Abstract
Background
The specific aim of this study was to conduct a systematic review of the literature to assess outcomes data on complications and aesthetic results associated with autologous tissue based breast reconstruction performed prior to or after chest wall irradiation.
Methods
Studies from a PubMed search that met predetermined inclusion criteria were identified. Complications of interest included partial or total flap loss, fat necrosis, thrombosis, infection, seroma, hematoma, delayed wound healing and flap fibrosis/contracture. Pooled complication rates were calculated.
Results
A total of 20 articles were included in the study for autologous reconstruction. These primary articles were selected after screening 897 publications, with 6 studies presenting data on pre-reconstruction radiation, 9 studies presenting data on post-reconstruction radiation and 5 studies presenting data on both patient groups. Comparison of pooled complication rates between flaps radiated before or after reconstruction were statistically similar, including total flap loss (1% versus 4%), wound healing complications (10% versus 14%), infection (4% versus 6%), hematoma (2% versus 1%), seroma (4% versus 4%) and fat necrosis (10% versus 13%). The pooled rate of flap contracture and fibrosis was 27% in flap reconstructions exposed to radiation therapy. Statistical evaluation of aesthetic outcomes was impossible due to variability in assessment and reporting methods.
Conclusions
Review of the current literature suggests similar rates of complications and success rates in autologous breast reconstruction patients exposed to pre-reconstruction or post-reconstruction radiation. Immediate autologous reconstruction should be considered as a viable option even in patients who are likely to require post-mastectomy radiation therapy.
Keywords: Breast Reconstruction, Autologous, Flaps, Radiation Therapy
INTRODUCTION
The full impact of chest wall irradiation (XRT) on breast reconstruction is not completely understood. XRT is often used as an adjunct treatment to surgery for breast cancer to further reduce the risk of locoregional recurrence.1, 2 Autologous tissue breast reconstruction is favored in the setting of radiation therapy but poses a unique set of challenges and potential complications when compared to other reconstructive techniques.
Previous studies have looked at the effects of XRT prior to or after flap reconstruction of the breast.3 Radiation therapy following immediate autologous breast reconstruction has historically been thought to result in suboptimal outcomes.4, 5 Based on evidence from some of these studies expert opinions and recommendations have been made favoring delayed autologous reconstruction after completion of post-mastectomy radiation therapy to improve outcomes.6 However, others have shown that with current radiation delivery, this dictum may no longer hold true and that women can have the same benefits of an autologous breast reconstruction without waiting an extended period of time without a breast.7 Moreover, most studies on this subject are based on limited study samples and the quality of evidence has not been adequate to assess the appropriate timing of reconstruction relative to delivery of chest wall XRT. High levels of evidence comparing this variation in autologous reconstruction strategies are lacking and possibly not feasible due to challenges associated with patient randomization in this setting. Nevertheless, clinical decisions regarding management of breast reconstruction patients needs to be based on the best available evidence. This evidence is most effectively evaluated through a rigorously designed systematic review.
The aim of this study was thus to perform a critical appraisal of currently available literature, using previously described methods of search integrity,8 to evaluate complication data and aesthetic outcomes on autologous tissue-based breast reconstruction prior to and after chest wall XRT. This would help guide the decision-making process for the timing of autologous reconstruction when radiation therapy is necessary.
PATIENTS AND METHODS
Search Criteria
A thorough literature search was conducted using PubMed in August of 2012 to identify citations reporting outcomes of autologous tissue flap based breast reconstruction in the setting of radiation therapy. The search terms used were “breast reconstruction”, “breast reconstruction and radiation” and “autologous flap and radiation.” Multiple authors independently examined the titles and abstracts of citations and generated a list of articles for review.
Inclusion and Exclusion Criteria
Studies were assessed against predetermined inclusion criteria (Table 1). This included primary data from prospective and retrospective observational studies. Only human studies that examined the outcomes of autologous flap-based breast reconstruction in women with pre-reconstruction or post-reconstruction XRT were considered eligible. The former included patients who underwent breast XRT as part of breast conservation therapy prior to mastectomy.
