Abstract
Background:
Plastic surgery has a well-known history of innovative procedures and products. However, with the rise in competition, such as aesthetic procedures being performed by other medical specialties, there is a need for continued innovation in plastic surgery to create novel treatments to advance this specialty. Although many articles introduce innovative technologies and procedures, there is a paucity of publications to highlight the application of principles of innovation in plastic surgery.
Methods:
We review the literature regarding business strategies for innovation.
Results:
We evaluate concepts of innovation, process of innovation (idea generation, idea evaluation, idea conversion, idea diffusion and adoption), ethical issues, and the application to plastic surgery.
Conclusions:
Adopting a business model of innovation is helpful to promote a new paradigm of progress to propel plastic surgery to new avenues of creativity.
Keywords: Innovation, creativity, plastic reconstructive surgery
Plastic surgery has a recognized history of innovation. This history includes innovative procedures and products, such as microvascular surgery, liposuction, and tissue expansion.1 Innovations in plastic surgery are not limited to academic centers; all plastic surgeons are willing and ready to seek creative solutions to improve current practice and to solve difficult clinical problems.2 In the 50 top-cited plastic surgery articles, almost half introduced a new or modified surgical technique that considerably changed clinical practice.3 Over the last two decades, advanced technology innovations such as virtual reality, simulators, and robotics have emerged in our medical learning and practice.4 Multiple computer-based learning curriculums have been integrated into resident training, preoperative planning and outcome evaluation. These interactive simulator technology innovations can substantially lower patients’ risk through increased precision and improve communication between plastic surgeons and patients to provide reasonable expectations of outcomes after treatment.5,6 Technique innovation is critically important for plastic surgeons because it stimulates basic and clinical research to develop novel procedures and original treatment approaches, such as fat transfer and laser liposuction.2 Innovation makes plastic surgery different and distinctive, and will be the key to the survival of the specialty.1,7
Although innovation has a rich tradition in plastic surgery, the study of innovation is new to this specialty. Published articles focus on the need for invention in the field or the introduction of innovative procedures or techniques without the “how to.” Attempts have been made to systematically evaluate approaches for industry and business innovation, but very few articles focus specifically on surgical innovation theory or strategies.8 The purpose of this article is to apply business concepts of innovation strategies to plastic surgery and to develop a systematic way to innovate in this specialty.
Concepts of Innovation
Innovation is defined as “an introduction of something new, including a new idea, method or device.”9 It is an improvement to something that is already existing. It is much more than just new techniques and technology; a new idea, a new perspective, or even a new question can be as valuable as a new device.10 Innovation must not be confused with invention, which is defined as a “discovery or finding” that is developed after study and experimentation.9 An innovation requires implementation in a way that captures its value (usefulness and profitability).11 Examples of innovation include the use of new devices in existing procedures, the introduction of new procedures that use new devices, and using existing devices in new procedures.12 For example, the invention of the microscope propelled innovation in the field of microsurgery, which ultimately led to replantation surgery and free tissue transfer. By taking an invention, plastic surgeons are able to innovate with a new surgical specialty.
A number of terms have been used to describe the impact of innovation. Incremental innovation refers to change that marginally improves currently available technology and does not lead to a major technological change.8 It is common in health care as organizations constantly seek ways to improve quality of care and eliminate unnecessary expenses.13 Enabling innovation refers to an innovation that supports further developments within a field.8 The development of endoscopy technology is such an example, as it has supported endoscopy surgery in general surgery, gynecology, otorhinolaryngology and plastic surgery. Disruptive innovation involves turning current practices completely upside down, such as the development of the electronic health record.13
Varkey et al.11 classified innovations in health care into three types: product, process, and structural. The product type represents innovations that the consumer pays for and typically consists of goods or services, such as new generation drugs, CT scan, and clinical procedure. Process innovations are innovations in the production or delivery method. Examples include digital imaging, telemedicine, and tissue engineering. Structural innovations usually “affect the internal and external infrastructure and create new business models,” such as health maintenance organizations.
