Abstract
The Papanicolaou Society of Cytopathology has developed a set of guidelines for pancreatobiliary cytology including indications for endoscopic ultrasound guided fine needle aspiration, terminology and nomenclature of pancreatobiliary disease, ancillary testing and post-biopsy management. All documents are based on the expertise of the authors, a review of the literature, discussion of the draft document at several national and international meetings and synthesis of selected online comments of the draft document. This document presents the results of these discussions regarding the use of ancillary testing in the cytologic diagnosis of biliary and pancreatic lesions. Currently, fluorescence in-situ hybridization (FISH) appears to be the most clinically relevant ancillary technique for cytology of bile duct strictures. The addition of FISH analysis to routine cytologic evaluation appears to yield the highest sensitivity without loss in specificity. Loss of immunohistochemical staining for the protein product of the SMAD4 gene and positive staining for mesothelin support a diagnosis of ductal adenocarcinoma. Immunohistochemical markers for endocrine and exocrine differentiation are sufficient for a diagnosis of endocrine and acinar tumors. Nuclear staining for beta-catenin supports a diagnosis of solid-pseudopapillary neoplasm. Cyst fluid analysis for amylase and carcinoembryonic antigen aids in the pre-operative classification of pancreatic cysts. A number of gene mutations (KRAS, GNAS, von Hippel-Lindau, RNF43 and CTNNB1) may be of aid in the diagnosis of cystic neoplasms. Other ancillary techniques do not appear to improve diagnostic sensitivity sufficiently to justify their increased costs.
Keywords: Ancillary studies, biliary tract, endoscopic ultrasound, fine-needle aspiration, molecular diagnosis, pancreas
INTRODUCTION
Ancillary testing of pancreatobiliary cytology specimens is becoming increasingly important as we learn more about the molecular mutations associated with pancreatobilary disease and develop clinically useful tests that aid in the diagnosis and management of patients. Management algorithms for pancreatic neoplasia are evolving; some are shifting from surgical intervention to conservative surveillance, while others are recommending pre-operative chemoradiation prior to surgery to improve the yield of R0 resections. As an invaluable member of the patient care team, the pathologist is at the forefront of diagnosis, both pre- and post-operatively. Ancillary tests are invaluable tools that assist in refining our diagnoses and include a wide range of biochemical and molecular tests with variable diagnostic and prognostic utility.
These proposed guidelines on ancillary testing of pancreatobiliary cytology specimens stems from the expertise of the authors, review of the literature, discussions with pathologists at several national and international meetings over an 18 month period and synthesis of selected online comments of the draft document on the Papanicolaou Society of Cytopathology web site [www.papsociety.org].
ANCILLARY TESTING OF PANCREATOBILIARY STRICTURES
Cytological evaluation of bile duct brushings is known to have suboptimal sensitivity ranging from 40% to 61%, respectively.[1,2,3,4,5,6,7,8,9,10,11] A number of approaches have been developed to augment the purely cytologic analysis of bile duct brushing specimens. These have included protocols to improve diagnostic sensitivity of brushing specimens designated as inconclusive, indeterminate or negative following on-site cytopathologic examination.[3,12,13,14] Ancillary procedures potentially useful in interpretation of negative and indeterminate cytological results include intraductal ultrasound examination, digital image analysis (DIA), immuno-labeling, fluorescence in-situ hybridization (FISH), genetic analysis for neoplasia-specific mutations and sequential mutational analysis.[1,2,3,4,5,6,7,8,9,10,11,12,13,15] Table 1 demonstrates markers which have been used in ancillary testing of brushing specimens.
Table 1.
Proposed ancillary testing for pancreatobiliary trictures
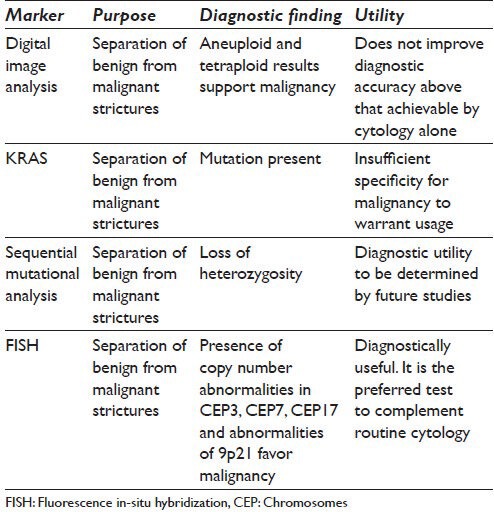
DIA of samples from duct strictures
At the level of the biosample, DIA has been used to identify abnormalities of nuclear deoxyribonucleic acid (DNA) content using spectrophotometric techniques.[16,17,18] DIA utilizes Feulgen reactions which hydrolyze DNA into constituent nucleic acids that stoichiometrically bind to the Feulgen dye. Using this technique, DNA ploidy can be assessed by a variety of commercially available image analyzers.[16,19] DNA ploidy status is assessed on the collected cells using a histogram generated by commercially available quantitative DNA analysis programs. Results are characterized as diploid, aneuploid or tetraploid. Aneuploid and tetraploid results are supportive of malignancy.[8]
The sensitivity of DIA does not appear to improve diagnostic accuracy beyond that achievable with routine cytology for patients with primary sclerosing cholangitis. However, in patients without primary sclerosing cholangitis, the technique does appear to improve diagnostic sensitivity. DIA has been reported to have excellent specificity for the diagnosis of carcinoma but only moderate sensitivity.[17,18] Thus, DIA has diagnostic characteristics similar to routine cytology in that a positive test is highly accurate but a negative result is of little clinical value.
Immunocytochemistry
A number of immunocytochemical markers have shown promise distinguishing benign from malignant biliary epithelium. These include S100P, von Hippel-Lindau gene product (pVHL), IMP3, and CD10. Tretiakova et al.[20] have demonstrated loss of CD10 immunopositivity in most examples of high-grade dysplasia and all cases of invasive carcinoma. CD10 positivity was present in the overwhelming majority of benign lesions. In a separate study, Levy et al.[21] have demonstrated strong staining for S100P in 90% of adenocarcinomas with 83% of adenocarcinomas showing diffuse staining for S100P. IMP3 staining was present in 77.5% of adenocarcinomas. Total loss of pVHL staining was seen in 37 of 40 adenocarcinomas. Nearly 70% of adenocarcinomas display the staining pattern S100P+/IMP3+/pVHL−.[21] Benign biopsies were invariably negative for S100P, but 94% were pVHL positive.[21] As discussed in the section on solid pancreatic lesions, adenocarcinomas frequently stain for MUC4 and mesothelin (MSLN) but demonstrate an absence of staining for clusterin-beta.[22]
MOLECULAR ANALYSIS OF DUCT STRICTURE BRUSHING SPECIMENS
KRAS mutational analysis
KRAS gene mutation analysis has been used as an ancillary testing procedure for analysis of pancreatic juice, bile duct brushings and fine-needle aspiration (FNA) of solid and cystic pancreatic masses.[23,24,25] The majority of studies involving KRAS mutations have investigated their relationship with pancreatic adenocarcinoma[26,27,28,29] and genetic progression in pancreatic duct lesions and intraductal papillary mucinous neoplasms (IPMN).[30,31,32] KRAS mutational analysis of pancreatic juice has even been used as a potential method for the early diagnosis of pancreatic carcinoma.[33,34,35] Although KRAS mutations can be found in pre-invasive dysplastic lesions and invasive carcinomas of the pancreas,[23,24,25,26,27,28,29,30] several studies have shown that KRAS mutation analysis is a sensitive test for pancreatic adenocarcinoma.[36,37,38] Sturm et al.[24] studied 312 consecutive patients with extrahepatic biliary stenosis and found that conventional cytology combined with KRAS mutational analysis was more sensitive than conventional cytology alone. Kipp et al.[39] studied 35 brushing cytology samples collected during endoscopic retrograde cholangiopancreatography and demonstrated a combined sensitivity of 86% for KRAS mutation and FISH analyses in the diagnosis of pancreatic adenocarcinoma. Hruban et al.[31] have described a progression model for pancreatic carcinoma in which both KRAS and telomere abnormalities are seen in low grade dysplasias as well as malignancies. KRAS mutations are, therefore, not specific for invasive cancer, and have been described in chronic pancreatitis.[27,28] Reicher et al.[23] have demonstrated some utility for KRAS mutational analysis in the evaluation of endoscopic ultrasound (EUS)-FNA specimens. They described KRAS mutations in approximately 9% of benign specimens and 56% of malignant specimens. Importantly, KRAS mutational analysis was helpful in dividing atypical specimens into benign and malignant categories. Neither of the two cases in Reicher's study considered cytologically atypical but on follow-up demonstrated to be benign, contained KRAS mutations.[23] On the other hand, in cases considered atypical with malignant follow-up, 20% of cases (2/10) demonstrated KRAS mutations. Additional studies will be necessary to determine the value of KRAS mutational analysis in both bile duct stricture cytology samples and FNA of solid pancreatic masses, but current data do not support KRAS testing of solid pancreatic masses and bile duct strictures as a useful ancillary test for diagnosis. The utility of KRAS mutational analyses in the evaluation of cystic lesions of the pancreas is discussed below.