Table 1. Predetermined Inclusion and Exclusion Criteria for literature Search.
| Inclusion Criteria |
|
|
| Primary data from prospective and retrospective observational studies |
| Human Studies |
| Studies that include data on autologous flap reconstruction |
| Studies that stratify results by delivery of radiation before or after initiation of reconstruction |
|
|
| Exclusion Criteria |
|
|
| Review, technique or case report articles |
| Studies with fewer than 10 total patients with pre or post reconstruction radiation therapy |
| Articles that did not undergo the peer review process |
| Studies focused solely on reconstruction with implants |
| Studies focused solely on the elderly (older than 65years) |
| Studies with no relevant extractable outcomes |
| Studies not published in English |
Studies were excluded if the timing of radiation therapy relative to the reconstruction could not be determined. Studies focused solely on implant-based reconstruction or mixed autologous-implant reconstruction combinations were also excluded.
Data Abstraction
The data were extracted from studies satisfying the inclusion criteria and verified by multiple authors. Any disagreements were resolved by consensus. Variables extracted included: study design, patient demographics, method and timing of reconstruction relative to radiation therapy, mean follow-up time, specific complication rates and aesthetic outcomes. Complications included seromas, hematomas, infections, delayed wound healing, flap fibrosis or contracture (flap fibrosis or contracture referred to as “fibrosis” in the rest of the article), vascular thrombosis, fat necrosis, partial flap necrosis, and total flap necrosis.
Complications were reported per breast reconstruction and limited only to the irradiated breast. As each operation on a breast had potential for complications, multiple complications could be recorded per breast depending on the study design. Reconstructions were considered failures if flaps underwent necrosis significant enough to require further reconstruction with a new flap or removal of the breast mound without further intervention. Reconstructions were considered successful and complete in patients who had viable flap reconstructions with only minor revision procedures for symmetry or aesthetic improvements.
Aesthetic outcomes presented in the selected studies were also documented in both study groups.
Statistical Analysis
Our outcomes of interest included complications related to autologous breast reconstruction. It was not possible to conduct a rigorous meta-analysis model of these studies because outcomes of interest were not reported with a uniform standard and there was significant heterogeneity between studies in terms of design, patient characteristics, and outcome estimates; this lack of uniformity made any statistical evaluation of aesthetic outcomes impossible. From extracted frequencies of the outcomes of interest and the number of autologous flaps for each outcome, we report the rates and 95% confidence interval for each possible complication using the variance-stabilizing Freeman-Tukey double arcsine transformation method. Overall estimates and 95% confidence intervals of each outcome of interest were pooled for studies according to whether reconstruction was performed pre- or post-radiation using a random effects meta-analysis of the Freeman-Tukey transformed proportion (Table 3). Variance between studies was estimated using the DerSimonian-Laird estimator with chi square tests of heterogeneity for all outcomes of interest, supplemented by the descriptive measure of I2. I2 is often used as a descriptive measure to represent the proportion of total variation in the estimates of treatment effects that is due to heterogeneity (difference between studies), rather than to chance. To explore comparisons between pre-reconstruction and post-reconstruction radiation therapy, we report the overall point estimates of each complication rate with confidence intervals, even with significant heterogeneity. We provide forest plots to visualize both the heterogeneity and trends in estimates. All quantitative analysis was performed in R using the meta and rmeta packages.
Table 3. Summary of Overall Estimates and 95% CI for Complication Rates.
