Abstract
It was examined whether the physiological effects of high-amylose maize starch (HAMS) are influenced by hydroxypropylation. Rats were fed one of the following three diets: an AIN-93-based diet with waxy maize starch (WMS) as a starch source, or this diet with 150 g of WMS replaced by either HAMS or hydroxypropylated HAMS (HP-HAMS). The activities of amylase in bile-pancreatic juice and sucrose, maltase and isomaltase of the jejunum and ileum were not affected by diet, but the digestibility of HAMS was decreased by hydroxypropylation. The amounts of SCFA in caecal content and H2 excreted in the breath and flatus for HAMS were decreased by hydroxypropylation. Plasma glucagon-like peptide-1 (GLP-1), glucose and insulin concentrations were not affected by diet. On the basis of PCR-denaturing gradient gel electrophoresis (DGGE) profiles, the similarity in caecal bacteria population of the HP-HAMS group and HAMS group was low, but that of the HP-HAMS and WMS groups was high. The amount of caecal IgA was not affected by hydroxypropylation, but those in the HAMS and HP-HAMS groups were greater than that in the WMS group. Plasma and liver concentrations of TAG and cholesterol for HAMS were not affected by hydroxypropylation. These results show that the small intestinal digestibility and fermentation-dependent parameters such as caecal SCFA and H2 productions and caecal bacterial profile of HAMS were affected by hydroxypropylation, but parameters of glucose metabolism such as GLP-1 and insulin, those of lipid metabolism such as plasma TAG and cholesterol and the amount of caecal IgA were not.
Key words: High-amylose maize starch, Hydroxypropylation, Digestibility, Fermentability
Abbreviations: DGGE, denaturing gradient gel electrophoresis; GLP-1, glucagon-like peptide-1; HAMS, high-amylose maize starch; HP-HAMS, hydroxypropylated HAMS; PYY, peptide YY; RS, resistant starch; WMS, waxy maize starch
Resistant starch (RS) is defined as starch that escapes digestion in the small intestine of healthy individuals. Potential food applications of RS are of interest to product developers and nutritionists for two reasons: (1) fibre-fortification and the potential physiological benefits of RS, which has fibre-like functions; and (2) the unique functional properties of RS, which can yield high-quality products that are not attainable otherwise with traditional insoluble fibre. RS has been classified into four types, termed RS-type 1 to RS-type 4, depending on the cause of digestive resistance( 1 , 2 ).
High-amylose starch usually comprises more than 50 % amylose( 3 ) and is classified as RS-type 2. High-amylose starch resists digestion due to its granular structure and molecular architecture. High-amylose maize starch (HAMS) has been reported to provide many health benefits( 4 ), and is often used as a source of dietary fibre( 5 ).
Starch can be widely modified by chemical methods and is subsequently classified as RS-type 4. Chemically modified starch includes starch esters, starch diethyl ethers and cross-bonded starches. Starch is primarily digested by pancreatic α-amylase and brush border disaccharide hydrolases in the small intestine. Chemically modified starch resists digestion in the small intestine, and starch with lower digestibility increases the amount of digesta in the distal gut( 6 ). A large amount of digesta provides a favourable environment for microbial growth in the distal gut by providing nutrients to gut bacteria. Therefore, having a greater digesta mass may increase the diversity and population of gut bacteria and the production of SCFA.
The glucose and insulin response is strongly related to the rate of starch hydrolysis( 7 ); however, other factors such as incretins and glucagon-like peptide-1 (GLP-1) may be important( 8 ). The incretins are hormones secreted from the gut in response to food and stimulate the secretion of insulin. Fermentation products, particularly SCFA, may be involved in a cascade of events that improve insulin sensitivity( 9 ).
It has been reported that HAMS lowers plasma cholesterol and TAG concentrations( 10 , 11 ) and influences the glycaemic and insulinaemic responses( 12 , 13 ).
Hydroxypropylated tapioca starch retarded the development of insulin resistance in genetically diabetic KKay mice fed a normal fat diet( 14 ) and a high-fat diet( 15 ). So, hydroxypropylation of HAMS would reduce the digestibility and show many physiological effects through changes of energy, glucose and lipid metabolism and fermentation. However, the physiological effects of hydroxypropylated HAMS (HP-HAMS) have not been researched. Hydroxypropylation is an etherification process that is commonly used to modify food starch. Therefore, we investigated whether the physiological effects of HAMS on fermentation-dependent parameters such as caecal SCFA and caecal bacterial profile, glycaemic control and plasma and hepatic lipid concentrations are influenced by hydroxypropylation.
Materials and methods
Materials
Waxy maize starch (WMS) (about 100 % amylopectin, Matsunorin A60M; Matsutani Chemical Industry Co., Ltd.), HAMS (about 70 % amylose, HS-class 7, J-Oil Mills Inc.) and HP-HAMS were used in this study.
Hydroxypropylation of high-amylose maize starch
HAMS was hydroxypropylated according to a previously described procedure( 16 ). The hydroxypropyl content was determined by the method of Johnson( 17 ). The degree of substitution for HP-HAMS was 0·21.
Animals and diets
Male Wistar rats (SLC) weighing 450–500 g for experiments 1 and 2 and 200–220 g for experiment 3 were housed individually in screen-bottomed, stainless-steel cages in a room maintained at 23 ± 1°C with a 12 h light:dark cycle (light on, 07.00–19.00 hours). The study was approved by the Laboratory Animal Care Committee of Ehime University. Rats were maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals of Ehime University.
Experiment 1: apparent starch digestibility in the small intestine
After acclimatisation on the American Institute of Nutrition (AIN)-93G-based purified diet without cellulose for 7 d, the rats were subjected to ileorectomy in which the distal ileum was anastomosed to the rectum. The rats were not allowed food for 24 h before and after the operation. They received a daily intramuscular injection of 10 µl of Mycillin sol (containing procaine penicillin G (200 g/l) and dihydrostreptomycin sulphate (250 g/l); Toyo Jozo) for the first 3 d after surgery to prevent infection. They were then freely fed the AIN-93-based purified diet without cellulose for 10 d. A constant growth rate (5–7 g body weight gain/d) was achieved with this diet after 5 d.
After post-operative recovery, the rats were divided into three groups (n 7) and given the WMS, HAMS or HP-HAMS diet (Table 1) and water containing neomycin sulphate (0·1 g/100 ml; Wako Pure Chemical Industries, Co.) for 10 d to inhibit the action of the intestinal bacteria which remain after ileorectomy. The ileorectomy effluent was collected from each rat on the final 3 d of the experimental period. The ileorectomy effluent was freeze-dried, weighed and stored at −50°C until analysis. The amount of protein in the diet and effluent was determined by the Kjeldahl method with an N to protein conversion factor of 6·25. The amount of lipid in the diet and effluent was determined by the Soxhlet method. The amount of ash in the diet and effluent was determined by combustion in a muffle furnace (FM-26Yamato Scientific Co. Ltd.) at 550°C overnight. The amount of starch in the diet and effluent was determined by subtracting the weight of crude protein, lipid, cellulose, moisture and ash from the total weight. The amounts of WMS and HAMS can be determined by an enzymatic assay, but the amounts of HP-HAMS can not. Therefore, the apparent starch digestibility (%) was calculated as (A − B)/A × 100, where A is the starch intake and B is the starch output in the effluent as follows:
Table 1.
