Abstract
It is known that Fe deficiency has a negative impact on cognitive function in children by altering brain energy metabolism and neurotransmitter function. It is unclear whether Fe deficiency has detrimental effects on cognition, mental health and fatigue in women of childbearing age. Our aim was to systematically review the literature to determine whether Fe deficiency in women of childbearing age affects cognition, mental health and fatigue, and whether a change in Fe status results in improvements in cognition, mental health and fatigue. Studies using Fe supplement interventions were reviewed to examine the effect of Fe deficiency in women of childbearing age (13–45 years) on their cognition, mental health and fatigue. English-language articles ranging from the earliest record to the year 2011 were sourced. The quality of retrieved articles was assessed and the Fe pathology, cognitive, mental health and fatigue data were extracted. Means and standard deviations from cognitive test data were included in meta-analyses of combined effects. Of the 1348 studies identified, ten were included in the review. Three studies showed poorer cognition and mental health scores and increased fatigue with Fe deficiency at baseline. Seven studies reported an improvement in cognitive test scores after Fe treatment. Results of three of these studies were included in meta-analyses of the effect of Fe supplement intervention on cognition. The results of the meta-analyses showed a significant improvement in Arithmetic scores after treatment (P < 0·01), but no effect on Digit Symbol, Digit Span or Block Design. While an improvement in cognition after Fe treatment was seen in seven out of ten studies, the evidence base is limited by poor study quality and heterogeneity across studies. Additional high-quality studies using consistent measures are warranted.
Key words: Iron deficiency, Women of childbearing age, Cognition, Mental health, Fatigue
Abbreviations: JBI, Joanna-Briggs Institute; RCT, randomised controlled trial; SF, serum ferritin; SMD, standardised mean difference
Fe deficiency is the most prevalent nutritional deficiency worldwide( 1 ). Internationally, rates of Fe deficiency are highest for infants and young children during their first 2 years of life and women of childbearing age( 2 ). Women of childbearing age are at particular risk of Fe deficiency owing to the increased demand for Fe during pregnancy, as well as the Fe losses resulting from menstruation and during childbirth( 3 ). Other possible causes of Fe deficiency include diets that are low in Fe and high in Fe absorption inhibitors such as phytates and polyphenols( 4 ).
Fe deficiency is characterised by a reduction in stored Fe, which is most commonly measured by the marker, serum ferritin (SF)( 5 ). Functional Fe is often measured by Hb( 6 ). For the adult female population, normal SF is usually defined as >20 µg/l and normal Hb as >120 g/l( 5 , 7 ). For the purposes of this review, participants with normal SF and Hb levels were considered as Fe sufficient. Non-anaemic Fe deficiency was classified as SF ≤ 20 µg/l, Hb > 120 g/l in conjunction with two other markers indicative of Fe deficiency (serum Fe < 10 µmol/l, total Fe-binding capacity >68 µmol/l, serum transferrin saturation <15 %)( 5 ). Fe deficiency anaemia is the most severe form of Fe deficiency and results in Hb ≤ 120 g/l, in addition to satisfying the markers for Fe deficiency( 8 ).
Up to two-thirds of women of childbearing age in developing countries suffer from Fe deficiency( 9 ). Fe deficiency in women of childbearing age is not merely a phenomenon of developing nations, with rates of 10–20 % found in the USA, Japan and Europe( 2 , 10 ). In Australia, prevalence estimates for women of childbearing age are limited, but recent data from the Queensland cohort of the Ausdiab study indicate that the rates are high, with one in five women aged 25–50 years having either mild (9·7 % with SF 12–20 µg/l) or moderate Fe deficiency (10·6 % with SF <12 µg/l)( 11 ). Another Australian study has shown high rates of Fe deficiency with 32 % of a convenience sample of women of childbearing age from the University of Sydney (mean age 22 years) having an SF of <15 µg/l, corresponding with depleted Fe stores( 12 ). Note that each of these studies used a different cut-off for SF. A New Zealand study assessed the dietary Fe intakes and biochemical Fe status of a nationally representative sample of women aged 15–49 years. Results indicated that the prevalence of Fe deficiency anaemia and non-anaemic Fe deficiency ranged from 1·4 to 5·5 %, and for Fe deficiency without anaemia, from 0·7 to 12·6 %( 13 ).
Objectives
The aim of this paper was to review the literature on the effects of Fe deficiency in women of childbearing age on cognitive functioning, mental health and fatigue, ranging from the earliest record to the year 2011.
This review considered two main questions:
-
1.
What is known about the effects of Fe deficiency in women of childbearing age on cognitive functioning, mental health and fatigue?
-
2.
Is a change in Fe status related to improvements in cognitive performance or mental health and fatigue?
The review provides a summary of the literature, including the measures used to assess Fe status, cognition, mental health and fatigue. For intervention studies included in the review, Fe dosages and consequent changes in Fe status are also reported, summarised by meta-analysis where appropriate. The review also provides recommendations for future research and practice in the area.
Methods
Protocol and registration
The protocol for this review was peer reviewed by the Joanna-Briggs Institute (JBI), and is registered in the JBI library of systematic review protocols (http://www.joannabriggs.edu.au/Search.aspx).
Eligibility criteria
Types of participants
Studies that include female human participants aged between 13 and 45 years were included.
Types of intervention
Studies that met the aforementioned criteria and included the assessment of cognition and any form of Fe treatment, for any time period, were considered.
Types of studies
This review considered, but was not limited to, randomised controlled trials (RCT). In the absence of RCT, other research designs such as non-RCT and before and after studies were considered, to enable the inclusion of the current best evidence regarding the effects of Fe deficiency in women of childbearing age on cognition, mental health and fatigue.
Terms
For the purposes of this review, cognitive functioning refers to the mental process by which we acquire and use knowledge and generally relates to concentration, attention and memory( 8 ). Cognitive functioning domains include verbal memory, working memory, sustained attention, information processing speed and impulsivity. Both validated and unvalidated measures of cognitive functioning were considered. Measures of mental health taken from general health perception measures were considered. We accepted assessment of fatigue by a range of methods, including self-report as described by Piper et al.( 14 ).
Types of outcome measures
Studies that included the following outcome measures were considered:
-
•
Assessment of Fe status (Fe deficiency, Fe-deficiency anaemia or Fe sufficiency) using standardised laboratory methods, for example, SF, Hb, serum transferrin receptor, serum Fe and markers of inflammation.
-
•
Fe treatment intervention (distribution of Fe supplementation), for a specified time period, to participants who have previously had Fe studies and cognition, mental health or fatigue measured.
-
•
Follow-up testing of Fe status in the post-supplement period in intervention studies.
-
•
Measures of cognitive function.
-
•
Measures of mental health and fatigue included the assessment of any aspect of mental health or fatigue when Fe status and cognition were also being measured.
Keywords used in search
Keywords used were: [women of childbearing age, young women, females, iron deficiency] AND cognition, iron status AND cognitive functioning, iron status AND attention, iron status AND memory, iron status AND concentration, iron status AND mental health, iron status AND fatigue.
Search strategy
The search was conducted in September 2010 and updated in December 2011. The search strategy aimed to find both published and unpublished studies written in the English language from the earliest record to the year 2011. A three-step search strategy was used. Databases searched were PRE-MEDLINE® and MEDLINE (Ovid), CINAHL, Scopus, Embase and PsycINFO. Following an initial search, analysis of the text contained in the title, abstract, index and reference list of retrieved articles was conducted. A second search using all the identified keywords and index terms was then undertaken across all the included databases. Thirdly, the reference list of all the identified reports and articles was searched for additional studies. The search for unpublished studies was conducted using Mednar, the online unpublished Australian Digital Theses Program.
