Abstract
Previous studies have shown that fish protein, as well as marine n-3 PUFA, may have beneficial effects on cardiovascular risk profile. The objectives of this study were to investigate the combined effects of fish gelatine (FG) and n-3 PUFA supplementation on (1) energy intake and body weight, (2) lipid profile and (3) inflammatory and CVD markers in free-living insulin-resistant males and females. Subjects were asked to consume, in a crossover study design with two experimental periods of 8 weeks each, an n-3 PUFA supplement and n-3 PUFA supplement plus FG (n-3 PUFA + FG). n-3 PUFA + FG led to an increase in protein intake and a decrease in carbohydrate intake compared with n-3 PUFA (P < 0·02) in males and females. Sex–treatment interactions were observed for TAG (P = 0·03) and highly sensitive C-reactive protein (hsCRP) (P = 0·001) levels. In females, n-3 PUFA reduced plasma TAG by 8 % and n-3 PUFA + FG by 23 %, whereas in males, n-3 PUFA reduced plasma TAG by 25 % and n-3 PUFA + FG by 11 %. n-3 PUFA increased serum hsCRP by 13 % and n-3 PUFA + FG strongly reduced hsCRP by 40 % in males, whereas in females, n-3 PUFA reduced serum hsCRP by 6 % and n-3 PUFA + FG increased hsCRP by 20 %. In conclusion, supplementation with FG may enhance the lipid-lowering effect of marine n-3 PUFA in females and beneficially counteract the effect of n-3 PUFA on serum hsCRP in males. Further studies are needed to identify the sex-dependent mechanisms responsible for the divergent effects of FG on TAG and hsCRP levels in females and males, respectively.
Key words: Fish gelatine, n-3 Fatty acids, TAG, Inflammatory markers, CVD
Abbreviations: FG, fish gelatine; hsCRP, highly sensitive C-reactive protein
Over the last few decades, dyslipidaemia and chronic low-grade inflammation associated with obesity have been described as key features of obesity-related insulin resistance, CVD and type 2 diabetes( 1 ).
Marine n-3 PUFA, EPA and DHA have been shown to contribute to cardiovascular protection by their ability to induce independent and beneficial effects on lipid profile by reducing plasma TAG and by increasing plasma HDL-cholesterol( 2 ). Furthermore, it has been proposed that dietary n-3 PUFA can reduce low-grade inflammatory state by modulating the eicosanoid system and the transcription factors NF-κB and PPAR( 3 ). Indeed, increasing evidence shows that the ingestion of EPA and DHA in human subjects may reduce the secretion of pro-inflammatory cytokines, e.g. IL-6, TNF-α, and monocyte chemoattractant protein-1, by adipocytes and macrophages( 4 ). However, other intervention trials have not confirmed these effects( 5 , 6 ) and these studies using n-3 fatty acid supplementation have failed to show any effect on the levels of systemic pro-inflammatory cytokine C-reactive protein( 5 , 6 ) even though the fish oil supplement was given at a high dose( 6 ).
CVD risk factors and type 2 diabetes have also been shown to be strongly influenced by energy intake and weight loss. Thus, the optimal macronutrient composition of a diet that facilitates lasting and safe weight loss and that improves markers of CVD risk factors remains an area of great controversy( 7 ). Interestingly, increasing the amount of protein may positively influence the regulation of food intake by improving satiety and modulating energy balance( 8 , 9 ). Besides, there has also been interest in the effect of various types of proteins on the modulation of CVD risk factors.
Fish protein has indeed been shown to induce beneficial effects on insulin sensitivity( 10 , 11 ), blood lipids( 12 – 15 ) and systemic inflammation( 16 , 17 ). In comparison with other animal proteins, fish protein has been shown to modulate lipid profile by decreasing VLDL TAG in pre- and post-menopausal females( 12 , 13 ) and increasing HDL2-cholesterol in normocholesterolaemic( 14 ) and hypercholesterolaemic males( 15 ). In addition, we( 16 ) and others( 17 ) recently observed that highly sensitive C-reactive protein (hsCRP) is reduced following a lean fish diet. The effects of fish protein on CVD risk factors have been proposed to be due, at least in part, to its unique amino acid profile rich in glycine, taurine and arginine. On the other hand, one of our previous studies( 18 ) showed that fish protein and menhaden oil exerted additive and independent effects on plasma TAG metabolism in the rat. In that study the combination of cod protein and menhaden oil lowered plasma TAG by 50 %. In recent years, there has been growing interest in studying the properties of fish gelatine (FG)( 19 ), composed of peptides and amino acids resulting from collagen hydrolysis. Indeed, there is increasing evidence for the use of collagen hydrolysate supplementation for patients with inflammatory disease( 20 ), and it has been shown that ingestion of collagen may have hypolipidaemic effects in animals( 21 , 22 ). However, there is little information about the effects of FG supplementation on lipid profile and inflammatory and CVD markers in human subjects.
Based on evidence indicating that marine n-3 PUFA and fish protein could independently reduce CVD markers, this study was designed to compare the combined effects of a FG and n-3 PUFA supplement with those of an n-3 PUFA supplement on (1) energy intake and body weight, (2) lipid profile and (3) inflammatory and CVD markers in free-living insulin-resistant subjects. We hypothesised that the FG supplement would reduce energy intake and body weight and improve the effects of marine n-3 PUFA on blood lipids, cytokines and CVD indicators in insulin-resistant males and females. As there is considerable evidence that sex hormones may modulate food intake, body weight( 23 ) and lipid and inflammatory metabolism( 24 ), we further explored possible sex differences in the effect of FG on those parameters.
Experimental methods
Subjects
A total of twenty-one subjects, ten males and eleven females, aged 35–70 years, were recruited in the Quebec City area by media advertising. All subjects completed a medical evaluation, including routine laboratory testing and a 75 g oral glucose tolerance test. Individuals were eligible if they were overweight or obese (BMI > 25 kg/m2) and were insulin-resistant with high fasting plasma insulin( 25 ) corresponding to the percentile above the 75th for fasting insulin levels in a sample from the adult Quebec population( 26 ), and/or had impaired fasting plasma glucose (5·6–6·9 mm), and/or had impaired glucose tolerance following 2 h post 75 g oral glucose tolerance test with plasma glucose corresponding to 7·8–11·0 mm( 27 ). Exclusion criteria included weight change ± 10 % within the 6 months that preceded the study, major surgery in the 3 months prior to study onset, diabetes, familial or primary hyperlipidaemia, hepatic or metabolic diseases, smoking, chronic hypertension (>160/100 mmHg), incompatibility with fish consumption (allergy, intolerance or dislike), and medications known to affect the lipid and glucose metabolism.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Clinical Research Ethical Committee of Laval University Hospital Center. After the experimental protocol was explained with precaution, written informed consent was obtained from all subjects.
