Abstract
Pulmonary dendritic cells (DCs) constantly sample the tissue and traffic inhaled antigens to the lung-draining lymph node where they normally orchestrate an appropriate immune response. The dynamic ability of these professional antigen-presenting cells to promote tolerance or immunity has been intensively studied by several groups, including ours. Distinct DC subsets in both lymphoid and non-lymphoid tissues have been described based on their surface molecule expression and location. Current efforts to unravel DC development and function are providing insight into the various roles each subset offers the immune system. Elucidating DC functions, particularly in the lung, may then allow use of the inherent ability of these cells for enhanced vaccine strategies and therapeutics for pulmonary infections and diseases.
Keywords: Dendritic cell, Lung-draining lymph node, Pulmonary, Antigen acquisition
Introduction
Dendritic cells (DCs) are the most potent professional antigen-presenting cells specialized in the ability to initiate, sustain, and regulate appropriate immune responses [1]. Constantly sampling their environment, DCs process captured proteins into peptides and present antigens via major histocompatibility complex (MHC) molecules, which are recognized by T cells [2]. The outcome of antigen presentation to T cells largely depends on the environment in which the antigen was captured. Under steady-state conditions, DCs tolerize T cells to innocuous antigens acquired in tissue including self-antigens [3]. However, in the presence of pathogen-derived substances, tissue damage, or adjuvants, DCs are activated to express co-stimulatory molecules and cytokines that promote the development of antigen-specific CD8 and CD4 T cells [4–7]. They also express pattern recognition receptors including members of the toll-like receptor (TLR) family that enables recognition of foreign pathogens and adjuvants [8]. The diversity of expression for pattern recognition receptors among the DC subsets suggests selectivity in pathogen recognition. Moreover, DC subsets differ in location, hematopoietic lineage, and function that directly influence the immunological outcome. This overall new appreciation of DC diversity has focused the field on understanding how various non-lymphoid and lymphoid DC subsets differentially acquire antigen in the tissues and present it in the draining lymph nodes (LNs). In the lung, four populations of DC have been described so far: plasmacytoid DCs (pDCs), monocyte-derived DC, and two migratory DC subsets. This review focuses on the migratory DCs of the mouse lung by outlining their development and specialized function in tissue antigen acquisition and presentation.
Dendritic cells of the lung
Myeloid cells in the lung
There are several cell types capable of antigen presentation in the lung, which include resident alveolar macrophages and DCs, of which both express high levels of the integrin CD11c. Other mononuclear myeloid populations present in the lung are pDCs and monocytes, which become more abundant during infection and inflammation [9–11]. PDCs can be detected by the intermediate expression of MHC II and CD11c with high levels of expression for B220 and Gr-1 (Ly6C) [12]. Of these various antigen-presenting cell types, non-lymphoid migratory DCs have the greatest capacity to initiate T cell-mediated immune responses due to their ability to capture antigens and migrate down afferent lymphatics into the draining LN where they present ferried antigens [13].
The two migratory DC subsets that exist in the lung can be distinguished using flow cytometry and microscopy through their differential expression of integrins: (1) CD11c+ CD11bloCD103+ and (2) CD11c+ CD11bhiCD103− DCs [12, 14], identified herein as CD103+ or CD11bhi DCs, respectively. Both DCs are present in the lungs of naïve mice with relatively similar frequencies as shown in Fig. 1a. In naïve mice, pulmonary migratory DCs represent approximately 1.5 % of all leukocytes in the lungs, while pDCs are less than 0.5 % [9].
Fig. 1.
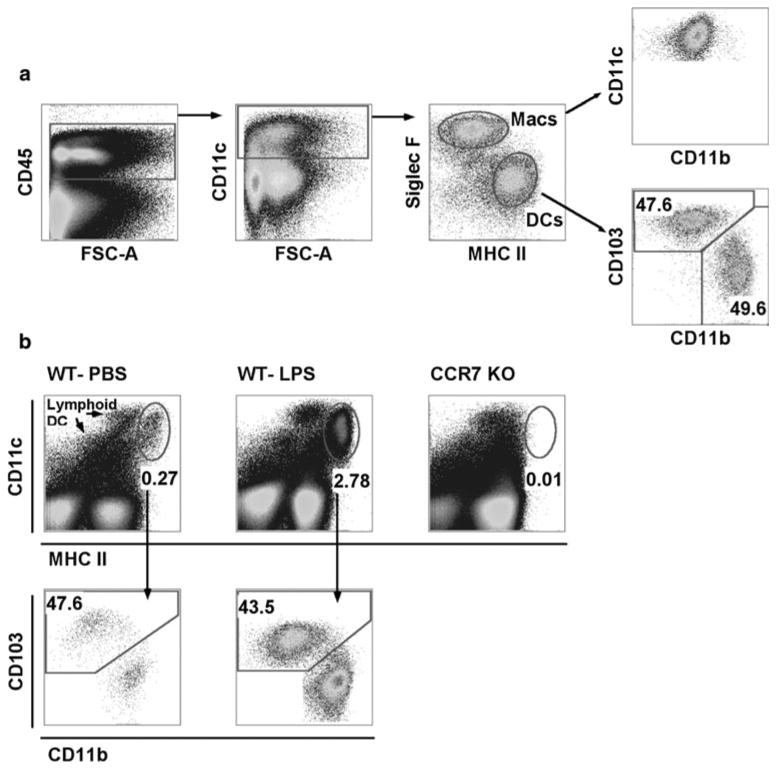
Gating strategy for the identification of DCs in lung and LLN. a Whole lung digest from a naïve mouse gated on live cells. CD45+ cells were plotted for CD11c expression. CD11c+ cells were plotted as Siglec-F versus MHC II to differentiate macrophages from DCs. Gated Siglec-F− MHCII+ DCs were plotted as CD103 versus CD11b to differentiate migratory DC subsets that are found in similar frequencies. b Live cells from single LLNs 24 h post-intranasal instillation of PBS or 2 μg LPS isolated from WT or CCR7−/− mice. Cells plotted as CD11c versus MHC II display lymphoid-resident and migratory DC populations. Migratory DCs have higher MHC II expression than lymphoid-resident DCs. CD11c+ MHCIIhi migratory DCs were plotted CD103 versus CD11b to demonstrate similar migration frequencies in WT mice
Location of pulmonary DCs