Study selection
Studies were screened for eligibility and articles were retrieved if information in the title, abstract and descriptor headings met eligibility criteria. Eligibility was independently assessed by two reviewers. Once retrieved, background and methods were examined. Investigators were contacted if articles contained insufficient information to meet inclusion criteria. If no reply was received, studies were excluded. Studies that met criteria then underwent a critical appraisal to examine the quality of the processes used in the study, assessing for the bias and strength of methodological techniques. Critical appraisal was achieved using the JBI critical appraisal tool (JBI-MAStARI).
Data extraction process
For studies that met the inclusion criteria, the following data were extracted: study details, Fe status assessment and measures of cognitive function, mental health and fatigue.
Assessing risk of bias in individual studies
The use of the JBI-MASTARI tool enabled a comprehensive assessment of bias within individual studies at both the study and outcome levels. The JBI provides researchers with an assessment and review instrument (JBI-MAStARI). This tool is designed to manage, appraise, extract and analyse quantitative data as part of a systematic review of evidence. JBI-MAStARI is a web-based database and incorporates a critical appraisal scale, data extraction forms and a data analysis function (built with JBI-CReMS). This information was used when examining the quality of the data synthesised. Studies that included unexplained bias were classified as lower-quality, with less emphasis placed on the data and outcomes of lower-quality studies. Publication bias was not measurable owing to the limited number of studies in the analysis. Given that many of the studies have negative findings, publication bias is less likely( 15 ). Having limited studies included in the review reduces the type II error, or the ability to distinguish chance from asymmetry( 16 ).
Analysis
Meta-analyses were conducted using STATA11( 17 ) to estimate the combined effect of Fe status on cognitive function across studies. Standard deviations and standard errors of means were calculated from the available data. The mean difference was then calculated if it was not provided by the authors, enabling a complete data set in preparation for analysis. This was calculated by dividing the difference in mean outcome scores between groups by the standard deviation of the outcome among participants. Standardised mean difference (SMD) was included as a summary statistic in the meta-analyses. The assumption of SMD is that studies included random samples and that the population distribution is normal. Differences between studies were measured using the I 2 statistic. A guide to the interpretation of the I 2 statistic is as follows: 0–40 % might not be important, 30–60 % may represent moderate heterogeneity, 50–90 % may represent substantial heterogeneity and 75–100 % may represent considerable heterogeneity( 18 ). The choice of the model used was based upon heterogeneity determined from the I 2 statistic. Both fixed and random effects models were examined in the meta-analysis to enable comparison of each model.
Results
Study characteristics
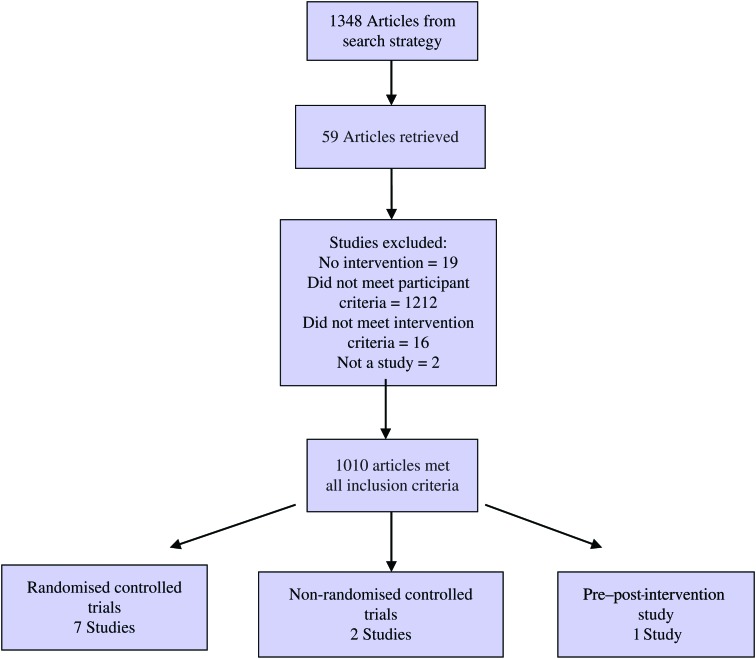
The flow diagram in Fig. 1 shows the studies screened, assessed for eligibility, and included in the review, with reasons being listed for those excluded. Ten studies satisfied eligibility criteria and were included in the review; the characteristics of these studies are summarised in Table 1. Of the included studies, there were seven RCT, two non-RCT and one pre-post intervention study. The pre–post-intervention study measured cognition after an Fe therapy intervention.
Fig. 1.
Flow diagram of the study screening process.
Table 1.
Characteristics of the included studies
| Source | Design | n, population | Dose and type of oral Fe | Fe supplementation duration (weeks) | Follow-up (months) | Study arms | Retention %* | Intention-to-treat analysis |
|---|---|---|---|---|---|---|---|---|
| Beard et al.( 19 ) | RCT | Ninety-five, mothers, 18–30 years | 125 mg FeSO4 tablets | 10 | 2·5 | IIT + CT + MV + Fe + FeSO4 | 85 | No |
| Murray-Kolb & Beard( 20 ) | RCT | 152, females, 18–35 years | 160 mg FeSO4 (60 mg Fe) tablets | 16 | 4 | IIT + CT + GPA/PA +Fe/Plac. | 74 | No |
| Bruner et al.( 21 ) | RCT | Eighty-one, adolescent girls, 13–18 years | 1300 mg FeSO4 (260 mg Fe) tablets | 8 | 2 | IIT + CT + Fe/Plac. | 90 | Yes |
| Ballin et al.( 22 ) | RCT | Fifty-nine, adolescent girls, 16–17 years | 10 ml Fe polystyrene sulphonate (105 mg elemental Fe) | 8 | 2 | IIT + CT + PA + HQ + Fe/Plac. | 27 | No |
| Elwood & Hughes( 23 ) | RCT | Forty-seven, females, ≥20 years | 150 mg FeCO3 tablets | 8 | 2 | IIT + CT + Fe/Plac. + HQ | 87 | No |
| Groner et al.( 24 ) | RCT | Thirty-eight, pregnant females, 14–24 years | 90 mg C4H2FeO4 (60 mg Fe) capsules | 4 | 1 | IIT + CT + Fe + MV | 75 | No |
| Patterson( 25 ) | RCT | Seventy-six females 18–35 years | 350 mg FeSO4 (105 mg Fe) | 12 | 3 | IIT + CT + Fe/Plac. + HQ + DT | 74 | No |
| Khedr et al.( 26 ) | Non-RCT | Fifty-three, adults, 16–28 years | 600 mg C4H2FeO4 (195 mg Fe) tablets | 12 | 3 | IIT + CT + Fe | 100 | No |
| Månsson et al.( 27 ) | Non-RCT | 75 375, students, 16–19 years | 100 mg FeSO4 tablets | 12 | 3 | IIT + CSR + HQ + Fe | 128 | No |
| Kretsch et al.( 28 ) | Pre–post-intervention | Twenty-four, obese dieting females, 25–42 years | 55 mg C4H2FeO4 (18 mg Fe) tablets | 20 | N/A | +CT | 58 | No |
RCT, randomised controlled trial; FeSO4, ferrous sulfate therapy; IIT, Fe testing; CT, cognition testing; MV, multivitamin; Fe, Fe therapy; GPA, grade point average assessed; PA, physical assessment; Plac., placebo therapy; HQ, health questionnaire; DT, diet therapy; CSR, cognition self-report; N/A, not applicable.