Experimental design
A crossover design with two experimental periods of 8 weeks was used. After a 4-week run-in period, either an n-3 PUFA supplement alone or an n-3 PUFA supplement plus FG incorporated into a broth (n-3 PUFA + FG) was administered randomly to the subjects. At the end of the first experimental period, participants returned to their usual diet for a washout period of 12 weeks, including a second 4-week run-in period. Then, the participants crossed over to the other experimental diet for an additional 8 weeks. During the 4-week run-in periods, the participants were asked not to consume products that contained marine n-3 PUFA, to maintain their regular food habits and their physical activity level and to refrain from alcohol consumption (not more than one drink per d for females and two drinks per d for males).
Diets
The n-3 PUFA supplement provided during the n-3 PUFA and the n-3 PUFA + FG supplements corresponded to 1·8 g of EPA and DHA per d in a 2:1 ratio (capsules provided by Ocean Nutrition Canada). The FG supplement provided during the n-3 PUFA + FG treatment corresponded to approximately 25 % of the participant's daily protein consumption and was incorporated into a chicken broth. A high-molecular-weight food/pharmaceutical-grade FG (Norland Products) produced from the hydrolysis of collagen was used to prepare the FG broth. It was produced from lean fish skins, mainly from cod, pollock and haddock. Amino acid analyses of the FG were performed at Quebec's Aquaculture and Fisheries Innovation Centre, Quebec, using a technique previously described by Beaulieu et al.( 28 ). Briefly, a pre-column derivatisation technique, the AccQ-Tag amino acid analysis procedure (Waters), was used for the measurement of amino acids resistant to acidic hydrolysis. The amino acids were separated by reversed-phase HPLC and quantified using fluorescence detection. The amino acid composition of FG is shown in Table 1.
Table 1.
| Amino acids | FG (g/100 g of amino acids)‡ |
|---|---|
| Aspartic acid | 6·40 |
| Serine | 6·06 |
| Glutamic acid | 10·0 |
| Glycine | 21·5 |
| Histidine | 2·09 |
| Taurine | 0·40 |
| Arginine | 7·50 |
| Threonine | 2·66 |
| Alanine | 8·57 |
| Proline | 9·90 |
| Cysteine | 0·67 |
| Tyrosine | 0·71 |
| Valine | 1·68 |
| Methionine | 1·93 |
| Lysine | 3·91 |
| Isoleucine | 1·01 |
| Leucine | 2·20 |
| Phenylalanine | 1·84 |
Provided by Norland Products Inc. (Kenney and Ross Limited).
Amino acid measurements were carried out at Quebec's Aquaculture and Fisheries Innovation Centre, Quebec, using the technique previously described by Beaulieu et al.( 28 ).
‡ Values are the means of two determinations.
The FG broth was prepared at the metabolic kitchen of the Institute of Nutraceuticals and Functional Foods of Laval University and was provided daily to the subjects. The FG broth was consumed in different recipes at home by the participants. Compliance was assessed from the return of capsules and containers and uneaten FG broth to determine the quantity consumed, from 24 h recalls every 2 weeks and from a FFQ administrated by a dietitian, at the beginning and at the end of each experimental period. The energy and nutrient intake information derived from compilation of the FFQ was analysed using nutrient composition values from the Nutrition Data System for Research software version 4.03 (University of Minnesota, Minneapolis, MN, Food and Nutrient Database, 2000). Moreover, participants were asked to fill in a 3-d diary (two weekdays and one weekend day) during each experimental period. All participants consumed more than 80 % of the FG broth and more than 85 % of the n-3 PUFA supplements. The compliance for FG and n-3 PUFA supplements was more than 95 % for fourteen subjects.
Clinical and laboratory measurements
Anthropometric measurements
Height was measured with a stadiometer at the beginning of the study. Before and after each treatment, waist circumference and hip circumference were measured in duplicate. Waist circumference was measured at the level midway between the lowest rib margin and the iliac crest, and hip circumference was measured at the broadest circumference below the waist. Weight was recorded at the beginning and at the end of the two experimental periods, and at mid-period during each experimental period using a digital scale while subjects were barefoot. BMI was calculated as weight (in kg) divided by height (in metres) squared.
Blood collection
Blood samples were drawn after a 12 h overnight fast from an antecubital vein, before and after each experimental treatment. Blood samples were centrifuged immediately at 3000 g for 10 min at 4°C to separate plasma and serum, which were stored at −80°C until further measurements of inflammatory marker and lipid concentrations.
Inflammatory and cardiovascular marker concentrations
Inflammatory and cardiovascular marker concentrations were measured in serum, at the Laval University Hospital Center, Quebec, using commercially available methods as follows: the immunoassay Luminex xMAP Multiplexing (Millipore) was used to measure IL-6, monocyte chemotactic protein-1, TNF-α, adiponectin and myeloperoxidase. IL-1β (Invitrogen) and IL-1 receptor antagonist (Invitrogen) were measured using an ultrasensitive ELISA kit. IL-10 (Invitrogen), serum amyloid A (Invitrogen) and asymmetric dimethylarginine (ALPCO) were measured using an ELISA kit.
Other biomarker concentrations were measured at the Atherosclerosis Specialty Laboratory at St Paul's Hospital, Vancouver, using commercially available methods as follows: leptin (R&D Systems) was measured using an ELISA kit in serum. Plasminogen activator inhibitor-1 (R&D Systems) and resistin (R&D Systems) were measured using ELISA kits in plasma. hsCRP was measured in serum using the CardioPhase hsCRP immunonephelometry technique (Siemens Healthcare Diagnostics Products). Homocysteine was measured in plasma using an ADVIA Centor HCY assay (Bayer Healthcare LLC), based on a competitive immunoassay and direct chemiluminescent technique.
Plasma lipid and lipoprotein concentrations
Plasma VLDL (<1·006 g/ml) were removed from plasma using a ultracentrifugation technique as previously reported by Moorjani et al.( 29 ). LDL was precipitated with heparin and MnCl2 and HDL particles were isolated from the bottom fraction (>1·006 g/ml)( 30 ). Cholesterol and TAG concentrations were determined enzymatically by using a Technicon RA-500 analyzer (Bayer).
Statistical analyses
The a posteriori power of the study was calculated using the collected data from this study. On the basis of those data, the present study had a power of 0·73 to detect a 20 % decrease in our main outcome plasma TAG (at a two-sided α-level of 0·05) in our fifteen subjects. Statistical analysis was performed using SAS, version 9.0 (SAS Institute, Inc.). Differences in baseline characteristics between males and females were assessed by the Student's t test. The PROC MIXED procedure for an ANOVA for crossover design with two periods was used to compare the effects of the two treatments. A statistically significant level of P ≤ 0·05 was applied for all tests and the results presented are means with their standard errors. The influence of body weight, BMI, sex, and waist and hip circumferences on the changes in various metabolic parameters measured in this study was tested by individual entry of terms and interaction terms into the ANOVA model. No residual effects of the first experimental period over the second period were observed for all inflammatory, cardiovascular and lipid markers. Variable serum amyloid A was not normally distributed, so a non-parametric test using a rank transformation was used( 31 ).
