses. For comparison of two groups, independent sample t-test or Mann-Whitney U-test were used. For more than two groups, we used one-way independent ANOVA or the Kruskal-Wallis H-test. Three-way independent ANOVA was computed to assess the additional impact of age and gender on PVT results. Factor age was represented by 3 levels (age ≤ 35, 36-55, ≥ 56). Bivariate correlation analysis was done by calculating correlation coefficients to explore the relationships in more detail. PVT data from controls served as reference to evaluate normative cutoff levels (mean ± 2 SD or 2.5th / 97.5th percentile, as appropriate) to analyze the fraction of patients outside this normal range. The χ2 test of independence was performed to examine categorical variables. Significance was accepted at p < 0.05. Data are presented as mean and standard deviation.
RESULTS
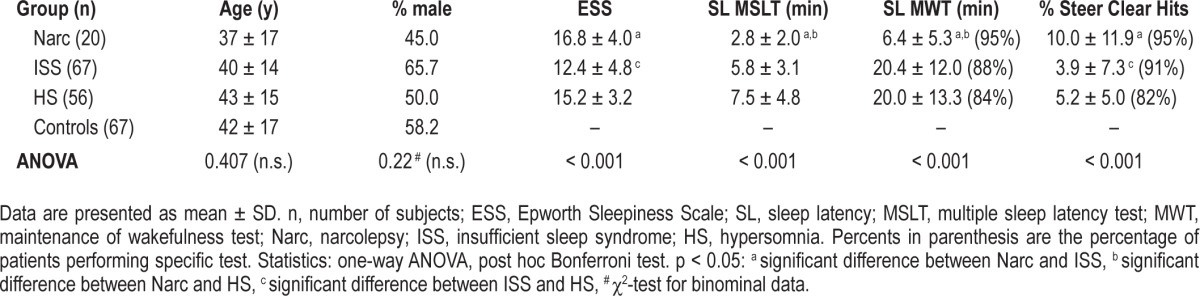
One-hundred forty three patients and 67 healthy controls were evaluated. Table 1 provides an overview on clinical and electrophysiological data. There were no differences in age and gender between patients with sleep-wake disorders and control subjects. Patient groups differed significantly in subjective sleepiness (ESS) and in objective measures such as sleep latency (SL) on MSLT, persistence staying awake during MWT, and performance on Steer Clear (Table 1). Patients with narcolepsy and those with HS indicated higher subjective sleepiness and performed significantly worse on Steer Clear than patients with ISS. Sleep latency on MSLT and the ability to stay awake during MWT were shorter in patients with narcolepsy than in ISS and HS patients.
Table 1.
Anthropometric and clinical data, organized by sleep diagnoses.
Impaired Vigilance in Disorders of Excessive Daytime Sleepiness
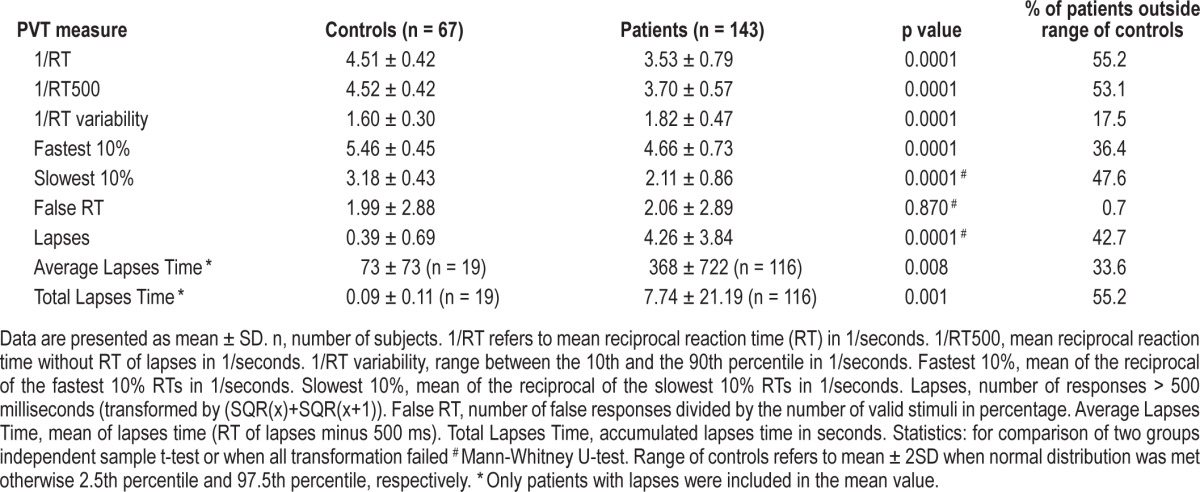
Patients showed significant deficits in vigilant attention compared to controls for almost all PVT measures: as shown in Table 2, patients had significantly slower speed, greater variability, more lapses, and longer lapses times. False RT was the only measure that was similar between controls and patients. Of the 143 patients, 55% were too slow, i.e., outside the normal range of the control group; this was still true for 53% when RT during lapses was not included in the speed measure (1/RT500). Forty-three percent of patients had more lapses than the control group and 55% of patients had longer total lapses times, whereas only 18% of patients were outside the range of variability of the control group. However, on the other side, 41% of patients had all PVT measures within the normal range of the control group.