The location of DCs in the lung has been examined in mice and rats [15]. In mice, the frequency of pulmonary DCs is greater in the conducting airways, that is trachea and major bronchi, compared to the lung parenchyma [16]. However, DCs were not detected in bronchoalveolar lavage fluid where the predominant myeloid cell is the CD11c expressing alveolar macrophage. This suggests that DCs in the airways are tightly associated with the epithelium. Sung and colleagues performed the first characterization of murine pulmonary CD103+ DCs in 2006. Immunohistochemistry staining revealed that CD103+ DCs represent 70–75 % of the CD11c+ MHCII+ DCs positioned on the basal side of airway epithelium and parenchymal side of vascular endothelial cells [12]. Subepithelial location of the CD103+ DCs is likely mediated by interaction of CD103 (alpha e integrin) and beta 7 integrin with E-cadherin expressed basally by epithelial cells. Moreover, CD103+ DCs express several tight junction proteins including Claudin-1, Claudin-7, and Z0-2 to a greater extent than CD11bhi DCs, which might suggest its capacity to extend protrusions through the epithelial barrier into the bronchiolar luminal space, granting access to external antigens or pathogens [12]. CD11bhi DCs are not seen as often in these locations and predominantly localize in the peribronchial regions and interstitial space [12].
Identification of pulmonary DCs
To identify pulmonary DCs using flow cytometry, single-cell suspensions need to be obtained from lung tissue [17]. Using the gating strategy shown in Fig. 1a, CD11c expressing DCs can be discriminated from macrophages in the lung by staining for sialic acid binding Ig-like lectin F, Siglec-F. Eosinophils also express Siglec-F but do not stain for CD11c [12]. Moreover, under steady-state conditions, macrophages do not express CD11b, like eosinophils. During inflammation, the use of Siglec-F becomes useful to distinguish macrophages from DCs and incoming monocytes because resident macrophages upregulate CD11b and MHC II on their surface [18–20].
Pulmonary DC turnover
Steady-state turnover of migratory DCs from the conducting airways has been shown to occur as often as every 2–3 days in rats and even more rapidly in mice using bone marrow-depletion studies to determine DC lifespan in the lung [21]. Other studies using parabiotic mice suggest DC turnover in the lung is much longer, with CD11bhi and CD103+ DCs having half-lives of 15 and 30 days, respectively [22]. These studies show that there is frequent cell turnover, that is migration from the tissue and then eventual death within the LN, supporting the critical role these cells have in immune surveillance of inhaled antigens.
Cytokines and transcription factors
Nearly two decades after the discovery of DCs by Steinman and Cohn, granulocyte/macrophage colony-stimulating factor (GM-CSF) was identified as a key cytokine for differentiating bone marrow progenitors into DCs in vitro [23]. These antigen-presenting, MHCII+ cells enabled cell biologists to investigate in detail the mechanisms of migration, antigen-processing, and antigen presentation [6, 24–26]. However, it has now become evident that these bone marrow-derived DCs do not accurately reflect DCs found in lymphoid and non-lymphoid tissues in vivo. Interestingly, GM-CSF is not required for the development of DCs in vivo: GM-CSF receptor-deficient mice have normal DC development in the lung with slightly reduced DC numbers in tissue and LNs [27].
More recent studies have provided a greater understanding of the developmental role that cytokines and transcription factors have in shaping the DC repertoire in lymphoid and non-lymphoid compartments. DC lineage commitment starts in the bone marrow with the macrophage and DC precursor (MCP) that has the potential to become monocytes or the common DC precursor (CDP) [28, 29]. CDPs can no longer differentiate into monocytes or macrophages but can give rise to pDCs and pre-cDCs, which are progenitors to lymphoid and some non-lymphoid DC subsets [30, 31]. Monocytes have been shown to give rise to some non-lymphoid DC subsets [14, 32–34], although adoptively transferred monocytes show minimal, if any, capacity to differentiate into defined DC subsets in the lung under steady-state conditions (unpublished data). Only in mice depleted of CD11c+ cells along with a transfer of large numbers of Gr1hiCD11b+ CD115+ BM monocytes did monocytes appear to differentiate into cells that expressed similar surface molecules as CD11bhi DCs. However, it is not yet clear that these cells become fully differentiated pulmonary DCs [22, 34]. During inflammation, monocyte-derived DCs are recruited into tissues, which derive from the blood-circulating CD115+ CCR2+ monocytes [33, 35]. The inflammatory monocyte-derived DCs (also known as TNF-iNOS producing DCs, TIP-DCs) express CD11c, MHC II, CD24, SIRPα, and DC-SIGN and lose expression of CD115 and Ly6C, making them difficult to distinguish from steady-state DCs [36, 37]. With these studies in mind, using the gating strategy in Fig. 1a, CD11bhi DCs are most likely comprised of a heterogeneous population, and more so during inflammation.
