Abstract
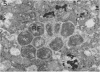
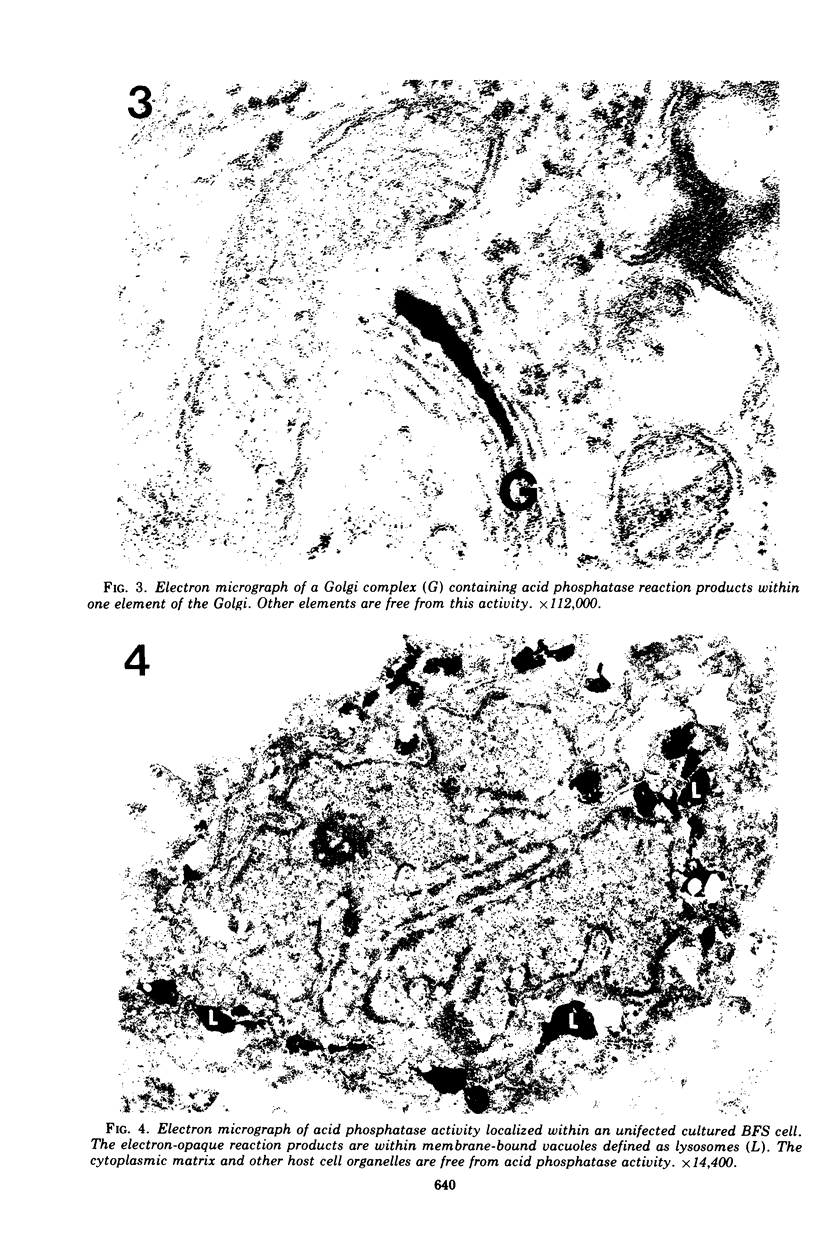
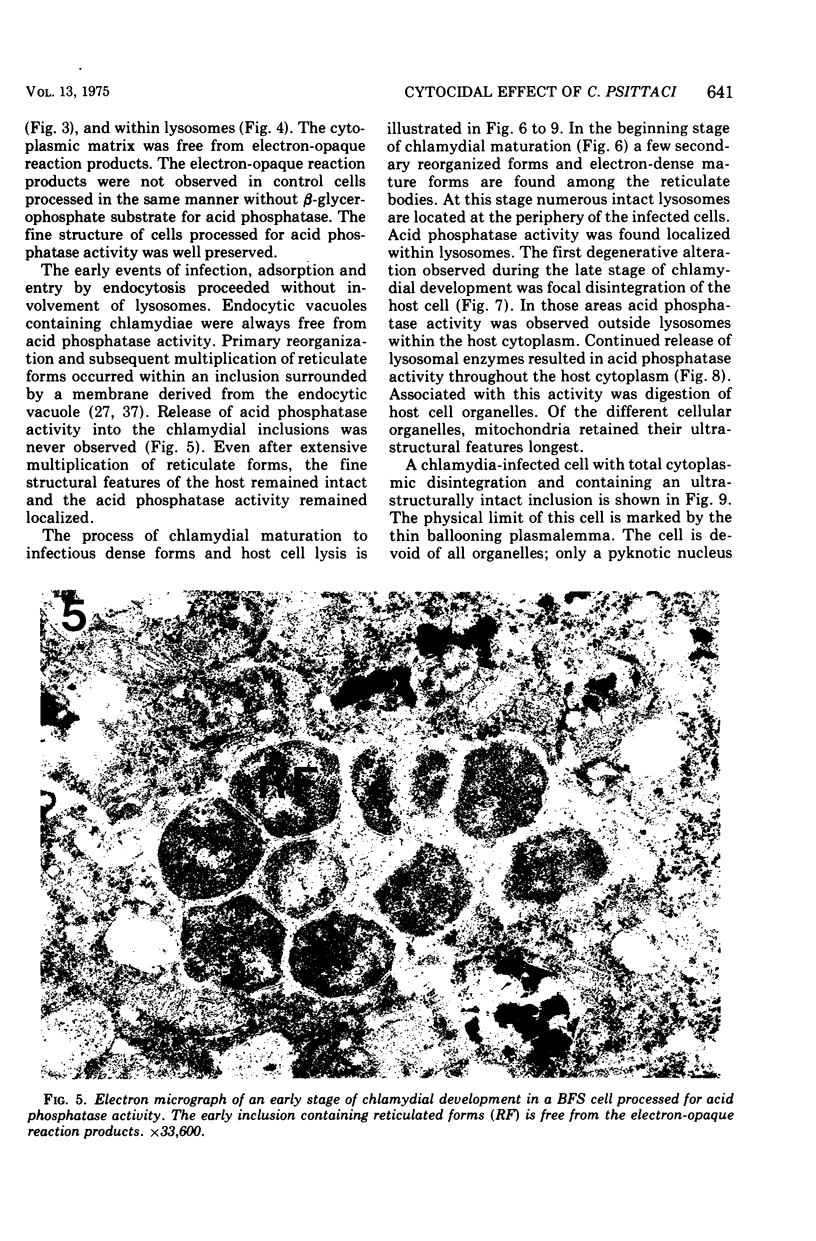
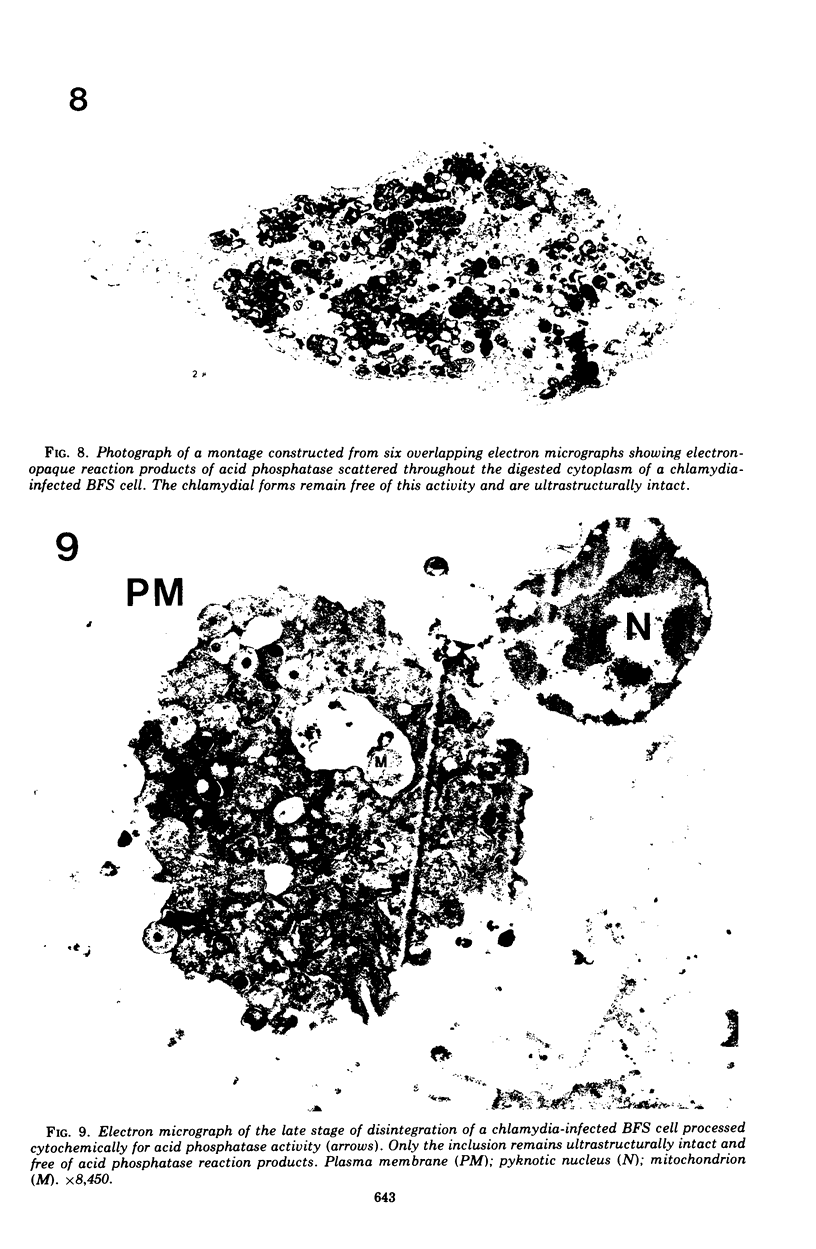
The cytopathic effect of the polyarthritis strain of Chlamydia psittaci was studied in cultured bovine fetal spleen cells and found to be mediated by the release of lysosomal enzymes into the host cytoplasm during the late stages of chlamydial development. Ultrastructural cytochemical analysis and cell fractionation studies of infected cells revealed a close relationship between the stage of chlamydial development, fine structural features of the host, and localization of lysosomal enzyme activities. After adsorption, chlamydiae entered the host cells by endocytosis. The endocytic vacuoles containing individual chlamydiae and later the inclusion vacuoles containing the different chlamydial developmental forms were always free from lysosomal enzyme activity. Even after extensive multiplication of chlamydiae, lysosomal enzymes remained localized within lysosomes or their precursors in the host cell. Coincident with the process of chlamydial maturation, lysosomal enzymes were released into the host cytoplasm and were always associated with disintegration of host cell constituents and lysis. The chlamydiae appeared to be protected from this lysosomal enzyme activity by the inclusion membrane. After release from the inclusion, elementary bodies maintained their fine structural features, whereas all other chlamydial developmental forms lost their ultrasturctural integrity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., MALLUCCI L. HISTOCHEMICAL STUDIES OF LYSOSOMES AND LYSOSOMAL ENZYMES IN VIRUS-INFECTED CELL CULTURES. J Exp Med. 1965 Mar 1;121:463–476. doi: 10.1084/jem.121.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLISON A. C., SANDELIN K. Activation of lysosomal enzymes in virus-infected cells and its possible relationship to cytopathic effects. J Exp Med. 1963 Jun 1;117:879–887. doi: 10.1084/jem.117.