Abstract
The renal cell carcinoma (RCC) is one of the top ten cancers in USA. The renal tumors are highly angiogenic and are resistant to conventional interventions, particularly radiotherapy. The advent of multispecific tyrosine kinase inhibitor sorafenib has improved the progression-free survival in RCC, but overall survival in recurrent and metastatic RCC is still a concern that has lead to characterization of combinatorial regimens. Hence, we studied the effect of combination of nutlin-3, an MDM2 inhibitor which increases p53 levels, and sorafenib in RCC. Sorafenib along with nutlin-3 synergistically inhibited the cell survival and enhanced caspase-3 cleavage leading to apoptosis in RCC. Nutlin-3 and sorafenib were more effective in reducing the migration of RCC, in combination than as single agents. Sorafenib and nutlin-3 decreased the phosphorylation of vascular endothelial growth factor receptor-2 (VEGFR-2) and ERK along with inducing p53 activity. The sorafenib and nutlin-3 co-treatment lead to enhanced levels of p53, p-p53 and increase in the levels of p53 pro-apoptotic effector PUMA, Bax and decrease in the anti-apoptotic Bcl-2 levels. Importantly, our studies revealed that sorafenib alone can activate p53 in a concentration dependent manner. Thus, co-treatment of nutlin-3 with sorafenib leads to increased half-life of p53, which in turn can be activated by sorafenib, to induce downstream pro-apoptotic and anti-proliferative effects. This is the first report showing the synergistic effect of sorafenib and nutlin-3 while providing a strong clinical-translational rationale for further testing of sorafenib and nutlin-3 combinatorial regimen in human RCC.
Keywords: Renal Cell Carcinoma, Nutlin-3, Sorafenib, p53, Chemotherapy
Introduction
Renal cell carcinoma (RCC) accounts for approximately 3% of all malignancies while patients with metastatic RCC have a median survival of 13 months. The most common form of RCC corresponds to the clear cell histology [clear cell renal cell carcinoma (CCRCC), 75% of RCC], which is highly aggressive and unresponsive to radiation or chemotherapy [1]. Surgery by radical or partial nephrectomy is the most effective choice for the treatment of localized RCC. However, in one third of the patients, tumors recur postoperatively as distant metastases. Only 4 to 6% of such recurrent and metastatic tumors respond to chemotherapy. Recently, clinical trials of receptor tyrosine kinase inhibitors such as the vascular endothelial growth factor receptor-2 (VEGFR-2) and platelet-derived growth factor (PDGF) receptor-β inhibitors like sorafenib (Nexavar) and sunitinib (Sutent), respectively, have shown positive results in prolonging progression-free survival in ∼70% of patients with CCRCC. However, neither of those newer drugs had a significant effect on overall tumor clearance and patient survival [1, 2].
Sorafenib is a small molecule multi-targeted kinase inhibitor that blocks the activation of C-RAF, both the wild-type and the activated V600E mutant of B-RAF, c-KIT, FLT-3, RET, VEGFR-2, VEGFR-3, and PDGFR-β [3, 4]. The pre-clinical studies have shown that sorafenib is active in a broad spectrum of tumor types [5-7]. In in vitro assays, sorafenib inhibits the ligand-induced auto-phosphorylation of VEGFR-1, VEGFR-2, VEGFR-3, and PDGFR-β [8]. Sorafenib is currently approved for the treatment of metastatic RCC as well as for advanced hepatocellular carcinoma, and is under investigation in phase II/III trials in other malignancies including NSCLC but the clinical outcomes warrant further testing of combinatorial regimens with sorafenib [9-12]. Hence, as the clinical application of sorafenib evolves, there is increasing interest in defining the mechanisms underlying its anti-proliferative activity as well as examining the effects of sorafenib in combination with other anti-cancer drugs [13-16].
MDM2 is an E3 ligase that binds to and ubiquitinates p53, leading to its proteasomal degradation [17, 18]. Both the p53 and MDM2 form an auto-regulatory feedback loop in which p53 transcriptionally activates the expression of MDM2, and MDM2 stimulates the degradation of p53, thereby efficiently regulating the levels of both proteins. Many cancer therapies depend on p53 induced apoptosis by activating the DNA damage response pathway and stress-responsive signaling pathways. Although these treatments can be effective, their genotoxic potential can lead to the development of secondary cancers, notably leukemias [19-21]. MDM2 inhibitors represent a new class of anti-cancer agents that can activate p53 in cancer cells without triggering DNA damage [22, 23]. Nutlin-3 is a cis-imidazoline compound that specifically binds to MDM2 and prevents the interaction of MDM2 with p53 [24]. Therefore, in the presence of nutlin-3, p53 does not undergo proteasomal degradation and accumulates in the cells leading to inhibition of proliferation and induction of cell death [24, 25]. Nutlin-3 treatment has been shown to inhibit the growth of human tumors that express wild-type p53 in nude mice xenograft models [26]. Though the p53 mutations are rare in RCC, p53 can be functionally inactivated [27]. A multivariate analysis of the human RCC has revealed a statistically significant association with co-expression of p53 and MDM2 with higher clinical stage, distant metastases and poor survival [28]. Thus, increasing the p53 expression or inhibition of its degradation by targeting MDM2 would be a mechanistically sound approach for developing targeted therapeutics for RCC. In this regard, we evaluated the efficacy of the combination of nutlin-3 and sorafenib with the aim of developing pre-clinical rationale for multi-targeted drug-combinations for aggressive stages of RCC.