The potential application of analytic techniques for evaluating sequential mutation accumulation in bile duct brushing specimens has been studied. Lapkus et al.[15] have shown that there is considerable overlap in the spectrum of mutational markers in pancreatic duct and biliary brushings, but the temporal profile of accumulation of these mutations differs significantly between pancreatic and biliary neoplasms. These authors studied the time course of mutation accumulation in pancreatic and biliary tract lesions by microdissecting cell clusters on the basis of cytomorphologic features and analyzed the cells for the loss of heterozygosity with a panel of 15 markers (1p, 3p, 5q, 9p, 10q, 17p, 17q, 21q, 22q) as well as point mutations in KRAS using polymerase chain reaction (PCR)/capillary electrophoresis. The prevalence of loss of heterozygsity (LOH) and KRAS mutations in these lesions varied.[15] While distinctive prevalences for sequential mutation accumulation were demonstrable, diagnostic utility of this approach is still to be determined.
FISH
FISH analysis of bile duct brushing specimens for polysomy using a commercially available DNA probe set (UroVysion; Abbot Molecular, USA) has been reported as a useful technique by a number of authors.[3,8,11,12,40,41,42] This commercial kit utilizes probes targeting the pericentromeric regions of chromosomes 3 (CEP3), 7 (CEP7), and 17 (CEP17) as well as chromosomal bands 9p21 (LSI 9p21). The method can be automated using the Bioview Imaging Duet system (Bioview, Ltd., Nes Zionu, Israel). Others[8] have developed probe sets of their own based on known chromosomal alterations in genes associated with pancreatic carcinoma including: TP53, CDKN2A/p16 and epidermal growth factor receptor. In a series of 93 pancreaticobiliary brushings, Barr Fritcher et al.[41] demonstrated a specificity of 100% and a sensitivity of approximately 60% for the identification of carcinoma using FISH probes targeting centromeric regions of chromosomes 3, 7, 17 and the 9p21 band. They considered a specimen as positive for malignancy by FISH when five or more cells showed polysomy (>2 signals in at least 2 of the 4 probes). In that study, FISH analysis outperformed routine cytology and review consensus cytology of the brushing specimens. Boldorini et al.[40] also reported similar findings with FISH of brushing specimens outperforming routine cytology. In that study, the sensitivity of FISH was 90% with 94% specificity while the positive predictive value was 98% and negative predictive value was 75%. Levy et al.[12] have reported similar success with FISH. In that study, the authors considered trisomy for chromosome 7 to be benign. In a series of morphologically negative cytology samples, FISH was able to suggest malignancy in 62% of cases.[12] Barr Fritcher et al.[41] investigated the utility of ancillary studies including DIA and FISH in a series of 498 consecutive patients with pancreaticobiliary strictures. They found that FISH had a sensitivity of 43% and was significantly better than the sensitivity of routine cytology (20%) when equivocal cytology samples were considered as negative. They concluded that FISH had a higher sensitivity than cytology without compromising specificity.[41]
Of all the ancillary techniques currently available for analysis of cytology specimens obtained by brushings from pancreaticobiliary strictures, FISH appears to improve diagnostic sensitivity the most over that achievable by routine cytology.[3,12,14,23,40,41,42] While not directly addressing bile duct brushings, Kubiliun et al.[13] recommended the use of FISH for the diagnosis of pancreatic carcinoma in inconclusive cytologic evaluations. It appears that a similar approach is successful for the evaluation of negative and inconclusive pancreaticobiliary tract brushing specimens.
Additional markers of malignancy in bile duct brushing are under investigation. While experience is limited, ribonucleic acid (RNA)-binding protein-3 (IMP3) shows promise as a marker for adenocarcinoma in bile duct brushings. In one study, IMP3 immunohistochemical expression demonstrated a sensitivity of 64% with a specificity of 100% for adenocarcinoma.[43]
ANCILLARY TESTING FOR PANCREATIC CYSTIC LESIONS
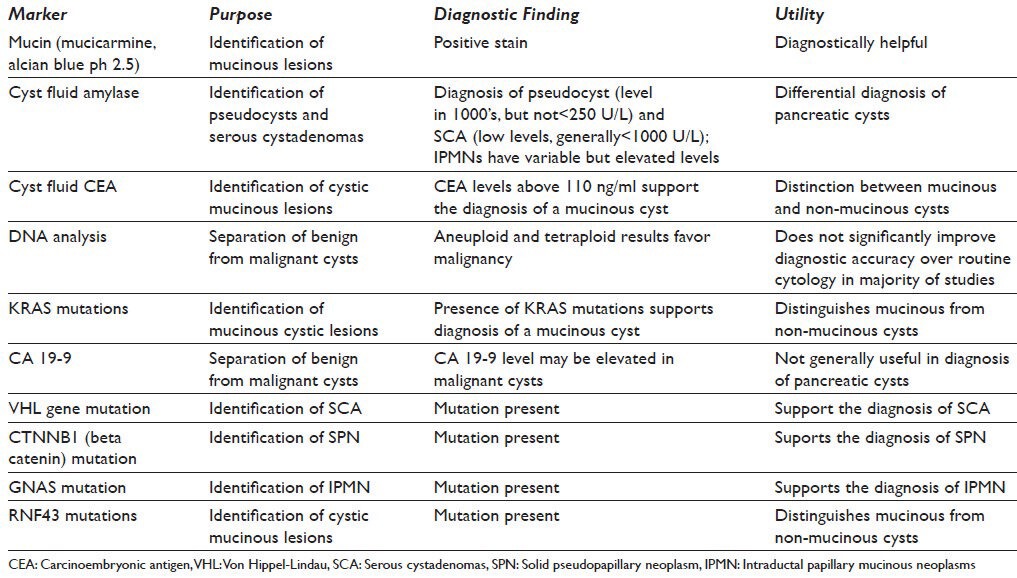
A number of ancillary tests have been proposed to aid in the diagnosis of cystic lesions of the pancreas. These vary from measuring carcinoembryonic antigen (CEA) and amylase levels in cyst fluid, to histochemical stains for mucin on cytologic smears, to mutational analysis (KRAS, GNAS, TP53, VHL, CTNNB1 and RNF43) and measures of polysomy.[32,37,44,45] Table 2 lists markers, which have been used in the diagnosis of pancreatic cystic lesions.
Table 2.
Proposed ancillary testing for cystic pancreatic lesions
Gross cyst fluid evaluation
A gross description such as “thick, white, viscous, sticky fluid” and cyst fluid that is difficult to pull into the needle and express from the needle clearly indicates a mucinous cyst fluid. These descriptions act as a surrogate marker for viscosity, a test that is not readily available in the biopsy suite. Leung et al.[45] examined the role of the “string sign” as a marker of viscosity. By placing the fluid between the thumb and index finger and gently pulling the fingers apart, the fluid would “string” to 3.5 mm if mucinous. Ancillary testing adds little to this simple visual test. Similarly, thin, non-mucoid, serosanguinous or frankly bloody cyst fluid is typical of serous cystadenoma due to the high vascularity of the septae in these cysts, which causes them to bleed internally and during aspiration.
Histochemical stains for mucin
Staining of direct smear preparations for mucin by a variety of techniques including the mucicarmine stain for neutral mucin and alcian blue pH 2.5 for acid mucin can be performed on direct smear preparations, but it is not diagnostically valid when performed on liquid-based preparations. Staining of smear preparations for mucin aids in the establishment of mucinous differentiation of the lining epithelium; however, it cannot separate benign from malignant lesions, and standardization of how much staining constitutes a positive result in EUS-FNA specimens with contamination from the gastrointestinal tract has not been determined. Gastrointestinal contamination of mucin can lead to a false positive interpretation. Thick colloid-like mucin with cellular debris floating in the mucin is consistent with mucinous cyst contents and not gastrointestinal contamination.[47] Documentation of mucinous differentiation in cystic pancreatic lesions is clinically helpful in that it points to either a mucinous cystic neoplasm or an IPMN.