Evidence of the differential need for growth factors by pulmonary DCs is supported by the disparity in their cytokine receptor expression [38]. Fms-like tyrosine kinase 3 ligand (Flt3 ligand) is an important growth factor for the expansion of lymphoid DCs, pDCs and CD103+ DCs [39]. Multiple studies use recombinant human Flt3 ligand to demonstrate the expansion of CD11c+ CD11b+/− MHCII+ cells [39]. However, when our group expanded pulmonary CD11c+ MHCII+ cells in vivo using recombinant human Flt3 ligand, we observed the expansion of an MHCII+ cell not phenotypically present in wild-type lungs. By contrast, when recombinant murine Flt3 ligand was used, pulmonary CD103+ DCs were preferentially expanded (unpublished data). In Flt3-deficient mice, CD103+ DCs were severely diminished compared to CD11bhi DCs [22, 38]. On the other hand, Flt3 ligand-deficient mice exhibit reduced development of both DC subsets in the lung and other tissues, suggesting both DCs require this cytokine but use separate receptors for their differentiation [22, 40].
M-CSF is a key cytokine involved in monocyte and macrophage differentiation [41]. It also appears to have a role in the development of CD11bhi DCs, which express M-CSFR to a greater extent than CD103+ DCs in the lung and other tissues [22, 38]. In contrast to CD103+ DCs that developed normally in the M-CSFR-deficient mice, CD11bhi DCs were partially reduced in numbers in the lung [22].
In addition to cytokines, lung DCs depend on transcription factors for their subset programming (Table 1). One unifying developmental requirement for all DC subsets is PU.1, which is the key regulator of Flt3 expression, and interferon-regulatory factor 8, IRF8 [42]. Deficiencies in these transcription factors render animals devoid of all DC lineages [43–46]. CDPs and pre-DCs express high levels of PU.1 and IRF8 and low levels of ID2. PDCs diverge from the conventional DC program by down-regulating PU.1 and upregulating E-protein E2-2 while maintaining IRF8 expression [36]. Conventional DCs commit to their lineage at the CDP stage by retaining high levels of PU.1 and gaining the expression of inhibitor of DNA binding 2, ID2, which is expressed highly on developmentally similar CD8α+ and CD103+ DCs. Unlike pDCs, conventional DCs do not gain E2-2 [36]. Recently, two groups have identified a unifying transcription factor, Zbtb46, which is selectively expressed by all cDCs and pre-DCs but not pDCs, monocytes, and macrophages [47, 48]. Although not required for cDC development, Zbtb46 regulates the silencing of the G-CSF receptor and leukemia inhibitory factor receptors that occurs during the course of normal cDC development [48]. Interestingly, DC differentiation from purified BM monocytes cultured with GM-CSF promoted Zbtb46 expression and high surface MHC II [48]. This datum suggests some caution in associating all Zbtb46+ immune cells with their derivation from preDCs, such that it is possible that recruited monocytes entering a GMCSF-rich lung environment upregulate Zbtb46 resulting in its acquisition of a DC-like phenotype, as observed in vitro.
Table 1. Developmental requirements of pulmonary DCs.
| DC subset | Transcription factors | Growth factor receptors | Growth factors | CD45+ lung compartment frequency (%) |
Refs. |
|---|---|---|---|---|---|
| Plasmacytoid DC (CD11cintB220+Ly6C+) | IRF8, E2-2 | Flt3 | Flt3 ligand | 0.3 | [18], [43–45] |
| CD103+ DC | Batf3, ID2, IRF8, Zbtb46 | Flt3 | Flt3 ligand | 0.75 | [18], [45–50] |
| CD11bhi DC | RELB, IRF2, IRF4, Zbtb46 | M-CSFR (CD115) | Flt3 ligand, M-CSF | 0.75 | [18], [47, 48], [51–55] |
In tissues, CD103+ and CD11bhi DCs differ in the requirement of transcription factors. CD103+ DCs rely heavily upon basic leucine zipper transcription factor, ATF-like 3, BATF3, for their development. In fact, CD103+ DCs are completely absent in the lung and other tissues in BATF3 knockout animals [38, 49]. Additionally, this subset depends on high expression of IRF8 and ID2 for normal cellularity [22, 50]. The transcription factors contributing to CD11bhi DC development are less well defined. However, this subset appears to depend less upon IRF8 and BATF3 such that in the BATF3-deficient mice, CD11bhi DCs are intact in the lung [38, 49]. Although not shown in the lung, in the spleen, lymphoid CD11bhi DCs require RELB, IRF2, and IRF4 for their development [51–55]. It seems likely that these transcription factors are also involved in the lung, as they appear to be selectively expressed in pulmonary CD11bhi DCs [38].
Understanding DC programming in humans will be critical as deficiencies and mutations in these transcription factors have been recently described [56–58]. These individuals have dysfunctional DC development, rendering them unable to control mycobacterial infections [56, 57]. Thus, identification of key transcription factors for the development of murine DCs is increasingly assisting in the identification of human orthologs of the murine DC subsets.