Composition of experimental diets
| Diet | |||
|---|---|---|---|
| Ingredient (g/kg) | WMS | HAMS | HP-HAMS |
| Casein | 200 | 200 | 200 |
| WMS | 532 | 382 | 382 |
| HAMS | – | 150 | – |
| HP-HAMS | – | – | 150 |
| Sucrose | 100 | 100 | 100 |
| Soyabean oil | 70 | 70 | 70 |
| Cellulose* | 50 | 50 | 50 |
| AIN-93 mineral mixture† | 35 | 35 | 35 |
| AIN-93 vitamin mixture†‡ | 10 | 10 | 10 |
| l-Cystine | 3 | 3 | 3 |
WMS, waxy maize starch; HAMS, high-amylose maize startch; HP-HAMS, hydroxypropylated HAMS.
* Cellulose powder, PC200 (Danisco Japan Ltd).
† Based on AIN-93G( 18 ).
‡ The vitamin mixture contained 20 g choline bitartrate per 100 g.
A = (food intake − cellulose) − sum of dietary protein, lipid, ash and water intake
B = (faecal dry weight − dietary cellulose intake) − sum of dietary protein, lipid and ash in faeces.
Experiment 2: measurement of hydrogen expired in rat breath
After acclimatisation on a commercial solid diet for 7 d, the rats were allowed free access to the AIN-93 diet without cellulose for 8 h per d from 09.00 to 17.00 hours for 2 weeks, and then for 4 h per d from 09.00 to 13.00 hours for 2 weeks. The rats were then divided into three groups (n 7) and given access to one of three kinds of test meal containing 70 g of soyabean oil, 130 g of sucrose and 800 g of test substance (WMS, HAMS or HP-HAMS) for 30 min.
Breath-test system
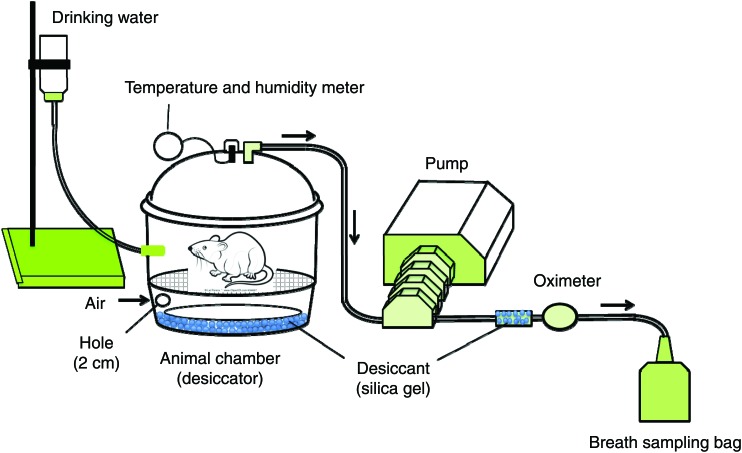
The system used for monitoring H2 in the air expired from the rats is shown in Fig. 1. In brief, the system was composed of an animal chamber with a volume of 9500 ml, a pump (Masterflex model 7524-50; Cole-Parmer Instrument Co. Ltd.) and a breath-sampling bag of 500 ml (Laboratory for Expiration Biochemistry Nourishment Metabolism Co., Ltd.). The air in the chamber was continuously aspirated during the experimental period. A desiccator was employed as the animal chamber so that the rats could move freely within the chamber and the expired air could be collected effectively in the breath-sampling bag.
Fig. 1.
System used for monitoring hydrogen amount in the air expired from rats. The system comprised a desiccator that was used as an animal chamber, a pump and a breath-sampling bag. Aspiration of the expired air through the aspiration tube caused fresh air to be automatically drawn through a hole in the side of the desiccator.
Hydrogen-breath test
The rats were placed in the chamber immediately after access to the test meal for 30 min in the cage described previously. The expired air was collected at 30 min intervals for 12 h after the rats were placed in the chamber. The expired air was obtained by a single-pass (as opposed to re-breathing) method, in which air was passed through the chamber containing the rats at a flow rate of 100 ml/min. Aspiration of the expired air through the aspiration tube caused fresh air to be automatically drawn through a hole (2 cm in diameter), through which the aspiration tube also passed, on the side of the desiccator (Fig. 1). The air in the chamber was continuously aspirated during the experimental period. The aspirated air was discharged outside the breath-test system, except for the period during which the expired air was collected in the breath-sampling bag. The temperature and moisture in the chamber were checked every 30 min, and were maintained at 22 ± 2°C and 50 ± 10 %, respectively. The H2 concentration of each sample was determined by means of a breath gas analyser (BGA-1000D; Laboratory for Expiration Biochemistry Nourishment Metabolism Co., Ltd).
Experiment 3: effects of hydroxypropylation of high-amylose maize starch on digestion and fermentation-dependent parameters
After acclimatisation on a commercial solid diet for 7 d, the rats were randomly divided into three groups (n 6), and allowed free access to one of the three experimental diets (Table 1) for 4 weeks. Body weight and food intake were recorded daily for each rat in the morning before the food was replaced.
Sampling and analytical procedures
Before the rats were killed, faeces were collected from each rat for the final 3 d of the experimental period. The faeces were freeze-dried, weighed and milled.
At the end of the experiment, rats were anaesthetised by intraperitoneal injection of sodium pentobarbital (40 mg/kg body weight), a mid-line laparotomy was performed and the bile-pancreatic duct was exposed and ligated distally. The bile-pancreatic duct was then cannulated with a PE-10 polyethylene tube (Clay Adams), and bile-pancreatic juice was collected into a pre-weighed tube that had been cooled on ice for 30 min. The volume of bile-pancreatic juice was determined gravimetrically (1·0 ml = 1·0 g).
After the bile-pancreatic juice was collected, the rats were killed by decapitation and a blood sample was taken from the carotid artery into a blood collection tube (Vacutainer; Becton Dickinson) containing heparin as an anticoagulant. Plasma was separated by centrifugation at 1400 g at 4°C for 15 min, and stored at −50°C until analysis. After blood collection, the liver was immediately perfused with cold saline, removed, washed with cold saline, blotted dry on filter paper, weighed and stored at −50°C until analysis. After the liver had been removed, the small intestine except for the duodenum portion (the first 15 cm distal from the pylorus) was removed, flushed with ice-cold saline (9 g NaCl/l), everted, washed again and divided into two segments: an upper half (jejunum) and a lower half (ileum). The caecum was then removed and weighed, and the content of the caecum was transferred into a pre-weighed tube. Subsequently, the caecal wall was flushed clean with ice-cold saline, blotted dry on filter paper and weighed. The caecal content was homogenised under CO2.