* Retention rates reported post-intervention if no follow-up or at latest point of follow-up.
Study aims
The predominant focus of the seven RCT was to investigate the effect of Fe status and Fe treatment on cognitive functioning in female participants. Both of the two non-RCT assessed the effects of Fe status and Fe treatment on cognitive functioning in Fe-deficient participants( 26 , 27 ). One of the two non-RCT also assessed changes in symptoms of mental health and fatigue in Fe-deficient participants after treatment( 27 ). The pre–post-intervention study measured the change in Fe status after an Fe therapy intervention during a weight loss trial and the consequent effect on cognitive functioning( 28 ).
Sample demographic information
Sample sizes ranged from twenty-four to 716. Sample populations were from geographically defined areas, and included pregnant women, mothers, university and secondary school students, healthy women, general practitioner-referred Fe-deficient women, Fe-deficient women from haematology outpatient clinics and obese dieting women.
Iron status at baseline
All ten included studies assessed Fe status at baseline. Fe status data from nine of the ten studies that conducted an intervention are summarised in Table 2. One intervention study did not provide sufficient data for inclusion in the table( 28 ). As shown in Table 2, there is substantial variation in the methods used for testing Fe status.
Table 2.
Iron status results
| Source | Fe status results | ||||||||||||
| Beard et al.( 19 ) | Control group | IDA group 1 | IDA group 2 | ||||||||||
| CN (n 30) | IDA-Pl (n 21) | IDA-Fe (n 30) | |||||||||||
| Mean | sd | Mean | sd | Mean | sd | ||||||||
| Hb (g/l) | |||||||||||||
| BL | >135 | 90–115 | 90–115 | ||||||||||
| 10 weeks | 136 | 5 | 109 | 7 | 108 | 9 | |||||||
| 9 months | 134 | 9 | 120 | 8 | 129 | 8 | |||||||
| MCV (fl) | |||||||||||||
| BL | >80 | <80 | <80 | ||||||||||
| 10 weeks | 90·7 | 12·4 | 84·4 | 6·7 | 87·0 | 7·6 | |||||||
| 9 months | 91·7 | 4·4 | 89·3 | 4·1 | 86·1 | 5·6 | |||||||
| TSAT (%) | |||||||||||||
| BL | >15 | <15 | <15 | ||||||||||
| 10 weeks | 27·4 | 10·4 | 8·9 | 4·2 | 8·4 | 4·3 | |||||||
| 9 months | 28·6 | 11·6 | 12·9 | 7·0 | 21·3 | 8·5 | |||||||
| Ft (μg/l) | |||||||||||||
| BL | >12 | <12 | <12 | ||||||||||
| 10 weeks | 56·0 | 28·0 | 11·9 | 5·1 | 10·6 | 6·6 | |||||||
| 9 months | 48·4 | 33·6 | 17·1 | 13·9 | 33·8 | 19·8 | |||||||
| Murray-Kolb & Beard( 20 ) | Control group | ID group | IDA group | ||||||||||
| CNPL (n 21, 16) | CNFE (n 21, 14) | IDPL (n 37, 28) | IDFE (n 36, 25) | IDAPL (n 15, 13) | IDAFe (n 19, 17) | ||||||||
| Mean | sd | Mean | sd | Mean | sd | Mean | sd | Mean | sd | Mean | sd | ||
| Hb (g/l) | |||||||||||||
| BL | 139 | 9 | 137 | 8 | 133 | 6 | 131 | 6 | 114 | 5 | 113 | 5 | |
| EP | 142 | 11 | 134 | 11 | 132 | 10 | 131 | 8 | 121 | 9 | 125 | 9 | |
| Hct (%) | |||||||||||||
| BL | 42 | 3 | 42 | 3 | 41 | 2 | 40 | 2 | 35 | 1 | 35 | 1 | |
| EP | 44 | 5 | 41 | 4 | 41 | 4 | 40 | 3 | 37 | 3 | 38 | 3 | |
| MCV (fl) | |||||||||||||
| BL | 90·4 | 2·2 | 90·8 | 2·0 | 88·2 | 3·4 | 88·0 | 3·5 | 79·7 | 8·0 | 78·6 | 8·7 | |
| EP | 90·2 | 3·3 | 89·8 | 3·9 | 87·5 | 4·1 | 89·6 | 4·1 | 81·8 | 6·4 | 82·0 | 7·0 | |
| RDW (%) | |||||||||||||
| BL | 12·7 | 0·5 | 12·9 | 0·5 | 13·4 | 1·0 | 13·3 | 1·0 | 14·4 | 1·1 | 14·2 | 1·4 | |
| EP | 13·0 | 0·7 | 13·0 | 0·8 | 14·0 | 0·9 | 13·0 | 1·0 | 15·0 | 1·0 | 15·0 | 1·9 | |
| Ft (g/l) | |||||||||||||
| BL | 45·3 | 20·1 | 50·0 | 19·7 | 8·9 | 3·4 | 8·8 | 4·0 | 5·7 | 4·5 | 7·2 | 5·7 | |
| EP | 42·2 | 20·7 | 70·3 | 44·4 | 14·7 | 14·2 | 24·6 | 22·4 | 9·2 | 7·7 | 22·8 | 17·7 | |
| sTFR (mg/l) | |||||||||||||
| EP | 4·6 | 1·0 | 4·6 | 1·4 | 6·5 | 2·0 | 5·9 | 1·8 | 9·1 | 2·7 | 8·2 | 4·3 | |
| BL | 5·3 | 1·8 | 4·0 | 1·5 | 6·4 | 2·6 | 5·6 | 2·3 | 7·9 | 1·3 | 6·6 | 2·5 | |
| TSAT (%) | |||||||||||||
| EP | 33 | 11 | 33 | 9 | 24 | 12 | 22 | 9 | 19 | 9 | 18 | 12 | |
| BL | 23 | 9 | 25 | 13 | 24 | 14 | 29 | 10 | 16 | 7 | 32 | 15 | |
| Body Fe (mg/kg) | |||||||||||||
| EP | 6·5 | 1·5 | 7·0 | 1·7 | −0·4 | 2·0 | −0·3 | 2·3 | −4·0 | 3·6 | −2·6 | 4·6 | |
| BL | 5·8 | 2·2 | 8·2 | 3·6 | 0·6 | 3·9 | 3·2 | 3·3 | −1·9 | 3·2 | 2·0 | 4·1 | |
| Bruner et al.( 21 ) | Control group treatment | ID group treatment | |||||||||||
| Mean | sd | Mean | sd | ||||||||||
| Hb (g/dl) | |||||||||||||
| BL | 13·0 | 0·7 | 13·1 | 0·7 | |||||||||
| EP | 12·7 | 0·7 | 13·5 | 0·8 | |||||||||
| MCV (fl) | |||||||||||||
| BL | 84·4 | 4·8 | 86·1 | 3·8 | |||||||||
| EP | 85·1 | 4·8 | 88·5 | 3·6 | |||||||||
| RCD (%) | |||||||||||||
| BL | 13·5 | 1·1 | 13·2 | 0·9 | |||||||||
| EP | 13·6 | 1·0 | 13·3 | 1·2 | |||||||||
| Ft (μg/l) | |||||||||||||
| BL | 8·5 | 2·6 | 9·1 | 2·2 | |||||||||
| EP | 12·1 | 7·6 | 27·3 | 13·2 | |||||||||
| Ballin et al.( 22 ) | ID group 1 placebo | ID group 2 treatment | |||||||||||
| Mean | sd | Mean | sd | ||||||||||
| Serum Fe (mmol/l) | |||||||||||||
| BL | 14·56 | 5·25 | 12·88 | 5·03 | |||||||||
| EP | −1·36 (mean change) | 3·2 (mean change) | |||||||||||
| TSAT (%) | |||||||||||||
| BL | 23·26 | 9·0 | 21·26 | 9·1 | |||||||||
| EP | |||||||||||||
| Ft (μg/l) | |||||||||||||
| BL | Whole figures | ||||||||||||
| EP | Not recorded | ||||||||||||
| Groner et al.( 24 ) | Control group (n 9) | Experimental group treatment (n 16) | |||||||||||
| Hb (g/dl) | |||||||||||||
| BL | 12·3 | 12·2 | |||||||||||
| EP | 11·1 | 11·6 | |||||||||||
| Hct (%) | |||||||||||||
| BL | 35·9 | 35·2 | |||||||||||
| EP | 33·4 | 33·6 | |||||||||||
| MCV (%) | |||||||||||||
| BL | 88·4 | 8·3 | |||||||||||
| EP | 86·8 | 88·2 | |||||||||||
| MCH (pg/l) | |||||||||||||
| BL | 30·6 | 30·4 | |||||||||||
| EP | 30·6 | 30·9 | |||||||||||
| Ft (μg/l) | |||||||||||||
| BL | 57·0 | 42·4 | |||||||||||
| EP | 36·7 | 36·8 | |||||||||||