Antigen acquisition and trafficking
Macrophage interaction with pulmonary DCs
The respiratory tract provides a large surface area where a single epithelial cell layer allows for efficient gas exchange with an underlying endothelial layer surrounding the pulmonary capillaries. This thin barrier presents a challenge to the mucosal immune system that must discriminate a pathogen from the constant bombardment of innocuous antigens inhaled from the environment. Prior to antigen presentation, antigens must get past both physical and cellular barriers before reaching tissue DCs including the mucociliary escalator, surfactants, and highly phagocytic macrophages.
The anatomical location of alveolar macrophages suggests that alveolar macrophages are the first line of defense during microbial airway challenges, which occurs daily. Patrolling the alveolar space, macrophages play an important role in suppressing DC function [59, 60]. These cells ingest most airway antigens, limiting underlying DCs from acquiring environmental particulates [61]. Thus, macrophages minimize lung tissue damage from an unnecessary immune response that could be initiated by pulmonary DCs. For pulmonary DC antigen acquisition, a threshold must be met where the macrophage population becomes fully saturated to permit antigen spillover into the DC compartment. One study found that the antigen delivery dose into the lungs requires approximately 109 organisms before bacterial ingestions are taken up by pulmonary DCs [61]. Therefore, when macrophages can no longer contain a microbial challenge, DCs will assist in eliciting an adaptive immune response. However, minimizing inflammation in the interstitial space of the lung is important since the main function of the lungs is gas exchange. Other studies supporting the role of macrophages as DC suppressers demonstrated that clodronate depletion of alveolar macrophages resulted in enhanced DC numbers in the airways and particle uptake by DCs [62]. The enhanced uptake in the lung resulted in elevated antigen-bearing DCs in the draining LN and amplified APC function [62, 63].
Pulmonary DC antigen acquisition
Microanatomical location within different lung compartments has been suggested to determine DC function. DCs residing in the conducting airways appear to have a greater capacity to endocytose soluble antigen ex vivo compared to parenchymal DCs [16]. In addition, the majority of the DCs in the conducting airways express CD205 (DEC-205), which are likely the CD103+ DCs shown by Sung and others to preferentially line the airway mucosa [12, 16]. The anatomical location of CD103+ DCs grants preferential access to airway antigens, although both migratory DC subsets are capable of acquiring soluble and non-cellular particulates as detected by trafficking studies to the lung-draining LN and whole lung digestion following intranasal instillation [38, 62]. Furthermore, 24 h post-intranasal delivery soluble antigen-containing CD11bhi DCs are present in greater frequency in the lung-draining LN than CD103+ DCs [38, 62]. However, CD103+ DCs contain larger quantities of antigen per cell than CD11bhi DCs, thus suggesting disparate antigen acquisition machineries and capacities between subsets [38, 64]. Another major difference between these subsets is that CD103+ DCs have the unique capacity to acquire and exclusively transport apoptotic cells to the lung-draining LN over their counterpart, CD11bhi DCs [38]. This may be in part because CD103+ DCs highly express candidate receptors for phosphatidylserine, which is exposed on apoptotic cells. A key question still remaining is which receptor(s) mediate efferocytosis by this subset. Phagocytosis, endocytosis, and pinocytosis involve distinct cellular processes for antigen uptake. Currently, it is unclear how pulmonary DC subsets acquire antigens and what mediators are involved in the process of presentation on MHC.
Using two-photon microscopy, it was demonstrated that most inhaled antigens are acquired by CD11c+ cells in the alveoli and not airways [65]. Imaging of CD11cGFP+ cell movement in both locations displayed sentinel-like DCs underlying the airways with minimal protrusions through the epithelium and relatively immobile DCs in the aleveoli with active dendrites constantly sampling the alveolar space. This would suggest DCs are granted access to antigens in the most distal lung compartments, limiting immunological responses to only the most invasive pathogens with the potential to either obstruct gas exchange in the alveoli or penetrate the airway epithelium. Using this methodology and the newly described Zbtb46GFP mice may assist in easily delineating antigen acquisition by lung DC subsets since Zbtb46 are not expressed by macrophages [48].
The majority of pulmonary antigens reaching the lung-draining LN are trafficked by migratory pulmonary DCs [62, 66–69]. During a viral infection, clearance of influenza virus-infected apoptotic cells is predominantly preformed by the langerin+ CD103+ DCs in the lung; however, both migratory pulmonary DCs can cross-present viral antigen [70]. Since CD11bhi DCs do not readily acquire apoptotic cells, it is conceivable that they acquire viral antigens directly or through infected cells that become necrotic. PDCs are not highly endocytic but do play an important role during viral infection by detecting viral constituents via TLR7 and TLR9 and secreting type I interferons at levels 100-fold higher than other cell types [71–74]. They also have a role in steady-state tolerogenic responses to inhaled antigens, such that in asthma, depletion of the pDCs resulted in exacerbated airway hyper-responsiveness [75]. PDCs appear to play a role in controlling immune responses locally at the site of antigen encounter. Afferent lymphatic trafficking by pDC to skin-draining LNs has been reported but has not been observed in intestinal or hepatic lymph [76, 77]. Our studies have revealed that the migratory CD103+ and CD11bhi DCs are the main cells trafficking cellular, particulate, and soluble antigen to the lung-draining LNs. Other studies have shown similar findings in viral and bacterial inoculations [38, 62, 66]. Therefore, it is unclear whether pDCs play a major role in antigen trafficking and presentation from the lung to the draining LN.
