INTRODUCTION
COPD is an important lung and airway disease with an increasing incidence particularly in developing countries. Worldwide, asthma and COPD affect the lives of ∼300 and 200 million people, respectively (1). COPD is a chronic inflammatory disease for which smoking is the major risk factor in the developed world and is currently the fourth leading cause of death worldwide. It is predicted to become the third ranked disease by the year 2030 (2, 3). Unfortunately, there are no effective treatments for severe asthma and COPD due to a lack of clarity in disease mechanisms. However, new observations in the areas of signal transduction and epigenetics may provide new understanding of the pathogenesis of lung diseases. Understanding the pathways and mechanisms leading to mediator release may lead to better therapeutic approaches for these diseases. The inflammatory mediators involved in COPD have not been clearly delineated but are thought to include many lipid mediators, inflammatory peptides, reactive oxygen species (ROS) and nitrogen species, chemokines, cytokines and growth factors. These are all involved in orchestrating the complex inflammatory process that results in small airway fibrosis and alveolar destruction occurring in COPD (4–6). Cigarette smoke contains over 4,700 chemical compounds, and both the tar and gas phases contain numerous free radicals and other oxidants present in high concentrations which contribute to the pathogenesis of this condition (7–10). Exposure to cigarette smoke activates an inflammatory cascade in the airways, resulting in the production of a number of potent cytokines and chemokines with accompanying damage to the lung epithelium leading to increased permeability and recruitment of macrophages and neutrophils (11). Free radicals in cigarette smoke activate inflammatory cells which, in turn, generate high levels of ROS and other toxic metabolites. Activation of immune cells by these radicals leads to the production of oxidants and cytokines, such as IL-8, IL-6 and TNF-α (12–19). IL-8 is a powerful chemotactic and paracrine mediator for neutrophils, and infiltration of activated neutrophils is the key in pulmonary inflammation and oxidative injury (20–23). Several inflammatory cells and their mediators, both of the innate and adaptive immune system, participate in the inflammatory response in COPD. Macrophages, neutrophils and CD8+ T cells are the cells usually considered the prime effector cells in pathogenesis of COPD (24, 25). The role of neutrophils and macrophages and their product IL-8 has been well established in the slow progression of the disease (26, 27). Besides IL-8, it has been shown that fragments of the extracellular matrix, such as collagen fragments, have chemotactic properties to the cells (28, 29). One of these fragments is N-acetylated Proline–Glycine–Proline (N-ac-PGP) and it is shown that injecting N-ac-PGP into the normal rabbit corneas resulted in rapid and severe neutrophil invasion and neutrophil infiltration in the injured eye (30). Interestingly, N-ac-PGP has been found in the sputum of COPD (31) and cystic fibrosis patients (32). Overbeek et al. demonstrated that N-ac-PGP stimulates the neutrophils to release IL-8, which in in vivo may lead to a self-perpetuating situation where N-ac-PGP and CXCL8 work in concert, leading to enhanced neutrophil inflammation and lung inflammation (33, 34).
Recent findings concerning the innate and acquired immune responses in COPD have led to the suggestion of a possible autoimmune component contributing to its pathogenesis. This notion is supported by similar pathophysiology between COPD and some autoimmune diseases (35). In this line, the T lymphocyte subset TH17 was shown to play a role in regulating neutrophilic and macrophage inflammation of the lungs (35), suggesting a potential role for TH17 cells in severe, steroid-insensitive COPD (36–39). Thus, the nature of the immune reaction in COPD and increased amounts of IL-17 raise the possibility of autoimmune hypothesis in its pathogenesis.
Potential role of signal transduction pathways as putative therapeutic targets in pathogenesis of COPD
Understanding the pathways and mechanisms leading to mediator release may lead to better therapeutic approaches for this disease. With the complexity of inflammatory signaling networks and the cross talk that occurs between them, it is important to develop a greater understanding of these networks in the pathogenesis of disease. In terms of lung disease, it is still debatable whether these diseases occur as a result of excessive inflammatory drive or a lack of inhibitory feedback loops. However, it is clear that many of these pathways/networks are abnormally activated in COPD and that interference with these signaling pathways could shed light on disease processes and provide novel therapeutic approaches. Among signaling pathways involved in pathogenesis of COPD, Toll Like Receptors (TLRs) and Inflammasome NALP3 signal transduction activation have been described (40–42).