| Complication | Pre or Post- Recon XRT Flaps |
Number of Studies |
Number of Flaps |
Weighted Point Estimate and 95% CI |
I2 | Test of Heterogeneity p-value |
|---|---|---|---|---|---|---|
| Total Flap Loss | Pre | 11 | 1011 | 0.01 (0.00-0.02) | 6.8 (0-62.9) | 0.38 |
| Post | 12 | 426 | 0.04 (0.00-0.04) | 0 (−) | 1.0 | |
| Partial Flap Loss |
Pre | 9 | 728 | 0.06 (0.03 -0.11) | 64.9 (28.3-82.8) | 0.004 |
| Post | 5 | 162 | 0.00 (0.00-0.02) | 0 (−) | 1.0 | |
| Thrombosis | Pre | 3 | 515 | 0.04 (0.03-0.06) | 0 (0-88.4) | 0.41 |
| Post | 3 | 81 | 0.00 (0.00-0.02) | 0 (−) | 0.98 | |
| Would Healing | Pre | 6 | 705 | 0.10 (0.03-0.20) | 92.3 (86.0-95.8) | <0.0001 |
| Post | 4 | 118 | 0.14 (0.00-0.38) | 89.0 (74.5-95.3) | <0.0001 | |
| Infection | Pre | 8 | 802 | 0.04 (0.02-0.06) | 0 (0-64.9) | 0.24 |
| Post | 7 | 213 | 0.06 (0.03-0.10) | 8.8 (0-73.4) | 0.36 | |
| Hematoma | Pre | 4 | 375 | 0.02 (0.01-0.04) | 0 (0-82.3) | 0.46 |
| Post | 5 | 160 | 0.01 (0.01-0.04) | 22.7 (0-68.0) | 0.27 | |
| Seroma | Pre | 6 | 583 | 0.04 (0.02-0.05) | 0 (0-69.6) | 0.53 |
| Post | 4 | 135 | 0.04 (0.00-0.12) | 61.9 (0-87.2) | 0.049 | |
| Fat Necrosis | Pre | 9 | 872 | 0.10 (0.06-0.14) | 68.9 (37.7-84.4) | 0.001 |
| Post | 12 | 463 | 0.13 (0.07-0.20) | 73.1 (52.1-84.9) | <0.0001 | |
| Contracture/ Fibrosis |
Pre | - | - | - | - | - |
| Post | 9 | 368 | 0.27 (0.12-0.45) | 92.2 (87.4-95.2) | <0.0001 |
XRT- Radiation Therapy
RESULTS
Study Retrieval and Characteristics
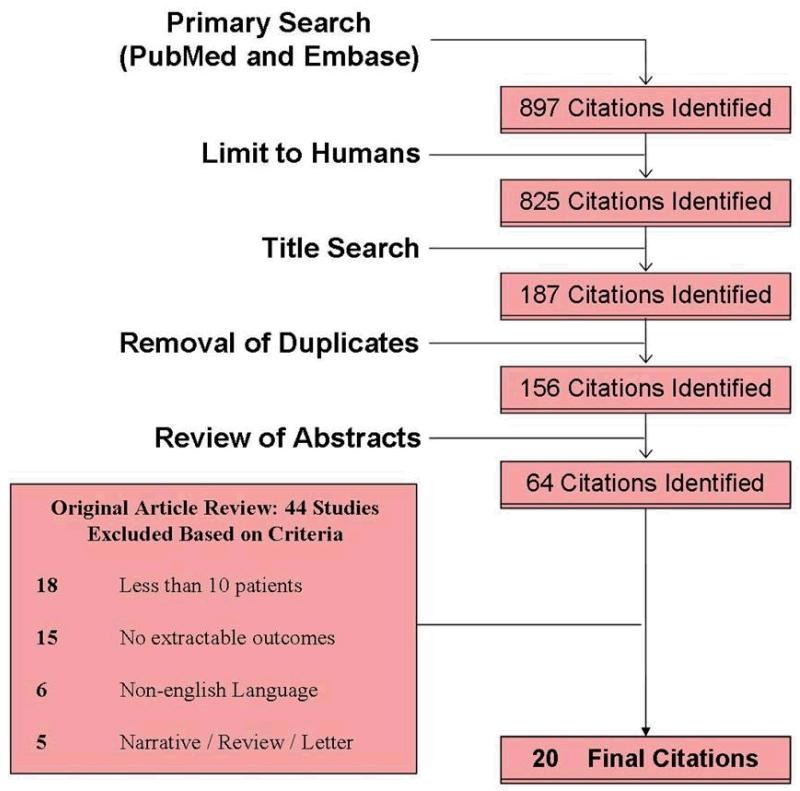
A total of 897 citations were identified from our initial PubMed search (Fig 1). The application of predetermined inclusion and exclusion criteria (Table 1) resulted in 20 selected articles to be utilized for the study.. Studies included were published in years ranging from 1994 to 2012. A summary of study characteristics is included in Table 2.
Figure 1. Attrition diagram.