Results
Subjects
A total of twenty-one insulin-resistant subjects completed the study. Of these, five subjects were excluded from the analysis because they became diabetic during the study, according to the American Diabetes Association criteria( 27 ) and, in addition, one female was pre-menopausal and was excluded from the analysis. The characteristics at baseline for the fifteen insulin-resistant subjects (seven male and eight female) are reported in Table 2. No significant differences were observed between males and females in all mean initial characteristics excepted for HDL-cholesterol (P = 0·01). All subjects were overweight or obese (BMI 26–39 kg/m2), fourteen subjects (eight female and six male) had abdominal adiposity (waist circumference >94 cm for males and >80 cm for females)( 32 ) and twelve subjects (75 %) met the National Heart, Lung, and Blood Institute/American Heart Association criteria for the metabolic syndrome( 33 ). According to the criteria for CVD risk of the Centers for Disease Control and Prevention and the American Heart Association( 34 ), five subjects (four male, one female) had baseline hsCRP concentrations <1·0 mg/l (low risk), six (three male, three female) had concentrations between 1 and 3 mg/l (moderate risk) and four females had concentrations ≥3·0 mg/l (high risk). Moreover, seven subjects (three male, four female) had optimal fasting TAG concentrations (<1·13 mm), four subjects (two male, two female) had desirable TAG concentrations (between 1·13 and 1·69 mm) and four subjects (two male, two female) had normal concentrations (between 1·7 and 2·26 mm)( 35 ). Baseline LDL-cholesterol concentrations were optimal (<2·6 mm) for one subject (one male), near or above optimal (2·6–3·3 mm) for five (four male, one female), borderline high (3·4–4·1 mm) for four females, high (4·15–4·9 mm) for three (one male, two female) and very high (≥4·9 mm) for two (one male, one female)( 36 ).
Table 2.
Subject characteristics at baseline
(Mean values with their standard errors)
| All (n 15) | Male (n 7) | Female (n 8) | ||||
|---|---|---|---|---|---|---|
| Mean | sem | Mean | sem | Mean | sem | |
| Age (years) | 57·6 | 2·3 | 54·1 | 2·9 | 61·0 | 3·2 |
| Body weight (kg) | 80·8 | 2·6 | 83·9 | 4·0 | 78·1 | 3·2 |
| BMI (kg/m2) | 29·7 | 0·9 | 28·4 | 1·0 | 30·9 | 1·5 |
| Waist circumference (cm) | 99·6 | 1·9 | 99·3 | 3·4 | 99·9 | 2·3 |
| Hip circumference (cm) | 108 | 2 | 104 | 3 | 112 | 3 |
| Cholesterol (mm) | ||||||
| Total | 5·60 | 0·31 | 5·26 | 1·44 | 5·89 | 0·34 |
| LDL | 3·74 | 0·26 | 3·52 | 1·28 | 3·93 | 0·27 |
| HDL | 1·26 | 0·05 | 1·14 | 0·18 | 1·37 | 0·04* |
| Total TAG (mm) | 1·29 | 0·11 | 1·29 | 0·46 | 1·29 | 0·16 |
| Total cholesterol:HDL-cholesterol ratio | 4·52 | 0·34 | 4·75 | 1·85 | 4·31 | 0·24 |
| Fasting plasma glucose (mm) | 6·07 | 0·11 | 5·98 | 0·12 | 6·14 | 0·18 |
| 2 h plasma glucose (mm) | 7·65 | 0·45 | 8·07 | 0·88 | 7·29 | 0·37 |
| Fasting plasma insulin (pmol/l) | 87·9 | 17·8 | 107·0 | 33·0 | 69·1 | 12·9 |
* Difference between males and females, P = 0·01.
Our subjects were all insulin-resistant. However, among them, ten subjects had hyperinsulinaemia (fasting plasma insulin >90 pmol/l)( 26 ). According to the American Diabetic Association for the Diagnosis and Classification of Diabetes Mellitus( 27 ), two subjects (one male and one female) had normal glucose tolerance, eight (four male and four female) had impaired fasting glucose (5·6–6·9 mm) and five (two male and three female) had both impaired fasting glucose and impaired glucose tolerance (7·8–11·0 mm: 2 h post 75 g oral glucose tolerance test).
Anthropometric measures
Changes in body weight (0·23 (sem 0·39) kg) for n-3 PUFA and −0·02 (sem 0·32) kg) for n-3 PUFA + FG), BMI (0·07 (sem 0·14) kg/m2 for n-3 PUFA and 0·01 (sem 0·11) kg/m2 for n-3 PUFA + FG), waist circumference (0·03 (sem 0·22) cm for n-3 PUFA and 0·47 (sem 0·36) cm for n-3 PUFA + FG) and hip circumference (−0·07 (sem 0·4) cm for n-3 PUFA and −0·03 (sem 0·21) cm for n-3 PUFA + FG) did not differ significantly between the periods.
Macronutrient intake
As shown in Table 3, n-3 PUFA + FG led to an increase in protein intake in males (21 %; P = 0·02) and females (12 %; P = 0·03) and a decrease in carbohydrate intake in males (−14 %; P = 0·01) and females (−13 %; P = 0·05) compared with the n-3 PUFA supplement. However, lipid and energy intakes did not differ during the experimentation, and no differences between the treatments were observed for these variables.
Table 3.