| Patterson( 25 ) | Control group (n 22) | Supplement group (n 22) | |||||||||||
| Mean | sd | Mean | sd | ||||||||||
| Ft (μg/l) | |||||||||||||
| BL | 49·4 | 6·1 | 9·0 | 0·8 | |||||||||
| 12 weeks | 44·5 | 5·7 | 24·8 | 2·1 | |||||||||
| 6 months | 51·1 | 6·6 | 24·2 | 2·1 | |||||||||
| Hb (g/l) | |||||||||||||
| BL | 135·9 | 1·4 | 125·2 | 1·9 | |||||||||
| 12 weeks | 134·0 | 1·3 | 130·4 | 1·4 | |||||||||
| 6 months | 134·9 | 1·2 | 131·4 | 1·4 | |||||||||
| Khedr et al. ( 26 ) | Control group (n 13) | ID group treatment (n 14) | |||||||||||
| Mean | sd | Mean | sd | ||||||||||
| Hb (g/dl) | |||||||||||||
| BL | 14·35 | 0·54 | 6·50 | 1·50 | |||||||||
| EP | 14·35 | 0·88 | 13·80 | 1·55 | |||||||||
| sFe (μg/l) | |||||||||||||
| BL | 86·50 | 30·75 | 45·50 | 22·30 | |||||||||
| EP | 89·90 | 22·52 | 98·50 | 30·35 | |||||||||
| TIBC (μg/dl) | |||||||||||||
| BL | 269·50 | 14·56 | 411·40 | 62·68 | |||||||||
| EP | 275·88 | 15·35 | 322·40 | 30·88 | |||||||||
| Månsson et al.( 27 ) | ID group treatment (n 129) | ||||||||||||
| sTRG (g/l) | |||||||||||||
| BL | 2·89 | ||||||||||||
| EP | 2·62 | ||||||||||||
| Ft (μg/l) | |||||||||||||
| BL | 11 | ||||||||||||
| EP | 22 | ||||||||||||
| FeSAT (%) | |||||||||||||
| BL | 21 | ||||||||||||
| EP | 28 | ||||||||||||
IDA, Fe deficiency anaemia; CN, control; Pl, placebo; BL, baseline; MCV, mean corpuscular volume; TSAT, transferrin saturation; Ft, ferritin; ID, Fe deficient; CNPL, control placebo; CNFE, control Fe supplementation; IDPL, Fe deficient placebo; IDFE, Fe deficient Fe supplementation; IDAPL, Fe deficiency anaemia placebo; IDAFe, Fe deficiency anaemia Fe supplementation; EP, end point; Hct, haematocrit; RDW, red blood cell distribution width; sTFR, soluble transferrin receptor; RCD, red cell distribution width; sFe, serum Fe, TIBC, total Fe binding capacity; sTFR, soluble transferrin receptor; Fe SAT, Fe saturation.
The markers used to assess iron status
The markers used to assess Fe status varied between studies. The most commonly used markers of Fe status were SF, Hb and serum Fe. However, more than nine other markers were used to assess Fe status across the included studies (see Table 2). Where a marker was used by more than one study, the reference range criteria were comparable. For example, the reference range for normal SF was defined as >15–20 µg/l and normal Hb as >120 g/l throughout studies.
Iron supplement interventions
All ten included studies conducted an Fe supplement intervention( 19 – 28 ). As shown in Table 1, the Fe supplements used varied across studies, and included ferrous sulphate, Fe polystyrene sulphonate (liquid Fe), ferrous carbonate and ferrous fumerate. The dosage and duration of the intervention also varied between studies. Dosage of elemental Fe supplementation ranged from 18 mg to 260 mg per d. The shortest Fe supplementation intervention was 4 weeks( 24 ), and the longest intervention was 20 weeks( 28 ).
Iron status after iron supplementation intervention
All studies that conducted Fe supplement interventions measured Fe status during follow-up testing. As shown in Table 2, Fe status improved with treatment in all except two studies( 24 , 28 ). However, not all of the studies reporting improved Fe status after Fe supplement intervention included a control group( 24 , 25 ). Hb improved in six of the seven studies reporting the measure, with an average improvement of 11 g/l after Fe treatment. Six studies measured SF levels, with improvements after treatment reported in five of the six studies( 24 ). The average improvement in SF levels was 26 µg/l.
The largest improvements were seen in the studies with the two longest interventions (10 and 16 weeks)( 19 , 20 ). The study reporting a decrease in SF and Hb after treatment( 24 ) was one of the shortest interventions (8 weeks). The study with the longest intervention (20 weeks) was conducted on dieting women, with energetic restriction imposed during the Fe intervention( 28 ). This study found an improvement in Hb of 6 g/l in 43 % of their fourteen participants, and a decrease of 6 g/l in 57 % of the participants( 28 ).
Three of the ten studies that included an intervention reported assessment of participant compliance( 21 , 24 , 27 ). Månsson et al. reported a 24 % compliance rate over a 3-month intervention period( 27 ). Groner reported an 88 % compliance rate over a 1-month intervention( 24 ). One study did not report the result of their assessment of compliance.
The effect of iron deficiency on cognitive functioning
Of the eight studies that included both Fe-deficient and Fe-sufficient participants at baseline( 19 , 20 , 22 , 24 – 28 ), four reported higher cognitive scores for Fe sufficient than Fe-deficient participants at baseline and improved scores after Fe treatment.
Ballin et al. showed that the self-reported ability to concentrate was lower in Fe-deficient participants at baseline compared with Fe-sufficient controls, and that the Fe-deficient participants reported a significant improvement in the ability to concentrate after Fe treatment( 22 ).
Murray-Kolb et al. showed that at baseline, Fe-sufficient participants performed better on cognitive tasks and completed them faster than Fe-deficient participants. After Fe treatment, learning, attention and memory scores all improved, and the time taken to complete tasks decreased. As the severity of Fe deficiency increased, cognition decreased and the time taken to complete tasks increased( 20 ).
Khedr et al. showed that at baseline, Fe-deficient participants performed poorer on cognitive tasks, including intelligence and memory (Wechsler memory scale-revised and Wechsler adult intelligence scale-revised), which significantly improved with Fe treatment( 26 ).