Plasma and liver lipids
Plasma total cholesterol, TAG and glucose concentrations were assayed spectrophotometrically with commercially available kits (Cholesterol-E test Wako, TAG-E test Wako and Glucose-test Wako; Wako Pure Chemical Industries). The concentration of liver total lipids was determined gravimetrically after extraction by the method of Folch et al.( 19 ). Liver TAG and cholesterol concentrations were determined enzymatically, as previously described( 20 ).
Plasma glucose, insulin and active glucagon-like peptide-1 concentration
The plasma glucose concentration was measured spectrophotometrically with a commercially available kit (Glucose CII Test Wako; Wako Pure Chemical Industry). The plasma insulin concentration was measured by a sandwich ELISA method (Rat Insulin Kit, Morinaga Institute of Biological Science). The plasma active gut hormone GLP-1 was measured by using a commercially available ELISA kit (LINCO Research).
Amylase assay in bile-pancreatic juice
Amylase activity in the bile-pancreatic juice was determined by using a QuantiChrom α-Amylase Assay Kit (DAMY-100; BioAssay Systems). Crystalline porcine amylase (type I-A; Sigma Chemical) was used as a standard.
Digestive enzymes in the brush border membrane
The mucosa of the upper half (jejunum) and the lower half (ileum) was scraped from each segment with a microscopic glass microscope slide. The intestinal mucosa was homogenised in ten volumes (v/w) of ice-cold 10 mmol/l potassium phosphate buffer (pH 7·0). Maltase, isomaltase and sucrase activities were assayed as described by Dahlqvist( 21 ). The protein concentration was measured according to the method of Lowry et al.( 22 ).
Caecal water content, pH and organic acids
The water content, pH and caecal organic acids (acetic, propionic, n-butyric, succinic and lactic acids) were measured by HPLC as described previously( 20 ).
IgA in the caecal content
The amount of IgA in the caecal content was determined by ELISA using a Rat IgA ELISA Quantitation Set (Bethyl Laboratories, Inc.).
Profile analysis of caecal microbiota by PCR-denaturing gradient gel electrophoresis
DNA was extracted from the caecal content using a faecal DNA isolation kit (TaKaRa Ex Taq Hot Start Version; Takara Bio Inc.) according to the manufacturer's instructions. DNA samples were used as a template to amplify fragments of the 16S rRNA gene with the universal primers HDA1-GC (5′-CGC CCG GGG CGC GCC CCG GGC GGG GCG GGG GCA CGG GGG GAC TCC TAC GGG AGG CAG CAG T-3′) and HDA2 (5′-GTA TTA CCG CGG CTG CTG GCA C-3′)( 23 ). PCR was performed as described by Walter et al.( 23 ). Denaturing gradient gel electrophoresis (DGGE) was performed with a DCode universal mutation detection system (Bio-Rad) as described by Walter et al.( 23 ). DGGE profiles were compared with Quantity One version 4.6.0 software (Bio-Rad Laboratories), and similarities were expressed with Dice's similarity coefficient.
Statistical analysis
Data were analysed by one-way ANOVA, and significant differences among means were discriminated by the Tukey–Kramer test. When variances were not homogeneous, the Bartlett test( 24 ) was used and the data were transformed logarithmically before analysis by ANOVA, followed by multiple comparisons, or were analysed by the Steel–Dwass test. Differences were considered significant at P < 0·05. The Tukey–Kramer test and the Bartlett test were performed with StatView 5.0 software (SAS Institute), and the Steel–Dwass test and linear regression analyses were performed with the Excel Statistics program (version 6.0; Esumi).
Results
Experiment 1
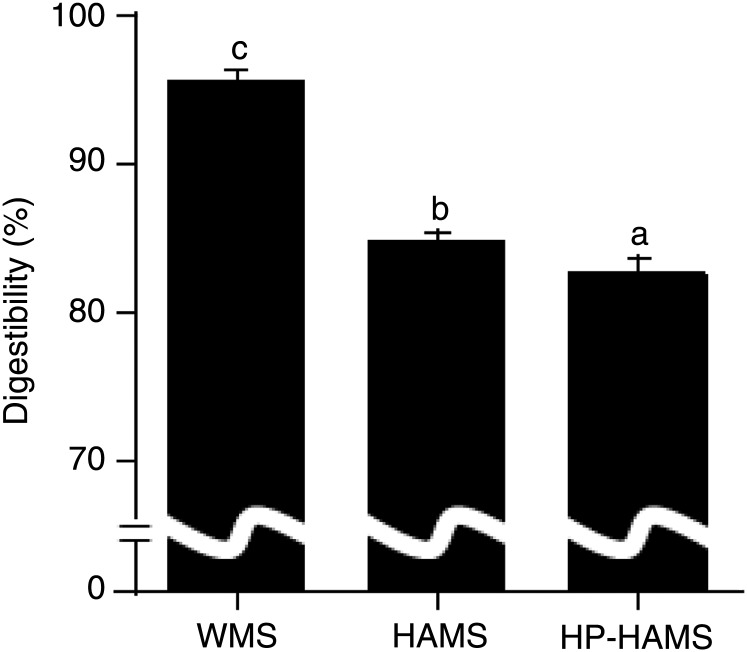
The apparent digestibility of starch was significantly lower in the HAMS and HP-HAMS groups than in the WMS group; furthermore, the apparent digestibility of starch in the HP-HAMS group was significantly lower than in rats fed the HAMS diet (Fig. 2).
Fig. 2.
Apparent digestibility of starch in rats fed the waxy maize starch (WMS), high-amylose maize starch (HAMS) or hydroxypropylated HAMS (HP-HAMS) diet. Values are means, with standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05; Tukey-Kramer test).
Experiment 2
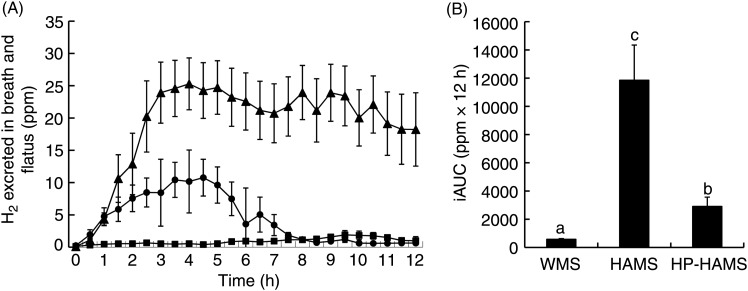
Food intake for 30 min was 3·7 (sem 0·1) g for rats fed WMS, 3·6 (sem 0·2) g for rats fed HAMS and 3·7 (sem 0·3) g for rats fed HP-HAMS. The excretion of H2 in breath was markedly increased in rats fed HAMS, reaching a peak at 4 h and then decreasing just a little, although a considerable amount of H2 was also excreted in breath at 12 h (Fig. 3). There was a more moderate increase in the excretion of H2 in breath after the ingestion of HP-HAMS, reaching a peak at 4 h and then decreasing towards the base value, which was reached after 8 h. The amount of H2 excreted in breath changed slightly after WMS feeding as compared with that at fasting. The incremental AUC for breath H2 expiration after HP-HAMS feeding was 75 % lower than that after HAMS feeding.