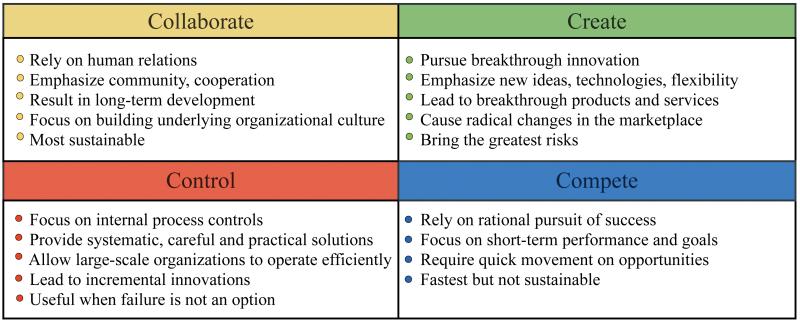
The innovation genome (Figure1) was published by DeGraff and Quinn of the University of Michigan Ross School of Business.14 The genome demonstrates three levels (purposes, practices and people) and four fundamental creative approaches (collaborate, create, compete and control) of innovation. The innovation starts with the outcomes the organization intends to create (purposes), and then works backwards to “organizational culture, competency and processes” (practices) and finally individuals especially leadership behaviors (people). Four approaches (competing values) work in different ways to produce innovation. The collaborate approach emphasizes community, cooperation and focuses on building the underlying organizational culture and competencies for innovation. The create approach produces breakthrough products and services. It provides the greatest level of innovation with also the greatest risk. The compete approach focuses on the rational pursuit of success. It pursues radical innovation and leads to disruptive and enabling innovation. The control approach focuses on continuity, elimination of errors, and leads to incremental innovation. It is a valuable system to combine innovation purpose, practices, approaches, leadership behaviors and stages together and has been used in business consultation services.
Process of Innovation
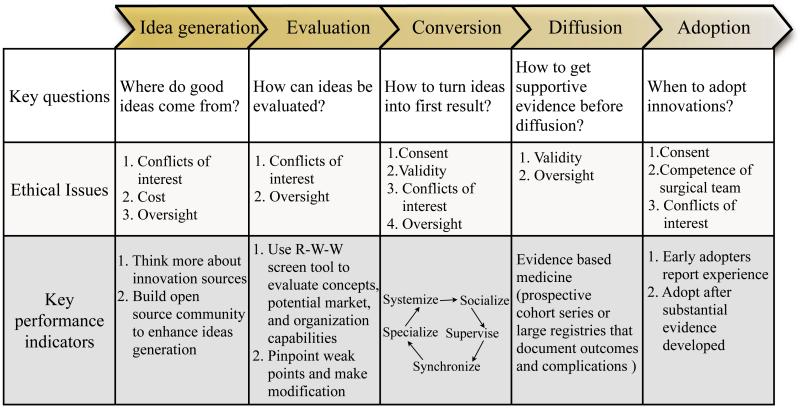
Innovations generally go through a five-phase process that involves idea generation, evaluation, development, diffusion and adoption15 (Figure 2).
Figure 2.
The process of innovation.
Idea generation-sources of innovation
Innovation starts with good ideas, but where do these concepts come from? There are innovations that are from a “light bulb” moment. Most innovations, however, result from conscious, purposeful searches for innovation opportunities.16,17 Six areas of opportunity exist around us: new knowledge, “follow up with a critical eye,”18 unexpected occurrence, incongruities, process needs, and changes in perception19 (Figure 3). Exploring new knowledge for unsolved questions is the traditional type of innovation. Plastic surgery researchers get new ideas for unsolved problems most commonly by literature searches, performing systematic literature reviews to evaluate existing data or studies, and attending conferences or discussions to comprehend current trends and issues.11 New knowledge from other people also gives us the opportunity to adapt it into our specialty or to transfer it into usable technology.