Table 2. Study and Patient Characteristics.
| Authors | Year | Study Design | No. of Autologous Flaps |
Mean Age (Years) |
Timing of XRT | Mean Months of Follow-Up (Range) |
|---|---|---|---|---|---|---|
| Momoh et al. | 2012 | Retrospective | 100 | 47.1 | Pre-Recon XRT | 33.3 |
| Ho et al. | 2012 | Retrospective | 30 | N/A | Pre-Recon XRT | 42.2 (12 - 113) |
| Monrigal et al. | 2011 | Retrospective | 81 | N/A | Pre-Recon XRT | N/A (12 - 240) |
| Baumann et al. | 2011 | Retrospective | 189 | 48 | Pre-Recon XRT | 10 |
| Fosnot et al. | 2011 | Retrospective | 226 | N/A | Pre-Recon XRT | N/A |
| Crisera et al. | 2011 | Retrospective | 69 | N/A | Post-Recon XRT | N/A |
| Albino et al. | 2010 | Retrospective | 76 | 47 | Post-Recon XRT | N/A |
| Nahabedian et al. | 2008 | Retrospective | 94 | N/A | Both | N/A |
| Jhaveri et al. | 2008 | Retrospective | 23 | N/A | Post-Recon XRT | N/A |
| Carlson et al. | 2008 | Retrospective | 55 | N/A | Both | N/A |
| Wong et al. | 2008 | Retrospective | 47 | N/A | Post-Recon XRT | 13 (2 - 58) |
| Huang et al. | 2006 | Retrospective | 82 | 42.7 | Post-Recon XRT | N/A |
| Foster et al. | 2005 | Retrospective | 35 | N/A | Post-Recon XRT | N/A |
| Spear et al. | 2005 | Retrospective | 72 | 46.6 | Both | N/A |
| Rogers et al. | 2002 | Retrospective | 30 | 48.2 | Post-Recon XRT | 19.9 |
| Tran et al. | 2001 | Retrospective | 102 | 49 | Both | N/A |
| Tran et al. | 2000 | Retrospective | 41 | 48 | Post-Recon XRT | N/A |
| Hunt et al. | 1997 | Retrospective | 19 | 47 | Post-Recon XRT | N/A (26 - 51) |
| Williams et al. | 1995 | Retrospective | 127 | N/A | Both | 53.2 |
| Kroll et al. | 1994 | Retrospective | 82 | N/A | Pre-Recon XRT | N/A |
XRT- Radiation Therapy
Summary of Complication Rates
Significant heterogeneity was noted between studies with overlap of confidence intervals for multiple complication rates. This overlap of confidence intervals is demonstrated in a selected forest plot diagram for wound healing complications (Fig. 2).
Figure 2. Forest Plot of Wound Healing Complication Rates. Diamonds represent the overall summary estimate.
Wound healing complications were reported in six studies in the pre-reconstruction group9-14 and four studies in the post-reconstruction group.4, 5, 13, 15 The pooled rate of wound healing complications was 10% (95% CI, 0.04 – 0.77) in 705 flap reconstructions performed after chest wall radiation versus 14% (95% CI, 0.00 – 0.38) in 118 flaps exposed to radiation.
Fat necrosis rates were reported in nine studies in the pre-reconstruction group9, 10, 12-14, 16-19 and twelve in the post-reconstruction group.4, 5, 13-15, 18-24 The pooled rate of fat necrosis was 10% (95% CI, 0.06 – 0.14) in 872 flap reconstructions preformed after chest wall radiation versus 13% (95% CI, 0.07 – 0.20) in 463 flaps exposed to radiation.
Postoperative infection rates were reported in eight studies in the pre-reconstruction group9, 10, 12, 13, 16-19 and seven in the post-reconstruction group.4, 5, 13, 18, 19, 22, 24 The pooled rate of postoperative infections was 4% (95% CI, 0.02 – 0.06) in 802 flap reconstructions performed after chest wall radiation versus 6% (95% CI, 0.03 – 0.10) in 213 flaps exposed to radiation.
Rates of hematoma occurrence were reported in four studies in the pre-reconstruction group10, 13, 17, 18 and five in the post-reconstruction group.4, 13, 15, 18, 24 The pooled rate of postoperative hematomas was 2% (95% CI, 0.01 – 0.04) in 375 flap reconstructions preformed after chest wall radiation versus 1% (95% CI, 0.01 – 0.04) in 160 flaps exposed to radiation.
Seroma rates were reported in six studies in the pre-reconstruction group10, 12, 13, 16, 17, 19 and four in the post-reconstruction group.4, 13, 15, 24 The pooled rate of postoperative seromas was 4% (95% CI, 0.02 – 0.05) in 583 flap reconstructions performed after chest wall radiation versus 4% (95% CI, 0.00 – 0.12) in 135 flaps exposed to radiation.
Rates of flap fibrosis were addressed in nine studies in only the post-reconstruction group.4, 5, 14, 15, 19, 20, 23-25 The pooled rate of flap fibrosis as a result of post-reconstruction radiation was 27% (95% CI, 0.12 – 0.45) in 368 flap reconstructions.