Fig. 3.
Time course of hydrogen in air expired from rats (A) and incremental AUC (iAUC) (B) after ingestion of waxy maize starch (WMS), high-amylose maize starch (HAMS) or hydroxypropylated HAMS (HP-HAMS). Values are means, with standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05; Tukey–Kramer test). -▲-, HAMS; -•-, HP-HAMS; -■-, WMS; ppm, parts per million.
Experiment 3
Body weight gain in the WMS group was significantly higher than that in the HAMS and HP-HAMS groups (Table 2). Food intake was significantly lower in the HAMS group than in the WMS group, but was not different from that in the HP-HAMS group. Liver weight, liver total lipid, liver and plasma cholesterol and plasma insulin concentrations were not affected by diet. Plasma TAG concentration in the HP-HAMS group was significantly lower than that in the WMS group, but was not different from that in the HAMS group. Plasma GLP-1 concentrations were significantly higher in the HAMS and HP-HAMS groups than in the WMS group. Plasma glucose concentration in the HP-HAMS group was significantly lower than in the HAMS group, but was not different from that in the WMS group.
Table 2.
Body weight gain, food intake, liver weight, liver lipids, plasma lipids, plasma glucose, plasma insulin and plasma glucagon-like peptide-1 (GLP-1) in rats fed the waxy maize starch (WMS), high-amylose maize starch (HAMS) and hydroxypropylated high-amylose maize starch (HP-HAMS) diets*
(Mean values and pooled standard errors for six rats per group)
| Diet | ||||
|---|---|---|---|---|
| WMS | HAMS | HP-HAMS | Pooled sem | |
| Body weight gain (g/27 d) | 99b | 81a | 76a | 3 |
| Food intake (g/27 d) | 479b | 433a | 445a,b | 11 |
| Liver | ||||
| Weight (g) | 9·73 | 9·02 | 8·41 | 0·35 |
| Liver lipids | ||||
| Total (mg/g liver) | 41·7 | 37·3 | 38·5 | 1·6 |
| TAG (μmol/g liver) | 65·7b | 44·4a | 54·5a,b | 3·4 |
| Cholesterol (μmol/g liver) | 12·6 | 12·5 | 13·5 | 0·7 |
| Plasma lipids (mmol/l) | ||||
| TAG | 1·31b | 1·04a,b | 0·73a | 0·10 |
| Cholesterol | 1·96 | 1·75 | 1·68 | 0·12 |
| Plasma glucose (mmol/l) | 11·9a,b | 12·5b | 11·1a | 0·3 |
| Plasma insulin (ng/ml) | 5·05 | 5·60 | 5·48 | 0·45 |
| GLP-1 (pmol/l) | 3·56a | 7·74b | 6·44b | 0·67 |
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Data were analysed by one-way ANOVA, followed by the Tukey–Kramer test.
The amount of bile-pancreatic juice secreted in the HAMS and HP-HAMS groups was significantly higher than that in the WMS group (Table 3). The activities of amylase in bile-pancreatic juice, maltase and isomaltase in the jejunum and maltase, isomaltase and sucrase in the ileum were not affected by diet. Sucrase activity in the jejunum was significantly lower in the HAMS group than in the WMS group, but was not different from that in the HP-HAMS group.
Table 3.
Secretion of bile-pancreatic juice, amylase activity of pancreatic juice, and activity of membrane digestive enzymes in rats fed the waxy maize starch (WMS), high-amylose maize starch (HAMS) and hydroxypropylated high-amylose maize starch (HP-HAMS) diets*
(Mean values and pooled standard errors for six rats per group)
| Diet | ||||
|---|---|---|---|---|
| WMS | HAMS | HP-HAMS | Pooled sem | |
| Bile-pancreatic juice | ||||
| Juice secretion (ml/30 min) | 0·492a | 0·583b | 0·594b | 0·021 |
| Amylase secretion (μg/30 min) | 64·2 | 66·0 | 69·8 | 5·5 |
| Membrane digestive enzymes (μmol glucose/min per mg protein) | ||||
| Jejunum | ||||
| Maltase | 1·79 | 1·84 | 1·73 | 0·19 |
| Isomaltase | 0·75 | 0·92 | 0·78 | 0·08 |
| Sucrase | 0·29b | 0·18a | 0·23a,b | 0·03 |
| Ileum | ||||
| Maltase | 0·97 | 0·74 | 0·94 | 0·10 |
| Isomaltase | 0·44 | 0·39 | 0·45 | 0·04 |
| Sucrase | 0·03 | 0·01 | 0·02 | 0·01 |
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Data were analysed by one-way ANOVA followed by the Tukey–Kramer test.
The wet weight of faeces excreted in the HP-HAMS group was significantly higher than that in the WMS and HAMS groups, but the number of faecal pellets was not affected by diet (Table 4). Faecal moisture was significantly higher in the HP-HAMS group than in the WMS group, but was not different from that in the HAMS group. The caecum wall weight of rats in the HAMS and HP-HAMS groups was significantly greater than that in the WMS group, and the caecum wall weight in the HP-HAMS group was significantly greater than that in the HAMS group. The wet weight of the caecal content and the moisture percentage of the caecal content were significantly greater in the HAMS and HP-HAMS groups than in the WMS group, but were not significantly different between the HAMS and HP-HAMS groups.
Table 4.
Faecal output, caecal tissue weight and wet weight, pH, IgA and organic acids in the caecal contents of rats fed the waxy maize starch (WMS), high-amylose maize starch (HAMS) and hydroxypropylated high-amylose maize starch (HP-HAMS) diets*
(Mean values and pooled standard errors for six rats per group)
| Diet | ||||
|---|---|---|---|---|
| WMS | HAMS | HP-HAMS | Pooled sem | |
| Faecal output | ||||
| Wet weight (g/d) | 1·53a | 1·55a | 2·44b | 0·09 |
| Moisture (%) | 21·6a | 28·1a,b | 34·3b | 2·6 |
| Number (no./d) | 16·2 | 14·0 | 14·3 | 0·7 |
| Caecum | ||||
| Tissue weight (g) | 0·48a | 1·02b | 1·45c | 0·04 |
| Contents | ||||
| Wet weight (g) | 2·47a | 13·23b | 15·62b | 0·61 |
| Moisture (%) | 63·6a | 72·9b | 77·5b | 0·4 |
| pH | 8·46c | 5·67a | 6·37b | 0·06 |
| IgA (μg/caecum) | 95a | 2817b | 2494b | 414 |
| Organic acids (μmol/caecum) | ||||
| SCFA | ||||
| Acetate | 93a | 378c | 221b | 22 |
| Propionate | 25a | 154b | 56a | 7 |
| n-Butyrate† | 1·18a | 1·64b | 1·65b | 0·39 |
| Total‡ | 133a | 576c | 322b | 34 |
| Succinate | 16a | 940b | 48 | 26 |
| Lactate§ | NA | NA | 31 | |
NA, not analysed statistically.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Data were analysed by one-way ANOVA, followed by the Tukey–Kramer test.