Daily dietary macronutrient intakes of males and females throughout the experimental periods
(Mean values with their standard errors)
| Male (n 7) | Female (n 8) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n-3 PUFA | n-3 PUFA + FG | n-3 PUFA | n-3 PUFA + FG | |||||||||||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |||||||||||
| Nutrient intakes | Mean | sem | Mean | sem | Mean | sem | Mean | sem | P value* | Mean | sem | Mean | sem | Mean | sem | Mean | sem | p* |
| Total energy (kJ) | 10137 | 1527 | 9159 | 1490 | 9678 | 1406 | 10514 | 1251 | 0·39 | 8204 | 597 | 8679 | 458 | 7836 | 684 | 8747 | 621 | 0·42 |
| Protein (% en) | 15·4 | 1·1 | 15·0 | 0·5 | 15·1 | 0·5 | 19·1 | 0·6 | 0·02 | 17·8 | 1·1 | 16·5 | 1·0 | 17·3 | 0·9 | 19·4 | 1·1 | 0·03 |
| Carbohydrates (% en) | 48·7 | 2·7 | 48·7 | 1·5 | 49·1 | 1·9 | 42·3 | 1·8 | 0·01 | 47·2 | 1·4 | 47·1 | 1·7 | 50·6 | 2·2 | 44·1 | 2·1 | 0·05 |
| Lipid (% en) | 36·7 | 2·6 | 36·3 | 1·6 | 35·8 | 1·6 | 39·3 | 1·7 | 0·19 | 35·8 | 1·7 | 37·3 | 1·5 | 33·3 | 1·9 | 37·5 | 2·0 | 0·28 |
| SFA | 33·1 | 6·5 | 28·3 | 5·5 | 32·4 | 6·0 | 34·6 | 4·5 | 0·16 | 28·1 | 4·2 | 30·5 | 3·9 | 25·6 | 3·6 | 30·4 | 3·9 | 0·58 |
| MUFA | 40·6 | 6·8 | 38·7 | 8·4 | 43·4 | 5·7 | 47·8 | 5·6 | 0·39 | 31·7 | 3·6 | 34·3 | 2·7 | 27·6 | 3·6 | 35·6 | 4·0 | 0·12 |
| PUFA | 17·6 | 3·1 | 17·5 | 3·8 | 17·1 | 2·0 | 18·5 | 2·5 | 0·72 | 13·1 | 1·6 | 15·2 | 1·1 | 12·1 | 1·9 | 15·5 | 2·1 | 0·68 |
| Fibre (g) | 25·7 | 4·0 | 20·9 | 1·4 | 25·1 | 2·1 | 22·4 | 0·8 | 0·70 | 21·0 | 1·7 | 22·0 | 1·9 | 21·1 | 1·3 | 22·6 | 1·4 | 0·76 |
FG, fish gelatine; en, energy.
* P values refer to comparisons between changes induced by the n-3 PUFA and n-3 PUFA + FG treatments (ANOVA for crossover design with two periods).
Plasma lipid concentrations, and inflammatory and CVD markers
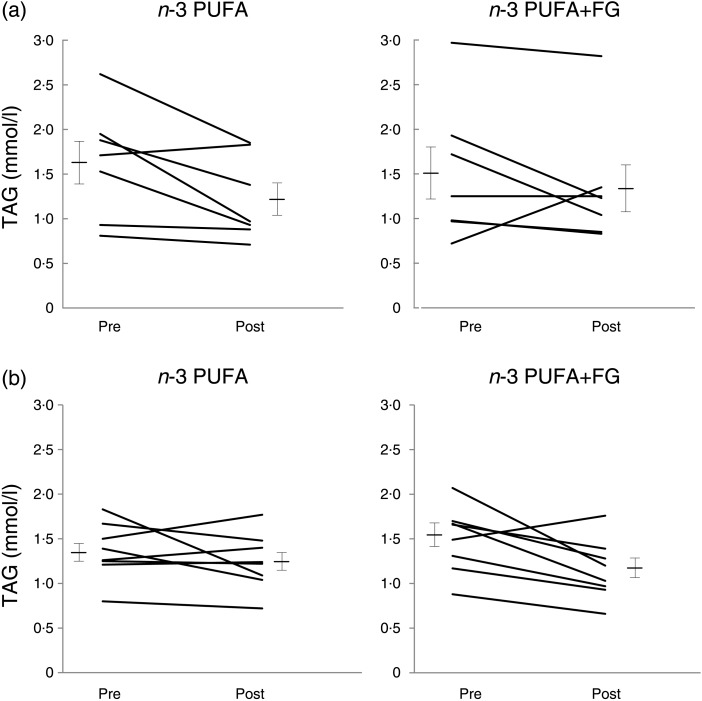
The plasma lipid concentrations as well as the inflammatory and CVD markers of the subjects are presented in Table 4. When all subjects were taken together, there were no differences in the observed changes for all lipid and lipoprotein markers between the n-3 PUFA and n-3 PUFA + FG supplements. Because a sex–treatment interaction was observed on plasma TAG concentrations (P = 0·03), we further analysed those lipid data in males and females. In males (Fig. 1(a)), 25 and 11 % decreases were observed in TAG concentrations following n-3 PUFA (1·63 (sem 0·24)–1·22 (sem 0·18) mm) and n-3 PUFA + FG (1·51 (sem 0·29)–1·34 (sem 0·29) mm), respectively. However, no difference was observed between changes induced by the two treatments (P = 0·14). In females (Fig. 1(b)), an 8 % reduction in TAG was observed after n-3 PUFA (1·36 (sem 0·11)–1·25 (sem 0·11) mm), whereas a 23 % reduction was observed after n-3 PUFA + FG (1·49 (sem 0·13)–1·15 (sem 0·12) mm), inducing a difference in the changes of TAG between the n-3 PUFA and n-3 PUFA + FG supplements (P = 0·04).
Table 4.
Concentrations of blood lipids, inflammatory and CVD markers before (pre) and after (post) consuming n-3 PUFA and n-3 PUFA + fish gelatine (FG) for 8 weeks in insulin-resistant males and females*
(Mean values with their standard errors)
| n-3 PUFA (n 15) | n-3 PUFA + FG (n 15) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | ∆ | Pre | Post | ∆ | ||||||||
| Mean | sem | Mean | sem | Mean | sem | Mean | sem | Mean | sem | Mean | sem | P† | |
| Blood lipids (mm) | |||||||||||||
| Total cholesterol | 5·78 | 0·34 | 5·55 | 0·28 | −0·23 | 0·12 | 5·45 | 0·28 | 5·41 | 0·30 | −0·04 | 0·10 | 0·22 |
| TAG | 1·49 | 0·13 | 1·23 | 0·10 | −0·26 | 0·10 | 1·50 | 0·15 | 1·24 | 0·13 | −0·26 | 0·10 | 0·98 |
| HDL-cholesterol | 1·07 | 0·04 | 1·12 | 0·05 | 0·05 | 0·03 | 1·03 | 0·05 | 1·13 | 0·06 | 0·09 | 0·03 | 0·18 |