Total flap loss rates, were addressed in eleven studies in the pre-reconstruction group9-14,16-19, 26 and twelve in the post-reconstruction group.4, 5, 13-15, 18, 19, 21-24, 26 The pooled total flap loss rate was 1% (95% CI, 0.00 – 0.02) in 1011 flap reconstructions performed after chest wall radiation versus 4% (95% CI, 0.00 – 0.04) in 426 flaps exposed to radiation.
Rates of partial flap necrosis were addressed in nine studies only in the pre-reconstruction group.9, 11-14, 16-19 The pooled rate of partial flap necrosis was 6% (95% CI, 0.03 – 0.11) in 728 flap reconstructions performed after chest wall radiation.
Rates of vascular thrombosis in free flaps, necessitating an intraoperative intervention or reoperation were addressed in three studies only in the pre-reconstruction XRT group.9, 10, 12 The pooled rate of vascular thrombosis was 4% (95% CI, 0.03 – 0.06) in 515 flap reconstructions performed after chest wall radiation.
Our post-hoc power calculation using fat necrosis as the outcome yielded 78% power to detect a difference of 10% between groups, based on the sample of 1355 reconstructed breasts. Less power was found for all other complication comparisons due to fewer flaps and estimates closer to 0%.
DISCUSSION
Breast reconstruction is a multifaceted process that ideally requires a multidisciplinary approach to patient management. It is well established that there is a survival advantage with the use of post-mastectomy XRT.27 However there are clearly detrimental effects of XRT on both chest wall soft tissue and autologous flaps utilized in reconstruction. The impact of XRT on implant based breast reconstruction in general has been documented in multiple studies, with clinically significant complications and implant loss rates with radiation exposure.28, 29 The resultant complications and overall outcomes with flap based surgery before or after radiation therapy are more subtle.
The decision-making process between immediate and delayed breast reconstruction for the patient requiring post-mastectomy radiation therapy takes into consideration oncologic factors as well as reconstructive outcomes. Delayed autologous reconstruction is traditionally favored when radiation is needed for multiple reasons including an avoidance of a potential delay in the time to radiation delivery, an avoidance of potentially compromising the actual delivery of radiation to the chest wall and finally an avoidance of radiation induced morbidity to an otherwise successful flap reconstruction.
From the oncologic standpoint, postoperative complications with immediate reconstruction may lead to delays in initiating post-mastectomy XRT.30 This concern is not limited to patients requiring post-mastectomy radiation therapy, it is also a concern for a greater number of patients who require adjuvant chemotherapy. However, with anticipated adjuvant chemotherapy, typically beginning 4 to 6 weeks after reconstruction, most would not hesitate to offer immediate reconstruction though delays are possible due to postoperative complications. It is reasonable to suggest that if immediate reconstruction can be done effectively with planned adjuvant chemotherapy, it can also be done effectively with planned post-mastectomy radiation therapy. Concerns about compromising the ability to radiate the chest wall and internal mammary lymph nodes without overly radiating the heart and lungs have been raised.30 However, a recent treatment planning study7 has showed that adequate radiation doses to the reconstructed breast are feasible with clinically acceptable doses to the heart and lungs even when the internal mammary nodes are included. Thus a major argument against immediate reconstruction may no longer be an issue with appropriate 3D planning.
This systematic review of the literature attempts to address questions related to complications and flap compromise as a result of radiation delivery before or after autologous breast reconstruction. In our review of 20 relevant articles with over 1500 flap reconstructions, pooled individual complication rates in patients exposed to radiation before or after reconstruction were found to be relatively low overall. No significant differences in measurable postoperative complications including total flap loss, wound healing complications, infection, hematoma, seroma and fat necrosis were found in comparing both groups of patients.