† Data for butyrate were transformed logarithmically before analysis. n-Butyrate was expressed as the log concentration.
‡ Total (μmol/caecum) = acetate (μmol/caecum) + propionic (μmol/caecum) + n-butyrate (μmol/caecum).
§ Lactate was detected in three of the six rats fed the WMS diet (3·6–5·3 µmol/caecum) and in one of the six rats fed the HAMS diet (479 µmol/caecum).
The amount of acetic acid and total SCFA in the caecal content were significantly greater in the HAMS and HP-HAMS groups than those in the WMS group, and were significantly greater in the HAMS group than those in the HP-HAMS group. The amount of propionic and succinic acids in the caecal content was significantly greater in the HAMS group than that in the WMS and HP-HAMS groups, but was not significantly different between the WMS and HP-HAMS groups. The amount of n-butyric acid in the caecal content was significantly greater in the HAMS and HP-HAMS groups than in the WMS group, but did not differ significantly between the HAMS and HP-HAMS groups.
The pH of the caecal content was significantly lower in the HAMS and HP-HAMS groups than that in the WMS group, and was significantly lower in the HAMS group than in the HP-HAMS group. The amount of IgA in the caecal content was significantly higher in the HAMS and HP-HAMS groups than that in the WMS group, but did not differ significantly between the HAMS and HP-HAMS groups. Linear regression analysis showed that the caecal amount of IgA positively correlated with the caecal amount of acetic acid (r 0·696), propionic acid (r 0·644), n-butyric acid (r 0·716), SCFA (acetic + propionic + n-butyric acids; r 0·710) and succinic acid (r 0·555).
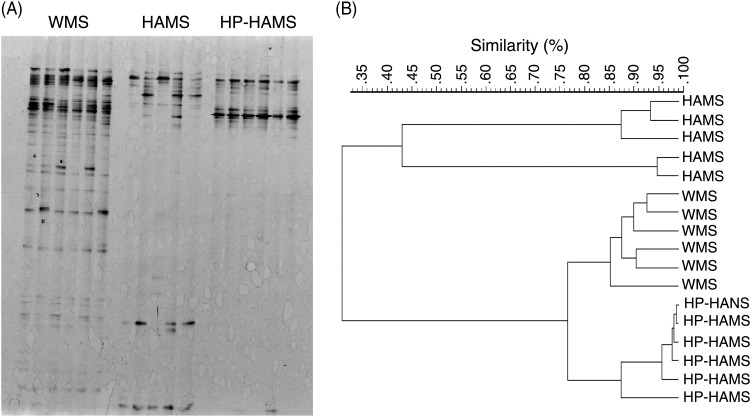
The DGGE patterns of the three dietary groups (six samples for rats fed the WMS and HP-HAMS diets, and five samples for rats fed the HAMS diet) are shown in Fig. 4(A). Bacterial diversity was divided into three distinct groups. Rats fed the WMS and HP-HAMS diets formed a group with high similarity (Dice's similarity coefficient, 78 %) (Fig. 4(B)). Rats fed the HAMS diet were segregated from those fed the WMS and HP-HAMS diets with low similarity (Dice's similarity coefficient, 31 %).
Fig. 4.
PCR-denaturing gradient gel electrophoresis (DGGE) analysis of the caecal microbiota, based on 16S rRNA gene sequences in rats fed the waxy maize starch (WMS), high-amylose maize starch (HAMS) or hydroxypropylated HAMS (HP-HAMS) diet for 4 weeks. (A) DGGE gel image stained with SYBR green. (B) DGGE banding patterns were analysed using FPQuest software (Bio-Rad). Similarity was visualised by dendograms using the unweighted pair group method using the arithmetic averages (UPGMA).
Discussion
The digestibility of HAMS in the small intestine was significantly decreased by hydroxypropylation. Although all of the three hydroxyl groups (at 2-O, 3-O and 6-O) are potentially available for substitution, the hydroxypropyl groups are predominantly located at position 2-O in the glucose units( 25 ). Hydroxypropyl groups at position 2-O sterically hinder the enzymatic hydrolysis of neighbouring glycoside bonds in hydroxypropylated starch( 26 ). Therefore, the decreased digestibility of HP-HAMS would be mainly due to enzyme inhibition by hydroxypropyl groups at position 2-O.
The digestibilities of cellulose, hemicellulose and pectin for ileosotmised human subjects were 16, 17 and 15 %, respectively( 27 – 29 ). The digestibility of HP-HAMS was 82 %, which shows that the digestibility of HP-HAMS is higher than that of cellulose.
Body weight gain was significantly lower in the HP-HAMS group than in the WMS group, while food intake was not. The structural modification of starch might decrease its digestibility, and hydroxypropylation has been shown to decrease the susceptibility of starch to digestive enzymes( 30 , 31 ). The small intestinal digestibility of starch was significantly lower in the HP-HAMS group than in the HAMS group, as calculated from the recovery of starch from effluent in neomycin-treated ileorectomised rats. Ingesting less energy is the major determinant towards weight loss. Therefore, the decreased body weight gain observed in the HP-HAMS group might be due to the lower digestibility of HP-HAMS.
HAMS reduces plasma TAG concentrations in rats( 10 , 32 ). Propionate inhibits fatty acids synthesis( 33 , 34 ) and decreases the concentration of fatty acids synthesis mRNA in cultured hepatocytes( 35 ). Propionate reduces serum and hepatic cholesterol amounts in rats( 36 ). In the present study, the amount of propionic acid in the caecal content was significantly higher in the HAMS group than in the WMS and HP-HAMS groups. The concentration of TAG in the liver was significantly lower in the HAMS group than in the WMS group. Therefore, feeding a diet rich in HAMS might reduce lipogenesis in the liver. However, the concentration of plasma TAG in the HAMS group was not lower than that in the WMS group. Conversely, the concentration of plasma TAG was significantly lower in the HP-HAMS group than in the WMS group, but the concentration of TAG in the liver was not. The concentration of plasma TAG is also controlled by the amount of TAG absorbed from the small intestine. Hydroxypropyl groups are hydrophilic in nature, and the solubility, viscosity and swelling power of starch are increased after hydroxypropylation( 37 ). Hydroxypropylation also decreases the temperature at peak viscosity( 38 ). Moreover, the addition of sucrose increased in viscosity( 38 ). As a result, the digestion of HP-HAMS may be lower than that of WMS. The slower digestion of HP-HAMS might in turn make the digestion of TAG slow, which might result in a lower concentration of plasma TAG in the HP-HAMS group.