In the LNs during steady-state conditions, lymphoid-resident DCs (CD8a+ and CD8a−) are the dominant DC populations present even though tissue-derived DCs are constitutively migrating to the LN in the absence of infection (Fig. 1b) [78]. With TLR-stimulated airways, DC migration from the lung to the draining LNs is enhanced, so that they then become the most numerous DC populations present in the nodes (Fig. 1b) [78, 79]. After exposure to a TLR agonist, the first wave of emigration from the lung reveals similar frequencies for both DC subsets in the draining LN (Fig. 1b) [38, 64]. After 24 h of an inflammatory response, the proportion of DC types in the lung tissue changes with CD11bhi DCs predominating (unpublished data, [9]). In fact, in some cases of bacterial infection, DC accumulation in the lung following TLR stimulation has been shown to be as rapid as neutrophil influx [80]. The influx of CD11bhi MHCII+ CD11c+ DC in the lung during inflammation most likely represents a heterogeneous population that contains precursor or mononuclear cells that still need to be characterized. By contrast, replenishment of the CD103+ population is delayed (unpublished data, [9]).
Potential role of pulmonary DCs in tolerance
The constitutive trafficking of pulmonary DCs most likely contributes to peripheral tolerance under steady-state conditions. Tolerance induction in the periphery requires trafficking of inhaled antigen by migratory DC from lung to the LLN. Using CCR7-deficient mice, Hintzen and colleagues showed antigen acquired by lymphoid-resident DCs from the lymph alone was not sufficient for T cell tolerization [81]. Moreover, these mice developed allergic airway disease to otherwise inert antigens in the absence of CCR7-mediated trafficking DCs from the lung.
CD103+ DCs in the gut have been shown to induce Foxp3+ regulatory T cells, which were dependent on the production of TGF-β and the vitamin A metabolite, retinoic acid [82]. These studies have suggested that CD103+ DCs are the tolerogenic DCs in the gut. It is unclear whether the same is true in the lungs. It has been shown that the expression of retinoic acid alone is not an indicator of tolerance [82–84]. It may be that self-acquiring DCs are better at promoting tolerance than non-self-acquiring DCs. Further investigations to support this concept would need to be performed. We and others have found that in the absence of TLR stimulation, CD103+ DC will promote tolerance to exogenously delivered antigens (unpublished data, [85]). However, this does not prove that other DCs are not as capable of promoting tolerance. Understanding how endogenous DCs can either promote tolerance or induce an active immune response will be critical to developing strategies to resolve allergic or autoimmune disease.
Pulmonary DCs in immune activation
Once migratory DCs reach the lung-draining LN, they present antigens to antigen-specific T cells. CD11bhi DCs present antigens predominantly via MHC II, thus promote CD4 T cell proliferation [38, 86]. On the other hand, CD103+ DCs preferentially cross-present soluble antigens to CD8 T cells as they express higher levels of MHC class I processing machinery [38, 86]. Interestingly, cell-associated antigens acquired by CD103+ DC are exclusively cross-presented to CD8 T cells in vivo [38]. In the absence of TLR stimulation, proliferating CD8 T cells do not gain cytotoxic properties; additional stimulation with intranasal immunization, such as TLR3 ligands, is required to differentiate proliferating CD8 T cells into cytotoxic T cells [38]. Although lung DC subsets can exhibit preferential antigen presentation, in other circumstances (for example with soluble antigens and selective adjuvant stimulation), both do have the capacity to directly present on MHC II as well as cross-present on MHC I.
During influenza infection, in addition to antigen presentation in the lung-draining LN, pulmonary DCs can present antigens locally in the lung to sustain effector CD8 T cells [87]. Which DC subsets contribute to CD8 T cell maintenance during this type of viral infection has not been elucidated as these studies utilized adoptive transfer of CD8α+ DCs into the lungs that are not normally found in lung tissue. The influx of CD11c+ MHCIIhi CD11bhi cells during inflammation may also function in this capacity, but this remains unclear. Regardless, studies such as these suggest that pulmonary DCs can present antigen to T cells both in the lung-draining LN and in the local lung environment.
Lymphoid-resident DCs
Lymphoid-resident DCs enter the lung-draining LNs as precursor cells via the high endothelial venules (HEV). It is believed that lymphoid DCs acquire antigen by either placing their dendrites into the conduits system, from lymphatic flow, from the blood through HEVs, or by migratory DC transport (antigen transfer, cross-dressing, or death) [81, 88, 89]. Lymphoid-resident DCs are capable of antigen presentation; however, their exact role has not been clearly defined. Two elegant studies suggest that lymphoid-resident DCs can either (1) differentiate memory T cells due to their low levels of antigen acquisition and presentation or (2) they can retain antigen-specific T cells in the LN during the early stage of infection prior to the arrival of migratory DCs, at which point the sequestered T cells would be clonally expanded by antigen-ferrying DCs [67, 90].