Table 2.
Psychomotor vigilance task (PVT) variables in controls and patients.
PVT Results in Different Sleep-Wake Disorders
Differences between the 3 patient groups were not as prominent as between controls and patients, yet a significant variation was found in speed, variability, false starts, and lapses (Table 3).
Table 3.
Psychomotor vigilance task (PVT) variables and different sleep-wake disorders.
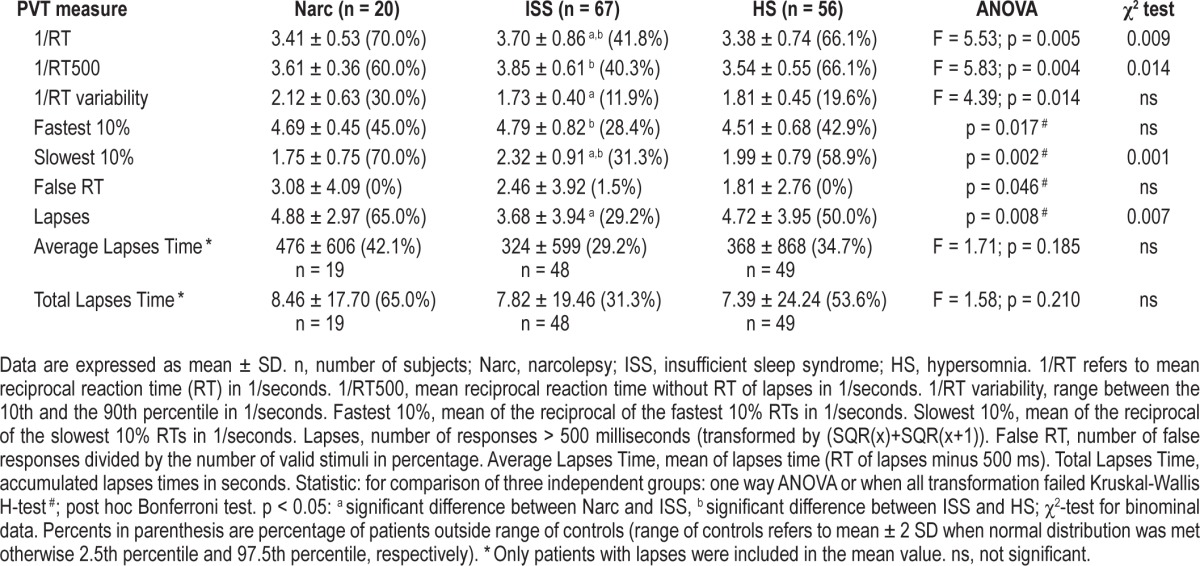
Among the different sleep-wake disorders, patients with ISS were significantly faster in their reaction than narcoleptics and HS, and ISS made significantly less lapses than narcoleptics. Patients with narcolepsy had a higher variability in reaction time compared to ISS. HS were significantly slower compared to ISS in the 10% slowest and 10% fastest RT, demonstrating a general slower RT in HS, whereas in narcoleptic patients only the 10% slowest RT were significantly different to ISS subjects, probably due to more lapses in narcoleptics than in ISS. None of the HS PVT measures differed significantly from those of patients with narcolepsy.
Abnormal PVT Measures
Chi-square analyses were conducted to examine whether there was a relationship between type of sleep disorder and incidence of deviant PVT measure (Table 3). The results revealed that there were significant relationships between the 2 variables.
Patients with narcolepsy or HS were more likely to be outside the normal PVT range of healthy subjects than patient with ISS. Patients with narcolepsy or HS were more likely to be slower in speed and had more lapses. However, the associations were only of weak to moderate strength.