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks J., Eddie B., Schachter J., Meyer K. F. Plaque formation by Chlamydia in L cells. Infect Immun. 1970 Mar;1(3):259–262. doi: 10.1128/iai.1.3.259-262.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz K., Egger D., Wolff D. A. Virus replication, cytopathology, and lysosomal enzyme response of mitotic and interphase Hep-2 cells infected with poliovirus. J Virol. 1973 Apr;11(4):565–574. doi: 10.1128/jvi.11.4.565-574.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman K. E., Bubel H. C. Poliovirus-induced Cellular Injury. J Virol. 1969 Sep;4(3):203–208. doi: 10.1128/jvi.4.3.203-208.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk U. T., Ericsson J. L. The demonstration of acid phosphatase in vitro cultured tissue cells. Studies on the significance of fixation, tonicity and permeability. Histochem J. 1972 Jul;4(4):349–363. doi: 10.1007/BF01005009. [DOI] [PubMed] [Google Scholar]
- DEFENDI V. Cytopathology of virus infection. Fed Proc. 1962 Nov-Dec;21:1113–1117. [PubMed] [Google Scholar]
- DOUNCE A. L. THE ISOLATION OF NUCLEI FROM TUMOR CELLS. Exp Cell Res. 1963;24:SUPPL9–SUPPL9:143. doi: 10.1016/0014-4827(63)90253-2. [DOI] [PubMed] [Google Scholar]
- Fan V. S., Jenkin H. M. Lipid metabolism of monkey kidney cells (LLC-MK-2) infected with Chlamydia trachomatis strain lymphogranuloma venereum. Infect Immun. 1974 Sep;10(3):464–470. doi: 10.1128/iai.10.3.464-470.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. F. Hydrolytic enzymes in KB cells infected with poliovirus and herpes simplex virus. J Bacteriol. 1966 Feb;91(2):789–797. doi: 10.1128/jb.91.2.789-797.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskey L. E., Smith P. C., Wolff D. A. Patterns of cytopathology and lysosomal enzyme release in poliovirus-infected HEp-2 cells treated with either 2-(alpha-hydroxybenzyl)-benzimidazole or guanidine HCl. J Gen Virol. 1970 Jan;6(1):151–161. doi: 10.1099/0022-1317-6-1-151. [DOI] [PubMed] [Google Scholar]