Figure 3.
The sources of innovation.
(Adapted from Henry Chesbrough. Open innovation and open business models: a new approach to industrial innovation. Available at: www.crp-eut.org/2010_Chesbrough.pdf.)
Critical follow-up can lead to innovations like technique modification or new research questions. For example, in breast reconstruction, bulky appearance and morbidity on the donor site stimulated surgeons to dissect indirect musculocutaneous perforators, which resulted in the application of the perforator flap.
Unexpected occurrences often include failures. Failure can be an opportunity to recognize drawbacks and turn a setback into victory. Incongruities inspire people to change what they used to do. For example, chronic, non-healing wounds in ill patients prompted the creative idea of using a vacuum-assisted wound closure technique. This technique led to an expanded indication for the battlefield to temporize traumatic wounds during transfer to a suitable treatment facility. An example of process needs includes the application of surgical safety checklists in order to reduce complication rates.
Not every idea has to be a blockbuster. Sufficient numbers of small or incremental innovations can lead to big profits.20 In addition, innovations do not have to only focus on new product development, like technique or technology. Good ideas can also come from process and structural innovations. Finally, to be effective, an innovation needs to be simple and focused. It should be directed towards a specific, clear, and carefully designed application.19 For example, in hand surgery, putting hand tables on recovery room beds in the operating room avoids the need for transferring patients onto operating room tables, which cuts down the time for bed transfer and increases surgical efficiency. Consider how evidence-based medicine changed our perspective and direction of clinical research.
Businesses also use networks to enhance idea generation, “by building external, internal cross-unit networks, or by developing a small, coordinated team working on the same problem.”21 Every important innovation is fundamentally constructed by a network. One can develop an open-source community, without economic purpose, in which ideas can freely circulate. The ideas can be refined and expanded by other minds in the network.21 For example, the innovations in immediate breast reconstruction come from connections between oncologists and plastic surgeons. This external network allows individual preoperative surgical technique planning for different patients, which improves both breast cancer management and aesthetic results. A new procedure using superiorly based single mastectomy flap with inferiorly based dermal flap and anchorage of inframammary crease has been introduced recently for immediate breast reconstruction in patients with ptosis. This procedure has improved aesthetic results by maintaining the resultant mastectomy scar in the inframammary crease.22
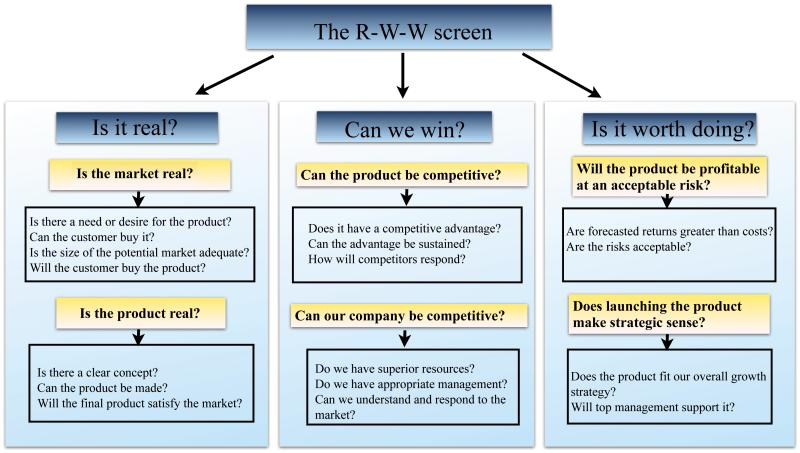
Idea evaluation
There may be more innovative ideas than available resources in the organization. If so, how can ideas be evaluated or screened for funding? The R-W-W (real, win, worth it) screen is a simple but powerful tool that has been used to evaluate business projects. The company 3M has used this tool for more than 1,500 projects.23 It is built on a series of questions about the innovative concept, its potential market, and the company’s capabilities and competition. We can also use this tool to evaluate surgical innovation and the capability of the organization, and pinpoint the weak points of the project and organization. Modifying the weak points may turn an infeasible mission to possible (Figure 4). For evaluation of a research project, we can think about these important questions: “Is there a need for the project? Is there a clear concept for the project? Can the project be competitive? Can our organization be qualified? Will the project be profitable at an acceptable risk? Does the project fit our overall strategy?” A definite no to any question argues strongly against proceeding with development.23 For example, we can apply this screening tool to the evaluation of the functional outcomes and patient satisfaction after silicone metacarpophalangeal arthroplasty (SMPA) for rheumatoid arthritis.24 First, there is a challenge to measure patient outcomes for procedures with low morbidity and mortality rates such as SMPA. Patient satisfaction can offer a perspective on the definition of therapeutic success, but prior research did not clarify the connection between objective functional recovery and patient satisfaction. There is a need for this research project. Second, the aim of the project is a clear concept “to identify the level of objective functional recovery that yields satisfaction after SMPA.” Third, compared with prior research, the study has competitive advantage in demonstrating outcome measurements in patient satisfaction. Fourth, our organization has the capability and resources to conduct a multicenter, cohort study. Finally, the risks are minimal and the study allows surgeons to set realistic and attainable postoperative goals.