Factors Influencing PVT Results
The PVT outcomes were subjected to 3-way independent ANOVA (group, age, gender). There was a main effect of group for speed (1/RT, p < 0.001) and 10% fastest RT (p < 0.001), but not for other PVT measures. The main effects of age and gender were nonsignificant. The interaction of group and age was significant, indicating that the influence of age on PVT results is different in patients and healthy controls (1/RT: p = 0.035, 10% fastest RT: p = 0.037). Other interactions were not found.
Additionally, there was a positive correlation between age and speed in controls (r = 0.620, p < 0.01) but not in patients. In other words, older control subjects had slower reaction time on PVT. There was also a positive correlation between age and speed for the 10% fastest RT among both subpopulations (patients: r = 0.190, p < 0.05; controls: r = 0.605, p < 0.01), although it was weaker among patients.
Comparing PVT Outcomes to Other Laboratory Tests
PVT outcomes strongly correlated with Steer Clear Hits (Lapses: r = 0.551, 1/RT: r = -0.521) and moderately with MWT sleep latency (Lapses: r = -0.357, 1/RT: r = 0.349).
DISCUSSION
The main findings of our study can be summarized as follows. First, PVT shows different patterns between healthy subjects and patients suffering from sleep-wake disorders. Second, PVT results differ between different groups of sleep-wake disorders. Third, patients with narcolepsy or patients with HS were more likely to be outside the normal PVT range of healthy subjects than patient with ISS. Forth, whereas age significantly influences PVT outcome in healthy subjects, this association gets lost in patients with sleep-wake disorders.
Our findings suggest that PVT may be helpful in the diagnostic work-up of sleep-wake disorders. Most importantly, PVT results are worse in patients with sleep-wake disorders than control subjects. Thus, PVT results may help to distinguish between people suffering from excessive daytime sleepiness and healthy controls. However, as recording conditions in controls differed significantly from those in patients—the latter performed PVT between MSLT or MWT recordings—future prospective studies may want to perform PVT in a uniform setting for patients and controls.
Even more so, PVT may contribute to distinguish among specific sleep-wake disturbances, e.g., between ISS and hypersomnia of other origin. This differentiation is not always simple. For example, both narcolepsy and ISS may present with increased REM sleep pressure on MSLT.25 Here we show that ISS and HS have similar MSLT and MWT results. Therefore, fast and cost-effective additional tests are welcome.
Our findings suggest that subjects with ISS perform better on PVT than patients with narcolepsy and hypersomnia (ISS were faster). Additionally, ISS and patients with narcolepsy were different in the amount of lapses (narcolepsy patients made more lapses) and RT variability (narcolepsy patients had greater variability in RT).
Along the same line, other studies using different vigilance tests (e.g., SART26 and Steer Clear27) demonstrated high variability in narcolepsy patients. A recent study analyzed SART in patients with different sleep wake disorders. In this study high SART error rate reflected vigilance impairment in excessive daytime sleepiness irrespective of its cause.15
PVT seems to be sensitive to distinguish specific sleep-wake disturbances. It would be interesting to calculate cutoff values for selected sleep-wake disorders; however, larger and more homogeneous groups of patients will be needed; these issues may be addressed by future studies. Our preliminary results suggest that PVT will be able to distinguish ISS from other hypersomnia patients.
A recent systematic study investigating power of performance outcomes to discriminate alert and sleep deprived subjects revealed mean reciprocal reaction time and the number of lapses as PVT primary outcomes.28 Several PVT performance outcomes were analyzed in our study. Along this line, in our study PVT response speed, lapses and variability were most sensitive and differed among sleep-wake disorders with excessive daytime sleepiness, whereas lapses duration and total lapses time did not.
Age influences PVT outcome. This has been shown by other groups.20,29–31 In our study, older control subjects had slower RT and higher variability, but this effect was lost in patients with any sleep-wake disorders included. The reason for this finding may be the fact that severity of disease differed from individual to individual in the diagnostic groups included in this study, irrespective of demographic data. Yet interestingly, a similar phenomenon was described in previous sleep-restriction studies.20,29 Young subjects were faster than older only under low, but not under high sleep pressure conditions. In older subjects, the relative PVT performance decline after sleep deprivation was significantly less pronounced than in the young,29 or even reversed20: young subjects performed more lapses and a higher variability in PVT results. Neurobiological mechanisms underlying this finding still need to be elucidated.