- Killington R. A., Lee D., Scott E. J., Osborne J. A. Studies on the lysosomes of L132 cells infected with either rhinovirus type 2 or poliovirus type 1. J Gen Virol. 1974 Feb;22(2):303–307. doi: 10.1099/0022-1317-22-2-303. [DOI] [PubMed] [Google Scholar]
- Kordová N., Poffenroth L., Wilt J. C. Lysosomes and the "toxicity" of rickettsiales. 3. Response of L cells infected with egg-attenuated C. psittaci 6BC strain. Can J Microbiol. 1972 Aug;18(8):1343–1348. doi: 10.1139/m72-206. [DOI] [PubMed] [Google Scholar]
- Kordová N., Poffenroth L., Wilt J. C. Lysosomes and the "toxicity" of rickettsiales. II. Non-cytocidal interactions of egg-grown C. psittaci 6BC and in vitro macrophages. Can J Microbiol. 1972 Jun;18(6):869–873. doi: 10.1139/m72-134. [DOI] [PubMed] [Google Scholar]
- Kordová N., Wilt J. C., Poffenroth L. Lysosomes and the "toxicity" of Rickettsiales. V. In vivo relationship of peritoneal phagocytes and egg-attenuated C. psittaci 6BC. Can J Microbiol. 1973 Nov;19(11):1417–1423. doi: 10.1139/m73-228. [DOI] [PubMed] [Google Scholar]
- Kordová N., Wilt J. C., Sadiq M. Lysosomes in L cells infected with Chlamydia psittaci 6BC strain. Can J Microbiol. 1971 Jul;17(7):955–959. doi: 10.1139/m71-152. [DOI] [PubMed] [Google Scholar]
- Koschel K., Aus H. M., ter Meulen V. Lysosomal enzyme activity in poliovirus-infected HeLa cells and vesicular stomatitis virus-infected L cells: biochemical and histochemical comparative analysis with computer-aided techniques. J Gen Virol. 1974 Dec;25(3):359–369. doi: 10.1099/0022-1317-25-3-359. [DOI] [PubMed] [Google Scholar]
- Malmquist W. A., Van der Maaten M. J., Boothe A. D. Isolation, immunodiffusion, immunofluorescence, and electron microscopy of a syncytial virus of lymphosarcomatous and apparently normal cattle. Cancer Res. 1969 Jan;29(1):188–200. [PubMed] [Google Scholar]
- McLIMANS W. F., DAVIS E. V., GLOVER F. L., RAKE G. W. The submerged culture of mammalian cells; the spinner culture. J Immunol. 1957 Nov;79(5):428–433. [PubMed] [Google Scholar]