In this regard, increased ROS production by cigarette smoke has been directly linked to oxidation of proteins, DNA, and lipids which may cause direct lung injury or induce a variety of cellular responses through the generation of highly reactive secondary metabolic entities. ROS may alter remodeling of extracellular matrix, apoptosis and mitochondrial respiration, cell proliferation, maintenance of surfactant and the antiprotease screen, effective alveolar repair response and immune modulation in the lungs (43, 44). ROS have also been implicated in initiating the lung inflammatory response through the activation of transcription factors such as NF-κB and AP-1 and the regulation of the expression and activity of histone modifying enzymes and thereby enhancing inflammatory gene expression (45). Activation of ROS pathways in pathogenesis of asthma (46–48) and COPD has been described (49–51).
Importantly, growing evidence indicates a role for ROS in the activation of TLR pathways (52–54) and NALP3 inflammasome (55–57).
Besides ROS, increased ATP levels in in-vitro, in-vivo models and preclinical smoke-exposure models have been reported (58, 59). ATP can activate the NLRP3 Inflammasome through the P2X7 receptor (60–62) and, in addition, regulate neutrophil chemotaxis and activation through P2Y receptors (63, 64).
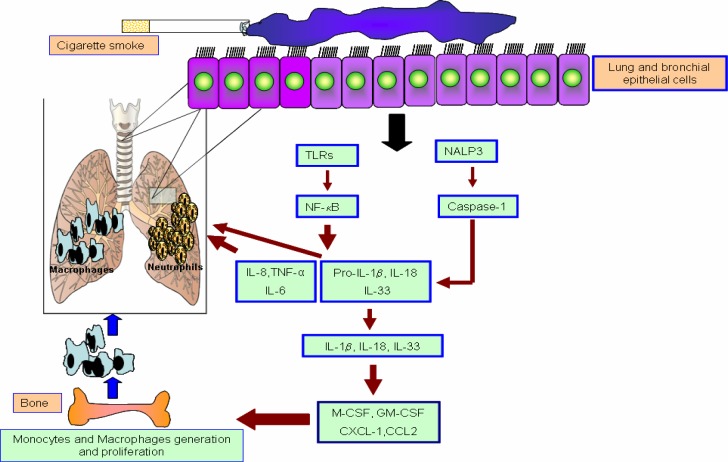
Finally, the expression of the NLRP3 Inflammasome-associated cytokines IL-1β and IL-18 is increased in the lungs of COPD patients and animals exposed to cigarette smoke (65, 66). IL-1β levels are increased in airway secretions during COPD exacerbations (66) and correlate significantly with disease severity and other inflammatory mediators such as TNF-α and IL-8 (67). Furthermore, IL-1β can induce the release of M-CSF and GM-CSF from inflammatory cells (68) which in turn potentiates induction of chronic inflammatory diseases (Figure 1).
Figure 1.
Role of inflammasome and TLRs signaling in pathogenesis of COPD. An overview of the signalling cascade associated with the NLRP3 Inflammasome and TLRs in pathogenesis of COPD. The Interactions between Epithelial cells (EP) and Dendritic cells (DC) in the airways and lungs when they exposed to allergen or cigarette smoke. DCs sample the airway lumen by forming dendritic extensions in between epithelial cells.
GENERAL CONCLUSIONS
Taken together, we conclude that a key process in the pathogenesis of COPD involves ROS/ATP-mediated activation of the NALP3 inflammasome and TLRs leading to prolonged, inflammatory responses.