| LDL-cholesterol | 4·03 | 0·30 | 3·87 | 0·26 | −0·16 | 0·08 | 3·73 | 0·23 | 3·72 | 0·26 | −0·02 | 0·09 | 0·22 |
| Total cholesterol:HDL-cholesterol ratio | 5·54 | 0·47 | 5·12 | 0·40 | −0·42 | 0·13 | 5·46 | 0·44 | 5·00 | 0·45 | −0·46 | 0·10 | 0·79 |
| Inflammatory and CVD markers | |||||||||||||
| MCP-1 (pg/ml)* | 672 | 42 | 685 | 46 | 13 | 53 | 597 | 58 | 651 | 42 | 54 | 36 | 0·17 |
| TNF-α (pg/ml)* | 6·83 | 1·41 | 5·88 | 0·71 | −0·96 | 0·88 | 4·92 | 0·50 | 5·34 | 0·49 | 0·42 | 0·45 | 0·23 |
| hsCRP (mg/l)* | 1·64 | 0·36 | 1·65 | 0·43 | 0·00 | 0·21 | 1·78 | 0·35 | 1·74 | 0·54 | −0·04 | 0·31 | 0·81 |
| IL-1ra (pg/ml) | 116 | 8 | 115 | 11 | −1 | 8 | 120 | 9 | 114 | 7 | −6 | 6 | 0·65 |
| IL-6 (pg/ml) | 123·6 | 39·7 | 86·8 | 28·7 | −36·8 | 39·0 | 105·9 | 30·8 | 63·0 | 25·5 | −42·9 | 31·1 | 0·99 |
| PAI-1 (ng/ml) | 2·49 | 0·45 | 3·69 | 0·74 | 1·20 | 0·65 | 1·96 | 0·47 | 3·41 | 0·54 | 1·45 | 0·37 | 0·76 |
| Adiponectin (μg/ml) | 14·1 | 1·3 | 14·7 | 1·6 | 0·56 | 0·73 | 14·3 | 1·4 | 14·2 | 1·57 | −0·06 | 0·51 | 0·42 |
| Homocysteine (μmol/l) | 10·24 | 0·45 | 9·69 | 0·46 | −0·55 | 0·39 | 9·57 | 0·58 | 9·93 | 0·43 | 0·36 | 0·46 | 0·12 |
| Leptin (ng/ml) | 16·2 | 2·7 | 14·8 | 2·5 | −1·4 | 1·0 | 17·7 | 3·7 | 15·9 | 2·7 | −1·8 | 2·0 | 0·79 |
| Cystatin C (mg/l) | 0·76 | 0·03 | 0·73 | 0·03 | −0·03 | 0·01 | 0·73 | 0·03 | 0·71 | 0·03 | −0·02 | 0·02 | 0·62 |
| Resistin (ng/ml) | 25·5 | 2·5 | 37·2 | 4·6 | 11·8 | 3·0 | 26·5 | 2·6 | 33·7 | 4·10 | 7·2 | 3·5 | 0·32 |
| Serum amyloid A (μg/ml) | 84·6 | 37·3 | 50·3 | 6·5 | −34·3 | 37·1 | 113·9 | 60·7 | 65·3 | 11·0 | −48·6 | 62·0 | 0·35 |
| Myeloperoxidase (pg/ml) | 40·8 | 11·6 | 79·1 | 29·4 | 38·3 | 19·4 | 43·0 | 9·0 | 65·5 | 15·9 | 22·5 | 15·9 | 0·42 |
| ADMA* (μg/l) | 86·5 | 2·6 | 112·2 | 14·8 | 25·7 | 14·4 | 82·9 | 4·4 | 98·9 | 10·8 | 15·9 | 11·6 | 0·47 |
MCP-1, monocyte chemotactic protein-1; hsCRP, highly sensitive C-reactive protein; IL-1ra, IL-1 receptor antagonist; PAI-1, plasminogen activator inhibitor 1; ADMA, asymmetric dimethylarginine.
n 14 for ADMA, MCP-1 and TNF-α, n 13 for hsCRP.
† P values refer to comparisons between changes induced by the n-3 PUFA and n-3 PUFA + FG treatments (ANOVA for crossover design with two periods).
Fig. 1.
Plasma TAG concentrations before (pre) and after (post) consuming n-3 PUFA and n-3 PUFA + fish gelatine (FG) (a) in males and (b) in females for 8 weeks. Values are means, with standard errors represented by vertical bars, n 7 for males, and n 8 for females. P value for sex–treatment interaction = 0·03. P value is 0·04 between changes induced by the n-3 PUFA and n-3 PUFA + FG supplements in females (ANOVA for crossover design with two experimental periods).
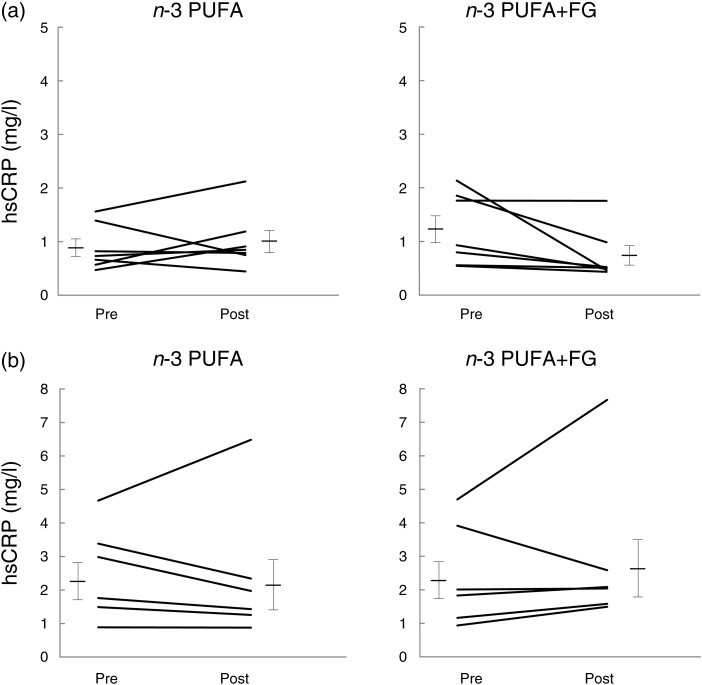
Changes in concentrations of inflammatory and CVD markers were not different between the two treatments (Table 4). However, a sex–treatment interaction was also observed for serum hsCRP concentrations (P = 0·001). In males, n-3 PUFA + FG reduced serum hsCRP by 40 % (1·23 (sem 0·25)–0·74 (sem 0·18) mg/l) whereas n-3 PUFA increased hsCRP by 13 % (0·89 (sem 0·16)–1·01 (sem 0·20) mg/l) (Fig. 2(a)), provoking a difference in hsCRP changes between n-3 PUFA and n-3 PUFA + FG (P = 0·03). In females, n-3 PUFA + FG increased serum hsCRP by 20 % (2·43 (sem 0·63)–2·91 (sem 0·97) mg/l) whereas the n-3 PUFA supplement induced a 6 % reduction only (2·53 (sem 0·57)–2·39 (sem 0·84) mg/l) (Fig. 2(b)). There was thus no difference between hsCRP changes induced by the two treatments in females (P = 0·69). In the present study, there was a sex–treatment interaction on monocyte chemotactic protein-1 (P = 0·04) but because it was attributed to two hyper-responders to the n-3 PUFA supplement, it does not seem to be of biological relevance in this study.
Fig. 2.