Biochemical tests of cyst fluid
Cyst fluid amylase
Amylase testing quantifies α-amylase using an enzymatic colorimetric assay to measure the formation of degradation products saccharogenically or kinetically with the aid of enzyme-catalyzed subsequent reactions. The color intensity of the degradation product formed is directly proportional to the α-amylase activity, which is determined by measuring the increase in absorbance. Cyst fluid amylase is elevated in pseudocysts (typically in the thousands) and in cysts that communicate with the pancreatic ductal system, such as IPMNs, but not in cystic lesions that do not, such as serous cystadenoma and cystic neuroendocrine tumors.[47] Although mucinous cystic neoplasms (MCNs) do not communicate with the pancreatic ductal system, amylase levels can be quite elevated in MCNs making the specificity of this test for distinguishing IPMN and MCN of little value. High amylase combined with low CEA is consistent with a pseudocyst, low amylase and low CEA is typical of a serous cystadenoma and high amylase and high CEA is consistent with cystic-mucinous neoplasm with rare exceptions. A pancreatic cyst fluid amylase below 250 U/L is associated with a low risk for a pseudocyst.[48]
Cyst fluid CEA
Cyst fluid CEA level is a reliable indicator of mucinous differentiation in a cyst, but unfortunately it does not reliably predict the presence or absence of malignancy.[49] Suggested cut-off values for distinguishing between non-neoplastic and neoplastic cysts vary, with early reports suggesting 192 ng/mL as a useful cut point.[49] More recent data suggests a cut point of 110 ng/mL. Increasing the cut-off point for support of a mucinous cyst increases specificity but at the expense of sensitivity. Early studies suggest that CEA level was helpful in separating benign neoplastic cysts from malignant neoplastic cysts,[44] but subsequent studies have made it clear that an elevated CEA is not a reliable test for malignancy.[48,49] In addition, a low CEA level does not exclude a mucinous cyst in general, nor malignant cyst in particular. However, in a single study, mean CEA levels of cyst fluid demonstrated a striking difference between benign and malignant cystic lesions.[44] CEA greater than 693 ng/mL predicted malignancy with a sensitivity of 80% and a specificity of 90%.[48]
CA 125 in pancreatic cyst fluid
A few studies have investigated cyst fluid CA 125 levels to assess the usefulness of this marker in determining the cyst type and discriminating between benign and malignant neoplasms.[50,51,52,53] These studies have demonstrated that while the CA 125 levels are generally low in a pseudocyst and high in a cystic neoplasm, significant overlap exists between serous cystadenoma and mucinous cystic neoplasm. Furthermore, low levels of CA 125 have been reported in cystic neuroendocrine tumors,[54] and high levels in ciliated enteric duplication cyst[55] and lymphoepithelial cyst.[56] For these reasons, cyst fluid CA 125 assay does not have an important role in the evaluation of pancreatic cysts.
MUC protein expression patterns in pancreatic cyst fluid
Mucin expression patterns demonstrate a correlation with the presence of dysplasia and carcinoma. Several studies have demonstrated that analysis of pancreatic cyst fluid or serum can predict the presence of dysplasia or malignancy in the surrounding pancreatic cyst lining.[22,57,58] Jhala et al.[22] showed that 91% of pancreatic ductal adenocarcinomas expressed MUC4 but no evidence of staining was found in reactive ductal epithelium. In a study of 90 patients, Carrara et al.[57] investigated the expression of MUC1, MUC2, MUC3, MUC4 MUC5A, MUC5B, MUC6 and MUC7 and found that MUC7 expression was a strong marker for adenocarcinoma and borderline for IPMN. However, MUC7 was expressed in 37% of cases of chronic pancreatitis.[57] Maker, et al.[58] showed that high-risk IPMNs demonstrated elevated concentrations of MUC2 and MUC4 in pancreatic cyst fluid. From these data, it appears that evaluation of cyst fluid for MUC2, MUC4 and MUC7 may be helpful in the recognition of dysplasia and malignancy in cystic neoplasms of the pancreas.
Immunohistochemical analysis of pancreatic cyst samples
Most cyst fluids are usually very scant and of insufficient volume and cellularity for cellblock preparation. Exceptions to this are secondarily cystic neoplasms such as pancreatic neuroendocrine tumors (PanNET) and solid-pseudopapillary neoplasms (SPNs) where ancillary testing is often vital to a specific diagnosis.[62] See discussion of immunohistochemical testing of solid pancreatic lesions.
DNA analysis of cyst fluid
DNA analysis may also aid in the separation of non-neoplastic and neoplastic, and benign from malignant neoplastic cystic lesions. The success of DNA analyses depends on the amount of recoverable DNA.[9] Measurements of cyst fluid DNA can be quantitated using a variety of commercially available techniques.[60] The concentration of DNA is correlated with optical density (OD) as measured at a wavelength 260/280. The mean concentration of DNA present within a fluid from a pancreatic cystic lesion documented by OD ranges from a low of 6.5 in benign cysts to 16.5 in malignant cysts.[60]
Molecular testing of cyst fluid and tissue
Molecular analysis for KRAS mutations and for LOH has been reported to be helpful in the separation of benign from malignant cysts.[36,37,49] Khalid et al.[37] studied cyst fluid aspirates from 36 pancreatic cysts with histologically confirmed pathology. KRAS gene mutations were not observed in the benign cysts (a mix of pseudocysts and benign cystic neoplasms such as serous cystadenoma), while 40% of the cysts in the “premalignant” group (neoplastic cysts with low or intermediate-grade dysplasia) had a KRAS mutation, as did the majority of “malignant” cysts (neoplastic cysts with high-grade dysplasia or an associated invasive carcinoma). Similar percentages were reported in an analysis of a larger number of pancreatic cysts in the “pancreatic cyst DNA analysis study.”[60] Of interest, in this latter study, all malignant cysts (those with either high-grade dysplasia or an associated invasive carcinoma) with negative cytologic evaluation could be diagnosed as malignant using DNA analyses.[60] Others[32] have had less success in using mutational analysis for the distinction of benign from malignant cystic lesions. Chadwick et al.[32] have demonstrated that while KRAS point mutations were more common in malignant lesions than in benign lesions, they also could be found in both benign and malignant intraductal papillary mucinous tumors. Others have had a similar experience with solid pancreatic neoplasms.[26,27]
Shen et al.[61] studied the utilization of a commercially available test that combines the detection of KRAS mutation, LOH and DNA quantity/quality in the diagnosis of pancreatic cystic lesions. The concordance between the clinical consensus diagnosis and the commercial test was high with the commercial test showing a sensitivity of 83% and specificity of 100% for a malignant cyst and a sensitivity of 86% and specificity of 93% for a benign mucinous cyst. The authors concluded that the molecular analysis of pancreatic cyst fluids adds diagnostic value to the pre-operative diagnosis.[61]
From the available data, it appears that the analysis of pancreatic cyst fluid for mucin by histochemical stains on direct smears and CEA level are helpful diagnostic adjuncts for the recognition of a cystic lesion showing mucinous differentiation. Cyst fluid amylase levels appear to be of great value for the recognition of pseudocysts. KRAS and other mutational analyses may aid in the characterization of pancreatic mucinous cysts.[39,44,49]
Recent whole exome sequencing of the four most common cystic neoplasms of the pancreas (serous cystadenoma, SPN, mucinous cystic neoplasm and IPMN) has identified a specific mutational profile in each cyst type. VHL mutations are seen in serous cystic neoplasms, CTNNB1 (beta-catenin) in SPNs, RNF43, KRAS TP53 and SMAD4 in MCN, and KRAS, RNF43, GNAS TP53 and SMAD4 in IPMN.[62,63] It has therefore been suggested that mutational analysis for GNAS, KRAS, VHL, CTNNB1, RNF43, TP53 and SMAD4 may aid in the differential diagnosis of cystic lesions of the pancreas.
Mutations in the GNAS gene appear to be an important marker for IPMNs. The GNAS gene encodes for stimulatory G-protein alpha subunit which is a component of many transduction pathways.[64] GNAS gene protein product is defective in McCune-Albright Syndrome and some forms of fibrous dysplasia.[64,65] GNAS mutations appear to be specific for IPMNs,[66,67] while KRAS and RNF43 mutations can also be seen in MCNs.
The VHL is a tumor suppressor gene linked to sporadic hemangioblastomas and clear cell renal cell carcinomas.[68] This gene is somatically mutated in serous cyst adenomas.[62,69] Mutations in VHL gene are not seen in other cystic lesions of the pancreas.
RNF43 (ring finger protein 43) product is a HAP95 binding ubiquitin ligase that promotes cell growth.[70] RNF43 has recently been linked to the beta-catenin pathway. RNF43 mutations have been shown to occur in MCN along with IPMN.[71] Unlike IPMN, MCN do not typically harbor GNAS mutations.[71]
Virtually all SPNs harbor a CTNNB1 gene mutation, and these mutations, in the absence of other mutations, appear to be relatively specific for SPNs in the pancreas. Because the beta-catenin protein abnormally accumulates in the nuclei of cells with CTNNB1 gene mutations, immunolabeling for the beta-catenin protein is also a useful aid in establishing the diagnosis on limited cytology samples.[72]
MicroRNA analysis
The expression of selected microRNA (miRNA) is disregulated in cystic neoplasms of the pancreas, and Matthaei et al.[73] and others[74,75] have shown that the patterns of miRNA expression can help classify cyst type and in some instances even suggest the degree of dysplasia. Some groups have used panels of miRNA, while others have used a more focused approach using selected miRNA such as miRNA 21 and miRNA 221.[74,75]
ANCILLARY TESTING FOR SOLID PANCREATIC NEOPLASMS
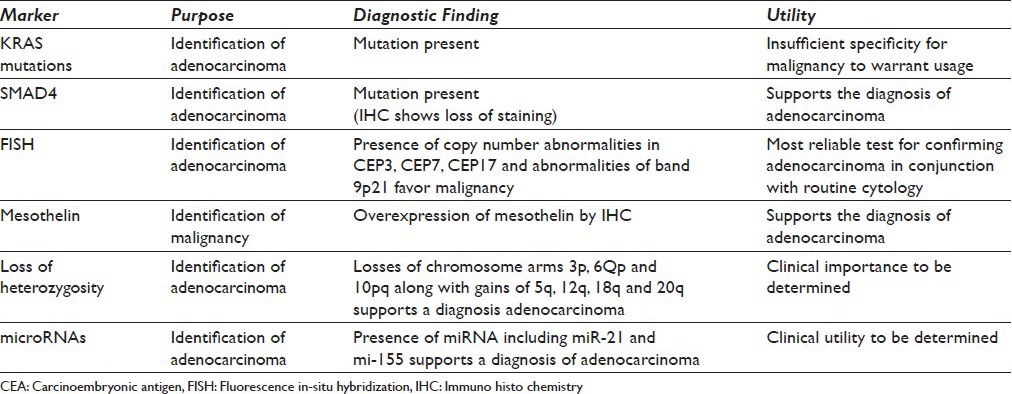
A number of ancillary procedures have been investigated for the detection of malignancy in solid pancreatic masses. Table 3 lists markers that have been used in the diagnosis of solid pancreatic lesions.