Patterson et al. included Fe-deficient and Fe-sufficient participants and found that there were significant differences on four tests overall between Fe deficient and Fe sufficient at baseline (Block Design, Digit Span, Digit Symbol and Arithmetic). After treatment, there was no improvement for the Fe-deficient participants on Digit Span. There was a learning effect for Digit Symbol, as Fe-deficient participants and controls both improved after treatment. There was an improvement for Fe-deficient participants on Arithmetic and Block Design( 25 ).
Three of the eight studies reported no difference in cognition between Fe-deficient participants and Fe-sufficient controls at baseline. These studies did show improvement in cognitive function in previously Fe-deficient participants after Fe treatment:
Kretsch et al. recruited participants based on BMI and not Fe status for a weight loss intervention. They showed that decreasing Hb occurred with dieting and that this correlated with decreased sustained attention, as measured by the Bakan Sustained Attention task( 28 ).
Groner et al. found a significant improvement in Arithmetic scores in Fe-deficient participants after treatment. On comparison of the changes between baseline and follow-up scores, the experimental group showed a significantly greater improvement than controls on tests of short-term memory and attention( 24 ).
Beard et al. included Fe-deficient participants and Fe-sufficient controls and found no difference in cognitive tasks at baseline. Fe treatment resulted in a significant improvement in previously Fe-deficient participants on intelligence and short-term memory scores( 19 ).
One study showed no difference in cognitive function between Fe-deficient and Fe-sufficient groups either at baseline or at follow-up, after Fe treatment.
Månsson et al. found no significant difference between the Fe levels of Fe-deficient and Fe-sufficient participants reporting an inability to concentrate at baseline and no difference after treatment which the study attributed to small sample size (n 375)( 27 ).
The two studies recruited only Fe-deficient participants( 21 , 23 ). One of these studies reported an improvement in cognitive tasks with Fe treatment:
Bruner et al. only included Fe-deficient participants and showed that verbal learning and memory improved with Fe treatment( 21 ).
And one study showed no difference in cognitive function after Fe treatment:
Elwood et al. showed no improvement in cognitive scores with treatment in Fe-deficient participants. The authors suggested that this may have been due to participants' Fe status not being low enough for an effect to be shown( 23 ).
Meta-analysis of the effects of iron supplement intervention on cognition
Results from three of the RCT that met the inclusion criteria were pooled in meta-analyses. The three studies included in the analyses were the only studies that provided sufficient data to do so( 24 – 26 ).
Heterogeneity between studies was tested using the I 2 statistic. The I 2 result was zero for each cognition test included in the analysis, indicating that there was no significant variation between the studies. There was no difference between the results of fixed or random effects models. A fixed-effects model was used as it was considered more reliable than the random effects model owing to very few studies being included in the analysis.
A range of tests was used to measure cognition, with a few of the included studies using the same tests. Digit Symbol, Digit Span, Arithmetic and Block Design (assessing the attention, working memory and visuo-spatial ability) were the only cognitive tests used in more than one study, and therefore were the ones included in the meta-analyses. The studies in which each of these tests was used are shown in Table 3. Digit Forward and Digit Backward are combined to form Digit Span. Total scores for Digit Span were included in the meta-analysis.
Table 3.
Cognitive tests used in the studies included in the meta-analysis
Digit Symbol
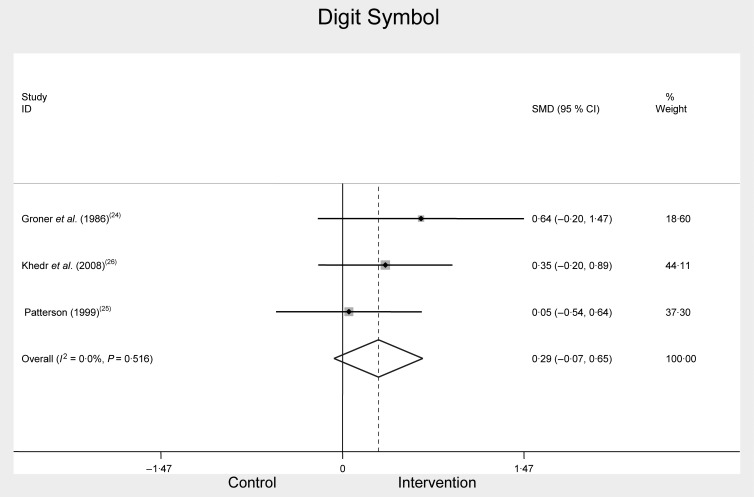
Digit Symbol was used in three of the included studies as a measure of the cognitive construct, attention. Two studies( 24 , 26 ) specified using the Wechsler adult intelligence scale-revised; the other( 19 ) did not specify which version of the test was used. The meta-analysis of Digit Symbol included pre- and post-Fe treatment intervention scores in Fe-deficient participants( 19 , 24 , 26 ) (Fig. 2). There was no significant difference between combined Digit Symbol test scores before and after treatment, SMD 0·29, 95 % CI −0·07, 0·65, n 186, P = 0·114, I 2 = 0·0 %.
Fig. 2.
Digit Symbol scores at baseline and after iron treatment intervention. SMD, standardised mean difference.
Digit Span and Arithmetic
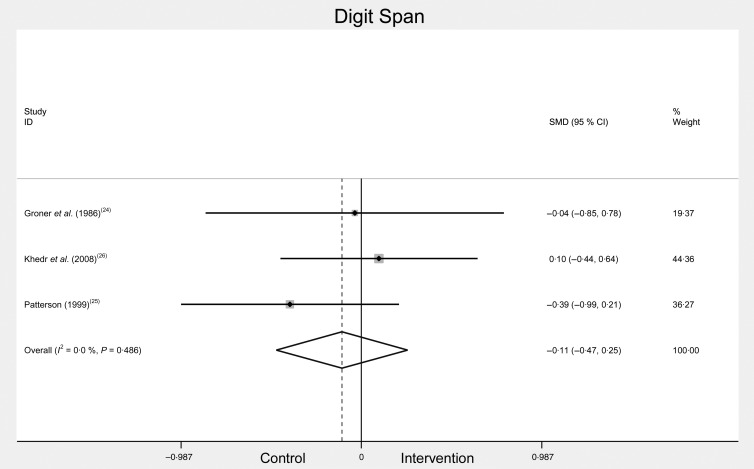
Digit Span and Arithmetic were used in three of the included studies as a measure of working memory. Working memory provides temporary storage and manipulation of the information required for complex cognitive tasks such as comprehension, learning and reasoning( 29 ). Two of these three studies reported using the Wechsler memory scale-revised battery( 24 , 26 ), while the third did not specify. Total scores for Digit Span (i.e. Digit Forward and Digit Backward) were included in the meta-analysis (as one study did not provide separate results for Digit Forward and Digit Backward). The meta-analysis of Digit Span included scores pre- and post-Fe treatment in Fe-deficient participants( 19 , 24 , 26 ) (see Fig. 3). Combined change scores for Digit Span were: SMD −0·11, 95 % CI −0·47, 0·25, n 186, P = 0·564, I 2 = 0·0 %. The analysis showed no significant difference in Digit Span scores before and after treatment.
Fig. 3.
Total Digit Span scores before and after iron treatment intervention. SMD, standardised mean difference.