Flap fibrosis was found to occur at a pooled rate of 27% in flap reconstructions exposed to radiation therapy. This sequelae of radiation therapy should really be expected in most flaps exposed to radiation. The clinically relevant question ultimately depends on the severity of the fibrosis and the subsequent effect on patient satisfaction and the aesthetic outcome of reconstruction. Flap fibrosis that ultimately ends up in a slightly firmer, less ptotic breast can be deemed less significant as opposed to fibrosis that requires an additional flap to augment or replace the existing reconstruction. Rogers et al.,5 in evaluating the effect of radiation therapy on DIEP flaps, found significantly higher rates of flap fibrosis, fat necrosis and shrinkage when compared to non-radiated flaps. In fact, 16.7% (5 of 30) of the radiated flaps had a contracture deemed severe enough to require a secondary flap to the area. However, they did not find that these radiation changes resulted in an increased number of flap revisions and contralateral mastopexies in comparison to the non-irradiated control group. In other words, radiated and non-irradiated patients needed similar numbers of flap revisions and contralateral procedures for symmetry. Tran et al.,23 in radiated pedicled and free TRAM flaps, also reported a 24% (10 of 41) flap contracture rate requiring an additional flap to create a breast mound. Findings from these studies are in contrast to a more recent study by Chang et al.,7 who in evaluating over 300 flaps found that none of the flaps exposed to postoperative radiation experienced significant shrinkage or volume loss requiring a secondary autologous flap procedure. They found that flaps with postoperative radiation exposure actually had a significantly lower incidence of ipsilateral revisions. This finding of fewer revisions was thought to be due to better baseline results gained from performing skin-sparing mastectomies with immediate reconstructions.
Our attempts to analyze aesthetic outcomes of both reconstructive approaches in this review were unsuccessful as a result of the lack of uniformity in methods of assessing aesthetic outcomes. Carlson et al. used four blinded reviewers and a 3-point grading system to evaluate aesthetic results from postoperative photographs of pedicled TRAM patients exposed to radiation before or after reconstruction.18 No significant differences in scores were noted in these two patient groups. Huang et al. evaluated aesthetic results in immediate TRAM flaps exposed to radiation after reconstruction by patient self-assessments, performed in clinic or over the phone.21 Using a 4-point grading scale, 70% of patients reported good to excellent results. Rogers and Allen reviewed before and after photographs of 10 irradiated DIEP flaps comparing them to similar non-irradiated flaps, with blinded evaluations from 8 judges ranging from a plastic surgeon to a layman.5 A 5-point grading scale was used to evaluate symmetry, the position of the superior pole and overall aesthetic proportion, with findings of decreased (aesthetically diminished) average scores for irradiated flaps compared to increased (aesthetically improved) average scores in the non-irradiated controls. Chang et al. assessed shrinkage and distortion in addition to overall cosmetic outcomes in microvascular breast reconstructions exposed to radiation.7 With three surgeon evaluators using a 4-point grading scale, no significant differences were found in distortion or cosmetic score when comparing patients exposed to radiation before or after reconstruction. As is illustrated in these selected studies, conflicting outcomes on flap fibrosis, flap contractures and aesthetic outcomes in the radiated patient contribute to the difficulty encountered when attempting to provide accurate reconstruction recommendations.
This study has a number of limitations. Most importantly, the results found are limited by the strength of the available evidence in the literature. Heterogeneity between included studies was significant likely due to clinical and methodological differences between studies. Additionally, there was an absence of randomized controlled trials exploring this important question. Pooled estimates had wide confidence intervals with overlap between groups due to the limited number of studies with extractable data. Outcomes reported by individual studies were also not uniform precluding rigorous statistical comparison of outcomes in patients exposed to pre-reconstruction or post-reconstruction XRT. Pooled estimates of aesthetic outcomes were not possible as a result of significant variability in methods of assessment and reporting.
Complications data from this systematic review in addition to evidence from multiple recent studies, challenges the traditional recommendation for delayed autologous reconstruction in the patient known to require post-mastectomy radiation. Though delayed autologous reconstruction represents a reasonable option, the benefits of immediate autologous reconstruction should not be overlooked, even in the patient known to require post-mastectomy radiation therapy. Women requiring post-mastectomy radiation should be fully apprised on what is understood on this subject, allowing them to make an informed choice on immediate or delayed autologous breast reconstruction.
Acknowledgments
Support for this study was provided in part by grants from the Plastic Surgery Foundation (to A.O.M) and by a Midcareer Investigator Award in Patient-Oriented Research (K24 AR053120) (to K.C.C.).
