Abstract
Pulmonary actinomycosis is a rare chronic pulmonary infection caused by actinomyces, a Gram–positive, microaerophilic bacterium. Pulmonary involvement other than cervicofacial or abdominopelvic actinomycosis is uncommon and often leads to a misdiagnosis of pulmonary tuberculosis or lung cancer. Endobronchial involvement is very rare in pulmonary actinomycosis. Here in, we describe the case of a 66–year–old male patient, referred with a history of massive hemoptysis since a few weeks ago. Plain chest radiograph and computerized tomographic scan revealed a dens consolidation in the right upper lobe; which was confirmed to be pulmonary actinomycosis with endobronchial involvement by transbronchial biopsy.
Keywords: Actinomycosis, Pulmonary, Sulphur granules
INTRODUCTION
Pulmonary actinomycosis is a rare but important condition. The causative microorganisms are found in the mouth and gastrointestinal tract, where they are usually harmless. Poor dental hygiene and dental abscess can predispose people to facial lesions and lung infections caused by this microorganism (1). Actinomycosis of the lungs causes cavities, lung nodules, peripheral consolidations, and pleural effusion (2). Although pulmonary actinomycosis is rare in the general population, it may be suspected when a middle-aged male patient presents with hemoptysis and cough together with the radiologic findings of a peripheral mass or chronic consolidation.
Even when the clinical suspicion is high, the disease is commonly confused with other chronic suppurative lung diseases and malignancies (2, 3). Sulphur granules are the pathological hallmark of actinomycosis. The objective of therapy is to control the infection; but response to treatment is usually slow (1). Pulmonary endobronchial actinomycosis is a very rare condition but should be considered in the differential diagnosis of air space consolidation or pulmonary mass. An early, accurate diagnosis will prevent the significant psychological and physical morbidity, including delayed diagnosis or unwarranted thoracic surgery (4).
We report a case of endobronchial pulmonary actinomycosis diagnosed by means of fiberoptic bronchoscopy and transbronchial biopsy. The purpose of reporting this case is its rare occurrence. The interesting finding in our patient was the rare endobronchial involvement by pulmonary actinomycosis, located in the right upper lobe. A discussion of the relevant literature is also presented.
Although endobronchial actinomycosis is not a common endobronchial lesion, it is included in the differential diagnosis of endobronchial especially bronchogenic carcinoma and endobronchial tuberculosis. In areas with high incidence of tuberculosis like Iran endobronchial actinomycosis can mimic its symptoms.
CASE SUMMARY
This is a case report of endobronchial actinomycosis. A 66 year–old previously healthy man was referred to Imam Reza-Hospital, Mashhad with a two-week history of mild cough and hemoptysis. The patient also reported a low-grade fever during a period of 3-4 months. He had never smoked. There was no history of recurrent infection or other evidence of immunodeficiency. On physical examination, he had poor dental hygiene and associated dental disease with a periapical abscess. Chest examination revealed crackles with mild expiratory wheezing over the right upper and middle lobes. His oral temperature was 37.8°C and he appeared ill. He had a blood pressure of 140/90 mmHg, a respiratory rate of 24 breaths/min, and a pulse rate of 78 beats/min. Laboratory values at admission included a white blood cell count of 9200Ul, a hemoglobin value of 12 g/Dl, and a platelet count of 240.000uLl. Differential cell count revealed 62% neutrophils, 30% lymphocytes, 6% monocytes and 2% eosinophils.