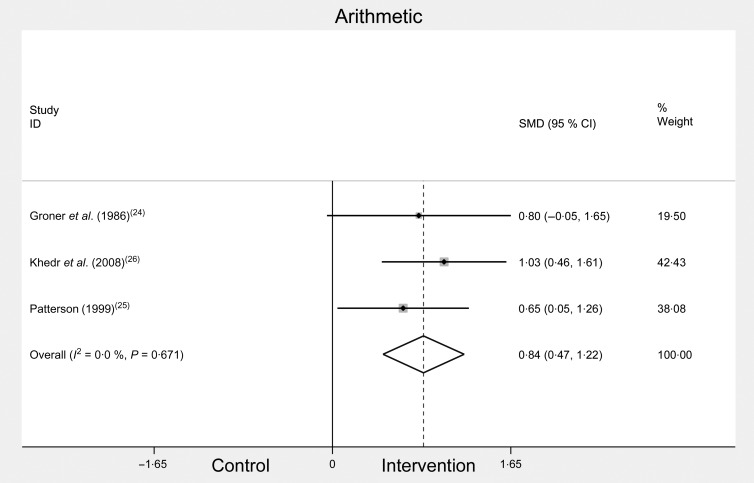
The meta-analysis of Arithmetic scores significantly improved after Fe treatment (see Fig. 4). Combined change scores for Arithmetic were: SMD 0·84, 95 % CI 0·47, 1·22, n 186, P = 0·01, I 2 = 0·0 %.
Fig. 4.
Arithmetic scores before and after iron treatment intervention. SMD, standardised mean difference.
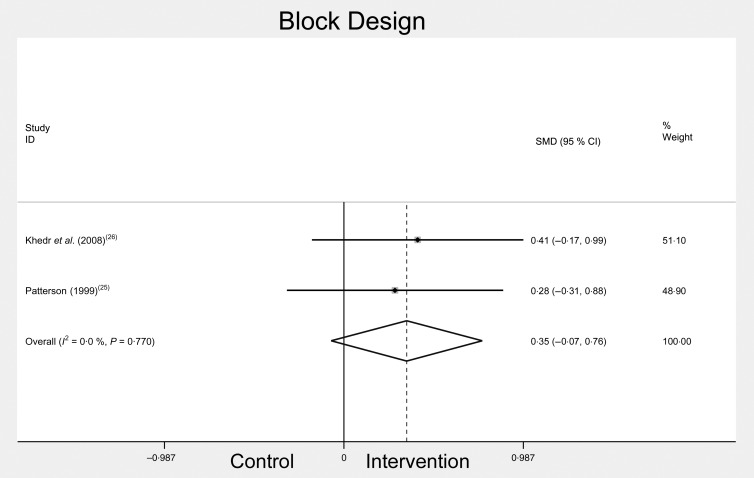
Block Design
Block Design was used by two of the included studies( 25 , 26 ), and each used the Wechsler adult intelligence scale-revised as a measure of the visuo-spatial ability. The meta-analysis of Block Design scores included pre- and post-Fe treatment interventions in Fe-deficient participants (Fig. 5). There was no significant difference in Block Design scores after treatment. Combined change scores for Block Design were: SMD 0·35, 95 % CI −0·07, 0·76, n 186, P = 0·103, I 2 = 0·0 %.
Fig. 5.
Block Design scores before and after iron treatment intervention. SMD, standardised mean difference.
The effect of iron deficiency on mental health and fatigue
In total, four of the ten included studies measured mental health( 22 , 23 , 27 , 30 ) and three studies measured fatigue( 23 , 27 , 30 ). Mental health was measured using the General Health Questionnaire and the Perceived Stress Scale. Fatigue was measured using the General Health Questionnaire, the Piper Fatigue Scale and the Edinburgh Postnatal Depression Scale.
Mental health
Three of the five studies that measured mental health reported lower scores for Fe-deficient participants compared with the controls, which improved with treatment( 22 , 27 , 30 ). Of the three studies, only one used validated tools to assess mental health: the General Health Questionnaire and the SF-36( 30 ). The other two studies that found significant results used unvalidated assessment tools( 22 , 27 ). The study that reported no improvement used an unvalidated, self-appraisal questionnaire( 23 ). This study only recruited anaemic participants( 23 ).
Fatigue
Of the three studies that measured fatigue, one reported a higher prevalence of self-reported fatigue in Fe-deficient participants at baseline, which significantly decreased with treatment( 27 ). This study used a standardised questionnaire consisting of thirty questions about different symptoms related to the quality of life. One study, which recruited only anaemic women, found no evidence of benefits of Fe therapy on fatigue, measured by self-report( 23 ). The third study that considered fatigue used the Piper Fatigue Scale and found higher Piper Fatigue Scale scores in Fe-deficient participants at baseline and reported significant improvements in Piper Fatigue Scale scores after Fe treatment( 30 ).
Overall study quality
Study quality was assessed using the JBI critical appraisal tool. Quality was low in four studies( 22 , 23 , 27 , 28 ), moderate in two( 24 , 26 ) and high in four( 19 – 21 , 25 ) (Table 4). The reasons for a low-quality rating were: not having follow-up testing; no report of handling participant withdrawals; no discussion of participant blinding; and no description of treatment intervention. Studies assessed as being of high quality were RCT, with comparable study groups, defined research questions and outcome measures, and they described the intervention adequately and used appropriate statistical analysis.
Table 4.
Study quality
| Author | Design | Quality* |
|---|---|---|
| Beard et al.( 19 ) | RCT | + |
| Murray-Kolb & Beard( 20 ) | RCT | + |
| Bruner et al.( 21 ) | RCT | + |
| Patterson( 25 ) | RCT | + |
| Groner et al.( 24 ) | RCT | 0 |
| Ballin et al.( 22 ) | RCT | − |
| Elwood & Hughes( 23 ) | RCT | − |
| Khedr et al.( 26 ) | Non-RCT | 0 |
| Månsson et al.( 27 ) | Non-RCT | − |
| Kretsch et al.( 28 ) | Cohort | − |
RCT, randomised controlled trial.
* Symbols: +, high quality; 0, moderate quality; −, low quality.
Discussion
Variation in the measurement and diagnosis of iron deficiency
The WHO recommends that the approach to assessing Fe status should be to measure SF and soluble transferrin receptor( 7 ). Hb concentration is also recommended to provide information about the severity of Fe deficiency( 31 ). This systematic review of the literature on the effects of Fe deficiency in women of childbearing age on cognition, mental health and fatigue has shown that there is a significant variation between studies in the methods used to evaluate Fe status( 32 ). The most common measures reported in the included studies were Hb and SF. These measures were often accompanied by soluble transferrin receptor, serum Fe, mean corpuscular volume, haematocrit, transferrin saturation and total Fe-binding capacity.
Relationship between iron status and cognitive function, mental health and fatigue
Most research on the effects of Fe deficiency on cognition has been conducted in children and infant populations( 33 ). This review demonstrates that relatively few studies have examined the relationship between Fe deficiency and cognition in women of childbearing age. While there does appear to be a relationship between Fe status and cognition in this population, it is difficult to specify which domains of cognitive function are, or whether cognition is, adversely impacted on, owing to the small number of studies that used comparable cognitive measures.
Eight of the ten studies in this review made a comparison of Fe-deficient participants with Fe-sufficient controls at baseline( 19 , 22 , 24 , 26 – 28 ). Four studies found that Fe-deficient participants had poorer results at baseline compared with controls, but this improved following Fe supplementation( 20 , 22 , 25 , 26 ). The studies found differences in the results of tests of attention, working memory and reaction time( 20 , 22 , 25 , 26 ). Not all the studies that included an Fe-sufficient control group found a relationship between Fe status and cognition at baseline( 19 , 24 , 28 ).