REFERENCES
- 1.Meretoja TJ, von Smitten KA, Leidenius MH, Svarvar C, Heikkila PS, Jahkola TA. Local recurrence of stage 1 and 2 breast cancer after skin-sparing mastectomy and immediate breast reconstruction in a 15-year series. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2007;33:1142–1145. doi: 10.1016/j.ejso.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. The New England journal of medicine. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 3.Chevray PM. Timing of breast reconstruction: immediate versus delayed. Cancer journal. 2008;14:223–229. doi: 10.1097/PPO.0b013e3181824e37. [DOI] [PubMed] [Google Scholar]
- 4.Foster RD, Hansen SL, Esserman LJ, et al. Safety of immediate transverse rectus abdominis myocutaneous breast reconstruction for patients with locally advanced disease. Archives of surgery. 2005;140:196–198. doi: 10.1001/archsurg.140.2.196. discussion 199-200. [DOI] [PubMed] [Google Scholar]
- 5.Rogers NE, Allen RJ. Radiation effects on breast reconstruction with the deep inferior epigastric perforator flap. Plastic and reconstructive surgery. 2002;109:1919–1924. doi: 10.1097/00006534-200205000-00022. discussion 1925-1916. [DOI] [PubMed] [Google Scholar]
- 6.Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: a critical review of the literature. Plastic and reconstructive surgery. 2009;124:395–408. doi: 10.1097/PRS.0b013e3181aee987. [DOI] [PubMed] [Google Scholar]
- 7.Chang EI, Liu TS, Festekjian JH, Da Lio AL, Crisera CA. Effects of radiation therapy for breast cancer based on type of free flap reconstruction. Plastic and reconstructive surgery. 2013;131:1e–8e. doi: 10.1097/PRS.0b013e3182729d33. [DOI] [PubMed] [Google Scholar]
- 8.Haase SC. Systematic reviews and meta-analysis. Plastic and reconstructive surgery. 2011;127:955–966. doi: 10.1097/PRS.0b013e318200afa9. [DOI] [PubMed] [Google Scholar]
- 9.Baumann DP, Crosby MA, Selber JC, et al. Optimal timing of delayed free lower abdominal flap breast reconstruction after postmastectomy radiation therapy. Plastic and reconstructive surgery. 2011;127:1100–1106. doi: 10.1097/PRS.0b013e3182043652. [DOI] [PubMed] [Google Scholar]
- 10.Fosnot J, Fischer JP, Smartt JM, Jr., et al. Does previous chest wall irradiation increase vascular complications in free autologous breast reconstruction? Plastic and reconstructive surgery. 2011;127:496–504. doi: 10.1097/PRS.0b013e3181fed560. [DOI] [PubMed] [Google Scholar]
- 11.Kroll SS, Schusterman MA, Reece GP, Miller MJ, Smith B. Breast reconstruction with myocutaneous flaps in previously irradiated patients. Plastic and reconstructive surgery. 1994;93:460–469. discussion 470-461. [PubMed] [Google Scholar]
- 12.Momoh AO, Colakoglu S, de Blacam C, Gautam S, Tobias AM, Lee BT. Delayed autologous breast reconstruction after postmastectomy radiation therapy: is there an optimal time? Annals of plastic surgery. 2012;69:14–18. doi: 10.1097/SAP.0b013e31821ee4b6. [DOI] [PubMed] [Google Scholar]
- 13.Spear SL, Ducic I, Low M, Cuoco F. The effect of radiation on pedicled TRAM flap breast reconstruction: outcomes and implications. Plastic and reconstructive surgery. 2005;115:84–95. [PubMed] [Google Scholar]
- 14.Tran NV, Chang DW, Gupta A, Kroll SS, Robb GL. Comparison of immediate and delayed free TRAM flap breast reconstruction in patients receiving postmastectomy radiation therapy. Plastic and reconstructive surgery. 2001;108:78–82. doi: 10.1097/00006534-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Hunt KK, Baldwin BJ, Strom EA, et al. Feasibility of postmastectomy radiation therapy after TRAM flap breast reconstruction. Annals of surgical oncology. 1997;4:377–384. doi: 10.1007/BF02305549. [DOI] [PubMed] [Google Scholar]
- 16.Ho AL, Tyldesley S, Macadam SA, Lennox PA. Skin-sparing mastectomy and immediate autologous breast reconstruction in locally advanced breast cancer patients: a UBC perspective. Annals of surgical oncology. 2012;19:892–900. doi: 10.1245/s10434-011-1989-4. [DOI] [PubMed] [Google Scholar]