Table 3.
Proposed ancillary tests for solid pancreatic lesions
Immunohistochemical testing
Immunohistochemical markers for adenocarcinoma
SMAD4 loss is observed in pancreatic intraepithelial neoplasia (PanIN)-3 high grade lesions and over half of pancreatic ductal adenocarcinomas.[76,77] SMAD4 loss also appears to be associated with a poor prognosis for pancreatic ductal adenocarcinoma.[78] Immunolabeling for SMAD4, the protein product of the SMAD4 gene, is a good surrogate for SMAD4 genetic alterations and is useful in cytologic material processed as cellblocks allowing for easy testing of atypical to suspicious cellular groups. Loss of SMAD4 staining supports a malignant diagnosis.
MSLN is a 40-kDa glycosylphosphatidylinositol-linked protein that has been demonstrated to be highly overexpressed in pancreatic adenocarcinoma[72] a finding corroborated in a subsequent study utilizing three platforms.[79] Differential expression of MSLN in pancreatic adenocarcinoma has also been documented in resected samples.[22] Three independent studies have shown that when applied to FNA material, immunolabeling for MSLN can serve as a useful marker for supporting the diagnosis of pancreatic adenocarcinoma.[22,80]
Immunohistochemical testing for PanNET
A variety of immunohistochemical markers are useful in the diagnosis and grading of PanNET. These include general markers of endocrine differentiation (chromogranin, synaptophysin, CD57, CD56 and neuron-specific enolase), cytokeratins CK8 and CK18 and CA 19-9.[81] The proliferation marker Ki-67 is of importance in the histologic classification of PanNET into low and high-grade tumors, but its utility in cytologic preparations including cell block material remains to be determined. Functional pancreatic endocrine neoplasms produce a number of hormones, which can be demonstrated by immunohistochemical techniques including insulin, glucagon, somatostatin, gastrin, vasoactive intestinal protein and pancreatic polypeptide.[82,83,84,85,86]
The separation of PanNET into high and low grade neoplasms based on mitotic count is proposed by the World Health Organization.[87] Some authors have shown that Ki-67 score appears to be a superior predictor of outcome than degree of tumor differentiation.[87,88] Application of Ki-67 staining to cell block material may have value in stratifying tumors into low and high grade forms.[89]
Immunohistochemical testing for acinar cell carcinoma
Immunohistochemical staining for enzymes produced by acinar cells aids in the distinction of these neoplasms from ductal carcinoma and PanNET. Immunolabeling for amylase, trypsin, chymotrypsin and lipase is most helpful in recognition of these neoplasms in cellblock material.[90,91] Expression of CK7 appears to aid in separation of acinar cell neoplasms (positive) from normal acinar cells (negative).[92] Abnormalities of beta-catenin can also be seen focally in acinar cell carcinomas.[93]
Immunohistochemical testing for pancreatoblastoma
Pancreatoblastoma displays diverse directions of differentiation as demonstrated by multiple patterns of antigen expression. The various components of differentiation-ductal, acinar and endocrine-will label with markers for these lines of differentiation as described above. The most common line of differentiation is along acinar cell differentiation.[98,99]
Immunohistochemical testing for SPN
SPNs express some markers seen in PanNET and acinar cell neoplasms including alpha-1-antitrypsin, neuron-specific enolase and CD56.[72,94,95] CD10 is also expressed in SPNs.[95] Beta-catenin abnormalities are also characteristic of SPNs and diffuse nuclear positivity for these markers usually suffices for the confirmation of the diagnosis.[96]
Immunohistochemical testing in pancreatic and biliary tract lymphoma
Both B-cell and T-cell lymphomas arise within the pancreas and biliary tract. The majority of these neoplasms will react with antibodies directed against common leukocyte antigen.
Immunohistochemical testing via either flow cytometry analysis or antibody panels performed on cellblock specimens is necessary for definitive classification of these neoplasms.[97] In depth discussion of these panels is beyond the scope of this document.
Molecular analysis
Molecular testing for pancreatic ductal adenocarcinoma
Mutational analysis
KRAS and p16 point mutation analysis, FISH and DNA ploidy analysis have been used as potential methods for separating benign and malignant masses by FNA.[3,26,27,28,49,61,70,71,100] Invasive pancreatic adenocarcinoma is believed to develop from two non-invasive precursor lesions, IPMN and PanIN by the accumulation of a series of mutations.[31] In this progression model for pancreatic carcinoma, activating point mutations in the KRAS oncogene and telomere shortening appear to be early events.[70,71,72,73,74,75,76,77,100,102,103] The prevalence of KRAS mutations in IPMN and PanIN increases along with increasing degrees of dysplasia.[100] Additional mutations affecting other genes occur later in the course of progression with increasing dysplasia, with p16/CDNK2A loss occurring in intermediate to high-grade lesions, and TP53 and SMAD4 inactivation occurring in high-grade precursors and in invasive cancers.[35,77,102,104] KRAS gene mutations can be found in 95% of invasive ductal adenocarcinomas of the pancreas, and p16/CDKN2A is inactivated in 90%, TP53 in 75%, and SMAD4 in 55%.[32]
A retrospective study by Khalid et al. demonstrated that testing of EUS-FNA material for LOH and KRAS mutations is useful in differentiating autoimmune pancreatitis from pancreatic cancer.[105] KRAS mutations were detected in 10 of 11 (91%) pancreatic cancer cases that yielded DNA amplification and in none of the autoimmune pancreatitis cases suggesting that KRAS mutation in a pancreatic mass FNA is associated with malignancy and may aid in the distinction from benign processes such as autoimmune pancreatitis.[105] However, KRAS mutations have been shown to occur in ductal hyperplasias, ductal metaplasias and chronic pancreatitis.[26,27,28,100]
FISH
FISH performed for evaluation of solid lesions has been shown to significantly improve diagnostic sensitivity without loss of specificity for ductal adenocarcinoma.[13,40] The probes utilized are directed against chromosomes 3, 7 and 17 as well as 9p21. Loss of 9p21 or alterations in copy number for 3, 7 and 17 are diagnostically important. The technique can be applied using commercially available probe sets. Disadvantages include cost, and that the method is time consuming and requires a fluorescence microscope. FISH analysis when combined with routine cytology has a sensitivity of approximately 85%.[13] Because routine cytology has an excellent specificity, FISH is most useful in improving diagnostic accuracy for cases reported as cytologically negative or inconclusive. Kubiliun et al.[13] have recommended the use of FISH for the evaluation of cases assessed on site as inconclusive or negative.
miRNA
Additional techniques for determining malignancy in pancreaticobiliary lesions include miRNA analysis. miRNAS are short 20-25 nucleotide RNA molecules containing regions complementary to various messenger RNAS (mRNA). Specific binding of a miRNA to a mRNA blocks translation. miRNA can be robustly quantified by real-time PCR and localized by in-situ hybridization. Steele et al.[106] have reviewed profiling studies of pancreatic adenocarcinoma. Some miRNA, like miR-21 and miR-155, are highly expressed in early lesions.[107,108,109] Most studies have analyzed resection specimens, one study analyzed FNA specimens,[110] but none have analyzed brushings. The clinical utility remains to be more firmly established, especially in comparison and conjunction with other techniques.
Molecular testing for PanNETs
Loss of heterozygosity
Nodit et al.[111] explored the utility of analysis of microsatellite loss in the diagnosis of PanNET. They concluded that microsatellite loss analysis of EUS-FNA material obtained from PanNET can be performed reliably and that losses of chromosome arms 3p, 6pq and 10pq along with gains of 5q, 12q, 18q and 20q were associated with malignant behavior.[111,112,113,114] LOH studies appear to have some technical limitations when using cytologic techniques, because these techniques require microdissection of small numbers of neoplastic cells followed by amplification of DNA and resultant stochastic effects may alter the validity of test results. In addition, the presence of polysomy or amplification in a cell population being analyzed by LOH may result in allelic imbalance due to other issues than loss of tumor suppressor genes. Polysomy of chromosome 17 might result in one TP53 allele appearing more prominent than the other in some of these assays. Thus, techniques must be used that normalize overall signals and control for neoplastic cellularity to assure validity.
A number of additional techniques have been investigated for their use in determining the presence of malignancy in pancreaticobiliary lesions. These include investigation of miRNAs.