Conclusions
Dendritic cells are the immune system's “professional” antigen-presenting cells and are distributed throughout the body. They can be simply classified as lymphoid and non-lymphoid, but it is only within the last decade that investigators have begun to appreciate the broader diversity and functional specialty of DC subsets. Because the airways frequently encounter foreign substances, the antigen-presenting role of DCs is critical for the maintenance of pulmonary health. Pulmonary DC subsets are differentially specialized for initiating and regulating the immune response. Immunologists have begun to appreciate the potential of DC-based vaccines and therapeutics for which cell-mediated immune responses are necessary. By investigating the functional role, antigen acquisition, and presentation properties of pulmonary DCs, cell-specific-targeted vaccines will be developed for optimal efficacy.
Acknowledgments
Grant support CJ and PH: HL81151, HL68864, and HL88138. AND was supported in part by National Institutes of Health NIAID Training Grant T32-A107045.
Abbreviations
- DC
Dendritic cells
- LN
Lymph node
Biography

Claudia V. Jakubzick Peter M. Henson A. Nicole Desch
Footnotes
Conflict of interest. The authors have no conflicting interests.
Contributor Information
A. Nicole Desch, Integrated Department of Immunology, University of Colorado School of Medicine, Denver, CO, USA.
Peter M. Henson, Department of Pediatrics, National Jewish Health, 1400 Jackson Street, Denver, CO 80206, USA, Integrated Department of Immunology, University of Colorado School of Medicine, Denver, CO, USA jakubzickc@njhealth.org
Claudia V. Jakubzick, Department of Pediatrics, National Jewish Health, 1400 Jackson Street, Denver, CO 80206, USA, jakubzickc@njhealth.org Integrated Department of Immunology, University of Colorado School of Medicine, Denver, CO, USA,.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 3.Tsitoura DC, DeKruyff RH, Lamb JR, Umetsu DT. Intranasal exposure to protein antigen induces immunological tolerance mediated by functionally disabled CD4+ T cells. J Immunol. 1999;163(5):2592–600. [PubMed] [Google Scholar]
- 4.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 5.Heath WR, Carbone FR. Immunology: dangerous liaisons. Nature. 2003;425(6957):460–1. doi: 10.1038/425460a. [DOI] [PubMed] [Google Scholar]
- 6.Inaba K, Turley S, Iyoda T, et al. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J Exp Med. 2000;191(6):927–36. doi: 10.1084/jem.191.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brimnes MK, Bonifaz L, Steinman RM, Moran TM. Influenza virus-induced dendritic cell maturation is associated with the induction of strong T cell immunity to a coadministered, normally nonimmunogenic protein. J Exp Med. 2003;198(1):133–44. doi: 10.1084/jem.20030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296(5566):298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 9.Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS ONE. 2009;4(1):e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lukens MV, Kruijsen D, Coenjaerts FE, Kimpen JL, van Bleek GM. Respiratory syncytial virus-induced activation and migration of respiratory dendritic cells and subsequent antigen presentation in the lung-draining lymph node. J Virol. 2009;83(14):7235–43. doi: 10.1128/JVI.00452-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randolph GJ, Jakubzick C, Qu C. Antigen presentation by monocytes and monocyte-derived cells. Curr Opin Immunol. 2008;20(1):52–60. doi: 10.1016/j.coi.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sung SS, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176(4):2161–72. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 13.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 14.Jakubzick C, Tacke F, Ginhoux F, Wagers AJ, van Rooijen N, Mack M, Merad M, Randolph GJ. Blood monocyte subsets differentially give rise to CD103+ and CD103− pulmonary dendritic cell populations. J Immunol. 2008;180(5):3019–27. doi: 10.4049/jimmunol.180.5.3019. [DOI] [PubMed] [Google Scholar]
- 15.Gong JL, McCarthy KM, Telford J, Tamatani T, Miyasaka M, Schneeberger EE. Intraepithelial airway dendritic cells: a distinct subset of pulmonary dendritic cells obtained by microdissection. J Exp Med. 1992;175(3):797–807. doi: 10.1084/jem.175.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Garnier C, Filgueira L, Wikstrom M, Smith M, Thomas JA, Strickland DH, Holt PG, Stumbles PA. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J Immunol. 2005;175(3):1609–18. doi: 10.4049/jimmunol.175.3.1609. [DOI] [PubMed] [Google Scholar]
- 17.Jakubzick C, Randolph GJ. Methods to study pulmonary dendritic cell migration. Methods Mol Biol. 2009;595:371–82. doi: 10.1007/978-1-60761-421-0_24. [DOI] [PubMed] [Google Scholar]
- 18.Kirby AC, Raynes JG, Kaye PM. CD11b regulates recruitment of alveolar macrophages but not pulmonary dendritic cells after pneumococcal challenge. J Infect Dis. 2006;193(2):205–13. doi: 10.1086/498874. [DOI] [PubMed] [Google Scholar]
- 19.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):547–60. doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermaelen K, Pauwels R. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A. 2004;61(2):170–7. doi: 10.1002/cyto.a.20064. [DOI] [PubMed] [Google Scholar]
- 21.Holt PG, Haining S, Nelson DJ, Sedgwick JD. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol. 1994;153(1):256–61. [PubMed] [Google Scholar]
- 22.Ginhoux F, Liu K, Helft J, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206(13):3115–30. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone-marrow cultures supplemented with granulocyte macrophage colony-stimulating factor. J Exp Med. 1992;176(6):1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307(5715):1630–4. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 25.Garrett WS, Chen LM, Kroschewski R, Ebersold M, Turley S, Trombetta S, Galan JE, Mellman I. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 2000;102(3):325–34. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 26.Turley SJ, Inaba K, Garrett WS, Ebersold M, Unternaehrer J, Steinman RM, Mellman I. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288(5465):522–7. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- 27.Vremec D, Lieschke GJ, Dunn AR, Robb L, Metcalf D, Shortman K. The influence of granulocyte/macrophage colony-stimulating factor on dendritic cell levels in mouse lymphoid organs. Eur J Immunol. 1997;27(1):40–4. doi: 10.1002/eji.1830270107. [DOI] [PubMed] [Google Scholar]