Faecal wet weight in the HP-HAMS group was higher than that in the WMS group, but did not differ significantly from that in the HAMS group. Faecal weight was found to be significantly higher in rats fed a diet with over 20 % HAMS than in those fed a normal maize starch diet( 11 , 39 ). The amount of HAMS in the diets used in this study was 150 g per 1 kg diet. The moisture content of the faeces in the HP-HAMS group was greater than that in the HAMS group, suggesting that HP-HAMS might possess water-holding properties. Hydroxypropylated waxy rice and maize starches have a higher water-holding capacity than unmodified starches( 40 ). The introduction of hydroxypropyl groups has been found to increase the water-holding capacity of all starches( 41 ).
An increase in caecal tissue weight in rats ingesting carbohydrates of low digestibility has been reported by various authors. Dietary HAMS and HP-HAMS increase the wet weight of the caecal content. Maldigested materials reaching the caecum are potential sources for intestinal bacteria, resulting in the production of SCFA. Here, an increase in caecal tissue weight was observed in rats fed the HAMS and HP-HAMS diets. This hypertrophic effect is mediated by butyrate, because butyrate has been recognised as the main source of energy for the caecal mucosa( 42 ). On the other hand, Oku et al.( 43 ) speculated that caecal enlargement depends on the amount of maldigested materials reaching the caecum. As a result, the increase in the caecal tissue weight in the HAMS and HP-HAMS groups appears to depend on a greater caecal content and a higher concentration of butyrate.
The increased propionate concentration measured in samples from the HAMS group was unexpected because dietary RS is reported to increase butyrate production, although propionate can be produced in preference to butyrate( 44 ). Succinic acid is converted to propionic acid by intestinal bacteria ( 45 ). In the caecal content, the amount of propionic acid increased with the amount of succinic acid (r 0·911, P < 0·001). On the basis of PCR-DGGE profiles, the similarity between the HAMS and HP-HAMS groups was low (Dice's similarity coefficient, 31 %). Therefore, the unexpected amount of propionic acid in the caecal content might be due to changes in the microbiota in the caecum.
The amount of caecal SCFA and the pH of the caecal content in the HP-HAMS group were significantly lower and higher, respectively, than those in the HAMS group. When undigested carbohydrates reach the large intestine, the intestinal bacteria that normally live in the large intestine break them down. This breakdown of carbohydrates by intestinal bacteria produces SCFA and H2. H2 is produced in the body only by intestinal bacteria. H2 excretion in breath in the HP-HAMS group was about 25 % of that in the HAMS group, suggesting that HP-HAMS is broken down by intestinal bacteria to a lesser extent than HAMS.
Organic acids may contribute to intestinal immune and barrier functions( 46 ). Here, large amounts of caecal organic acids and caecal IgA were manifested in rats fed the HAMS and HP-HAMS diets. The amount of caecal IgA positively correlated with the amount of caecal acetic acid, propionic acid, n-butyric acid and total SCFA and positively with caecal pH. The amount of caecal IgA correlates with the caecal amount of lactic acid( 47 – 49 ), propionic acid( 47 ), n-butyric acid( 47 ) and total SCFA( 47 ). Higher production of butyrate in the rat caecum results in more immune cells in the gut epithelial layer cells( 50 ). In this study, the caecal amount of IgA correlated with the amount of succinic acid in the caecal content, in contrast with the findings of Ito et al.( 47 – 49 ). However, the amount of caecal IgA positively correlated with the pH of the caecal content, which is in agreement with the result of Sung et al.( 50 ). Therefore, it appears that SCFA production was connected with the IgA production.
HAMS can reduce insulin resistance( 9 , 51 ), although the exact mechanism underlying improved insulin sensitivity has not been elucidated. One hypothesis is that SCFA produced by the fermentation of RS in the large intestine may lead to improved insulin sensitivity( 9 ). SCFA triggers secretion of GLP-1 from mixed colonic cultures in vitro( 52 ). GLP-1 improves insulin sensitivity in human subjects and rodents( 53 ).
Food intake, amylase and digestive enzymes in brush border membrane activities and plasma insulin and gastric inhibitory peptide concentrations were not significantly different between the HAMS group and the HP-HAMS group. However, the plasma glucose concentration of the HP-HAMS group was significantly lower than that of the HAMS group. HP-HAMS might interfere with the digestion and absorption of starch and sucrose in the diet as the viscosity of starch increases after hydroxypropylation.
The digestibility of carbohydrate in ileorectomised rats fed HAMS and HP-HAMS was about 80 %, which suggests that about 20 % of ingested carbohydrate reach the ileum. Under normal physiological situations, undigested nutrients can reach the ileum and induce activation of the so-called ‘ileal brake’. The ileal brake is a neurohormonal feedback mechanism that delays gastric and intestinal transit time and reduces food intake in response of unabsorbed nutrients in the distal ileum. Carbohydrate is a significant stimulus for the ileal brake mechanism( 54 ). Activation of the ileal brake is associated with the secretion of gut peptides, such as peptide YY (PYY) and GLP-1( 55 ). A high intake of amylose-resistant maize starch in rodents has also been found to consistently increase GLP-1 and peptide YY( 56 , 57 ). GLP-1 is an intestinal hormone that has potent effects on glucose-mediated insulin secretion, insulin gene expression, and β-cell growth and differentiation( 58 ). The plasma GLP-1 concentration was significantly higher in the HAMS and HP-HAMS groups than in the WMS group; however, the plasma insulin concentration was not affected by diet. GLP-1 secretion is dependent on the presence of nutrients in the distal intestine. HAMS and HP-HAMS might stimulate the secretion of GLP-1 by increasing the delivery of nutrients to the distal intestine. GLP-1 acts to reduce food intake( 59 ). In the present study, food intake decreased as the concentration of GLP-1 increased (r −0·685, P < 0·002).
Species composition of the microbiota and substrate availability largely determine the amount and types of SCFA produced( 60 ). On the basis of the PCR-DGGE profiles, the similarity in caecal bacteria population of the HAMS group and HP-HAMS group was 31 %, which indicates that the caecal bacteria population was markedly changed by hydroxypropylation of HAMS. On the other hand, the similarity of caecal bacteria population of the WMS group and HP-HAMS group was 78 %, which indicates that the microbial population of the HP-HAMS group was similar to that of the WMS group. In ileorectomised rats, the apparent digestibility of HAMS was significantly decreased by hydroxypropylation. However, the amount of total SCFA and succinic acid in the caecal content of rats fed the HP-HAMS diet decreased to about 1/2 and 1/20, respectively, of that detected in rats fed the HAMS diet. Therefore, the part of HP-HAMS that escaped digestion in the small intestine was hardly utilised by caecal bacteria and did not affect the bacterial population.