Mutational analysis
A number of chromosomal alterations occur in PanNET but do not appear to play a role in the etiology of these neoplasms.[114,115,116] PanNETS arising in association with multiple endocrine neoplasia type 1 (MEN1) syndrome demonstrate germline mutations in the MEN1 gene characterized by losses at the 11q13 locus.[117] PanNETs do not characteristically harbor mutations in KRAS, TP53, p16 and SMADA4/DPC4.[117]
Molecular testing of acinar cell carcinoma
Acinar cell carcinomas appear to be associated with losses in the chromosome arm 11p.[92,93] Mutations in the APC gene have also been reported.[92,93] The diagnostic significance of these alterations is not yet clear.
Molecular testing of SPNs
The majority (>95%) of SPNs have a somatic point mutation in the beta-catenin gene (exon3).[94,95,96] Mutations in KRAS, p16 and SMAD4/DPC4 genes have not been reported in SPNs.
SUMMARY
Currently, FISH is the most clinically relevant ancillary technique applicable to FNA material from pancreatic lesions, because the addition of FISH analysis to routine cytologic evaluation appears to yield the highest sensitivity without loss in specificity. Loss of immunohistochemical staining for the protein product of SMAD4 and positive staining for MSLN support a diagnosis of ductal adenocarcinoma. Immunohistochemical markers for endocrine and exocrine differentiation are sufficient for a diagnosis of endocrine and acinar tumors, respectively. Nuclear staining for beta-catenin supports a diagnosis of solid-pseudopapillary neoplasm. Cyst fluid analysis for amylase and CEA also appears of diagnostic utility for classification of pancreatic cysts. A number of gene mutations (KRAS, GNAS, VHL, RNF43 and CTNNB1) may be of aid in the identification of specific cystic neoplasms. Other ancillary techniques do not appear to improve diagnostic sensitivity sufficiently to justify their increased cost for the evaluation of EUS-FNA and brushing specimens.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author has participated sufficiently in the work and take public responsibility for appropriate portions of the content of this article. Each author acknowledges that this final version was read and approved.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, general manuscripts undergo a double blind peer review process (authors are blinded for reviewers and vice versa) through automatic online system. However, the current article is part of guidelines by Papanicolaou Society of Cytopathology (PSC) submitted by the committee members as authors and is being published after due copy-editing without peer review. Because it is agreed to be published as Open Access article under Creative Commons Legal Code (http://creativecommons.org/licenses/by/2.0/), this intellectual property (IP) would be retained in public domain.
Contributor Information
Lester J. Layfield, Email: layfieldl@health.missouri.edu.
Hormoz Ehya, Email: hormoz.eya@fccc.edu.
Armando C. Filie, Email: afilie@mail.nih.gov.
Ralph H. Hruban, Email: rhruban@jhmi.edu.
Nirag Jhala, Email: Nirag.Jhala@uphs.upenn.edu.
Loren Joseph, Email: ljlj@midway.uchicago.edu.
Philippe Vielh, Email: vielh@igr.fr.
Martha B. Pitman, Email: mpitman@mgh.harvard.edu.
REFERENCES
- 1.Mahmoudi N, Enns R, Amar J, AlAli J, Lam E, Telford J. Biliary brush cytology: Factors associated with positive yields on biliary brush cytology. World J Gastroenterol. 2008;14:569–73. doi: 10.3748/wjg.14.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber A, von Weyhern C, Fend F, Schneider J, Neu B, Meining A, et al. Endoscopic transpapillary brush cytology and forceps biopsy in patients with hilar cholangiocarcinoma. World J Gastroenterol. 2008;14:1097–101. doi: 10.3748/wjg.14.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fritcher EG, Kipp BR, Halling KC, Oberg TN, Bryant SC, Tarrell RF, et al. A multivariable model using advanced cytologic methods for the evaluation of indeterminate pancreatobiliary strictures. Gastroenterology. 2009;136:2180–6. doi: 10.1053/j.gastro.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 4.Kipp BR, Stadheim LM, Halling SA, Pochron NL, Harmsen S, Nagorney DM, et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am J Gastroenterol. 2004;99:1675–81. doi: 10.1111/j.1572-0241.2004.30281.x. [DOI] [PubMed] [Google Scholar]
- 5.Kocjan G, Smith AN. Bile duct brushings cytology: Potential pitfalls in diagnosis. Diagn Cytopathol. 1997;16:358–63. doi: 10.1002/(sici)1097-0339(199704)16:4<358::aid-dc11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Layfield LJ, Wax TD, Lee JG, Cotton PB. Accuracy and morphologic aspects of pancreatic and biliary duct brushings. Acta Cytol. 1995;39:11–8. [PubMed] [Google Scholar]
- 7.Logrono R, Kurtycz DF, Molina CP, Trivedi VA, Wong JY, Block KP. Analysis of false-negative diagnoses on endoscopic brush cytology of biliary and pancreatic duct strictures: The experience at 2 university hospitals. Arch Pathol Lab Med. 2000;124:387–92. doi: 10.5858/2000-124-0387-AOFNDO. [DOI] [PubMed] [Google Scholar]
- 8.Moreno Luna LE, Kipp B, Halling KC, Sebo TJ, Kremers WK, Roberts LR, et al. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064–72. doi: 10.1053/j.gastro.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponchon T, Gagnon P, Berger F, Labadie M, Liaras A, Chavaillon A, et al. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: Results of a prospective study. Gastrointest Endosc. 1995;42:565–72. doi: 10.1016/s0016-5107(95)70012-9. [DOI] [PubMed] [Google Scholar]
- 10.Volmar KE, Vollmer RT, Routbort MJ, Creager AJ. Pancreatic and bile duct brushing cytology in 1000 cases: Review of findings and comparison of preparation methods. Cancer. 2006;108:231–8. doi: 10.1002/cncr.21842. [DOI] [PubMed] [Google Scholar]
- 11.Rupp M, Hawthorne CM, Ehya H. Brushing cytology in biliary tract obstruction. Acta Cytol. 1990;34:221–6. [PubMed] [Google Scholar]
- 12.Levy MJ, Baron TH, Clayton AC, Enders FB, Gostout CJ, Halling KC, et al. Prospective evaluation of advanced molecular markers and imaging techniques in patients with indeterminate bile duct strictures. Am J Gastroenterol. 2008;103:1263–73. doi: 10.1111/j.1572-0241.2007.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubiliun N, Ribeiro A, Fan YS, Rocha-Lima CM, Sleeman D, Merchan J, et al. EUS-FNA with rescue fluorescence in situ hybridization for the diagnosis of pancreatic carcinoma in patients with inconclusive on-site cytopathology results. Gastrointest Endosc. 2011;74:541–7. doi: 10.1016/j.gie.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 14.Lee JG, Leung JW, Baillie J, Layfield LJ, Cotton PB. Benign, dysplastic, or malignant – Making sense of endoscopic bile duct brush cytology: Results in 149 consecutive patients. Am J Gastroenterol. 1995;90:722–6. [PubMed] [Google Scholar]
- 15.Lapkus O, Gologan O, Liu Y, Swalsky PA, Wilson MM, Finkelstein SD, et al. Determination of sequential mutation accumulation in pancreas and bile duct brushing cytology. Mod Pathol. 2006;19:907–13. doi: 10.1038/modpathol.3800545. [DOI] [PubMed] [Google Scholar]
- 16.Sebo TJ. Digital image analysis. Mayo Clin Proc. 1995;70:81–2. doi: 10.1016/S0025-6196(11)64670-3. [DOI] [PubMed] [Google Scholar]
- 17.Baron TH, Harewood GC, Rumalla A, Pochron NL, Stadheim LM, Gores GJ, et al. A prospective comparison of digital image analysis and routine cytology for the identification of malignancy in biliary tract strictures. Clin Gastroenterol Hepatol. 2004;2:214–9. doi: 10.1016/s1542-3565(04)00006-0. [DOI] [PubMed] [Google Scholar]
- 18.Rumalla A, Baron TH, Leontovich O, Burgart LJ, Yacavone RF, Therneau TM, et al. Improved diagnostic yield of endoscopic biliary brush cytology by digital image analysis. Mayo Clin Proc. 2001;76:29–33. doi: 10.4065/76.1.29. [DOI] [PubMed] [Google Scholar]
- 19.Bacus JW, Grace LJ. Optical microscope system for standardized cell measurements and analyses. Appl Opt. 1987;26:3280–93. doi: 10.1364/AO.26.003280. [DOI] [PubMed] [Google Scholar]