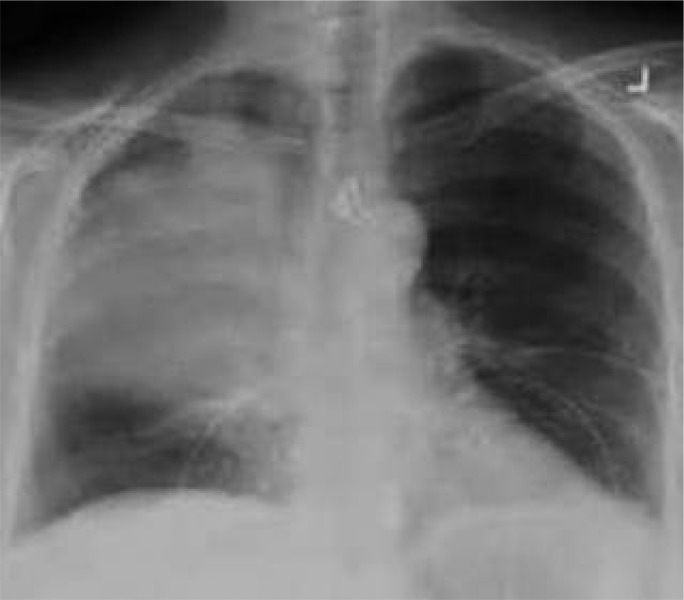
Plain chest radiograph at the first visit revealed a dense consolidation in the right lung. There was no other abnormality on the chest radiograph (Figure 1). Thoracic computed tomography (CT) scan showed air space consolidation in the right upper lobe (Figure 2). There was also evidence of mild peribronchial thickening at the right upper lobe bronchus. CT scan was negative for mediastinal lymphadenopathy, pleural effusion or chest wall abnormality. The left lung was normal.
Figure 1.
Chest X ray displaying consolidation in the right upper lobe.
Figure 2.
Axial CT scan in lung window setting showing air space consolidation with air bronchogram in the right upper lobe.
Sputum examination was also carried out. Gram staining showed many Gram-positive cocci, but AFB smear was negative.
Fiberoptic bronchoscopy showed partial occlusion of the right upper lobe bronchus by an irregular, yellow-white, granular thickening that was surrounded by inflamed and edematous bronchial mucosa. Biopsy was performed. Giemsa and Gram staining of the specimen and cytology of bronchial washing were performed.
Microscopic examination of the specimens obtained by bronchoalveolar lavage (BAL) and bronchial suctioning showed neutrophilic inflammation (BAL differential: 72% macrophages, 20% neutrophils, 1.5% eosinophils and 6.5% lymphocytes). Quantitative cultures of BAL showed mixed throat flora (including aerobic cultures). Fungal and mycobacterial smears were negative.
Histological examination of the biopsy specimen revealed inflammatory cellular infiltration and large colonies of Actinomyces SPP (Sulphur granules) surrounded by necrotic tissue and inflammatory cells (Figure 3). The colonies contained a radiating network of filaments intensely stained with hematoxylin. Gram-staining also demonstrated a thin, filamentous, Gram-positive branching organism.
Figure 3.
Histological examination of the specimen demonstrating a filamentous microorganism with sulphur granules indicating actinomycosis (Giemsa staining).
Bioptic material was cultured as well and Actinomyces SPP was identified. Further classification was not carried out due to insufficient growth on repeated subcultures.
The histological pattern was compatible with the diagnosis of endobronchial actinomycosis. Medical treatment was initiated with 1.5 million units of penicillin intravenously every six hours for two weeks, followed by oral ampicillin four times a day for 12 months. Reduction in the pulmonary opacity was seen on chest X ray within three months. Follow up of the patient, six months later, showed no abnormality on the chest X ray and thoracic CT, and the patient was completely free of symptoms or any clinical signs. At the end of medical treatment a fiberoptic bronchoscopic examination was performed which did not show any abnormality.
DISCUSSION
Pulmonary actinomycosis is a bacterial lung infection caused by actinomycosis or propionibacteria. Actinomyces SPP are higher prokaryotic bacteria belonging to the family Actinomycetaceae (1). The first published clinical description of the human form of the disease appeared in 1857. The thoracic form was described 25 years later; but it was not until 1897 that Actinomycosis israelii, the main species responsible for the human disease, was isolated (1).