Eight studies included in this review reported an improvement in cognitive function with Fe treatment in Fe-deficient women( 19 – 22 , 24 – 26 , 28 ). Improvements were shown in Digit Symbol, Arithmetic and Digit Span scores. The largest impact was on Arithmetic (assumed to assess the working memory), which was tested in three studies and included in the meta-analysis( 24 – 26 ). Two of the studies that were not included in the meta-analysis showed no improvements in cognitive tests( 23 , 27 ). Of the studies showing no effect of Fe treatment on cognition, one study relied on self-report of cognitive symptoms( 27 ). The other study that showed no effect had a short duration intervention of 4 weeks and hence is not likely to have been long enough to improve performance on cognitive measures( 23 ).
These results indicate a substantial amount of variation across the included studies. Performance on cognitive tests at baseline was not consistently poorer in Fe-deficient participants compared with Fe-sufficient controls. Similarly, not all studies reported an improvement in test scores after treatment. It is difficult to quantify the clinical importance due the limited number of studies using the same cognitive tests. Factors that may confound the relationship between Fe status and cognition in women of childbearing age include the level of education, parity, dietary intake, sleep patterns, menstruation and waist-to-hip ratio( 34 – 36 ). However, none of the studies included in this review reported on potential confounding factors. Inconsistency in cognition testing methods and Fe status markers used in the studies included in this review hinders the comparison of results. Future research requires consistency across the markers used to assess Fe status and across tests used for assessing cognitive function in order to further characterise any relationship between Fe deficiency and cognition in women of childbearing age.
There was an adequate amount of literature only supporting a meta-analysis for cognition, while the literature on fatigue and mental health was limited and heterogeneous that only a narrative review of these areas was feasible. Results for mental health and fatigue assessments varied, with some studies finding that Fe deficiency was related to poorer mental health scores and higher levels of fatigue at baseline( 22 , 27 , 30 ), and one study finding no difference( 23 ). Three studies found improved mental health after treatment( 22 , 27 , 30 ) and two showed reduced fatigue scores after Fe treatment( 27 , 30 ). One study that measured both mental health and fatigue showed no improvement for either after Fe treatment( 23 ). Study quality appeared to account for the difference in results between studies. The study that reported no improvement relied on self-report to measure mental health and fatigue, whereas the studies that reported an effect used validated assessment tools. Another factor that may affect the results is the method of recruitment used (e.g. volunteers v.. random sampling). Those volunteering for a study on Fe deficiency and fatigue are particularly likely to self-select based on their own perceived fatigue levels. Fatigue and poor mental health or vitality may be the result of numerous causes and may be completely unrelated to Fe status, and this would impact on the results of any trial where Fe supplementation is the only treatment. Hence comprehensive assessment of potential confounders is recommended.
Two recently conducted RCT measuring the effects of Fe deficiency on fatigue in young women were not eligible to be included in the review owing to no measure of cognitive function. One study, conducted in 2012, found that fatigue levels decreased by 47·7 % in the Fe treatment group and 28·8 % in the placebo group( 37 ). The other was conducted in 2011 and examined the effect of intravenous Fe in the treatment of fatigue in premenopausal women. This study reported that fatigue decreased during their Fe intervention in 82 % of participants compared with 47 % of controls( 38 ).
Effect of iron treatment on iron status in women of childbearing age
It is generally recognised by medical practitioners that 3 months supplementation is required to improve Fe status significantly( 27 ). Various forms and doses of Fe treatment were used in the included studies. The duration of Fe treatment interventions in the studies included in the present review ranged from 4 weeks to 20 weeks. All but one of the included studies found that Fe treatment successfully improved markers of Fe status. The greatest improvements in Fe status were seen for interventions of at least 10 weeks duration.
Limitations
This review was affected by a number of limitations that need to be acknowledged. Firstly, a large range of measures was used to assess Fe status across the included studies, and there was a lack of consistency both in the type of tests and in the reference ranges chosen. Most studies did not consider factors that may be related to, and thus be confounded with, Fe status and cognition, such as socio-economic status, pregnancy and dietary intake patterns. The use of a range of tests to measure cognition, mental health and fatigue made it difficult to compare studies( 21 , 22 , 27 ). The form of Fe supplementation varied, as did the dose of Fe used. In fact, the dosage range was quite extreme and varied from 18 to 260 mg, making it difficult to compare the impact of supplementation on Fe status. The length of supplementation also varied, and short duration interventions may have had insufficient time for alterations in Fe status to occur, especially with respect to brain Fe. There was limited assessment of compliance with Fe supplementation. Only three studies assessed compliance, and only two of these reported their findings( 21 , 24 ). A limited number of studies on the effects of Fe deficiency on cognitive function in women of childbearing age were available to inform the review. The small numbers of studies informing the meta-analysis means that their utility for clarifying the results is limited and the results need to be interpreted with caution( 18 ).
Implications for practice
The results of this review indicate that short-term (<8 weeks) Fe treatment had the lowest impact on Fe status. Health practitioners should therefore prescribe Fe supplements to women of childbearing age with low Fe stores for longer than 8 weeks and ideally for at least 3 months. This is the consensus standard treatment for Fe deficiency in Australia, to ensure a sufficient amount of time for Fe stores (as measured by SF and soluble transferrin receptor) to be replenished( 25 ).
Implications for research
This review highlights the variation in methodology used for testing cognition, mental health and fatigue in Fe-deficient women of childbearing age. This variation makes it difficult to adequately compare the results, and therefore indicates that high-quality RCT with a similar study design and methodology are needed to enable a more conclusive determination of an effect. A standardised approach to measuring cognition, mental health and fatigue, including the use of validated assessment tools, will enable benchmarking.
Conclusion
Relatively few published studies have examined the relationship between Fe deficiency in women of childbearing age and cognition, mental health and fatigue. In Fe-deficient participants, small improvements in fatigue and moderate improvements in mental health scores were seen after supplemental Fe treatment. The majority of the included studies showed some evidence of improvement in cognitive function after Fe supplementation. However, a few studies used the same measures of cognitive functioning, thus making a comparison of results difficult. Meta-analysis of four cognitive tasks (Digit Symbol, Digit Span, Arithmetic and Block Design) revealed a significant improvement following Fe treatment in only one task, Arithmetic, which is a measure of working memory.
Many of the included studies are limited by short treatment interventions and a poor assessment of compliance. Studies also varied in their focus on different aspects of cognition (e.g. memory or intelligence) and in the tests they used to measure these constructs. Further high-quality RCT of a similar design and using similar evaluation methods to determine Fe status and cognitive functioning would help in clarifying the relationship between Fe status and cognition in women of childbearing age.
Acknowledgements
This research was supported by a grant from Meat and Livestock Australia to A. P., K. C. and C. C. A. J. G. received an Australian postgraduate award and top-up scholarship from Meat and Livestock Australia. C. E. C. was supported by a National Health and Medical Research Council Australian Career Development Fellowship. The authors thank Dr Patrick McElduff of the University of Newcastle, Australia, for his advice and guidance with the statistical analyses; Debbie Booth, medical librarian at the University of Newcastle, for conducting the database searches; and Hannah Lucas, Accredited Practising Dietician for assistance with identifying the studies to be included. A. G. is the first author, conducted statistical analysis, extracted data from the studies and prepared the manuscript. A. P. K. C. and C. C. designed the review and participated in the data extraction and quality assessment and the drafting and reviewing of the manuscript. All authors have contributed to the systematic review and approved the final version of the manuscript. The authors state that there are no conflicts of interest.