Figure 4.
The R-W-W (real, win, and worth it) screen.
(Adapted from George S. Day. Is it real? Can we win? Is it worth doing? Managing risk and reward in an innovation protfolio. In: HBR's 10 Must Reads on Innovation. Boston: Harvard Business School Publishing Corporation; 2011:119.)
Idea conversion
In this phase, ideas are developed into first result, such as viable products, services, or business. During the development process, leaders first establish a shared vision and values in the leadership team (socialize); second, leaders develop process experts to sustain change and innovation (supervise); third, members of the organization come together to put pieces together and connect the dots (synchronize); fourth, leaders and process experts execute change and innovation project teams (specialize); finally, leaders review and revise projects, adjust organizational practice, and learn what works and what does not (systemize).14 For example, we can apply this process to the preparation for a distal radius fracture multicenter clinical trial.25 In the first step, the purpose is established and agreed upon: prepare to obtain funding for a multicenter clinical trial. In the second step, research teams are established and collaborative centers are recruited. In the third stage, collaborators participate in face-to-face meetings to design and obtain consensus on the final study protocols. The whole project has been synchronized though the organization. After that, we successfully submit an R01 grant and the study moves to the execution phase. In the last stage, the experience of conducting a multicenter clinical trial has been derived and can be reviewed. This experience will serve as the foundation to implement sustainable innovations.
During this process, tight controls may strangle innovation. On the other hand, one should accept that constraints will always exist. Adding flexibility to planning and control systems can make development move more smoothly.20 For example, reserved funds can support unplanned inspirations. Another easily made mistake involves connections between innovators and team members being too loose. Strengthening the human connections throughout the organization is important for a successful development process. For example, in the distal radius study outlined above, frequent conference calls among the study sites that include both the surgeons and study site coordinators as well as other team members such as hand therapists and data managers are critical to the process.
Idea diffusion and adoption
Innovation requires people to take risks and face failure. However, for surgical innovation, the first responsibility is to do no harm. “Physicians always need to balance patient interest with the larger societal goal--the discovery of better ways to help future patients.”26 Diffusion and adoption of innovations before having supportive evidence is not an option for surgical innovation. There are many examples of our overenthusiastic adoption of innovations. Injection of polyacrylamide hydrogel (PAAG), which initially started in the Ukraine, attracted a lot of interest as a new, ideal, injectable filler and has been widely used for injection augmentation mammoplasty and tissue contour correction in Russia, China, Iran and other Asian countries.27-29 However, complications associated with PAAG injection have been gradually reported, such as induration, lumps, gel migration and delayed diagnosis of breast cancer. In 2006, the Chinese Food and Drug Administration (FDA) withdrew the product from the market. Before this happened, it was reported that nearly 200,000 patients in 9 years received PAAG injection for breast augmentation in China.27 Since that time, increasing numbers of patients are seeking to have PAAG removed from their breasts regardless of whether they suffer from symptoms. The unique aspect of surgical innovation requires innovators to minimize risks as much as possible and to avoid wide spread adoption before risks have been fully evaluated.