The infection can involve any organ or body site. Pulmonary actinomycosis constitutes 15% of the total burden of disease, although estimates up to 50% have been reported (1, 5). It is now a rare infection, particularly in the developed world. The presentation of pulmonary actinomycosis has also changed ant it now appears less aggressive in nature compared with the pre-antibiotic era (5, 6).
Pulmonary actinomycosis occurs at all ages. A bimodal age distribution with an earlier peak at ages 11 – 20 has been described, but most series describe a clear peak incidence in the 4th and 5th decades of life. The incidence of infection is two to four times greater in males compared with females. A higher incidence of pulmonary actinomycosis has also been reported in patients with poor oral hygiene, underlying respiratory disorders, such as empyema, chronic bronchitis, bronchiectasis, and in alcoholics. Our patient was a 66 year-old man with very poor oral hygiene and dental disease. Pulmonary actinomycosis probably results from the aspiration of oropharyngeal or gastrointestinal secretions into the respiratory tract (1, 2, 7). Infection as a result of distant hematogenous seeding, lymphatic spread or spread from the neck through the mediastinum is very rare (8).
Pulmonary actinomycosis probably starts with an acute inflammation followed by the characteristic chronic, indolent phase that generates fibrosis, local necrosis and pulmonary cavities (1, 7). The disease usually progresses slowly and if left untreated, the infection may invade the pleura, chest wall, soft tissues and bony structures. Sinus tracts may also form, opening and closing spontaneously (8, 9). The disease symptoms are still quite nonspecific and similar to those of other chronic suppurative chest diseases and malignancies. Shortness of breath, lethargy, weight loss, fever, cough with sputum, night sweats, chest pain and hemoptysis are the main symptoms. Marked weight loss, malaise and high fever may be more suggestive of disseminated disease (1, 3). In a previous review of thoracic actinomycosis in five health regions in the UK, the three commonest complaints were cough, sputum and chest pain (1). Physical signs are nonspecific, expect in advanced, untreated disease, when sinus and fistula formation may reveal the diagnosis (8). Our patient presented with a massive hemoptysis and he reported a low-grade fever during a period of 1-4 months. On physical examination, there was no abnormality expect for poor oral hygiene and dental disease. When the disease does occur in HIV/AIDS patients, its clinical presentation appears similar in pattern to that in immunocompetent people and it appears to respond to the conventional treatment regimens (10, 11).
Basic laboratory tests reflect the nonspecific nature of this chronic disease.
Imaging modalities are not diagnostic. Irrespective of the imaging modality, a few general principles apply. First, the radiological findings depend on the duration of the disease; secondly, the disease usually shows a lower lobe and peripheral predominance, probably reflecting the role of aspiration in its pathogenesis. Finally, the infection usually shows reduction in size within 4 weeks of starting treatment (1, 12).
Plain chest radiographic findings are nonspecific and can resemble those of other benign pulmonary infections, lung carcinoma, and metastatic tumor. A non-segmental pneumonia, usually in the lower zone, tends to occur peripherally crossing fissures. However, the spectrum of radiographic changes may be wide from pulmonary infiltrate to cavitating mass lesions involving the pleura and chest wall (13). The main problem is distinguishing the disease from lung carcinoma on the chest radiograph.
A range of CT findings have been reported in pulmonary actinomycosis, including patchy air space consolidation, multifocal nodular appearances, solitary pulmonary mass lesion, cavitation, pleural thickening, pleural effusions and hilar, and/or mediastinal lymphadenopathy. Consolidation with involvement of the adjacent pleura and chest wall, and pulmonary infiltrates with air bronchogram or so–called “air sign” may be more suggestive of pulmonary actinomycosis (14). CT is probably more helpful than plain chest radiography particularly in evaluation of bony changes. Pulmonary actinomycosis may mimic a malignant tumor in both plain chest radiographs and CT scans (15). Associated pleural effusion tends to be small or moderate rather than massive. Occasionally, pericardial effusion may result from pericardial involvement (16). Jae described a peripherally located mass or nodule and consolidation as the main radiological features in their study (3). There is limited information on magnetic resonance imaging (MRI) findings in pulmonary actinomycosis. Part of the reason may be the attendant problems associated with imaging the chest using MRI (17).