Abbreviations
ATP, adenosine triphosphate
COPD, Chronic Obstructive Pulmonary Disease
CS, Cigarette smoke
IgE, Immunoglobulin E
IL-1β, Interleukin 1 beta
IL-18, Interleukin 18
NLR, Nod-like receptor
NLRP3, NACHT, LRR and PYD domains-containing protein 3
ROS, reactive oxygen species
TLR, Toll-like receptor
REFERENCES
- 1.WHO. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach; World Health Organisation; 2007. ISBN 978 92 4 156346 8. [Google Scholar]
- 2.Stockley RA, Mannino D, Barnes PJ. Burden and pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6(6):524–6. doi: 10.1513/pats.200904-016DS. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ, Kleinert S. COPD--a neglected disease. Lancet. 2004;364(9434):564–5. doi: 10.1016/S0140-6736(04)16866-9. [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56(4):515–48. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22(4):672–88. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro SD. Evolving concepts in the pathogenesis of chronic obstructive pulmonary disease. Clin Chest Med. 2000;21(4):621–32. doi: 10.1016/s0272-5231(05)70172-6. [DOI] [PubMed] [Google Scholar]
- 7.Barnes PJ. Chronic obstructive pulmonary disease: effects beyond the lungs. PLoS Med. 2010;7(3):e1000220. doi: 10.1371/journal.pmed.1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, Feng S, van Heemst J, McAdam KG. New insights into the formation of volatile compounds in mainstream cigarette smoke. Anal Bioanal Chem. 2010;396(5):1817–30. doi: 10.1007/s00216-010-3457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scian MJ, Oldham MJ, Miller JH, Kane DB, Edmiston JS, McKinney WJ. Chemical analysis of cigarette smoke particulate generated in the MSB-01 in vitro whole smoke exposure system. Inhal Toxicol. 2009;21(12):1040–52. doi: 10.1080/08958370802712705. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi Y, Kagota S, Haginaka J, Kunitomo M. Peroxynitrite-generating species: good candidate oxidants in aqueous extracts of cigarette smoke. Jpn J Pharmacol. 2000;82(1):78–81. doi: 10.1254/jjp.82.78. [DOI] [PubMed] [Google Scholar]
- 11.Valenca SS, Castro P, Pimenta WA, Lanzetti M, Silva SV, Barja-Fidalgo C, et al. Light cigarette smoke-induced emphysema and NFkappaB activation in mouse lung. Int J Exp Pathol. 2006;87(5):373–81. doi: 10.1111/j.1365-2613.2006.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan FM, Maini RN, Feldmann M. Cytokine expression in chronic inflammatory disease. Br Med Bull. 1995;51(2):368–84. doi: 10.1093/oxfordjournals.bmb.a072967. [DOI] [PubMed] [Google Scholar]
- 13.Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000;16(3):534–54. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- 14.Barnes PJ. The cytokine network in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2009;41(6):631–8. doi: 10.1165/rcmb.2009-0220TR. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev. 2007;87(3):1047–82. doi: 10.1152/physrev.00048.2006. [DOI] [PubMed] [Google Scholar]
- 16.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8(3):183–92. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 17.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153(2):530–4. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 18.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22(4):672–88. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 19.Barczyk A, Pierzchala W, Kon OM, Cosio B, Adcock IM, Barnes PJ. Cytokine production by bronchoalveolar lavage T lymphocytes in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2006;117(6):1484–92. doi: 10.1016/j.jaci.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura H, Yoshimura K, Jaffe HA, Crystal RG. Interleukin-8 gene expression in human bronchial epithelial cells. J Biol Chem. 1991;266(29):19611–7. [PubMed] [Google Scholar]
- 21.DeForge LE, Preston AM, Takeuchi E, Kenney J, Boxer LA, Remick DG. Regulation of interleukin 8 gene expression by oxidant stress. J Biol Chem. 1993;268(34):25568–76. [PubMed] [Google Scholar]
- 22.Kwon OJ, Au BT, Collins PD, Adcock IM, Mak JC, Robbins RR, et al. Tumor necrosis factor-induced interleukin-8 expression in cultured human airway epithelial cells. Am J Physiol. 1994;267(4 Pt 1):L398–405. doi: 10.1152/ajplung.1994.267.4.L398. [DOI] [PubMed] [Google Scholar]
- 23.Johnston SL, Papi A, Bates PJ, Mastronarde JG, Monick MM, Hunninghake GW. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J Immunol. 1998;160(12):6172–81. [PubMed] [Google Scholar]
- 24.Barnes PJ. Alveolar macrophages in chronic obstructive pulmonary disease (COPD) Cell Mol Biol (Noisy-le-grand) 2004;50 Online Pub: OL627-37. [PubMed] [Google Scholar]