Evidence-based medicine is used for patient care decision-making and to control health care spending. Ahn et al.12 published an article to assess how new technology can be adopted safely while maintaining an environment that allows for surgical innovation through an evidence-based approach. In his view, although randomized controlled trials are considered to be the best evidence of the efficacy of a treatment, they may not be the most effective form of evaluating a new technology. He recommended a prospective cohort series or large registries that document outcomes and adverse events as being more effective and more practical. Furthermore, early adopters should report their experience and outcomes. After a substantial proportion of surgeons have adopted the new technology and when there is ample evidence regarding the results of treatment, the great majority of surgeons may adopt the innovation.
Ethical Issues and Regulation in Surgical Innovation
McKneally30 made a list of ethical issues specific to innovative surgery, including consent, validity, competence, conflicts of interests, cost and oversight. The issue of consent can be easily mismanaged. It may be covered when the researchers apply for Institutional Review Board (IRB) approval, which will probably require a written informed consent form. Participants undergoing an innovative intervention should be explicitly informed about the novelty and lack of a proven record of effectiveness. Second, competent professionals should ethically justify the probable validity of introducing an innovation. Validating evidence can be developed if the intervention is founded to be feasible and effective. Third, competence means the ability of the surgical team to perform the appropriate surgical intervention with a high probability of success and a low risk of complications. Innovative interventions challenge the competence of the surgical team. As innovators, surgeons are always dealing with the learning curve during their careers. How can it be managed more effectively? Damiano26 addressed three components: personal, institutional, and professional. From a personal standpoint, a surgeon must be adequately trained before performing surgery on a patient. Within the institutions, a number of ways can be used to manage the learning curve, such as conferences or case-by-case analysis. Another institutional responsibility is to develop simulation facilities to move at least part of the learning curve out of the operating room. Our society needs to better define appropriate training programs for specific procedures. The next ethical issue is conflicts of interest. Attention should be paid to financial, personal, or reputational interests of innovative surgeons, which may compete with their fiduciary duty to put patients’ interests first. Professional oversight can help to manage this risk. The issue of cost arises because innovations can lead to a rapid rise in the cost of health care. It is not only a hospital management problem, also a societal justice. With greater understanding of the ethical issues of surgical innovation, surgeons will be better prepared to address these issues openly when implementing new technique or technologies.31
Research conducted by Reitsma32 showed that, different from FDA regulation for development of new drugs and devices, most surgical innovation is not clearly defined nor formally regulated by governing bodies. Existing guidelines and regulations still appear insufficient. His study showed that except for the FDA and local IRBs, most surgeons are not very familiar with governmental bodies regulating clinical research, such as the Office for Human Research Protections (OHRP). For some forms of human subject research, such as retrospective reviews or cohort studies, surgeons show a lower level of appreciation for federal rules. Surgeons could benefit greatly from additional education about basic regulatory requirements.