- 20.Tretiakova M, Antic T, Westerhoff M, Mueller J, Himmelfarb EA, Wang HL, et al. Diagnostic utility of CD10 in benign and malignant extrahepatic bile duct lesions. Am J Surg Pathol. 2012;36:101–8. doi: 10.1097/PAS.0b013e31822fbc95. [DOI] [PubMed] [Google Scholar]
- 21.Levy M, Lin F, Xu H, Dhall D, Spaulding BO, Wang HL. S100P, von Hippel-Lindau gene product, and IMP3 serve as a useful immunohistochemical panel in the diagnosis of adenocarcinoma on endoscopic bile duct biopsy. Hum Pathol. 2010;41:1210–9. doi: 10.1016/j.humpath.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Jhala N, Jhala D, Vickers SM, Eltoum I, Batra SK, Manne U, et al. Biomarkers in Diagnosis of pancreatic carcinoma in fine-needle aspirates. Am J Clin Pathol. 2006;126:572–9. doi: 10.1309/cev30be088cbdqd9. [DOI] [PubMed] [Google Scholar]
- 23.Reicher S, Boyar FZ, Albitar M, Sulcova V, Agersborg S, Nga V, et al. Fluorescence in situ hybridization and K-ras analyses improve diagnostic yield of endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses. Pancreas. 2011;40:1057–62. doi: 10.1097/MPA.0b013e3182200201. [DOI] [PubMed] [Google Scholar]
- 24.Sturm PD, Rauws EA, Hruban RH, Caspers E, Ramsoekh TB, Huibregtse K, et al. Clinical value of K-ras codon 12 analysis and endobiliary brush cytology for the diagnosis of malignant extrahepatic bile duct stenosis. Clin Cancer Res. 1999;5:629–35. [PubMed] [Google Scholar]
- 25.Sturm PD, Hruban RH, Ramsoekh TB, Noorduyn LA, Tytgat GN, Gouma DJ, et al. The potential diagnostic use of K-ras codon 12 and p53 alterations in brush cytology from the pancreatic head region. J Pathol. 1998;186:247–53. doi: 10.1002/(SICI)1096-9896(1998110)186:3<247::AID-PATH179>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 26.Caldas C, Hahn SA, Hruban RH, Redston MS, Yeo CJ, Kern SE. Detection of K-ras mutations in the stool of patients with pancreatic adenocarcinoma and pancreatic ductal hyperplasia. Cancer Res. 1994;54:3568–73. [PubMed] [Google Scholar]
- 27.Tada M, Ohashi M, Shiratori Y, Okudaira T, Komatsu Y, Kawabe T, et al. Analysis of K-ras gene mutation in hyperplastic duct cells of the pancreas without pancreatic disease. Gastroenterology. 1996;110:227–31. doi: 10.1053/gast.1996.v110.pm8536861. [DOI] [PubMed] [Google Scholar]
- 28.Yanagisawa A, Ohtake K, Ohashi K, Hori M, Kitagawa T, Sugano H, et al. Frequent c-Ki-ras oncogene activation in mucous cell hyperplasias of pancreas suffering from chronic inflammation. Cancer Res. 1993;53:953–6. [PubMed] [Google Scholar]
- 29.Terhune PG, Phifer DM, Tosteson TD, Longnecker DS. K-ras mutation in focal proliferative lesions of human pancreas. Cancer Epidemiol Biomarkers Prev. 1998;7:515–21. [PubMed] [Google Scholar]
- 30.Hruban RH, Wilentz RE, Kern SE. Genetic progression in the pancreatic ducts. Am J Pathol. 2000;156:1821–5. doi: 10.1016/S0002-9440(10)65054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–72. [PubMed] [Google Scholar]
- 32.Chadwick B, Willmore-Payne C, Tripp S, Layfield LJ, Hirschowitz S, Holden J. Histologic, immunohistochemical, and molecular classification of 52 IPMNs of the pancreas. Appl Immunohistochem Mol Morphol. 2009;17:31–9. doi: 10.1097/PAI.0b013e31817c02c6. [DOI] [PubMed] [Google Scholar]
- 33.Berthélemy P, Bouisson M, Escourrou J, Vaysse N, Rumeau JL, Pradayrol L. Identification of K-ras mutations in pancreatic juice in the early diagnosis of pancreatic cancer. Ann Intern Med. 1995;123:188–91. doi: 10.7326/0003-4819-123-3-199508010-00005. [DOI] [PubMed] [Google Scholar]
- 34.Tada M, Omata M, Kawai S, Saisho H, Ohto M, Saiki RK, et al. Detection of ras gene mutations in pancreatic juice and peripheral blood of patients with pancreatic adenocarcinoma. Cancer Res. 1993;53:2472–4. [PubMed] [Google Scholar]
- 35.Wilentz RE, Chung CH, Sturm PD, Musler A, Sohn TA, Offerhaus GJ, et al. K-ras mutations in the duodenal fluid of patients with pancreatic carcinoma. Cancer. 1998;82:96–103. doi: 10.1002/(sici)1097-0142(19980101)82:1<96::aid-cncr11>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 36.Maluf-Filho F, Kumar A, Gerhardt R, Kubrusly M, Sakai P, Hondo F, et al. Kras mutation analysis of fine needle aspirate under EUS guidance facilitates risk stratification of patients with pancreatic mass. J Clin Gastroenterol. 2007;41:906–10. doi: 10.1097/MCG.0b013e31805905e9. [DOI] [PubMed] [Google Scholar]
- 37.Khalid A, McGrath KM, Zahid M, Wilson M, Brody D, Swalsky P, et al. The role of pancreatic cyst fluid molecular analysis in predicting cyst pathology. Clin Gastroenterol Hepatol. 2005;3:967–73. doi: 10.1016/s1542-3565(05)00409-x. [DOI] [PubMed] [Google Scholar]
- 38.Hosoda W, Takagi T, Mizuno N, Shimizu Y, Sano T, Yamao K, et al. Diagnostic approach to pancreatic tumors with the specimens of endoscopic ultrasound-guided fine needle aspiration. Pathol Int. 2010;60:358–64. doi: 10.1111/j.1440-1827.2010.02527.x. [DOI] [PubMed] [Google Scholar]
- 39.Kipp BR, Fritcher EG, Clayton AC, Gores GJ, Roberts LR, Zhang J, et al. Comparison of KRAS mutation analysis and FISH for detecting pancreatobiliary tract cancer in cytology specimens collected during endoscopic retrograde cholangiopancreatography. J Mol Diagn. 2010;12:780–6. doi: 10.2353/jmoldx.2010.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boldorini R, Paganotti A, Sartori M, Allegrini S, Miglio U, Orsello M, et al. Fluorescence in situ hybridisation in the cytological diagnosis of pancreatobiliary tumours. Pathology. 2011;43:335–9. doi: 10.1097/PAT.0b013e32834642c0. [DOI] [PubMed] [Google Scholar]
- 41.Barr Fritcher EG, Caudill JL, Blue JE, Djuric K, Feipel L, Maritim BK, et al. Identification of malignant cytologic criteria in pancreatobiliary brushings with corresponding positive fluorescence in situ hybridization results. Am J Clin Pathol. 2011;136:442–9. doi: 10.1309/AJCPDULIOEOTUZ5H. [DOI] [PubMed] [Google Scholar]
- 42.Huddleston BJ, Lamb RD, Gopez EV, Adler DG, Collins BT. Cholangiocarcinoma in a 17-year-old boy with primary sclerosing cholangitis and UroVysion™ fluorescent in situ hybridization. Diagn Cytopathol. 2012;40:337–41. doi: 10.1002/dc.21629. [DOI] [PubMed] [Google Scholar]
- 43.Hart J, Parab M, Mandich D, Cartun RW, Ligato S. IMP3 immunocytochemical staining increases sensitivity in the routine cytologic evaluation of biliary brush specimens. Diagn Cytopathol. 2012;40:321–6. doi: 10.1002/dc.21571. [DOI] [PubMed] [Google Scholar]
- 44.Zhan XB, Wang B, Liu F, Ye XF, Jin ZD, Li ZS. Cyst fluid carcinoembryonic antigen concentration and cytology by endosonography-guided fine needle aspiration in predicting malignant pancreatic mucinous cystic neoplasms. J Dig Dis. 2013;14:191–5. doi: 10.1111/1751-2980.12027. [DOI] [PubMed] [Google Scholar]
- 45.Leung KK, Ross WA, Evans D, Fleming J, Lin E, Tamm EP, et al. Pancreatic cystic neoplasm: The role of cyst morphology, cyst fluid analysis, and expectant management. Ann Surg Oncol. 2009;16:2818–24. doi: 10.1245/s10434-009-0502-9. [DOI] [PubMed] [Google Scholar]
- 46.Pitman MB. Cytology of the pancreas. In: Gray W, Kocjan G, editors. Diagnostic Cytopathology. London: Churchill Livingstone; 2010. [Google Scholar]
- 47.Yoon WJ, Daglilar ES, Pitman MB, Brugge WR. Cystic pancreatic neuroendocrine tumors: Endoscopic ultrasound and fine-needle aspiration characteristics. Endoscopy. 2013;45:189–94. doi: 10.1055/s-0032-1325990. [DOI] [PubMed] [Google Scholar]
- 48.van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: A pooled analysis. Gastrointest Endosc. 2005;62:383–9. doi: 10.1016/s0016-5107(05)01581-6. [DOI] [PubMed] [Google Scholar]
- 49.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, et al. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 50.Lewandrowski KB, Southern JF, Pins MR, Compton CC, Warshaw AL. Cyst fluid analysis in the differential diagnosis of pancreatic cysts. A comparison of pseudocysts, serous cystadenomas, mucinous cystic neoplasms, and mucinous cystadenocarcinoma. Ann Surg. 1993;217:41–7. doi: 10.1097/00000658-199301000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sperti C, Pasquali C, Guolo P, Polverosi R, Liessi G, Pedrazzoli S. Serum tumor markers and cyst fluid analysis are useful for the diagnosis of pancreatic cystic tumors. Cancer. 1996;78:237–43. doi: 10.1002/(SICI)1097-0142(19960715)78:2<237::AID-CNCR8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 52.Sperti C, Pasquali C, Perasole A, Liessi G, Pedrazzoli S. Macrocystic serous cystadenoma of the pancreas: Clinicopathologic features in seven cases. Int J Pancreatol. 2000;28:1–7. doi: 10.1385/IJGC:28:1:01. [DOI] [PubMed] [Google Scholar]