Plasma highly sensitive C-reactive protein (hsCRP) concentrations before (pre) and after (post) consuming n-3 PUFA and n-3 PUFA + fish gelatine (FG) (a) in males and (b) in females for 8 weeks. Values are means, with standard errors represented by vertical bars, n 7 for males, and n 6 for females. P value for sex–treatment interaction is 0·001. P value is 0·03 between changes induced by the n-3 PUFA and n-3 PUFA + FG supplements in males (ANOVA for crossover design with two experimental periods).
Discussion
The present free-living study investigated the effects of 8-week interventions with n-3 PUFA and FG on lipid profile and inflammatory and CVD markers in males and females with insulin resistance, a population with a known risk for CVD. The marine n-3 PUFA supplement was used as the control. The main findings of the study are that FG may enhance the lipid-lowering effect of fish n-3 PUFA in females and counteract the effect of n-3 PUFA on plasma hsCRP in males. The concept that n-3 PUFA and fish protein could induce combined reducing effects on TAG levels has emerged from an animal study( 18 ), but few studies have examined these effects on blood lipid profile in human subjects. Because it has been shown that plasma TAG is one of the strongest predictors for the risk of atherosclerosis and cardiovascular events in subjects with and without CVD, this marker was chosen as the primary endpoint in the present study. Note that our study had a posteriori power of 70–80 % to detect a 20 % decrease in plasma TAG concentrations. This percentage decrease was considered relevant because optimisation of nutrition-related practices recommended by the American Heart Association can result in a marked TAG-lowering effect that ranges between 20 and 50 %( 35 ).
Fish oil consumption has well-documented and potent TAG-lowering effects( 2 ). A comparable dose of n-3 PUFA to the one used in the present study (1·8 g/d) has been shown to reduce plasma fasting TAG by approximately 20 % (28-d intervention)( 37 ). In the present study, an average 17 % decrease in plasma TAG was observed in response to the n-3 PUFA treatment in our fifteen subjects. In males, the n-3 PUFA supplementation decreased TAG concentrations by 25 %, whereas in females, it decreased TAG by only 8 %. These results are in good agreement with those of the FINGEN Study( 38 ), showing a sex-treatment interaction in 312 adults aged 20–70 years consuming a moderate EPA/DHA (1·8 g EPA–DHA/d) in a double-blind placebo-controlled crossover study for an 8-week intervention period. As in the present study, the FINGEN Study indicated a greater TAG-lowering action of n-3 PUFA in males than in females.
It is well accepted that dietary protein intake contributes more strongly than carbohydrate or fat intake to short-term satiety which, in turn, can have reducing effects on energy intake, body weight and plasma lipids, although data in human subjects are still inconclusive( 8 , 9 ). In this study, an increase in consumed energy from protein and a decrease in energy intake from carbohydrate in both males and females were observed with FG supplementation without a change in the total energy intake, body weight, BMI and waist circumference. No changes were observed in fibre intake, a nutrient known to affect satiety and energy intake( 39 ). There is thus a possibility that the effect upon satiety and energy adjustment of dietary protein in liquid form may evoke weaker satiety signals than in the solid form because of more rapid stomach emptying and transit of fluids through the digestive tract( 40 ).
Wolfe & Piche( 41 ) and Wolfe & Giovannetti( 42 ) have reported that the replacement of dietary carbohydrate by protein could lower plasma TAG, but in the present study, the reducing effect of this substitution on TAG was observed in females only. The present results thus indicate that the consumption of n-3 PUFA with or without FG reduced plasma TAG, and the magnitude of response depended on the treatment and on sex. Indeed in the present study, females had significantly lower plasma TAG following n-3 PUFA + FG (−23 %) than n-3 PUFA (−8 %) (P = 0·04). By contrast, males had a more pronounced plasma TAG reduction following n-3 PUFA (−25 %) than n-3 PUFA + FG (−11 %), albeit without reaching the level of significance. The higher variability of TAG changes and the low number of subjects could explain the absence of significant differences between the two treatments in males. According to Hokanson & Austin( 43 ), reductions in moderately elevated TAG levels decrease cardiovascular risk more in females than in males. Nevertheless, the marked TAG-lowering response either to n-3 PUFA in males or to n-3 PUFA + FG in females was in good agreement with the 20–50 % TAG reduction expected from American Heart Association nutritional recommendations( 35 ). These results suggest that n-3 PUFA in males and n-3 PUFA + FG in females can be effective in helping to reduce plasma TAG.
The TAG-lowering response to FG in females and not in males is consistent with the results of previous human studies showing a decreasing effect of lean white fish diet on TAG metabolism in pre-menopausal( 12 ) and post-menopausal females( 13 ), but not in normolipidaemic males( 14 ). The observed TAG sex-dependent response in the present study could be associated with an increase of sex hormone-binding globulin following the consumption of fish protein in post-menopausal females( 13 ). Increased testosterone and decreased sex hormone-binding globulin have been linked to adverse cardiovascular risk factors, such as adiposity, increased TAG and decreased HDL levels in both pre-menopausal and post-menopausal females( 44 ). Furthermore, lower sex hormone-binding globulin levels predict non-insulin-dependent diabetes mellitus in females but not in males( 45 ). Additionally, a previous study conducted in our laboratory demonstrated that fish protein can reduce insulin resistance( 11 ). Thus, the present results suggest that the divergent effects of FG supplementation on plasma TAG between males and females may be mediated by variations in the status of plasma sex hormones, which are related to glucose tolerance and insulin sensitivity. Further studies on the mechanisms involved in the hypotriacylglycerolaemic effects of FG in females are needed.
FG per se could also have modulated plasma TAG levels in the present study. Our data are in line with those of Saito et al.( 21 ) who reported a decrease in plasma TAG concentrations in rats fed hydrolysates of skin collagen. They observed that TAG levels were inversely correlated with plasma total hydroxyproline, total glycine and total proline concentrations, three major amino acids derived from low-molecular-weight collagen fish peptides( 21 ). Hafidi et al.( 46 ) and Ratnayake et al.( 47 ) have also reported a decrease in TAG levels following a diet rich in glycine in rats fed a high-sucrose diet, suggesting that glycine could have contributed to the observed TAG-lowering effects following the consumption of the FG broth in this study. The lower lysine:arginine ratio of fish protein and gelatine compared with casein may also have been involved( 48 ). Moreover based on studies on fish protein in rats( 18 ) and rabbits( 49 ), there is a possibility that FG per se may have contributed to decrease plasma TAG by increasing lipoprotein lipase activity.
Apart from the primary endpoint, inflammatory and other CVD markers (hsCRP, TNF-α, Il-6, adiponectin and others) were assessed. Our data showed a sex–treatment interaction for hsCRP (P = 0·001). In males, while n-3 PUFA increased plasma hsCRP concentrations by 13 %, n-3 PUFA + FG reduced these levels by 40 %. This reduction was considered to be of biological significance because, on average, the CVD risk based on plasma C-reactive protein concentrations decreased from moderate to low risk in males( 34 ). The C-reactive protein-lowering effect of FG in males is consistent with our previous observations showing that short-term consumption of cod protein compared with other animal proteins led to a decrease in hsCRP in insulin-resistant subjects, mostly males( 16 ). However, in the present study, the serum hsCRP concentration was increased in females by 20 % following n-3 PUFA + FG and decreased by 6 % following n-3 PUFA.