Applying the Innovation Principles to Plastic Surgery
Plastic surgery is facing more and more competition today, because unlike other medical specialties, plastic surgery does not treat one specific area of the body. The increased revenue of aesthetic surgery especially attracts competition from many different specialties.7 One study found that there were 1867 cosmetic practitioners offering hyaluronic acid injections in Southern California, but only 495 (27%) were trained in plastic surgery. The results also showed that there are growing numbers of non-surgery-trained individuals providing surgical cosmetic treatments.33 How can we separate ourselves from other practitioners? Our strategy should continue to emphasize innovation within plastic surgery.7 This is our competitive advantage compared to other specialties and also it is the only strategy to lead to novel therapies. Referring back to the innovation process, during the idea generation phase, aesthetic surgery was derived from plastic and reconstructive surgery. Plastic surgery innovators have more sources to generate new concepts, such as generating new ideas from relevant reconstructive surgeries or by accessing up-to-date peer-reviewed information from clinical work, conferences or journals. In the evaluation of project and organizations, we also have the capabilities to promote investment in both clinical care and research and to provide appropriate resources and management in carrying out new projects for the development phase. New technology, trends and information also can be rapidly disseminated through our cooperative networks and stimulate further innovations.7
The future innovative areas in aesthetic surgery can also be classified as product, process and structural innovations. For example, product innovation refers to new technique and technologies, which can be translated into novel therapies. One author predicted that total cosmetic surgery volume will exceed 55 million annual procedures by 2015. However, the future demand in cosmetic surgery will be driven largely by nonsurgical procedures.34 Innovations in technology and technique will also pursue optimization of existing practice and exploration for more non-operative technologies. Process innovations represent how patients get the service. For providers of aesthetic services, communication with the public is very important. Plastic surgeons should become familiar with the available Internet tools and aggressively utilize these tools for effective, but truthful practice building35 and also innovate new methods to improve service by using a documentary-style format to depict patients before, during, and after various surgical procedures to help patients better understand the procedure.36
Conclusions
Innovation, whether clinical or through research, is a “sustainable competitive advantage”7 for plastic surgery, which is the key to our current and future success. The application of valuable business innovation strategies into plastic surgery can provide us a systematic way to conceive and practice innovation. Innovation development, diffusion and adoption should be managed correctly to make it ethically acceptable and evidence-based to minimize patients’ risks and reduce costs.
Figure 1.
The innovation genome has two basic structural components. Three levels: people, practice, purpose; four competing values: collaborate, create, compete, control. (Adapted from Jeff DeGraff. How you innovate is what you innovate. Available at: http://competingvalues.com/competingvalues.com/wp-content/uploads/2009/07/How-You-Innovate-Is-What-You-Innovate.pdf.)
Acknowledgments
Supported in part by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging (R01 AR062066) and from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR047328) and a Midcareer Investigator Award in Patient-Oriented Research (K24 AR053120) (to Dr. Kevin C. Chung).
Footnotes
Financial Disclosures
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
References
- 1.Gurtner GC, Rohrich RJ, Longaker MT. From bedside to bench and back again: technology innovation in plastic surgery. Plastic and reconstructive surgery. 2009;124:1355–6. doi: 10.1097/PRS.0b013e3181b8901a. [DOI] [PubMed] [Google Scholar]
- 2.Mathes SJ. Innovation. Plastic and reconstructive surgery. 2007;120:2110–1. doi: 10.1097/01.prs.0000293498.73273.52. [DOI] [PubMed] [Google Scholar]
- 3.Loonen MP, Hage JJ, Kon M. Plastic Surgery Classics: characteristics of 50 top-cited articles in four Plastic Surgery Journals since 1946. Plastic and reconstructive surgery. 2008;121:320e–7e. doi: 10.1097/PRS.0b013e31816b13a9. [DOI] [PubMed] [Google Scholar]
- 4.Grunwald T, Krummel T, Sherman R. Advanced technologies in plastic surgery: how new innovations can improve our training and practice. Plastic and reconstructive surgery. 2004;114:1556–67. doi: 10.1097/01.prs.0000138242.60324.1d. [DOI] [PubMed] [Google Scholar]
- 5.Rosen JM, Long SA, McGrath DM, Greer SE. Simulation in plastic surgery training and education: the path forward. Plastic and reconstructive surgery. 2009;123:729–38. doi: 10.1097/PRS.0b013e3181958ec4. discussion 39-40. [DOI] [PubMed] [Google Scholar]
- 6.Flores RL, Deluccia N, Grayson BH, Oliker A, McCarthy JG. Creating a virtual surgical atlas of craniofacial procedures: Part I. Three-dimensional digital models of craniofacial deformities. Plastic and reconstructive surgery. 2010;126:2084–92. doi: 10.1097/PRS.0b013e3181f526f6. [DOI] [PubMed] [Google Scholar]
- 7.Longaker MT, Rohrich RJ. Innovation: a sustainable competitive advantage for plastic and reconstructive surgery. Plastic and reconstructive surgery. 2005;115:2135–6. doi: 10.1097/01.prs.0000168495.95560.eb. [DOI] [PubMed] [Google Scholar]
- 8.Riskin DJ, Longaker MT, Gertner M, Krummel TM. Innovation in surgery: a historical perspective. Annals of surgery. 2006;244:686–93. doi: 10.1097/01.sla.0000242706.91771.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dictionary and Thesaurus-Merriam-Webster Online. Innovation. (Accessed November 18, 2012, at http://www.merriam-webster.com/dictionary/innovation.