In conclusion, digestibility of HAMS in the small intestine and its fermentability in the caecum of rats were significantly decreased by hydroxypropylation, and the microbial population of the caecal content was markedly changed. However, the microbial population of the caecal content of rats fed HP-HAMS was similar to that of rats fed WMS. Food intake, plasma and hepatic cholesterol and TAG concentrations, plasma GLP-1 concentration, amylase activity in bile-pancreatic juice and the activity of membrane digestive enzymes were not affected by hydroxypropylation of HAMS. Thus, hydroxypropylation of HAMS changes digestion and fermentation-dependent parameters, but does not affect glucose and lipid metabolism.
Acknowledgements
This work was supported by a research grant from the Japanese Ministry of Education, Science and Culture (16580101). K. E. designed the study and wrote the manuscript. M. T. and N. K. planned the study, summarised the results of the study and discussed with the other researchers the results of the study. T. K. provided advice on all aspects of the study.
None of the authors has any conflicts of interest.
References
- 1.Englyst N, Kingman SM & Cummings JH (1992) Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr 46, S33–S50 [PubMed] [Google Scholar]
- 2.Topping DL, Morell MK, King KA, et al. (2003) Resistant starch and health – Himalaya 292, a novel barley cultivar to deliver benefits to consumers. Starch/Stärke 55, 539–545 [Google Scholar]
- 3.Li L, Jiang H, Campbell M, et al. (2008) Characterization of maize amylose-extender (AE) mutant starches. Part I: relationship between resistant starch contents and molecular structures. Carbohydr Polym 74, 396–404 [Google Scholar]
- 4.Anderson JW (2009) All fibers are not created equal. J Med 2, 87–91 [Google Scholar]
- 5.Morita T, Ohhashi A, Ikai M, et al. (1996) Physiological function of resistant starch and its use (article in Japanese). Shokuhinn to Kaihatsu 31, 34–38 [Google Scholar]
- 6.Topping DL, Gooden JM, Brown IL, et al. (1997) A high amylose (amylomaize) starch raises proximal large bowel starch and increases colon length in pigs. J Nutr 127, 615–622 [DOI] [PubMed] [Google Scholar]
- 7.Holm J, Lundquist J, Björck I, et al. (1988) Degree of starch gelatinization, digestion rate of starch in vitro, and metabolic response in rats. Am J Clin Nutr 47, 1010–1016 [DOI] [PubMed] [Google Scholar]
- 8.Holst JJ & Gromada J (2004) Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab 287, E199–E206 [DOI] [PubMed] [Google Scholar]
- 9.Maki KC, Pelkman CL, Finocchiaro ET, et al. (2012) Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obese men. J Nutr 142, 717–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Kishida T & Ebihara K (2006) High amylose cornstarch decrease plasma triacylglycerol concentration, but not plasma cholesterol, in a dose-dependent manner. J Food Sci 71, S379–S384 [Google Scholar]
- 11.Lopez HW, Levrat-Verny MA, Coudray C, et al. (2001) Class 2 resistant starches lower plasma and liver lipids and improve mineral retention in rats. J Nutr 131, 1283–1289 [DOI] [PubMed] [Google Scholar]
- 12.Behall KM & Hallfrisch J (2002) Plasma glucose and insulin reduction after consumption of breads varying in amylose content. Eur J Clin Nutr 56, 913–920 [DOI] [PubMed] [Google Scholar]
- 13.Britesa CM, Trigoa MJ, Carrapiçob B, et al. (2011) Maize and resistant starch enriched breads reduce postprandial glycemic responses in rats. Nutr Res 31, 302–308 [DOI] [PubMed] [Google Scholar]
- 14.Kato R, Tachibe M, Sugano S, et al. (2009) High-hydroxypropylated tapioca starch improves insulin resistance in genetically diabetic KKAy mice. J Food Sci 73, H89–H96 [DOI] [PubMed] [Google Scholar]
- 15.Tachibe M, Kato R, Sugano S, et al. (2009) Hydroxypropylated tapioca starch retards the development of insulin resistance in KKAy mice, a type 2 diabetes model, fed a high-fat diet. J Food Sci 74, H232–H236 [DOI] [PubMed] [Google Scholar]
- 16.Tachibe M, Kato R, Nishibata T, et al. (2010) Evaluation of nondigested carbohydrates in hydroxypropylated tapioca starch. J Food Sci 75, H1–H4 [DOI] [PubMed] [Google Scholar]
- 17.Johnson DP (1969) Spectrophotometric determination of the hydroxypropyl group in starch esters. Anal Chem 41, 859–860 [Google Scholar]
- 18.Reeves PG, Nielsen FH & Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123, 1939–1951 [DOI] [PubMed] [Google Scholar]
- 19.Folch J, Lees M & Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissue. J Biol Chem 226, 497–509 [PubMed] [Google Scholar]
- 20.Ebihara K, Shiraishi R & Okuma K (1998) Hydroxypropyl-modified potato starch increases faecal bile acid excretion in rats. J Nutr 128, 848–854 [DOI] [PubMed] [Google Scholar]
- 21.Dahlqvist A (1968) Assay of intestinal disaccharides. Anal Biochem 22, 99–107 [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, et al. (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193, 265–275 [PubMed] [Google Scholar]
- 23.Walter J, Tannock GW, Tilsala-Timisjarvi A, et al. (2000) Detection and identification of gastrointestinal Lactobacillus species by using denaturing gradient gel electrophoresis and species-specific PCR primers. Appl Environ Microbiol 66, 297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichihara K (2001) Bartlett's test In Statistics for Bioscience, pp. 155–157 Tokyo: Nankodo [Google Scholar]
- 25.Xu A & Seib PA (1997) Determination of the level and position of substitution in hydroxypropylated starch by high-resolution 1H-MNR spectroscopy of alpha-limit dextrins. J Cereal Sci 25, 17–26 [Google Scholar]
- 26.Richardson S, Nilsson GS, Bergquit K-E, et al. (2000) Characterisation of the substituent distribution in hydroxypropylated potato amylopectin starch. Carbohydr Res 328, 365–373 [DOI] [PubMed] [Google Scholar]
- 27.Holloway WD & Tasman-Jones C (1978) Digestion of certain fractions of dietary fiber in humans. Am J Clin Nutr 31, 927–930 [DOI] [PubMed] [Google Scholar]
- 28.Holloway WD & Tasman-Jones C (1983) The hemicellulose component of dietary fiber. Am J Clin Nutr 32, 260–263 [DOI] [PubMed] [Google Scholar]
- 29.Holloway WD & Tasman-Jones C (1983) Pectin digestion in humans. Am J Clin Nutr 37, 253–255 [DOI] [PubMed] [Google Scholar]