- 28.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311(5757):83–7. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 29.Liu K, Victora GD, Schwickert TA, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324(5925):392–7. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+ M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8(11):1207–16. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 31.Naik SH, Sathe P, Park HY, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8(11):1217–26. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- 32.Ginhoux F, Tacke F, Angeli V, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7(3):265–73. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varol C, Landsman L, Fogg DK, et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204(1):171–80. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol. 2007;179(6):3488–94. doi: 10.4049/jimmunol.179.6.3488. [DOI] [PubMed] [Google Scholar]
- 35.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19(1):59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 36.Belz GT, Nutt SL. Transcriptional programming of the dendritic cell network. Nat Rev Immunol. 2012;12(2):101–13. doi: 10.1038/nri3149. [DOI] [PubMed] [Google Scholar]
- 37.Cheong C, Matos I, Choi JH, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143(3):416–29. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desch AN, Randolph GJ, Murphy K, et al. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J Exp Med. 2011;208(9):1789–97. doi: 10.1084/jem.20110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masten BJ, Olson GK, Kusewitt DF, Lipscomb MF. Flt3 ligand preferentially increases the number of functionally active myeloid dendritic cells in the lungs of mice. J Immunol. 2004;172(7):4077–83. doi: 10.4049/jimmunol.172.7.4077. [DOI] [PubMed] [Google Scholar]
- 40.Waskow C, Liu K, Darrasse-Jeze G, et al. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9(6):676–83. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carotta S, Dakic A, D'Amico A, Pang SH, Greig KT, Nutt SL, Wu L. The transcription factor PU.1 controls dendritic cell development and Flt3 cytokine receptor expression in a dose-dependent manner. Immunity. 2010;32(5):628–41. doi: 10.1016/j.immuni.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Schiavoni G, Mattei F, Sestili P, Borghi P, Venditti M, Morse HC, III, Belardelli F, Gabriele L. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8alpha(+) dendritic cells. J Exp Med. 2002;196(11):1415–25. doi: 10.1084/jem.20021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsujimura H, Tamura T, Ozato K. Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of type I IFN-producing plasmacytoid dendritic cells. J Immunol. 2003;170(3):1131–5. doi: 10.4049/jimmunol.170.3.1131. [DOI] [PubMed] [Google Scholar]
- 45.Tailor P, Tamura T, Morse HC, III, Ozato K. The BXH2 mutation in IRF8 differentially impairs dendritic cell subset development in the mouse. Blood. 2008;111(4):1942–5. doi: 10.1182/blood-2007-07-100750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiavoni G, Mattei F, Borghi P, Sestili P, Venditti M, Morse HC, III, Belardelli F, Gabriele L. ICSBP is critically involved in the normal development and trafficking of Langerhans cells and dermal dendritic cells. Blood. 2004;103(6):2221–8. doi: 10.1182/blood-2003-09-3007. [DOI] [PubMed] [Google Scholar]
- 47.Meredith MM, Liu K, Darrasse-Jeze G, et al. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med. 2012;209(6):1153–65. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satpathy AT, Wumesh KC, Albring JC, Edelson BT, Kretzer NM, Bhattacharya D, Murphy TL, Murphy KM. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209(6):1135–52. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edelson BT, Wumesh KC, Juang R, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207(4):823–36. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson JT, Hu Y, Liu R, et al. Id2 expression delineates differential checkpoints in the genetic program of CD8alpha+ and CD103+ dendritic cell lineages. EMBO J. 2011;30(13):2690–704. doi: 10.1038/emboj.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ichikawa E, Hida S, Omatsu Y, Shimoyama S, Takahara K, Miyagawa S, Inaba K, Taki S. Defective development of splenic and epidermal CD4+ dendritic cells in mice deficient for IFN regulatory factor-2. Proc Natl Acad Sci USA. 2004;101(11):3909–14. doi: 10.1073/pnas.0400610101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O'Shea JJ, Singh H, Ozato K. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J Immunol. 2005;174(5):2573–81. doi: 10.4049/jimmunol.174.5.2573. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki S, Honma K, Matsuyama T, et al. Critical roles of interferon regulatory factor 4 in CD11bhighCD8alpha− dendritic cell development. Proc Natl Acad Sci USA. 2004;101(24):8981–6. doi: 10.1073/pnas.0402139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu L, D'Amico A, Winkel KD, Suter M, Lo D, Shortman K. RelB is essential for the development of myeloid-related CD8alpha− dendritic cells but not of lymphoid-related CD8alpha+ dendritic cells. Immunity. 1998;9(6):839–47. doi: 10.1016/s1074-7613(00)80649-4. [DOI] [PubMed] [Google Scholar]