There is, however, one limitation that needs to be addressed regarding the present study. This limitation concerns the absence of no intervention and FG treatments to clearly highlight the effects of FG. Although it has already been shown that fish protein and fish oil have independent and additive effects on plasma TAG in rats( 18 ) and thus that n-3 PUFA supplement can be used as the control group, firm conclusions in this respect cannot be drawn in the present human study. Therefore, in this study, the findings cannot be generalised beyond the comparison between the effects of marine n-3 PUFA and those of n-3 PUFA plus FG.
In conclusion, our data showed that, in free-living conditions, FG supplementation and a concomitant decrease in carbohydrate intake enhanced the lipid-lowering effect of n-3 PUFA supplementation in insulin-resistant females, and beneficially counteracted the effect of n-3 PUFA on plasma C-reactive protein in insulin-resistant males. These results suggest that FG may exert a beneficial effect on CVD risk by lipid-lowering and anti-inflammatory effects in free-living insulin-resistant females and males, respectively. Further studies are needed to elucidate the physiological mechanisms underlying the divergent effects of n-3 PUFA and FG on TAG and hsCRP levels between males and females, and to identify specific bioactive peptides and amino acids that contribute to these effects.
Acknowledgements
This research was supported by a grant from The Advanced Foods and Materials Network. There are no conflicts of interest in this research. Ocean Nutrition Canada provided marine n-3 PUFA capsules, and Norland Products provided fish gelatine. The authors thank Simon Pelletier, Patricia Pelletier, Marc Majaron, Marie-Élise Carbonneau and Nadine Leblanc for technical and skilful assistance. As to the contribution of each author to the manuscript, H. J. designed the experiment and discussed the experimental results with all the other co-authors, supervised E. P. D. and J. B. and assisted E. P. D. in writing the manuscript. S. J. W. helped in designing the experiment, was responsible for the medical follow-up of the participants and discussed the experimental results. As graduate students, E. P. D. and J. B. carried out the experiment under the supervision of H. J., S. J. W., J. M. and C. L. and discussed the experimental results. As a research coordinator, J.M. helped in designing the study and the experiment plan, supervised and advised E. P. D and J. B. on recruitment and nutritional aspects of the experiment, and discussed the experimental results. C. L. and A. M. helped E. P. D. to carry out measurements of inflammatory markers and to interpret data during the experiment. As a doctoral student, E. C. supervised by J. S. H. helped E. P. D. to carry out measurements of cardiovascular markers. E. C., J. S. H. and J. F. discussed the experimental results and supervised E. P. D.'s training in their laboratory. B. H. helped in designing the study and to interpret the data.
References
- 1.Schenk S, Saberi M & Olefsky JM (2008) Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 118, 2992–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris WS, Michael M, Ann PT, et al. (2008) Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis 197, 12–24 [DOI] [PubMed] [Google Scholar]
- 3.Calder PC (2006) n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 83, 1505S–1519S [DOI] [PubMed] [Google Scholar]
- 4.Schacky C (2007) n-3 PUFA in CVD: influence of cytokine polymorphism. Proc Nutr Soc 66, 166–170 [DOI] [PubMed] [Google Scholar]
- 5.Vega-Lopez S, Kaul N, Devaraj S, et al. (2004) Supplementation with omega-3 polyunsaturated fatty acids and all-rac alpha-tocopherol alone and in combination failed to exert an anti-inflammatory effect in human volunteers. Metabolism 53, 236–240 [DOI] [PubMed] [Google Scholar]
- 6.Mori TA, Woodman RJ, Burke V, et al. (2003) Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radical Biol Med 35, 772–781 [DOI] [PubMed] [Google Scholar]
- 7.Klein S, Sheard NF, Pi-Sunyer X, et al. (2004) Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. A statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Am J Clin Nutr 80, 257–263 [DOI] [PubMed] [Google Scholar]
- 8.Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, et al. (2009) Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr 29, 21–41 [DOI] [PubMed] [Google Scholar]
- 9.Halton LT & Hu FB (2004) The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr 23, 373–385 [DOI] [PubMed] [Google Scholar]
- 10.Lavigne C, Tremblay F, Asselin G, et al. (2001) Prevention of skeletal muscle insulin resistance by dietary cod protein in high fat-fed rats. Am J Physiol Endocrinol Metab 281, E62–E71 [DOI] [PubMed] [Google Scholar]
- 11.Ouellet V, Marois J, Weisnagel SJ, et al. (2007) Dietary cod protein improves insulin sensitivity in insulin-resistant men and women. Diabetes Care 30, 2816–2821 [DOI] [PubMed] [Google Scholar]
- 12.Gascon A, Jacques H, Moorjani S, et al. (1996) Plasma lipoprotein profile and lipolytic activities in response to the substitution of lean white fish for other animal protein sources in premenopausal women. Am J Clin Nutr 63, 315–321 [DOI] [PubMed] [Google Scholar]
- 13.Jacques H, Noreau L & Moorjani S (1992) Effects on plasma lipoproteins and endogenous sex hormones of substituting lean white fish for other animal protein sources in diets of postmenopausal women. Am J Clin Nutr 55, 896–901 [DOI] [PubMed] [Google Scholar]
- 14.Lacaille B, Julien P, Deshaies Y, et al. (2000) Responses of plasma lipoproteins and sex hormones to the consumption of lean fish incorporated in a prudent-type diet in normolipidemic men. J Am Coll Nutr 19, 745–753 [DOI] [PubMed] [Google Scholar]
- 15.Beauchesne-Rondeau E, Gascon A, Bergeron J, et al. (2003) Plasma lipids and lipoproteins in hypercholesterolemic men fed a lipid-lowering diet containing lean beef, lean fish, or poultry. Am J Clin Nutr 77, 587–593 [DOI] [PubMed] [Google Scholar]
- 16.Ouellet V, Weisnagel J, Marois J, et al. (2008) Dietary cod protein reduces plasma C-reactive protein in insulin-resistant men and women. J Nutr 138, 2386–2391 [DOI] [PubMed] [Google Scholar]
- 17.Pot GK, Geelen A & Majsak-Newman G (2010) Increased consumption of fatty and lean fish reduces serum C-reactive protein concentrations but not inflammation markers in feces and in colonic biopsies. J Nutr 140, 371–376 [DOI] [PubMed] [Google Scholar]
