Abstract
The present review discusses the role of tri-peptide Proline –Glycine –Proline (PGP) as a potential player, biomarker and therapeutic target in this process.
Keywords: Chemotactic factors, Cystic fibrosis, Chronic obstructive pulmonary disease, Extracellular matrix, Neutrophil activation praline, Interleukin-8 A and B, Serine ednopeptidases
INTRODUCTION
Respiratory diseases such as COPD, CF and asthma are leading causes of mortality by non-communicable diseases, with 4.2 million deaths in 2008 (1). The most important environmental risk factor for COPD is cigarette smoke, responsible for more than 50% of cases worldwide (2). Mutations in the Cystic fibrosis trans membrane conductance regulator (CFTR) gene are the cause of thick mucus in CF which subsequently leads to respiratory failure (3). To treat and possibly prevent these diseases most effectively, it is essential to be able to assess the patient's disease stage, evaluate response to therapy and define their prognosis. Biomarkers can be a powerful means of diagnosing COPD and CF at an early stage, thereby possibly minimizing lung damage. The search for biomarkers with both high sensitivity and selectivity has therefore received considerable interest (4).
Respiratory manifestations of COPD and especially CF include airway inflammation with neutrophils, also known as polymorphonuclear leukocytes (PMNs), as major players (2, 5). Influx of these neutrophils is caused by the production of chemoattractants, some of which are in turn produced by neutrophils themselves causing infiltration of even more neutrophils and chronic inflammation. Recently, the tri-peptide Pro-Gly-Pro (PGP) has been proposed as a potential player, biomarker and therapeutic target in this process (6).
CXCL8 and the generation and effect of PGP
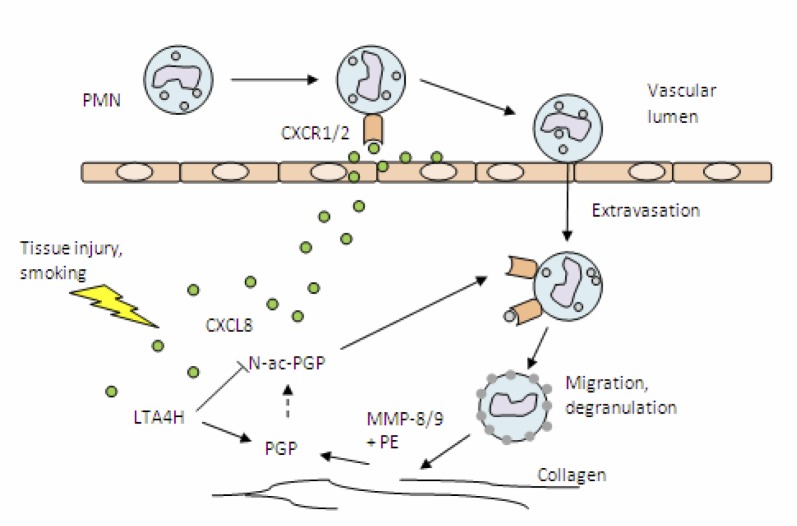
During inflammation, several factors are produced by various cell types in response to stress, such as chemokines and cytokines. In the early phase of inflammation these mediators attract primarily neutrophils. These neutrophils are the hallmark of acute inflammation and one of the first cellular lines in host defense against injury and infection (7). The chemokine CXCL8 (previously known as IL-8) has been identified as a key player in the recruitment of neutrophils (8). CXCL8 is one of the best-characterized members of the chemokine family. Production of CXCL8 is not restricted to leukocytes, such as neutrophils and macrophages, and it can also be synthesized by endothelial and epithelial cells and fibroblasts (9). After secretion at the site of production, CXCL8 diffuses through the tissue and can be transcytosed by endothelial cells to their luminal surface, where it is displayed on cell-surface glycosaminoglycans (GAGs). Chemokines also adhere to the extracellular matrix (ECM) and establish a concentration gradient. The GAG-bound CXCL8 can activate CXCR1 and CXCR2, specific G-protein coupled receptors, on travelling leukocytes. This activation induces extravasation of neutrophils and subsequent migration, dependent on CXCL8 concentration gradients (8). During extravasation and migration, secretory vesicles are partially exocytosed and matrix-degrading enzymes, such as matrix metalloproteases (MMPs), are released (Figure 1) (10). Chemokines bound to the ECM are released allowing further diffusion through the tissue and intensifying the chemotactic response (11). MMP-8 and MMP-9, both produced by neutrophils, digest collagen which is an important component of the ECM (12). Peptide fragments produced in this process function as a substrate for prolylendopeptidase (PE), an 80kDa serine protease present in the brain, serum and other tissues and is implicated in neurological disease (13). PE forms the tri-peptide PGP, which is a potent chemoattractant. Moreover, acetylation of PGP to N-ac-PGP dramatically increases its chemoattractant activity (14). Pfister et al, first noticed the chemotactic enhancing property of alkali-degraded cornea and in 1995 identified N-ac-PGP to be primarily responsible for this action (15, 16).