- 53.Räty S, Sand J, Alfthan H, Haglund C, Nordback I. Cyst fluid tumor-associated trypsin inhibitor may be helpful in the differentiation of cystic pancreatic lesions. J Gastrointest Surg. 2004;8:569–74. doi: 10.1016/j.gassur.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Weissmann D, Lewandrowski K, Godine J, Centeno B, Warshaw A. Pancreatic cystic islet-cell tumors. Clinical and pathologic features in two cases with cyst fluid analysis. Int J Pancreatol. 1994;15:75–9. [PubMed] [Google Scholar]
- 55.Pins MR, Compton CC, Southern JF, Rattner DW, Lewandrowski KB. Ciliated enteric duplication cyst presenting as a pancreatic cystic neoplasm: Report of a case with cyst fluid analysis. Clin Chem. 1992;38:1501–3. [PubMed] [Google Scholar]
- 56.Centeno BA, Stockwell JW, Lewandrowski KB. Cyst fluid cytology and chemical features in a case of lymphoepithelial cyst of the pancreas: A rare and difficult preoperative diagnosis. Diagn Cytopathol. 1999;21:328–30. doi: 10.1002/(sici)1097-0339(199911)21:5<328::aid-dc6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 57.Carrara S, Cangi MG, Arcidiacono PG, Perri F, Petrone MC, Mezzi G, et al. Mucin expression pattern in pancreatic diseases: Findings from EUS-guided fine-needle aspiration biopsies. Am J Gastroenterol. 2011;106:1359–63. doi: 10.1038/ajg.2011.22. [DOI] [PubMed] [Google Scholar]
- 58.Maker AV, Katabi N, Gonen M, DeMatteo RP, D’Angelica MI, Fong Y, et al. Pancreatic cyst fluid and serum mucin levels predict dysplasia in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg Oncol. 2011;18:199–206. doi: 10.1245/s10434-010-1225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang SC, Ng KF, Yeh TS, Chang HC, Su CY, Chen TC. Clinicopathological analysis of β-catenin and Axin-1 in solid pseudopapillary neoplasms of the pancreas. Ann Surg Oncol. 2012;19(Suppl 3):S438–46. doi: 10.1245/s10434-011-1930-x. [DOI] [PubMed] [Google Scholar]
- 60.Khalid A, Zahid M, Finkelstein SD, LeBlanc JK, Kaushik N, Ahmad N, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: A report of the PANDA study. Gastrointest Endosc. 2009;69:1095–102. doi: 10.1016/j.gie.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 61.Shen J, Brugge WR, Dimaio CJ, Pitman MB. Molecular analysis of pancreatic cyst fluid: A comparative analysis with current practice of diagnosis. Cancer. 2009;117:217–27. doi: 10.1002/cncy.20027. [DOI] [PubMed] [Google Scholar]
- 62.Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A. 2011;108:21188–93. doi: 10.1073/pnas.1118046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kanda M, Knight S, Topazian M, Syngal S, Farrell J, Lee J, et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut. 2013;62:1024–33. doi: 10.1136/gutjnl-2012-302823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tabareau-Delalande F, Collin C, Gomez-Brouchet A, Decouvelaere AV, Bouvier C, Larousserie F, et al. Diagnostic value of investigating GNAS mutations in fibro-osseous lesions: A retrospective study of 91 cases of fibrous dysplasia and 40 other fibro-osseous lesions. Mod Pathol. 2013;26:911–21. doi: 10.1038/modpathol.2012.223. [DOI] [PubMed] [Google Scholar]
- 65.Weinstein LS. G (s) alpha mutations in fibrous dysplasia and McCune-Albright syndrome. J Bone Miner Res. 2006;21(Suppl 2):P120–4. doi: 10.1359/jbmr.06s223. [DOI] [PubMed] [Google Scholar]
- 66.Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Furukawa T, Kuboki Y, Tanji E, Yoshida S, Hatori T, Yamamoto M, et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep. 2011;1:161. doi: 10.1038/srep00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22:4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 69.van Asselt SJ, de Vries EG, van Dullemen HM, Brouwers AH, Walenkamp AM, Giles RH, et al. Pancreatic cyst development: Insights from von Hippel-Lindau disease. Cilia. 2013;2:3. doi: 10.1186/2046-2530-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ryland GL, Hunter SM, Doyle MA, Rowley SM, Christie M, Allan PE, et al. RNF43 is a tumour suppressor gene mutated in mucinous tumours of the ovary. J Pathol. 2013;229:469–76. doi: 10.1002/path.4134. [DOI] [PubMed] [Google Scholar]
- 71.Macgregor-Das AM, Iacobuzio-Donahue CA. Molecular pathways in pancreatic carcinogenesis. J Surg Oncol. 2013;107:8–14. doi: 10.1002/jso.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burford H, Baloch Z, Liu X, Jhala D, Siegal GP, Jhala N. E-cadherin/beta-catenin and CD10: A limited immunohistochemical panel to distinguish pancreatic endocrine neoplasm from solid pseudopapillary neoplasm of the pancreas on endoscopic ultrasound-guided fine-needle aspirates of the pancreas. Am J Clin Pathol. 2009;132:831–9. doi: 10.1309/AJCPVT8FCLFDTZWI. [DOI] [PubMed] [Google Scholar]
- 73.Matthaei H, Wylie D, Lloyd MB, Dal Molin M, Kemppainen J, Mayo SC, et al. miRNA biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin Cancer Res. 2012;18:4713–24. doi: 10.1158/1078-0432.CCR-12-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ryu JK, Matthaei H, Dal Molin M, Hong SM, Canto MI, Schulick RD, et al. Elevated microRNA miR-21 levels in pancreatic cyst fluid are predictive of mucinous precursor lesions of ductal adenocarcinoma. Pancreatology. 2011;11:343–50. doi: 10.1159/000329183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas RM, Fleming JB. MicroRNA dissects out dangerous pancreatic cysts from all the rest. Clin Cancer Res. 2012;18:4482–4. doi: 10.1158/1078-0432.CCR-12-2089. [DOI] [PubMed] [Google Scholar]
- 76.Wilentz RE, Iacobuzio-Donahue CA, Argani P, McCarthy DM, Parsons JL, Yeo CJ, et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: Evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000;60:2002–6. [PubMed] [Google Scholar]
- 77.Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–3. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 78.Tascilar M, Skinner HG, Rosty C, Sohn T, Wilentz RE, Offerhaus GJ, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001;7:4115–21. [PubMed] [Google Scholar]
- 79.Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: Identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–8. [PubMed] [Google Scholar]
- 80.Agarwal B, Ludwig OJ, Collins BT, Cortese C. Immunostaining as an adjunct to cytology for diagnosis of pancreatic adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6:1425–31. doi: 10.1016/j.cgh.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 81.Hruban RH, Pitman MB, Klimstra DS. Washington, DC: The American Registry of Pathology; 2007. AFIP Atlas of Tumor Pathology Series 4: Tumors of the Pancreas; pp. 267–9. [Google Scholar]
- 82.Roth J, Klöppel G, Madsen OD, Storch MJ, Heitz PU. Distribution patterns of proinsulin and insulin in human insulinomas: An immunohistochemical analysis in 76 tumors. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;63:51–61. doi: 10.1007/BF02899244. [DOI] [PubMed] [Google Scholar]
- 83.Hamid QA, Bishop AE, Sikri KL, Varndell IM, Bloom SR, Polak JM. Immunocytochemical characterization of 10 pancreatic tumours, associated with the glucagonoma syndrome, using antibodies to separate regions of the pro-glucagon molecule and other neuroendocrine markers. Histopathology. 1986;10:119–33. doi: 10.1111/j.1365-2559.1986.tb02468.x. [DOI] [PubMed] [Google Scholar]
- 84.Dayal Y, Oberg K, Perren A, Komminoth P. Pancreatic endocrine tumours: somatostatinoma. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. Lyon: IARC Press; 2004. pp. 189–90. [Google Scholar]
- 85.Komminoth P, Perreu A, Öberg K, Rindi G, Bordi C, Klöppel G, et al. Pancreatic endocrine tumours: Gastronoma. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. Lyon: IARC Press; 2004. pp. 191–4. [Google Scholar]