- 18.Demonty I, Deshaies Y, Lamarche B, et al. (2003) Cod protein lowers the hepatic triglyceride secretion rate in rats. J Nutr 133, 1398–1402 [DOI] [PubMed] [Google Scholar]
- 19.Karim AA & Bhat R (2009) Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocolloids 23, 563–576 [Google Scholar]
- 20.Bello AE & Oesser S (2006) Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders: a review of the literature. Curr Med Res Opin 22, 2221–2232 [DOI] [PubMed] [Google Scholar]
- 21.Saito M, Kiyose C, Higuchi T, et al. (2009) Effect of collagen hydrolysates from salmon and trout skins on the lipid profile in rats. J Agric Food Chem 57, 10477–10482 [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Fujioka M, Sugimoto K et al. (2004) Assessment of effectiveness of oral administration of collagen peptides on bone metabolism in growing and mature rats. J Bone Miner Metab 22, 547–553 [DOI] [PubMed] [Google Scholar]
- 23.Shi H & Clegg DJ (2009) Sex differences in the regulation of body weight. Physiol Behav 97, 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilote L, Dasgupta K, Guru V, et al. (2007) A comprehensive view of sex-specific issues related to cardiovascular disease. Can Med Assoc J 176, S1–S44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahn SE, McCulloch DK & Porte D (1997) Insulin secretion in the normal and diabetic human In International Textbook of Diabetes Mellitus, 2nd ed., pp. 337–354 [Alberti KGMM, Zimmet P, Defronzo RA and H Keen, editors]. New York: John Wiley & Sons [Google Scholar]
- 26.Scarsella C, Almeras N, Mauriege P, et al. (2000) Determination of reference values for fasting insulin levels in a representative sample of the adult Quebec population. Atherosclerosis 151, 101. [Google Scholar]
- 27.American Diabetes Association (2010) Standards of medical care in diabetes (position statement). Diabetes Care 33, S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beaulieu L, Thibodeau J, Bryl P, et al. (2009) Proteolytic processing of Atlantic mackerel (Scomber scombrus) and biochemical characterisation of hydrolysates. Int J Food Sci Tech 44, 1609–1618 [Google Scholar]
- 29.Moorjani S, Gagné C, Lupien PJ, et al. (1986) Plasma triglycerides related decrease in high-density lipoprotein cholesterol and its association with myocardial infarction in heterozygous familial hypercholesterolemia. Metabolism 35, 311–316 [DOI] [PubMed] [Google Scholar]
- 30.Burstein M & Samaille J (1960) Sur un dosage rapide du cholestérol lié aux B-lipoproteins du sérum (On a rapid determination of cholesterol bound to serum alpha- and beta-lipoproteins). Clin Chim Acta 5, 609–610 [DOI] [PubMed] [Google Scholar]
- 31.Conover WJ (1999) Some methods based on ranks In Practical Nonparametric Statistics, 3rd ed., pp. 419–420 [Wiley B II, O'Sullivan M and Perea J, editors]. New York: John Wiley & Sons [Google Scholar]
- 32.Alberti KG, Zimmet P & Shaw J (2006) Metabolic syndrome – a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med 23, 469–480 [DOI] [PubMed] [Google Scholar]
- 33.Grundy SM, Bryan HB, Cleeman JI, et al. (2004) Definition of metabolic syndrome report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109, 433–438 [DOI] [PubMed] [Google Scholar]
- 34.Pearson TA, Mensah GA, Alexander RW, et al. (2003) Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107, 499–511 [DOI] [PubMed] [Google Scholar]
- 35.Miller M, Stone NJ, Ballantyne C, et al. (2011) Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 123, 2292–2333 [DOI] [PubMed] [Google Scholar]
- 36.National Cholesterol Education Program (2002) Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) Final Report. Circulation 106, 3143–3421 [PubMed] [Google Scholar]
- 37.Stark KD & Holub BJ (2004) Differential eicosapentaenoic acid elevations and altered cardiovascular disease risk factor responses after supplementation with docosahexaenoic acid in postmenopausal women receiving and not receiving hormone replacement therapy. Am J Clin Nutr 79, 765–773 [DOI] [PubMed] [Google Scholar]
- 38.Caslake MJ, Miles EA, Kofler BM, et al. (2008) Effect of sex and genotype on cardiovascular biomarker response to fish oils: the FINGEN Study. Am J Clin Nutr 88, 618–629 [DOI] [PubMed] [Google Scholar]
- 39.Burton-Freeman B (2000) Dietary fiber and energy regulation. J Nutr 130, 272S–275S [DOI] [PubMed] [Google Scholar]
- 40.DiMeglio DP & Mattes RD (2000) Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes 24, 794–800 [DOI] [PubMed] [Google Scholar]
- 41.Wolfe BM & Piche LA (1999) Replacement of carbohydrate by protein in a conventional-fat diet reduces cholesterol and triglyceride concentrations in healthy normolipidemic subjects. Clin Invest Med 22, 140–148 [PubMed] [Google Scholar]
- 42.Wolfe BM & Giovannetti PM (1991) Short-term effects of substituting protein for carbohydrate in the diets of moderately hypercholesterolemic human subjects. Metabolism 40, 338–343 [DOI] [PubMed] [Google Scholar]
- 43.Hokanson JE & Austin MA (1996) Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk 3, 213–219 [PubMed] [Google Scholar]
- 44.Pugeat M, Moulin P, Cousin P, et al. (1995) Interrelations between sex hormone-binding globulin (SHBG), plasma lipoproteins and cardiovascular risk. J Steroid Biochem Mol Biol 53, 567–572 [DOI] [PubMed] [Google Scholar]
- 45.Haffner SM, Valdez RA, Morales PA, et al. (1993) Decreased sex hormone-binding globulin predicts noninsulin-dependent diabetes mellitus in women but not in men. J Clin Endocrinol Metab 77, 56–60 [DOI] [PubMed] [Google Scholar]
- 46.Hafidi M, Perez I, Zamora J, et al. (2004) Glycine intake decreases plasma free fatty acids, adipose cell size, and blood pressure in sucrose-fed rats. Am J Physiol Regul Integr Comp Physiol 287, R1387–R1393 [DOI] [PubMed] [Google Scholar]
- 47.Ratnayake N, Sarwar G & Laffey P (1997) Influence of dietary protein and fat on serum lipids and metabolism of essential fatty acids in rats. Br J Nutr 78, 459–467 [DOI] [PubMed] [Google Scholar]
- 48.Pfeuffer M & Barth CA (1992) Dietary sucrose but not starch promotes protein-induced differences in rates of VLDL secretion and plasma lipid concentrations in rats. J Nutr 122, 1582–1586 [DOI] [PubMed] [Google Scholar]
- 49.Bergeron N, Deshaies Y & Jacques H (1992) Dietary fish protein modulates high density lipoprotein cholesterol and lipoprotein lipase activity in rabbits. J Nutr 122, 1731–1737 [DOI] [PubMed] [Google Scholar]