- 25.Cosio MG, Majo J, Cosio MG. Inflammation of the airways and lung parenchyma in COPD: role of T cells. Chest. 2002;121(5 Suppl):160S–165S. doi: 10.1378/chest.121.5_suppl.160s. [DOI] [PubMed] [Google Scholar]
- 26.Barnes PJ. Medicine. Neutrophils find smoke attractive. Science. 2010;330(6000):40–1. doi: 10.1126/science.1196017. [DOI] [PubMed] [Google Scholar]
- 27.Guo RF, Ward PA. Mediators and regulation of neutrophil accumulation in inflammatory responses in lung: insights from the IgG immune complex model. Free Radic Biol Med. 2002;33(3):303–10. doi: 10.1016/s0891-5849(02)00823-7. [DOI] [PubMed] [Google Scholar]
- 28.Laskin DL, Kimura T, Sakakibara S, Riley DJ, Berg RA. Chemotactic activity of collagen-like polypeptides for human peripheral blood neutrophils. J Leukoc Biol. 1986;39(3):255–66. doi: 10.1002/jlb.39.3.255. [DOI] [PubMed] [Google Scholar]
- 29.Pfister RR, Haddox JL, Sommers CI, Lam KW. Identification and synthesis of chemotactic tripeptides from alkali-degraded whole cornea. A study of N-acetyl-proline-glycine-proline and N-methyl-proline-glycine-proline. Invest Ophthalmol Vis Sci. 1995;36(7):1306–16. [PubMed] [Google Scholar]
- 30.O'Reilly P, Jackson PL, Noerager B, Parker S, Dransfield M, Gaggar A, et al. N-alpha-PGP and PGP, potential biomarkers and therapeutic targets for COPD. Respir Res. 2009;10:38. doi: 10.1186/1465-9921-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, et al. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12(3):317–23. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 32.Gaggar A, Jackson PL, Noerager BD, O'Reilly PJ, McQuaid DB, Rowe SM, et al. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180(8):5662–9. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Overbeek SA, Henricks PA, Srienc AI, Koelink PJ, de Kruijf P, Lim HD, et al. N-acetylated Proline-Glycine-Proline induced G-protein dependent chemotaxis of neutrophils is independent of CXCL8 release. Eur J Pharmacol. 2011 doi: 10.1016/j.ejphar.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braber S, Koelink PJ, Henricks PA, Jackson PL, Nijkamp FP, Garssen J, et al. Cigarette smoke-induced lung emphysema in mice is associated with prolyl endopeptidase, an enzyme involved in collagen breakdown. Am J Physiol Lung Cell Mol Physiol. 2011;300(2):L255–65. doi: 10.1152/ajplung.00304.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agustí A, MacNee W, Donaldson K, Cosio M. Hypothesis: does COPD have an autoimmune component? Thorax. 2003;58(10):832–4. doi: 10.1136/thorax.58.10.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179(11):7791–9. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 37.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 38.Doe C, Bafadhel M, Siddiqui S, Desai D, Mistry V, Rugman P, et al. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest. 2010;138(5):1140–7. doi: 10.1378/chest.09-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eustace A, Smyth LJ, Mitchell L, Williamson K, Plumb J, Singh D. Identification of cells expressing IL-17A and IL-17F in the lungs of patients with COPD. Chest. 2011;139(5):1089–100. doi: 10.1378/chest.10-0779. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest. 2006;116(11):3050–9. doi: 10.1172/JCI28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mortaz E, Henricks PA, Kraneveld AD, Givi ME, Garssen J, Folkerts G. Cigarette smoke induces the release of CXCL-8 from human bronchial epithelial cells via TLRs and induction of the inflammasome. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbadis.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Pons J, Sauleda J, Regueiro V, Santos C, López M, Ferrer J, et al. Expression of Toll-like receptor 2 is up-regulated in monocytes from patients with chronic obstructive pulmonary disease. Respir Res. 2006;7:64. doi: 10.1186/1465-9921-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birrell MA, Eltom S. The role of the NLRP3 Inflammasome in the pathogenesis of airway disease. Pharmacol Ther. 2011;130(3):364–70. doi: 10.1016/j.pharmthera.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Rahman I, MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. Am J Physiol. 1999;277(6 Pt 1):L1067–88. doi: 10.1152/ajplung.1999.277.6.L1067. [DOI] [PubMed] [Google Scholar]
- 45.Edeas M, Attaf D, Mailfert AS, Nasu M, Joubet R. Maillard reaction, mitochondria and oxidative stress: potential role of antioxidants. Pathol Biol (Paris) 2010;58(3):220–5. doi: 10.1016/j.patbio.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Rahman I, MacNee W. Role of transcription factors in inflammatory lung diseases. Thorax. 1998;53(7):601–12. doi: 10.1136/thx.53.7.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vachier I, Chanez P, Le Doucen C, Damon M, Descomps B, Godard P. Enhancement of reactive oxygen species formation in stable and unstable asthmatic patients. Eur Respir J. 1994;7(9):1585–92. doi: 10.1183/09031936.94.07091585. [DOI] [PubMed] [Google Scholar]