Figure 1.
Neutrophil influx and degranulation cause production of potent chemoattractants PGP and N-ac-PGP.
After the occurrence of tissue injury, chemokines are produced attracting leukocytes, such as PMNs. These PMNs secrete enzymes capable of breaking down collagen in the ECM, thereby producing PGP and N-ac-PGP.
A subsequent experiment, where N-ac-PGP was injected in normal rabbit corneas caused a rapid and severe neutrophil infiltration, confirming this property in-vivo (17). The chemoattractive effect of N-ac-PGP is thought to be caused by agonism of CXCR1 and -2, possibly a result of the occurrence of the sequence PGP in several chemokines or the structurally highly similar SGP in CXCL8 (13). Overbeek et al. showed that the effect of N-ac-PGP is independent of CXCL8 release (18). In their study, incubation of freshly isolated neutrophils with α-CXCL8 did not diminish chemotaxis induced by N-ac-PGP, while α-CXCL8 blocked CXCL8-induced chemotaxis. In addition, N-ac-PGP promoted CXCL8 release in a time- and dose-dependent manner, a CXCR2 antagonist attenuated chemotaxis demonstrating interaction of N-ac-PGP with CXCR2 (18). Gaggar et al. showed that neither administration of MMP-8, -9 nor PE alone into mouse airways did produce PGP to any significant amount, showing that MMP-8 or -9 in combination with PE is necessary for PGP generation (12). PE is synthesized by neutrophils themselves, indicating that these cells possess all enzymes needed to produce PGP effectively. This is confirmed by the large amount of both PGP and N-ac-PGP generated by human peripheral blood neutrophils incubated with collagen and lipopolysaccharide (LPS) (19).
Accumulation of PGP in the lungs is prevented in acute neutrophil-driven inflammation, facilitating the resolution of inflammation. This is due to Leukotriene A4 hydrolase (LTA4H), an enzyme known for producing proinflammatory mediator leukotriene B4. Recently, PGP has been identified as a physiological substrate for LTA4H's aminopeptidase activity, with previously unknown substrate and physiological importance (20). Although LTA4H has a potent capacity to degrade PGP, the more chemotactic N-ac-PGP is insensitive for this activity. The mechanism through which acetylation occurs is still unknown, but cigarette smoke condensate can N-terminally acetylate PGP. Consequently, cigarette smoking, a major risk factor in developing COPD, could enhance PGP acetylation, protecting it from degradation. In addition, cigarette smoke condensate is also an inhibitor for LTA4H, thus reducing degradation of nonacetylated PGP (20).
PGP as a biomarker
As PGP and N-ac-PGP are produced in considerable amounts during inflammation due to collagen degradation, it stands to reason this may also happen in chronic inflammation in COPD and CF. Local concentrations of PGP and N-ac-PGP can then be used as biomarkers of disease and/or disease stage. Direct measurement of local concentrations in the airways may however be unreasonably difficult or uncomfortable. O'Reilly et al, therefore explored if measurement of N-ac-PGP and PGP in sputum is an effective method (6). Concentrations were determined in samples from COPD and asthma patients compared to healthy controls. None of the controls or asthma patients showed N-ac-PGP in sputum above the detection limit of 10 pg/mL, while 13 out of 16 COPD patients did. All COPD sputum samples contained PGP, while in healthy controls only 3 of 10 had increased PGP levels. O'Reilly et al. did not report on PGP levels in sputum from asthma patients, but PGP levels in COPD samples were significantly higher than controls. In the same study, the potential of serum PGP levels as biomarker was explored (6). PGP serum levels were shown to be significantly higher in COPD patients compared to healthy controls. Interestingly, serum samples from patients were also able to generate PGP ex-vivo when incubated with collagen. It would be interesting to determine whether and to what extent PGP levels in sputum of non-COPD smoking individuals have a prognostic value. Weathington et al. explored the use of BAL fluid for the same purpose (14). Although the COPD group only consisted of 5 patients, the detected PGP concentrations in BAL were significantly higher in the COPD patients (363 pg/mL) than control subjects (22 pg/mL). It should be noted however that 2 of 18 control subjects showed PGP levels slightly above the detection limit, while in the COPD patients in 2 of 5 samples PGP concentrations were below the detection limit (14).
Similar findings were reported for CF patients (12). Sputum samples collected within 48h of admission from patients treated in the hospital for CF exacerbation showed a significantly higher level of PGP compared to healthy controls. At discharge the mean PGP level had lowered considerably, but still was 5-fold higher compared to controls (12).