- 55.Burkly L, Hession C, Ogata L, et al. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373(6514):531–6. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 56.Dickinson RE, Griffin H, Bigley V, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118(10):2656–8. doi: 10.1182/blood-2011-06-360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hambleton S, Salem S, Bustamante J, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011;365(2):127–38. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bigley V, Haniffa M, Doulatov S, et al. The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. J Exp Med. 2011;208(2):227–34. doi: 10.1084/jem.20101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holt PG, Schon-Hegrad MA, Oliver J. MHC class II antigen-bearing dendritic cells in pulmonary tissues of the rat. Regulation of antigen presentation activity by endogenous macrophage populations. J Exp Med. 1988;167(2):262–74. doi: 10.1084/jem.167.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lipscomb MF, Pollard AM, Yates JL. A role for TGF-beta in the suppression by murine bronchoalveolar cells of lung dendritic cell initiated immune responses. Reg Immunol. 1993;5(3–4):151–7. [PubMed] [Google Scholar]
- 61.MacLean JA, Xia W, Pinto CE, Zhao L, Liu HW, Kradin RL. Sequestration of inhaled particulate antigens by lung phagocytes. A mechanism for the effective inhibition of pulmonary cell-mediated immunity. Am J Pathol. 1996;148(2):657–66. [PMC free article] [PubMed] [Google Scholar]
- 62.Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J Immunol. 2006;176(6):3578–84. doi: 10.4049/jimmunol.176.6.3578. [DOI] [PubMed] [Google Scholar]
- 63.Holt PG, Oliver J, Bilyk N, McMenamin C, McMenamin PG, Kraal G, Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993;177(2):397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jakubzick C, Helft J, Kaplan TJ, Randolph GJ. Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J Immunol Methods. 2008;337(2):121–31. doi: 10.1016/j.jim.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thornton EE, Looney MR, Bose O, Sen D, Sheppard D, Locksley R, Huang X, Krummel MF. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. J Exp Med. 2012;209(6):1183–99. doi: 10.1084/jem.20112667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001;193(1):51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belz GT, Bedoui S, Kupresanin F, Carbone FR, Heath WR. Minimal activation of memory CD8+ T cell by tissue-derived dendritic cells favors the stimulation of naive CD8+ T cells. Nat Immunol. 2007;8(10):1060–6. doi: 10.1038/ni1505. [DOI] [PubMed] [Google Scholar]
- 68.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18(2):265–77. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 69.Belz GT, Smith CM, Kleinert L, Reading P, Brooks A, Shortman K, Carbone FR, Heath WR. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci USA. 2004;101(23):8670–5. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.GeurtsvanKessel CH, Willart MA, van Rijt LS, et al. Clearance of influenza virus from the lung depends on migratory langer-in+CD11b− but not plasmacytoid dendritic cells. J Exp Med. 2008;205(7):1621–34. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 72.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e Sousa C. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33(4):827–33. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 73.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5(12):1219–26. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 74.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8(8):594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 75.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasma-cytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200(1):89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pascale F, Contreras V, Bonneau M, et al. Plasmacytoid dendritic cells migrate in afferent skin lymph. J Immunol. 2008;180(9):5963–72. doi: 10.4049/jimmunol.180.9.5963. [DOI] [PubMed] [Google Scholar]
- 77.Yrlid U, Cerovic V, Milling S, Jenkins CD, Zhang J, Crocker PR, Klavinskis LS, MacPherson GG. Plasmacytoid dendritic cells do not migrate in intestinal or hepatic lymph. J Immunol. 2006;177(9):6115–21. doi: 10.4049/jimmunol.177.9.6115. [DOI] [PubMed] [Google Scholar]
- 78.Jakubzick C, Bogunovic M, Bonito AJ, Kuan EL, Merad M, Randolph GJ. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J Exp Med. 2008;205(12):2839–50. doi: 10.1084/jem.20081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jahnsen FL, Strickland DH, Thomas JA, et al. Accelerated antigen sampling and transport by airway mucosal dendritic cells following inhalation of a bacterial stimulus. J Immunol. 2006;177(9):5861–7. doi: 10.4049/jimmunol.177.9.5861. [DOI] [PubMed] [Google Scholar]
- 80.McWilliam AS, Nelson D, Thomas JA, Holt PG. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med. 1994;179(4):1331–6. doi: 10.1084/jem.179.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hintzen G, Ohl L, del Rio ML, et al. Induction of tolerance to innocuous inhaled antigen relies on a CCR7-dependent dendritic cell-mediated antigen transport to the bronchial lymph node. J Immunol. 2006;177(10):7346–54. doi: 10.4049/jimmunol.177.10.7346. [DOI] [PubMed] [Google Scholar]
- 82.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204(8):1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204(8):1765–74. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191(3):411–6. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.del Rio ML, Rodriguez-Barbosa JI, Kremmer E, Forster R. CD103− and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J Immunol. 2007;178(11):6861–6. doi: 10.4049/jimmunol.178.11.6861. [DOI] [PubMed] [Google Scholar]
- 87.McGill J, Van Rooijen N, Legge KL. IL-15 trans-presentation by pulmonary dendritic cells promotes effector CD8 T cell survival during influenza virus infection. J Exp Med. 2010;207(3):521–34. doi: 10.1084/jem.20091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Allan RS, Waithman J, Bedoui S, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25(1):153–62. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 89.Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471(7340):629–32. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Allenspach EJ, Lemos MP, Porrett PM, Turka LA, Laufer TM. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity. 2008;29(5):795–806. doi: 10.1016/j.immuni.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