- 10.Damiano RJ., Jr. What is Innovation? Innovations (Phila) 2011;6:65. doi: 10.1097/IMI.0b013e3182162bcf. [DOI] [PubMed] [Google Scholar]
- 11.Varkey P, Horne A, Bennet KE. Innovation in health care: a primer. American journal of medical quality : the official journal of the American College of Medical Quality. 2008;23:382–8. doi: 10.1177/1062860608317695. [DOI] [PubMed] [Google Scholar]
- 12.Ahn H, Bhandari M, Schemitsch EH. An evidence-based approach to the adoption of new technology. The Journal of bone and joint surgery American volume. 2009;91(Suppl 3):95–8. doi: 10.2106/JBJS.H.01593. [DOI] [PubMed] [Google Scholar]
- 13.Jarousse LA. Making innovation a core competency. Trustee : the journal for hospital governing boards. 2012;65:26–7. 1. [PubMed] [Google Scholar]
- 14.DeGraff J. How you innovate is what you innovate. A systematic approach to effectively leading enterprise innovation. (Accessed November 18, 2012, at http://competingvalues.com/competingvalues.com/wp-content/uploads/2009/07/How-You-Innovate-Is-What-You-Innovate.pdf.
- 15.Hansen MT. HBR's 10 Must Reads on Innovation. Harvard Business School Publishing Corporation; Boston: 2011. The innovation value chain; p. 89. [Google Scholar]
- 16.Harvard business review on the innovative enterprise. Harvard Business School Pub.; Boston: 2003. [Google Scholar]
- 17.Drucker PF. Classic Drucker : essential wisdom of Peter Drucker from the pages of Harvard Business Review. Harvard Business Review Book; Boston: 2006. [Google Scholar]
- 18.Millard DR. Principalization of plastic surgery. Little Brown and Company; Boston/ Toronto: 1986. [Google Scholar]
- 19.Drucker PF. HBR's 10 Must Reads on Innovation. Harvard Business School Publishing Corporation; Boston: 2011. The Discipline of Innovation; p. 207. [Google Scholar]
- 20.Kanter RM. HBR's 10 Must Reads on Innovation. Harvard Business School Publishing Corporation; Boston: 2011. Innovation: the classic traps; p. 149. [Google Scholar]
- 21.Johnson S. Where good ideas come from: the natural history of innovation. Penguin Group; New York: 2010. [Google Scholar]
- 22.Halls MJ. Superiorly based single mastectomy flap with inferiorly based dermal flap and anchorage of the inframammary crease procedure for immediate breast reconstruction in patients with ptosis. Plastic and reconstructive surgery. 2012;130:632e–3e. doi: 10.1097/PRS.0b013e318262f682. [DOI] [PubMed] [Google Scholar]
- 23.Day GS. HBR's 10 Must Reads on Innovation. Boston: Harvard Business School Publishing Corporation. 2011. Is it real? Can we win? Is it worth doing? p. 119. [PubMed] [Google Scholar]