We did not observe an impact of sex on PVT results, as it had been shown by other groups.29
When comparing PVT outcomes to other sleep laboratory tests, the highest correlation was found with Steer Clear. Thus, it appears that these two vigilance tests measure similar aspects of vigilance and alertness. Steer Clear hits showed a significant difference between narcolepsy and ISS as well as between HS and ISS, while there was no significant difference between narcolepsy and HS. The PVT measures 1/RT and slowest 10% showed a similar pattern. The Steer Clear lasts longer and simulates the situation of a driver. The benefit of PVT is that the test is much shorter and still being sensitive to analyze vigilance. Both tests demand sustained attention and fast reactions. In many countries, the MWT is the gold standard to assess the ability to stay awake under non-stimulating conditions, and thus for the judgement of the ability to drive. In our study, there was only a mild correlation between PVT and MWT. Based on these findings, we might conclude that the two tests measure partially different aspects of vigilance. We still do not know which test is best for the medicolegal judgement of the fitness to drive.
The gold standard test to assess excessive daytime sleepiness, the MSLT, does not correlate with PVT results.16,17 In the same direction, subjective (ESS) and objective (MWT, MSLT) tests to assess sleep propensity may not assess the same aspects of alertness.32,33 Different pathophysiological and motivational pathways may underlie vigilance impairment and sleepiness. Thus, measuring vigilance with the PVT may be of clinical relevance because it provides important extra information in the diagnostic process which would otherwise be missed.
This study has significant limitations. It is based on retrospective data from patients referred to a sleep clinic. Furthermore, the conditions for PVT performances were different in patients and controls. Patients performed PVT mainly during MSLT days, whereas controls took their tests on regular days. The potential of PVT in aiding in the diagnostic work-up of patients presenting with sleep-wake disorders needs to be studied in future prospective studies including larger and more homogeneous groups of patients. Still, our study showed clearly that PVT has the potential to help in the diagnostic work-up of sleep-wake disorders, adding the important aspect of impaired vigilant attention in patients suffering from sleep-wake disorders.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENT
The authors thank Judith Meier and Sandra Weber for helping with data collection. Ms. Thomann and Dr. Baumann contributed equally to this study.
ABBREVIATIONS
- ESS
Epworth Sleepiness Scale
- HS
hypersomnia disorder
- ICSD-2
International Classification of Sleep Disorders 2nd Edition
- ISS
insufficient sleep syndrome
- MSLT
multiple sleep latency test
- MWT
maintenance of wakefulness test
- OSA
obstructive sleep apnea
- PVT
psychomotor vigilance task
- REM
rapid eye movement
- RT
reaction time
- SART
Sustained Attention to Response Task
- SL
sleep latency
- SQR
square root
- Steer Clear
Steer Clear Test (vigilance test)
REFERENCES
- 1.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Beh Res Meth Instr Comp. 1985;17:652–5. [Google Scholar]
- 2.Oken BS, Salinsky MC, Elsas SM. Vigilance, alertness, or sustained attention: physiological basis and measurement. Clin Neurophysiol. 2006;117:1885–901. doi: 10.1016/j.clinph.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 4.Mackworth JF. Vigilance, arousal, and habituation. Psychol Rev. 1968;75:308–22. doi: 10.1037/h0025896. [DOI] [PubMed] [Google Scholar]
- 5.Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 6.Graw P, Krauchi K, Knoblauch V, Wirz-Justice A, Cajochen C. Circadian and wake-dependent modulation of fastest and slowest reaction times during the psychomotor vigilance task. Physiol Behav. 2004;80:695–701. doi: 10.1016/j.physbeh.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Zhou X, Ferguson SA, Matthews RW, et al. Dynamics of neurobehavioral performance variability under forced desynchrony: evidence of state instability. Sleep. 2011;34:57–63. doi: 10.1093/sleep/34.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 9.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 10.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–29. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30:1309–16. doi: 10.1093/sleep/30.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinges DF, Weaver TE. Effects of modafinil on sustained attention performance and quality of life in OSA patients with residual sleepiness while being treated with nCPAP. Sleep Med. 2003;4:393–402. doi: 10.1016/s1389-9457(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 13.Sforza E, Haba-Rubio J, De Bilbao F, Rochat T, Ibanez V. Performance vigilance task and sleepiness in patients with sleep-disordered breathing. Eur Respir J. 2004;24:279–85. doi: 10.1183/09031936.04.00091903. [DOI] [PubMed] [Google Scholar]