References
- 1.World Health Organization (2012) Micronutrient Deficiencies. Geneva, Switzerland: World Health Organization; http://www.who.int/nutrition/topics/ida/en/index.html (accessed 2 October 2012). [Google Scholar]
- 2.World Health Organization (1992) The Prevalence of Anaemia in Women: a Tabulation of Available Information. Geneva, Switzerland: World Health Organization [Google Scholar]
- 3.Lee C, Dobson AJ, Brown WJ, et al. (2005) Cohort profile: the Australian longitudinal study on women's health. Int J Epidemiol 34, 987–991 [DOI] [PubMed] [Google Scholar]
- 4.Samman S (2007) Iron. Nutr Diet 64, S126–S130 [Google Scholar]
- 5.World Health Organization (2001) Iron Deficiency Anaemia: Assessment, Prevention, and Control. A Guide for Programme Managers. Geneva, Switzerland: World Health Organization; http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf [Google Scholar]
- 6.Cook JD (2005) Diagnosis and management of iron-deficiency anaemia. Best Pract Res Clin Haematol 18, 319–332 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization (2004) Assessing the Iron Status of Populations: Including Literature Reviews. Geneva, Switzerland: Department of Nutrition for Health and Development [Google Scholar]
- 8.Falkingham M, Abdelhamid A, Curtis P, et al. (2010) The effects of oral iron supplementation on cognition in older children and adults: a systematic review and meta-analysis. Nutr J 9, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scrimshaw NS (1991) Iron deficiency. Sci Am 265, 46–52 [DOI] [PubMed] [Google Scholar]
- 10.Scrimshaw NS (1984) Functional consequences of iron-deficiency in human-populations. J Nutr Sci Vitaminol (Tokyo) 30, 47–63 [DOI] [PubMed] [Google Scholar]
- 11.Ahmed F, Coyne T, Dobson A, et al. (2008) Iron status among Australian adults: findings of a population based study in Queensland, Australia. Asia Pac J Clin Nutr 17, 40–47 [PubMed] [Google Scholar]
- 12.Fayet F, Samman S & Truswell S (2007) Eating behaviour and biomarkers of nutritional status in female university students. Nutrition Society of Australia Abstracts.
- 13.Ferguson EL, Morison IM, Faed JM, et al. (2001) Dietary iron intakes and biochemical iron status of 15–49 year old women in New Zealand: is there a cause for concern? N Z Med J 114, 134–138 [PubMed] [Google Scholar]
- 14.Piper B, Lindsey A, Dodd M, et al. (1989) The development of an instrument to measure the subjective dimension of fatigue In Key Aspects of Comfort: Management of Pain, Fatigue and Nausea, pp. 199–208 [Funk S, Tornquist E, Champagne M, Coop L and Wiese R, editors]. New York, NY: Springer Publishing Company [Google Scholar]
- 15.Dwan K, Altman DG, Arnaiz JA, et al. (2008) Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS ONE 3, e3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterne JA, Sutton AJ, Ioannidis JP, et al. (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343, d4002. [DOI] [PubMed] [Google Scholar]
- 17.Sterne JAC, Bradburn MJ & Egger M (2008) Systematic Reviews in Health Care: Meta-Analysis in Context, 2nd ed. [Egger M, Smith GD and Altman DG, editors]. London, UK: BMJ Publishing Group [Google Scholar]
- 18.Cochrane Statistical Methods Group (2008) Analysing Data and Undertaking Meta-analyses [Deeks JJ, Higgins JP and Altman DG, editors]. Oxford: Cochrane Statistical Methods Group [Google Scholar]
- 19.Beard JL, Hendricks MK, Perez EM, et al. (2005) Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr 135, 267–272 [DOI] [PubMed] [Google Scholar]
- 20.Murray-Kolb LE & Beard JL (2007) Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr 85, 778–787 [DOI] [PubMed] [Google Scholar]
- 21.Bruner AB, Joffe A, Duggan AK, et al. (1996) Randomised study of cognitive effects of iron supplementation in non-anaemic iron-deficient adolescent girls. Lancet 348, 992–996 [DOI] [PubMed] [Google Scholar]
- 22.Ballin A, Berar M, Rubinstein U, et al. (1992) Iron state in females adolescents. Am J Dis Child 146, 803–805 [DOI] [PubMed] [Google Scholar]
- 23.Elwood PC & Hughes D (1970) Clinical trial of iron therapy on psychomotor function in anaemic women. Br Med J 3, 254–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groner JA, Holtzman NA, Charney E, et al. (1986) A randomized trial of oral iron on tests of short-term memory and attention span in young pregnant-women. J Adolesc Health 7, 44–48 [DOI] [PubMed] [Google Scholar]
- 25.Patterson AJ (1999) Iron deficiency in Australian women: development, implications and treatment. PhD Thesis, University of Newcastle, NSW [Google Scholar]
- 26.Khedr E, Hamed SA, Elbeih E, et al. (2008) Iron states and cognitive abilities in young adults: neuropsychological and neurophysiological assessment. Eur Arch Psychiatry Clin Neurosci 258, 489–496 [DOI] [PubMed] [Google Scholar]
- 27.Månsson J, Johansson G, Wiklund M, et al. (2005) Symptom panorama in upper secondary school students and symptoms related to iron deficiency. Screening with laboratory tests, questionnaire and interventional treatment with iron. Scand J Prim Health Care 23, 28–33 [DOI] [PubMed] [Google Scholar]
- 28.Kretsch MJ, Fong AK, Green MW, et al. (1998) Cognitive function, iron status, and hemoglobin concentration in obese dieting women. Eur J Clin Nutr 52, 512–518 [DOI] [PubMed] [Google Scholar]
- 29.Baddeley A (1992) Working memory. Science 255, 556–559 [DOI] [PubMed] [Google Scholar]
- 30.Patterson AJ, Brown WJ & Roberts DC (2001) Dietary and supplement treatment of iron deficiency results in improvements in general health and fatigue in Australian women of childbearing age. J Am Coll Nutr 20, 337–342 [DOI] [PubMed] [Google Scholar]
- 31.World Health Organisation (2004) Assessing the Iron Status of Populations: Including Literature Reviews: Report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level, 2nd ed.Geneva, Switzerland: Department of Nutrition for Health and Development, WHO [Google Scholar]
- 32.Baynes RD (1996) Assessment of iron status. Clin Biochem 29, 209–215 [DOI] [PubMed] [Google Scholar]
- 33.Lozoff B & Brittenham GM (1986) Behavioral aspects of iron deficiency. Prog Hematol 14, 23–53 [PubMed] [Google Scholar]
- 34.Laessle RG, Platte P, Schweiger U, et al. (1996) Biological and psychological correlates of intermittent dieting behavior in young women. A model for bulimia nervosa. Physiol Behav 60, 1–5 [DOI] [PubMed] [Google Scholar]
- 35.Lassek WD & Gaulin SJC (2008) Waist-hip ratio and cognitive ability: is gluteofemoral fat a privileged store of neurodevelopmental resources? Evol Hum Behav 29, 26–34 [Google Scholar]
- 36.Maki PM, Rich JB & Rosenbaum RS (2002) Implicit memory varies across the menstrual cycle: estrogen effects in young women. Neuropsychologia 40, 518–529 [DOI] [PubMed] [Google Scholar]
- 37.Vaucher P, Druais PL, Waldvogel S, et al. (2012) Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. Can Med Assoc J 184, 1247–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krayenbuehl PA, Battegay E, Breymann C, et al. (2011) Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood 118, 3222–3227 [DOI] [PubMed] [Google Scholar]