- Moulder J. W. Intracellular parasitism: life in an extreme environment. J Infect Dis. 1974 Sep;130(3):300–306. doi: 10.1093/infdis/130.3.300. [DOI] [PubMed] [Google Scholar]
- Ogier G., Chardonnet Y., Gazzolo L. Role of lysosomes during infection with Shope fibroma virus of primary rabbit kidney tissue culture cells. J Gen Virol. 1974 Feb;22(2):249–253. doi: 10.1099/0022-1317-22-2-249. [DOI] [PubMed] [Google Scholar]
- Poste G. Mechanisms of virus-induced cell fusion. Int Rev Cytol. 1972;33:157–252. doi: 10.1016/s0074-7696(08)61451-5. [DOI] [PubMed] [Google Scholar]
- Poste G. The role of lysosomes in virus-induced cell fusion. 2. Modification of the cell surface. Microbios. 1971 Mar;3(10):105–112. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Righthand F., Karzon D. T. Effect of host cell on distribution of a lysosomal enzyme during virus infection. J Virol. 1971 Apr;7(4):467–472. doi: 10.1128/jvi.7.4.467-472.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter E. M. Synthesis of nucleic acid and protein in L cells infected with the agent of meningopneumonitis. J Bacteriol. 1966 May;91(5):2069–2080. doi: 10.1128/jb.91.5.2069-2080.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes G. V. Cycloheximide-resistant glycosylation in L cells infected with Chlamydia psittaci. Infect Immun. 1974 Mar;9(3):497–499. doi: 10.1128/iai.9.3.497-499.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes G. V. Formation and destruction of internal membranes in L cells infected with Chlamydia psittaci. Infect Immun. 1973 Feb;7(2):173–177. doi: 10.1128/iai.7.2.173-177.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J., Smart R. A., Marriott M. E., Davis R. V. Polyarthritis of calves: isolation of psittacosis agents from affected joints. Am J Vet Res. 1966 May;27(118):633–641. [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFF D. A., BUBEL H. C. THE DISPOSITION OF LYSOSOMAL ENZYMES AS RELATED TO SPECIFIC VIRAL CYTOPATHIC EFFECTS. Virology. 1964 Nov;24:502–505. doi: 10.1016/0042-6822(64)90196-5. [DOI] [PubMed] [Google Scholar]