Fiberoptic bronchoscopy is usually not diagnostic unless there is endobronchial actinomycosis on which biopsy can be performed (1, 18). Endobronchial actinomycosis may manifest as irregular granular thickening and partial occlusion of bronchi, resembling a submucosal tumor. Bronchoscopy may show an exophytic mass with a purulent exudate and characteristic histology with presence of Sulphur granules (3).
With early detection and proper treatment, this condition has excellent prognosis. Penicillin has remained the drug of choice over the past 50 years for treatment of pulmonary actinomycosis (1). The thoracic form appears to require longer treatment courses compared to commoner forms. Generally, 18-24 million units of penicillin per day are given for 2-6 weeks followed by oral penicillin or amoxicillin for 8-12 months (19).
Tetracycline and erythromycin are the alternatives especially in penicillin–allergic patients or pregnant women. Response to treatment should be monitored radiologically with plain radiographs or CT scan (1, 13, 19). Reduction in the shadowing on chest radiograph is expected within 9 weeks. Coexistent bronchial carcinoma should be suspected in case of medical treatment failure.
Surgery is particularly useful if there are complications, such as pulmonary abscesses and empyema, or where discharging fistulas and sinuses may need to be opened up, or in very rare instances, to control life threatening hemoptysis that can occur with pulmonary actinomycosis (20, 21). There are two unique features in our case. The first is the endobronchial location of the mass; which is rare, with the diagnosis made by bronchoscopic biopsy. The second is its unusual clinical presentation with a massive hemoptysis and diagnosis in an early stage.
In conclusion, endobronchial actinomycosis is a rare type of pulmonary actinomycosis; but radiologists and respiratory physicians should be aware of this chronic pulmonary condition, when investigating patients for persistent pulmonary shadowing on plain chest radiographs or CT scans. An early diagnosis will prevent the significant physical and psychological morbidity, including unwarranted surgery associated with delayed diagnosis.
REFERENCES
- 1.Mabeza GF, Macfarlane J. Pulmonary actinomycosis. Eur Respir J. 2003;21(3):545–51. doi: 10.1183/09031936.03.00089103. [DOI] [PubMed] [Google Scholar]
- 2.Broquetas J, Arán X, Moreno A. Pulmonary actinomycosis with endobronchial involvement. Eur J Clin Microbiol. 1985;4(5):508. doi: 10.1007/BF02014435. [DOI] [PubMed] [Google Scholar]
- 3.Barikbin P, Grosser K, Hahn G, Fischer R, Suttorp M. Thoracic actinomycosis imitating a malignant chest wall tumor. Diagnosis: pulmonary actinomycosis. J Pediatr Hematol Oncol. 2007;29(5):345–6. doi: 10.1097/MPH.0b013e318055642b. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh MJ, Liu HP, Chang JP, Chang CH. Thoracic actinomycosis. Chest. 1993;104(2):366–70. doi: 10.1378/chest.104.2.366. [DOI] [PubMed] [Google Scholar]
- 5.Lee JP, Rudoy R. Pediatric thoracic actinomycosis. Hawaii Med J. 2003;62(2):30–2. [PubMed] [Google Scholar]