- 86.Solcia E, Capella C, Riva C, Rindi G, Polak JM. The morphology and neuroendocrine profile of pancreatic epithelial VIPomas and extrapancreatic, VIP-producing, neurogenic tumors. Ann N Y Acad Sci. 1988;527:508–17. doi: 10.1111/j.1749-6632.1988.tb27004.x. [DOI] [PubMed] [Google Scholar]
- 87.Faggiano A, Mansueto G, Ferolla P, Milone F, del Basso de Caro ML, Lombardi G, et al. Diagnostic and prognostic implications of the World Health Organization classification of neuroendocrine tumors. J Endocrinol Invest. 2008;31:216–23. doi: 10.1007/BF03345593. [DOI] [PubMed] [Google Scholar]
- 88.Scarpa A, Mantovani W, Capelli P, Beghelli S, Boninsegna L, Bettini R, et al. Pancreatic endocrine tumors: Improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23:824–33. doi: 10.1038/modpathol.2010.58. [DOI] [PubMed] [Google Scholar]
- 89.Larghi A, Capurso G, Carnuccio A, Ricci R, Alfieri S, Galasso D, et al. Ki-67 grading of nonfunctioning pancreatic neuroendocrine tumors on histologic samples obtained by EUS-guided fine-needle tissue acquisition: A prospective study. Gastrointest Endosc. 2012;76:570–7. doi: 10.1016/j.gie.2012.04.477. [DOI] [PubMed] [Google Scholar]
- 90.Kuerer H, Shim H, Pertsemlidis D, Unger P. Functioning pancreatic acinar cell carcinoma: Immunohistochemical and ultrastructural analyses. Am J Clin Oncol. 1997;20:101–7. doi: 10.1097/00000421-199702000-00023. [DOI] [PubMed] [Google Scholar]
- 91.Klimstra DS, Heffess CS, Oertel JE, Rosai J. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol. 1992;16:815–37. doi: 10.1097/00000478-199209000-00001. [DOI] [PubMed] [Google Scholar]
- 92.Zamboni G, Terris B, Scarpa A, Kosmahl M, Capelli P, Klimstra DS, et al. Acinar cell cystadenoma of the pancreas: A new entity? Am J Surg Pathol. 2002;26:698–704. doi: 10.1097/00000478-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 93.Abraham SC, Wu TT, Hruban RH, Lee JH, Yeo CJ, Conlon K, et al. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: Frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol. 2002;160:953–62. doi: 10.1016/s0002-9440(10)64917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miettinen M, Partanen S, Fräki O, Kivilaakso E. Papillary cystic tumor of the pancreas. An analysis of cellular differentiation by electron microscopy and immunohistochemistry. Am J Surg Pathol. 1987;11:855–65. doi: 10.1097/00000478-198711000-00004. [DOI] [PubMed] [Google Scholar]
- 95.Notohara K, Hamazaki S, Tsukayama C, Nakamoto S, Kawabata K, Mizobuchi K, et al. Solid-pseudopapillary tumor of the pancreas: Immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol. 2000;24:1361–71.s. doi: 10.1097/00000478-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 96.Tanaka Y, Kato K, Notohara K, Hojo H, Ijiri R, Miyake T, et al. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001;61:8401–4. [PubMed] [Google Scholar]
- 97.Bouvet M, Staerkel GA, Spitz FR, Curley SA, Charnsangavej C, Hagemeister FB, et al. Primary pancreatic lymphoma. Surgery. 1998;123:382–90. [PubMed] [Google Scholar]
- 98.Silverman JF, Holbrook CT, Pories WJ, Kodroff MB, Joshi VV. Fine needle aspiration cytology of pancreatoblastoma with immunocytochemical and ultrastructural studies. Acta Cytol. 1990;34:632–40. [PubMed] [Google Scholar]
- 99.Nishimata S, Kato K, Tanaka M, Ijiri R, Toyoda Y, Kigasawa H, et al. Expression pattern of keratin subclasses in pancreatoblastoma with special emphasis on squamoid corpuscles. Pathol Int. 2005;55:297–302. doi: 10.1111/j.1440-1827.2005.01829.x. [DOI] [PubMed] [Google Scholar]
- 100.Lüttges J, Schlehe B, Menke MA, Vogel I, Henne-Bruns D, Klöppel G. The K-ras mutation pattern in pancreatic ductal adenocarcinoma usually is identical to that in associated normal, hyperplastic, and metaplastic ductal epithelium. Cancer. 1999;85:1703–10. [PubMed] [Google Scholar]
- 101.Day JD, Digiuseppe JA, Yeo C, Lai-Goldman M, Anderson SM, Goodman SN, et al. Immunohistochemical evaluation of HER-2/neu expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasms. Hum Pathol. 1996;27:119–24. doi: 10.1016/s0046-8177(96)90364-0. [DOI] [PubMed] [Google Scholar]
- 102.Moskaluk CA, Hruban RH, Kern SE. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res. 1997;57:2140–3. [PubMed] [Google Scholar]
- 103.Yamano M, Fujii H, Takagaki T, Kadowaki N, Watanabe H, Shirai T. Genetic progression and divergence in pancreatic carcinoma. Am J Pathol. 2000;156:2123–33. doi: 10.1016/S0002-9440(10)65083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilentz RE, Geradts J, Maynard R, Offerhaus GJ, Kang M, Goggins M, et al. Inactivation of the p16 (INK4A) tumor-suppressor gene in pancreatic duct lesions: Loss of intranuclear expression. Cancer Res. 1998;58:4740–4. [PubMed] [Google Scholar]
- 105.Khalid A, Whitcomb DC. The importance of autoimmune pancreatitis. Gastroenterology. 2001;121:1518–20. doi: 10.1016/s0016-5085(01)83856-7. [DOI] [PubMed] [Google Scholar]
- 106.Steele CW, Oien KA, McKay CJ, Jamieson NB. Clinical potential of microRNAs in pancreatic ductal adenocarcinoma. Pancreas. 2011;40:1165–71. doi: 10.1097/MPA.0b013e3182218ffb. [DOI] [PubMed] [Google Scholar]
- 107.du Rieu MC, Torrisani J, Selves J, Al Saati T, Souque A, Dufresne M, et al. MicroRNA-21 is induced early in pancreatic ductal adenocarcinoma precursor lesions. Clin Chem. 2010;56:603–12. doi: 10.1373/clinchem.2009.137364. [DOI] [PubMed] [Google Scholar]
- 108.Nagao Y, Hisaoka M, Matsuyama A, Kanemitsu S, Hamada T, Fukuyama T, et al. Association of microRNA-21 expression with its targets, PDCD4 and TIMP3, in pancreatic ductal adenocarcinoma. Mod Pathol. 2012;25:112–21. doi: 10.1038/modpathol.2011.142. [DOI] [PubMed] [Google Scholar]
- 109.Ryu JK, Hong SM, Karikari CA, Hruban RH, Goggins MG, Maitra A. Aberrant MicroRNA-155 expression is an early event in the multistep progression of pancreatic adenocarcinoma. Pancreatology. 2010;10:66–73. doi: 10.1159/000231984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Szafranska AE, Doleshal M, Edmunds HS, Gordon S, Luttges J, Munding JB, et al. Analysis of microRNAs in pancreatic fine-needle aspirates can classify benign and malignant tissues. Clin Chem. 2008;54:1716–24. doi: 10.1373/clinchem.2008.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nodit L, McGrath KM, Zahid M, Jani N, Schoedel KE, Ohori NP, et al. Endoscopic ultrasound-guided fine needle aspirate microsatellite loss analysis and pancreatic endocrine tumor outcome. Clin Gastroenterol Hepatol. 2006;4:1474–8. doi: 10.1016/j.cgh.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 112.Speel EJ, Scheidweiler AF, Zhao J, Matter C, Saremaslani P, Roth J, et al. Genetic evidence for early divergence of small functioning and nonfunctioning endocrine pancreatic tumors: Gain of 9Q34 is an early event in insulinomas. Cancer Res. 2001;61:5186–92. [PubMed] [Google Scholar]
- 113.Barghorn A, Komminoth P, Bachmann D, Rütimann K, Saremaslani P, Muletta-Feurer S, et al. Deletion at 3p25.3-p23 is frequently encountered in endocrine pancreatic tumours and is associated with metastatic progression. J Pathol. 2001;194:451–8. doi: 10.1002/path.886. [DOI] [PubMed] [Google Scholar]
- 114.Barghorn A, Speel EJ, Farspour B, Saremaslani P, Schmid S, Perren A, et al. Putative tumor suppressor loci at 6q22 and 6q23-q24 are involved in the malignant progression of sporadic endocrine pancreatic tumors. Am J Pathol. 2001;158:1903–11. doi: 10.1016/S0002-9440(10)64658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Speel EJ, Richter J, Moch H, Egenter C, Saremaslani P, Rütimann K, et al. Genetic differences in endocrine pancreatic tumor subtypes detected by comparative genomic hybridization. Am J Pathol. 1999;155:1787–94. doi: 10.1016/S0002-9440(10)65495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stumpf E, Aalto Y, Höög A, Kjellman M, Otonkoski T, Knuutila S, et al. Chromosomal alterations in human pancreatic endocrine tumors. Genes Chromosomes Cancer. 2000;29:83–7. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1011>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 117.Hruban RH, Pitman MB, Klimstra DS. Tumors of the Pancreas. Washington, DC: The American Registry of Pathology; 2007. AFIP Atlas of Tumor Pathology Series 4; p. 271. [Google Scholar]