- 24.Waljee JF, Chung KC. Objective functional outcomes and patient satisfaction after silicone metacarpophalangeal arthroplasty for rheumatoid arthritis. The Journal of hand surgery. 2012;37:47–54. doi: 10.1016/j.jhsa.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 25.Chung KC, Song JW. A guide to organizing a multicenter clinical trial. Plastic and reconstructive surgery. 2010;126:515–23. doi: 10.1097/PRS.0b013e3181df64fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damiano RJ., Jr. Surgical innovation in the information age: the heavy burden of great potential. Innovations (Phila) 2011;6:283–8. doi: 10.1097/IMI.0b013e318237131f. [DOI] [PubMed] [Google Scholar]
- 27.Wang ZX, Luo DL, Dai X, Yu P, Tao L, Li SR. Polyacrylamide hydrogel injection for augmentation mammaplasty: loss of ability for breastfeeding. Annals of plastic surgery. 2012;69:123–8. doi: 10.1097/SAP.0b013e318225931c. [DOI] [PubMed] [Google Scholar]
- 28.Yu L, Wang J, Zhang B, Zheng DN, Zhu C. Treatment of Breast Injection with Polyacrylamide Hydrogel with Infiltrated Fascia Capsule Removal: Report on 104 Cases. Aesthetic plastic surgery. 2012 doi: 10.1007/s00266-012-9928-8. [DOI] [PubMed] [Google Scholar]
- 29.Ono S, Ogawa R, Hyakusoku H. Complications after polyacrylamide hydrogel injection for soft-tissue augmentation. Plastic and reconstructive surgery. 2010;126:1349–57. doi: 10.1097/PRS.0b013e3181ead122. [DOI] [PubMed] [Google Scholar]
- 30.McKneally MF. The ethics of innovation: Columbus and others try something new. The Journal of thoracic and cardiovascular surgery. 2011;141:863–6. doi: 10.1016/j.jtcvs.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Angelos P. The ethical challenges of surgical innovation for patient care. Lancet. 2010;376:1046–7. doi: 10.1016/s0140-6736(10)61474-2. [DOI] [PubMed] [Google Scholar]
- 32.Reitsma AM, Moreno JD. Ethics of innovative surgery: US surgeons' definitions, knowledge, and attitudes. Journal of the American College of Surgeons. 2005;200:103–10. doi: 10.1016/j.jamcollsurg.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 33.Camp MC, Wong WW, Wong RY, Camp JS, Son AK, Gupta SC. Who is providing aesthetic surgery? A detailed examination of the geographic distribution and training backgrounds of cosmetic practitioners in Southern California. Plastic and reconstructive surgery. 2010;125:1257–62. doi: 10.1097/PRS.0b013e3181d0accf. [DOI] [PubMed] [Google Scholar]
- 34.Liu TS, Miller TA. Economic analysis of the future growth of cosmetic surgery procedures. Plastic and reconstructive surgery. 2008;121:404e–12e. doi: 10.1097/PRS.0b013e318170818d. [DOI] [PubMed] [Google Scholar]
- 35.Camp MC, Wong WW, Mussman JL, Gupta SC. The battle for hearts and minds: who is communicating most effectively with the cosmetic marketplace? Aesthetic surgery journal / the American Society for Aesthetic Plastic surgery. 2010;30:614–7. doi: 10.1177/1090820X10371433. [DOI] [PubMed] [Google Scholar]
- 36.Crockett RJ, Pruzinsky T, Persing JA. The influence of plastic surgery "reality TV" on cosmetic surgery patient expectations and decision making. Plastic and reconstructive surgery. 2007;120:316–24. doi: 10.1097/01.prs.0000264339.67451.71. [DOI] [PubMed] [Google Scholar]