- 17.Monrigal E, Dauplat J, Gimbergues P, et al. Mastectomy with immediate breast reconstruction after neoadjuvant chemotherapy and radiation therapy. A new option for patients with operable invasive breast cancer. Results of a 20 years single institution study. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2011;37:864–870. doi: 10.1016/j.ejso.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Carlson GW, Page AL, Peters K, Ashinoff R, Schaefer T, Losken A. Effects of radiation therapy on pedicled transverse rectus abdominis myocutaneous flap breast reconstruction. Annals of plastic surgery. 2008;60:568–572. doi: 10.1097/SAP.0b013e31815b6ced. [DOI] [PubMed] [Google Scholar]
- 19.Williams JK, Bostwick J, 3rd, Bried JT, Mackay G, Landry J, Benton J. TRAM flap breast reconstruction after radiation treatment. Annals of surgery. 1995;221:756–764. doi: 10.1097/00000658-199506000-00014. discussion 764-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albino FP, Koltz PF, Ling MN, Langstein HN. Irradiated autologous breast reconstructions: effects of patient factors and treatment variables. Plastic and reconstructive surgery. 2010;126:12–16. doi: 10.1097/PRS.0b013e3181da878f. [DOI] [PubMed] [Google Scholar]
- 21.Huang CJ, Hou MF, Lin SD, et al. Comparison of local recurrence and distant metastases between breast cancer patients after postmastectomy radiotherapy with and without immediate TRAM flap reconstruction. Plastic and reconstructive surgery. 2006;118:1079–1086. doi: 10.1097/01.prs.0000220527.35442.44. discussion 1087-1078. [DOI] [PubMed] [Google Scholar]
- 22.Jhaveri JD, Rush SC, Kostroff K, et al. Clinical outcomes of postmastectomy radiation therapy after immediate breast reconstruction. International journal of radiation oncology, biology, physics. 2008;72:859–865. doi: 10.1016/j.ijrobp.2008.01.055. [DOI] [PubMed] [Google Scholar]
- 23.Tran NV, Evans GR, Kroll SS, et al. Postoperative adjuvant irradiation: effects on tranverse rectus abdominis muscle flap breast reconstruction. Plastic and reconstructive surgery. 2000;106:313–317. doi: 10.1097/00006534-200008000-00011. discussion 318-320. [DOI] [PubMed] [Google Scholar]
- 24.Wong JS, Ho AY, Kaelin CM, et al. Incidence of major corrective surgery after post-mastectomy breast reconstruction and radiation therapy. The breast journal. 2008;14:49–54. doi: 10.1111/j.1524-4741.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- 25.Crisera CA, Chang EI, Da Lio AL, Festekjian JH, Mehrara BJ. Immediate free flap reconstruction for advanced-stage breast cancer: is it safe? Plastic and reconstructive surgery. 2011;128:32–41. doi: 10.1097/PRS.0b013e3182174119. [DOI] [PubMed] [Google Scholar]
- 26.Nahabedian MY, Momen B. The impact of breast reconstruction on the oncologic efficacy of radiation therapy: a retrospective analysis. Annals of plastic surgery. 2008;60:244–250. doi: 10.1097/SAP.0b013e31811ff91b. [DOI] [PubMed] [Google Scholar]
- 27.Shirvani SM, Pan IW, Buchholz TA, et al. Impact of evidence-based clinical guidelines on the adoption of postmastectomy radiation in older women. Cancer. 2011;117:4595–4605. doi: 10.1002/cncr.26081. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch EM, Seth AK, Dumanian GA, et al. Outcomes of tissue expander/implant breast reconstruction in the setting of prereconstruction radiation. Plastic and reconstructive surgery. 2012;129:354–361. doi: 10.1097/PRS.0b013e31823ae8b1. [DOI] [PubMed] [Google Scholar]
- 29.Spear SL, Seruya M, Rao SS, et al. Two-stage prosthetic breast reconstruction using AlloDerm including outcomes of different timings of radiotherapy. Plastic and reconstructive surgery. 2012;130:1–9. doi: 10.1097/PRS.0b013e3182547a45. [DOI] [PubMed] [Google Scholar]
- 30.Motwani SB, Strom EA, Schechter NR, et al. The impact of immediate breast reconstruction on the technical delivery of postmastectomy radiotherapy. International journal of radiation oncology, biology, physics. 2006;66:76–82. doi: 10.1016/j.ijrobp.2006.03.040. [DOI] [PubMed] [Google Scholar]