- 30.Hoover R, Hannouz D & Sosulski FW (1988) Effects of hydroxypropylation on thermal properties, starch digestibility and freeze-thaw stability of field pea (Pisum sativum cv Trapper) starch. Starch/Stärke 40, 383–387 [Google Scholar]
- 31.Wootton M & Chaudhry MA (1981) In vitro digestion of hydroxypropyl derivatives of wheat starch. I. Digestibility and action pattern using porcine pancreatic alpha-amylase. Starch/Stärke 33, 135–137 [Google Scholar]
- 32.Goda T, Urakawa T, Watanabe M, et al. (1994) Effect of high-amylose starch on carbohydrate digestive capability and lipogenesis in epididymal adipose tissue and liver of rats. J Nutr Biochem 5, 256–560 [Google Scholar]
- 33.Lin Y, Vonk RJ, Slooff MJ, et al. (1995) Differences in propionate-induced inhibition of cholesterol and triacylglycerol synthesis between human and rat hepatocytes in primary culture. Br J Nutr 74, 197–207 [DOI] [PubMed] [Google Scholar]
- 34.Wright RS, Anderson JW & Briges SR (1990) Propionate inhibits hepatocyte lipids synthesis. Proc Soc Exp Biol Med 195, 26–29 [DOI] [PubMed] [Google Scholar]
- 35.Cheng H & Lai M (2000) Fermentation of resistant starch produces propionate reducing serum and hepatic cholesterol in rats. J Nutr 130, 1991–1995 [DOI] [PubMed] [Google Scholar]
- 36.Chen WJL, Anderson JW & Jennings D (1984) Propionate may mediate the hypocholesterolemic effects of certain soluble plant fibers in cholesterol fed rats. Proc Soc Exp Biol Med 175, 215–218 [DOI] [PubMed] [Google Scholar]
- 37.Liu H, Ramsden L & Corke H (1999) Physical properties and enzymatic digestibility of hydroxypropylated ae, wx, and normal maize starch. Carbo Polymer 40, 175–182 [DOI] [PubMed] [Google Scholar]
- 38.Takahashi S, Suzuki M, Yoshida E, et al. (1998) Cooking and processing properties of gelatinized hydroxypropylated wheat starch. J Home Econ Jpn 49, 1099–1108 (in Japanese). [Google Scholar]
- 39.Kishida T, Nogami H, Ogawa H, et al. (2002) The hypocholesterolemic effect of high-amylose corn starch in rats is mediated by an enlarged bile acid pool and increased fecal bile acid excretion, not by caecal fermented products. J Nutr 132, 2519–2524 [DOI] [PubMed] [Google Scholar]
- 40.Han J-A, Lee B-H, Lim WJ, et al. (2005) Utilization of hydroxypropylated waxy rice and corn starches in Korean waxy rice cake to retard retrogradation. AACC Int 82, 88–92 [Google Scholar]
- 41.Onofre FO & Wang Y-J (2010) Hydroxypropylated starches of varying amylase contents as sustained release matrices in tablets. Int J Pharm 385, 104–112 [DOI] [PubMed] [Google Scholar]
- 42.Le Blay GM, Michel CD, Blottière HM, et al. (1999) Prolonged intake of fructo-oligosaccharides induces a short-term elevation of lactic acid-producing bacteria and a persistent increase in cecal butyrate in rats. J Nutr 129, 2231–2235 [DOI] [PubMed] [Google Scholar]
- 43.Oku T, Konishi F & Hosoya K (1981) Effect of various carbohydrate and administration periods on several physiological functions of rats (article in Japanese). J Jpn Soc Nutr Food Sci 34, 437–443 [Google Scholar]
- 44.Topping DL & Clifton PM (2001) Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81, 1031–1064 [DOI] [PubMed] [Google Scholar]
- 45.Johns AT (1950) The mechanism of propionic acid formation by propionibacteria. J Gen Microbiol 5, 337–345 [DOI] [PubMed] [Google Scholar]
- 46.Ishizuka S, Tanaka S, Xu H, et al. (2004) Fermentable dietary fiber potentiates the localization of immune cells in the rat large intestinal crypts. Exp Biol Med (Maywood) 229, 876–884 [DOI] [PubMed] [Google Scholar]
- 47.Ito H, Wada T, Ohguchi M, et al. (2008) The degree of polymerization of inulin-like fructans affects caecal mucin and immunoglobulin A in rats. J Food Sci 73, H36–H41 [DOI] [PubMed] [Google Scholar]
- 48.Ito H, Takemura N, Sonoyama K, et al. (2011) Degree of polymerization of inulin-type fructans differentially affects number of lactic acid bacteria, intestinal immune fructans, and imunoglobulin A secretion in the rat caecum. J Agric Food Chem 59, 5771–5578. [DOI] [PubMed] [Google Scholar]
- 49.Ito H, Tanabe H, Kawagishi H, et al. (2009) Short-chain inulin-like fructans reduce endotoxin and bacterial translocation and attenuate development of TNBS-induced colitis in rats. Dig Dis Sci 54, 2100–2108 [DOI] [PubMed] [Google Scholar]
- 50.Sung HY, Jeong HJ & Choi YS (2004) Effects of fructans and isomaltooligosaccharide on large bowel mass and plasma and fecal immunoglobulin A in rat. Nutr Sci 7, 196–200 [Google Scholar]
- 51.Robertson MD, Bickerton AS, Dennis AL, et al. (2005) Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr 82, 559–567 [DOI] [PubMed] [Google Scholar]
- 52.Tolhurst G, Heffron H, Lam YS, et al. (2012) Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parlevliet ET, de Leeuw van Weenen JE, Romijn JA, et al. (2010) GLP-1 treatment reduces endogenous insulin resistance via activation of central GLP-1 receptors in mice fed a high-fat diet. Am J Physiol Endocrinol Metab 299, E318–E324 [DOI] [PubMed] [Google Scholar]
- 54.Spiller RC, Trotman IF, Adrian TE, et al. (1988) Further characterisation of the ‘ileal brake' reflex in man – effect of ileal infusion of partial digests of fat, protein, and starch on jejunal motility and release of neurotensin, enteroglucagon, and peptide YY. Gut 29, 1042–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strader AD (2006) Ileal transposition provides insight into the effectiveness of gastric bypass surgery. Physiol Behav 88, 277–282 [DOI] [PubMed] [Google Scholar]
- 56.Keenan M, Zhou J, McCutcheon K, et al. (2006) Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity 14, 1523–1534 [DOI] [PubMed] [Google Scholar]
- 57.Prigeon RL, Quddusi S, Paty B, et al. (2003) Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. AJP-Endocrinol Metab 285, E701–E707 [DOI] [PubMed] [Google Scholar]
- 58.Kjems LL, Holst JJ, Vølund A, et al. (2003) The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 52, 380–386 [DOI] [PubMed] [Google Scholar]
- 59.Zhang J & Ritter RC (2012) Circulating GLP-1 and CCK-8 reduce food intake by capsaicin-insensitive, nonvagal mechanisms. Am J Physiol Regul Integr Comp Physiol 302, R264–R273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macfarlane S & Macfarlane GT (2012) Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int 95, 50–60 [DOI] [PubMed] [Google Scholar]