- 6.Brook I. Actinomycosis: diagnosis and management. South Med J. 2008;101(10):1019–23. doi: 10.1097/SMJ.0b013e3181864c1f. [DOI] [PubMed] [Google Scholar]
- 7.Brown JR. Human actinomycosis. A study of 181 subjects. Hum Pathol. 1973;4(3):319–30. doi: 10.1016/s0046-8177(73)80097-8. [DOI] [PubMed] [Google Scholar]
- 8.Watt AJ. Chest wall lesions. Paediatr Respir Rev. 2002;3(4):328–38. [PubMed] [Google Scholar]
- 9.Herrak L, Msougar Y, Ouadnouni Y, Bouchikh M, Benosmane A. Thoracic actinomycosis: three cases. Rev Pneumol Clin. 2007;63(4):268–72. doi: 10.1016/s0761-8417(07)92651-6. [DOI] [PubMed] [Google Scholar]
- 10.Cendan I, Klapholz A, Talavera W. Pulmonary actinomycosis. A cause of endobronchial disease in a patient with AIDS. Chest. 1993;103(6):1886–7. doi: 10.1378/chest.103.6.1886. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhry SI, Greenspan JS. Actinomycosis in HIV infection: a review of a rare complication. Int J STD AIDS. 2000;11(6):349–55. doi: 10.1258/0956462001916047. [DOI] [PubMed] [Google Scholar]
- 12.Han JY, Lee KN, Lee JK, Kim YH, Choi SJ, Jeong YJ, et al. An overview of thoracic actinomycosis: CT features. Insights Imaging. 2013;4(2):245–52. doi: 10.1007/s13244-012-0205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poey C, Giron J, Verhaegen F, Levenes H, Gruels S, Fajadet P, et al. X-ray computed tomographic and radiographic aspects of thoracic actinomycosis. J Radiol. 1996;77(3):177–83. [PubMed] [Google Scholar]
- 14.Kim TS, Han J, Koh WJ, Choi JC, Chung MJ, Lee JH, et al. Thoracic actinomycosis: CT features with histopathologic correlation. AJR Am J Roentgenol. 2006;186(1):225–31. doi: 10.2214/AJR.04.1749. [DOI] [PubMed] [Google Scholar]
- 15.Ganesan K, Khan R, Eisenhut M. Pulmonary actinomycosis masquerading as a malignant lung tumor in a 9-year-old boy. Clin Pediatr (Phila) 2005;44(2):181–3. doi: 10.1177/000992280504400211. [DOI] [PubMed] [Google Scholar]
- 16.Datta JS, Raff MJ. Actinomycotic pleuropericarditis. Am Rev Respir Dis. 1974;110(3):338–41. doi: 10.1164/arrd.1974.110.3.338. [DOI] [PubMed] [Google Scholar]
- 17.Wand A, Gilbert HM, Litvack B, Markisz JA. MRI of thoracic actinomycosis. J Comput Assist Tomogr. 1996;20(5):770–2. doi: 10.1097/00004728-199609000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Oki Y, Morishita M, Kato H, Tokudome M, Sanji H, Kamasawa R, et al. Pulmonary actinomycosis diagnosed through transbronchial lung biopsy (TBLB) Nihon Kokyuki Gakkai Zasshi. 2003;41(3):202–6. [PubMed] [Google Scholar]
- 19.Choi J, Koh WJ, Kim TS, Lee KS, Han J, Kim H, et al. Optimal duration of IV and oral antibiotics in the treatment of thoracic actinomycosis. Chest. 2005;128(4):2211–7. doi: 10.1378/chest.128.4.2211. [DOI] [PubMed] [Google Scholar]
- 20.Endo S, Murayama F, Yamaguchi T, Yamamoto S, Otani S, Saito N, et al. Surgical considerations for pulmonary actinomycosis. Ann Thorac Surg. 2002;74(1):185–90. doi: 10.1016/s0003-4975(02)03616-0. [DOI] [PubMed] [Google Scholar]
- 21.Boudaya MS, Smadhi H, Marghli A, Mouna M, Charmiti F, Ismail O, et al. Surgery in thoracic actinomycosis. Asian Cardiovasc Thorac Ann. 2012;20(3):314–9. doi: 10.1177/0218492312439310. [DOI] [PubMed] [Google Scholar]