Considering all the above, higher levels of PGP and/or N-ac-PGP in sputum or BAL fluid are indicative of chronic inflammation. However, these levels seem to be specific for COPD and CF patients, since they are not significantly elevated in asthmatics. PGP levels in sputum from CF patients are associated with severity of symptoms. Whether such association exists for COPD patients, needs further studies. In conclusion, to prove the usefulness of PGP levels for staging disease progress and treatment effectiveness, additional large-scale trials are necessary. In addition, cohort studies that follow-up symptom-free smoking individuals could provide insight into the usefulness of assessing PGP levels in sputum for early diagnosis.
REFERENCES
- 1.World Health Organization. Burden: Mortality, morbidity and risk factors; 2011. Global status report on noncommunicable diseases2010; pp. 9–31. Chapter 1. [Google Scholar]
- 2.Sin D, van Eeden SF. Neutrophil-mediated lung damage: a new COPD phenotype? Respiration. 2012;83(2):103–5. doi: 10.1159/000334178. [DOI] [PubMed] [Google Scholar]
- 3.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352(19):1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 4.O'Reilly P, Bailey W. Clinical use of exhaled biomarkers in COPD. Int J Chron Obstruct Pulmon Dis. 2007;2(4):403–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Conese M, Copreni E, Di Gioia S, De Rinaldis P, Fumarulo R. Neutrophil recruitment and airway epithelial cell involvement in chronic cystic fibrosis lung disease. J Cyst Fibros. 2003;2(3):129–35. doi: 10.1016/S1569-1993(03)00063-8. [DOI] [PubMed] [Google Scholar]
- 6.O'Reilly P, Jackson PL, Noerager B, Parker S, Dransfield M, Gaggar A, Blalock JE. N-alpha-PGP and PGP, potential biomarkers and therapeutic targets for COPD. Respir Res. 2009;10:38. doi: 10.1186/1465-9921-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56(5):559–64. [PubMed] [Google Scholar]
- 8.Das ST, Rajagopalan L, Guerrero-Plata A, Sai J, Richmond A, Garofalo RP, Rajarathnam K. Monomeric and dimeric CXCL8 are both essential for in vivo neutrophil recruitment. PLoS One. 2010;5(7):e11754. doi: 10.1371/journal.pone.0011754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukaida N. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol. 2003;284(4):L566–77. doi: 10.1152/ajplung.00233.2002. [DOI] [PubMed] [Google Scholar]
- 10.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5(14):1317–27. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Smit JJ, Lukacs NW. The missing link: chemokine receptors and tissue matrix breakdown in COPD. Trends Pharmacol Sci. 2006;27(11):555–7. doi: 10.1016/j.tips.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Gaggar A, Jackson PL, Noerager BD, O'Reilly PJ, McQuaid DB, Rowe SM, et al. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180(8):5662–9. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morain P, Lestage P, De Nanteuil G, Jochemsen R, Robin JL, Guez D, et al. S 17092: a prolyl endopeptidase inhibitor as a potential therapeutic drug for memory impairment. Preclinical and clinical studies. CNS Drug Rev. 2002;8(1):31–52. doi: 10.1111/j.1527-3458.2002.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, et al. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12(3):317–23. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 15.Pfister RR, Haddox JL, Sommers CI. Alkali-degraded cornea generates a low molecular weight chemoattractant for polymorphonuclear leukocytes. Invest Ophthalmol Vis Sci. 1993;34(7):2297–304. [PubMed] [Google Scholar]
- 16.Pfister RR, Haddox JL, Sommers CI, Lam KW. Identification and synthesis of chemotactic tripeptides from alkali-degraded whole cornea. A study of N-acetyl-proline-glycine-proline and N-methyl-proline-glycine-proline. Invest Ophthalmol Vis Sci. 1995;36(7):1306–16. [PubMed] [Google Scholar]
- 17.Pfister RR, Haddox JL, Sommers CI. Injection of chemoattractants into normal cornea: a model of inflammation after alkali injury. Invest Ophthalmol Vis Sci. 1998;39(9):1744–50. [PubMed] [Google Scholar]
- 18.Overbeek SA, Henricks PA, Srienc AI, Koelink PJ, de Kruijf P, Lim HD, et al. N-acetylated Proline-Glycine-Proline induced G-protein dependent chemotaxis of neutrophils is independent of CXCL8 release. Eur J Pharmacol. 2011;668(3):428–34. doi: 10.1016/j.ejphar.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Reilly PJ, Hardison MT, Jackson PL, Xu X, Snelgrove RJ, Gaggar A, et al. Neutrophils contain prolyl endopeptidase and generate the chemotactic peptide, PGP, from collagen. J Neuroimmunol. 2009;217(1-2):51–4. doi: 10.1016/j.jneuroim.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snelgrove RJ, Jackson PL, Hardison MT, Noerager BD, Kinloch A, Gaggar A, et al. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science. 2010;330(6000):90–4. doi: 10.1126/science.1190594. [DOI] [PMC free article] [PubMed] [Google Scholar]