- 48.Henricks PA, Nijkamp FP. Reactive oxygen species as mediators in asthma. Pulm Pharmacol Ther. 2001;14(6):409–20. doi: 10.1006/pupt.2001.0319. [DOI] [PubMed] [Google Scholar]
- 49.Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic Biol Med. 2003;35(3):213–25. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 50.Loukides S, Bakakos P, Kostikas K. Oxidative stress in patients with COPD. Curr Drug Targets. 2011;12(4):469–77. doi: 10.2174/138945011794751573. [DOI] [PubMed] [Google Scholar]
- 51.Langen RC, Korn SH, Wouters EF. ROS in the local and systemic pathogenesis of COPD. Free Radic Biol Med. 2003;35(3):226–35. doi: 10.1016/s0891-5849(03)00316-2. [DOI] [PubMed] [Google Scholar]
- 52.Rahman I. The role of oxidative stress in the pathogenesis of COPD: implications for therapy. Treat Respir Med. 2005;4(3):175–200. doi: 10.2165/00151829-200504030-00003. [DOI] [PubMed] [Google Scholar]
- 53.Xiang M, Fan J, Fan J. Association of Toll-like receptor signaling and reactive oxygen species: a potential therapeutic target for posttrauma acute lung injury. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/916425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen K, Huang J, Gong W, Iribarren P, Dunlop NM, Wang JM. Toll-like receptors in inflammation, infection and cancer. Int Immunopharmacol. 2007;7(10):1271–85. doi: 10.1016/j.intimp.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 55.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–49. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40(3):616–9. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- 57.Roland L, Gagné A, Bélanger MC, Boutet M, Julien P, Bilodeau JF. Plasma interleukin-18 (IL-18) levels are correlated with antioxidant vitamin coenzyme Q(10) in preeclampsia. Acta Obstet Gynecol Scand. 2010;89(3):360–6. doi: 10.3109/00016340903576020. [DOI] [PubMed] [Google Scholar]
- 58.Mortaz E, Kraneveld AD, Smit JJ, Kool M, Lambrecht BN, Kunkel SL, et al. Effect of cigarette smoke extract on dendritic cells and their impact on T-cell proliferation. PLoS One. 2009;4(3):e4946. doi: 10.1371/journal.pone.0004946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lucattelli M, Cicko S, Müller T, Lommatzsch M, De Cunto G, Cardini S, et al. P2X7 receptor signaling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am J Respir Cell Mol Biol. 2011;44(3):423–9. doi: 10.1165/rcmb.2010-0038OC. [DOI] [PubMed] [Google Scholar]
- 60.Mortaz E, Braber S, Nazary M, Givi ME, Nijkamp FP, Folkerts G. ATP in the pathogenesis of lung emphysema. Eur J Pharmacol. 2009;619(1-3):92–6. doi: 10.1016/j.ejphar.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 61.Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol. 2010;22(1):28–33. doi: 10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wewers MD, Sarkar A. P2X(7) receptor and macrophage function. Purinergic Signal. 2009;5(2):189–95. doi: 10.1007/s11302-009-9131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mortaz E, Folkerts G, Nijkamp FP, Henricks PA. ATP and the pathogenesis of COPD. Eur J Pharmacol. 2010;638(1-3):1–4. doi: 10.1016/j.ejphar.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 64.Kukulski F, Ben Yebdri F, Lecka J, Kauffenstein G, Lévesque SA, Martín-Satué M, et al. Extracellular ATP and P2 receptors are required for IL-8 to induce neutrophil migration. Cytokine. 2009;46(2):166–70. doi: 10.1016/j.cyto.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang MJ, Homer RJ, Gallo A, Lee CG, Crothers KA, Cho SJ, et al. IL-18 is induced and IL-18 receptor alpha plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. J Immunol. 2007;178(3):1948–59. doi: 10.4049/jimmunol.178.3.1948. [DOI] [PubMed] [Google Scholar]
- 66.Pauwels NS, Bracke KR, Dupont LL, Van Pottelberge GR, Provoost S, Berghe TV, et al. Role of IL-1{alpha} and the Nlrp3/caspase-1/IL-1{beta} axis in cigarette smoke-induced pulmonary inflammation and COPD. Eur Respir J. 2011 doi: 10.1183/09031936.00158110. [DOI] [PubMed] [Google Scholar]
- 67.Fujita M, Shannon JM, Irvin CG, Fagan KA, Cool C, Augustin A, et al. Overexpression of tumor necrosis factor-alpha produces an increase in lung volumes and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2001;280(1):L39–49. doi: 10.1152/ajplung.2001.280.1.L39. [DOI] [PubMed] [Google Scholar]
- 68.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]