- 14.Findley L, Unverzagt M, Guchu R, Fabrizio M, Buckner J, Suratt P. Vigilance and automobile accidents in patients with sleep apnea or narcolepsy. Chest. 1995;108:619–24. doi: 10.1378/chest.108.3.619. [DOI] [PubMed] [Google Scholar]
- 15.Van Schie MK, Thijs RD, Fronczek R, Middelkoop HA, Lammers GJ, Van Dijk JG. Sustained attention to response task (SART) shows impaired vigilance in a spectrum of disorders of excessive daytime sleepiness. J Sleep Res. 2012;21:390–5. doi: 10.1111/j.1365-2869.2011.00979.x. [DOI] [PubMed] [Google Scholar]
- 16.Frey DJ, Badia P, Wright KP., Jr Inter- and intra-individual variability in performance near the circadian nadir during sleep deprivation. J Sleep Res. 2004;13:305–15. doi: 10.1111/j.1365-2869.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- 17.Franzen PL, Siegle GJ, Buysse DJ. Relationships between affect, vigilance, and sleepiness following sleep deprivation. J Sleep Res. 2008;17:34–41. doi: 10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathis J, Hess CW. Sleepiness and vigilance tests. Swiss Med Wkly. 2009;139:214–9. doi: 10.4414/smw.2009.12498. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2005. International Classification of Sleep Disorders, 2nd ed: Diagnostic and Coding Manual. [Google Scholar]
- 20.Adam M, Retey JV, Khatami R, Landolt HP. Age-related changes in the time course of vigilant attention during 40 hours without sleep in men. Sleep. 2006;29:55–7. doi: 10.1093/sleep/29.1.55. [DOI] [PubMed] [Google Scholar]
- 21.Baumann CR, Werth E, Stocker R, Ludwig S, Bassetti CL. Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain. 2007;130:1873–83. doi: 10.1093/brain/awm109. [DOI] [PubMed] [Google Scholar]
- 22.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 24.Sunwoo BY, Jackson N, Maislin G, Gurubhagavatula I, George CF, Pack AI. Reliability of a single objective measure in assessing sleepiness. Sleep. 2012;35:149–58. doi: 10.5665/sleep.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marti I, Valko PO, Khatami R, Bassetti CL, Baumann CR. Multiple sleep latency measures in narcolepsy and behaviourally induced insufficient sleep syndrome. Sleep Med. 2009;10:1146–50. doi: 10.1016/j.sleep.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Fronczek R, Middelkoop HA, van Dijk JG, Lammers GJ. Focusing on vigilance instead of sleepiness in the assessment of narcolepsy: high sensitivity of the Sustained Attention to Response Task (SART) Sleep. 2006;29:187–91. [PubMed] [Google Scholar]
- 27.Findley LJ, Suratt PM, Dinges DF. Time-on-task decrements in “steer clear” performance of patients with sleep apnea and narcolepsy. Sleep. 1999;22:804–9. doi: 10.1093/sleep/22.6.804. [DOI] [PubMed] [Google Scholar]
- 28.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34:581–91. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blatter K, Graw P, Munch M, Knoblauch V, Wirz-Justice A, Cajochen C. Gender and age differences in psychomotor vigilance performance under differential sleep pressure conditions. Behav Brain Res. 2006;168:312–7. doi: 10.1016/j.bbr.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Philip P, Taillard J, Quera-Salva MA, Bioulac B, Akerstedt T. Simple reaction time, duration of driving and sleep deprivation in young versus old automobile drivers. J Sleep Res. 1999;8:9–14. doi: 10.1046/j.1365-2869.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- 31.Parasuraman R, Nestor P, Greenwood P. Sustained-attention capacity in young and older adults. Psychol Aging. 1989;4:339–45. doi: 10.1037//0882-7974.4.3.339. [DOI] [PubMed] [Google Scholar]
- 32.Chervin RD, Aldrich MS, Pickett R, Guilleminault C. Comparison of the results of the Epworth Sleepiness Scale and the Multiple Sleep Latency Test. J Psychosom Res. 1997;42:145–55. doi: 10.1016/s0022-3999(96)00239-5. [DOI] [PubMed] [Google Scholar]
- 33.Sangal RB, Mitler MM, Sangal JM. Subjective sleepiness ratings (Epworth sleepiness scale) do not reflect the same parameter of sleepiness as objective sleepiness (maintenance of wakefulness test) in patients with narcolepsy. Clin Neurophysiol. 1999;110:2131–5. doi: 10.1016/s1388-2457(99)00167-4. [DOI] [PubMed] [Google Scholar]