Materials and Methods
Materials
Sorafenib was kindly provided by Bayer Schering (Italy). Nutlin-3 was purchased from Cayman Chemical (Ann Arbor, MI). Bradford reagent, acrylamide, bis-acrylamide, and SDS for SDSPAGE were obtained from Bio-Rad (Hercules, CA). Western blot stripping buffer was purchased from Pierce Co. (Rockford, IL). The apoptosis detection system (CaspACE FITC-VAD-FMK in situ marker) was purchased from Promega Inc. (Madison, WI). The cell culture medium RPMI and fetal bovine serum were from GIBCO (Invitrogen, Carlsbad, CA). All other reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Cell lines
Human RCCs (Caki-1 and Caki-2) were purchased from ATCC, Manassas, VA. All cells were cultured at 37°C in a humidified atmosphere of 5 % CO2 in RPMI-1640 medium supplemented with 10 % FBS and 1% P/S solution. The cells were trypsinized and passaged every 3-4 days.
Cytotoxicity (MTT) assay
Approximately 20,000 cells were seeded into each well of 96-well plates containing 180 μl medium. Post 24 h incubation, 10 μl aliquots of drug concentrations ranging from 1 μM to 50 μM was then added to eight replicate wells to assess the IC50 of drugs alone and in combination. After 72 h incubation, 10 μl of 5 mg/ml MTT was introduced to each well and incubated for 2 h. The plates were centrifuged and cells were subsequently dissolved in 100 μl DMSO with gentle shaking for 2 h at room temperature, followed by measurement of OD at 570 nm.
Annexin Staining
The caki-1 and caki-2 cells (1×105) were plated on glass cover slips in 12 well plates. After 48 h when cells became confluent, they were cultured in the presence or absence of sorafenib (20 μmol/L), nutlin-3 (20 μmol/L), or their combination for 4 h. Control and treated cells were washed and then incubated with AF488-annexin V in the binding buffer at room temperature for 15 min. The cells were then washed, fixed, and immuno-stained cells were imaged using LSM510 Meta confocal system equipped with Axio Observer Z1 microscope (Zeiss, Germany).
In situ caspase-3 assay for apoptosis
Caki-2 cells (1×105) were plated on glass cover slips in 12 well plates. After 24 h, cells were cultured in the presence or absence of sorafenib (20 μmol/L), nutlin-3 (20 μmol/L), or their combination for 24 h. Apoptotic cells were detected by staining with 5μM Caspase FITC-VAD-FMK (Promega) in situ marker for 30 min in the dark. The slides were fixed with 4% paraformaldehyde for 30 min, rinsed with PBS (with calcium and magnesium) thrice for 10 min, mounted in a medium containing DAPI (1.5μg/ml). Images were taken on Olympus AX70 fluorescence microscope.
Evaluation of protein expression by Western blotting
RCC cells were seeded into 100 mm plates for 24 h before treatment with each drug or drug combination as described above. The antibodies against p53 (DO-1) sc-126, p21 (F-5) sc-6246, ERK1 (C-16) sc-93, p-ERK (E-4) sc-7383, VEGFR (1005) sc-03, Bax (N-20) sc-493, Bcl-2 (C-2) sc7382, and GAPDH (6C5) sc-32233 were procured from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies for pVEGFR (Y1175), p-p53 (S15), caspase, and PUMA were obtained from Cell Signaling Technology, Inc. (Boston, MA). Horseradish peroxidase (HRP)-conjugated secondary antibodies and those against GAPDH were purchased from Southern Biotech (Birmingham, AL). Immuno-reactive proteins were visualized by enhanced chemiluminescence (Amersham International). Differences in protein band intensities were assessed by densitometry analysis.
Wound-healing assay
Wound healing assay was performed as described earlier [29]. Briefly Caki-1 and caki-2 cells (1×105) were plated on glass cover slip in 12 well plates. After 48 h when 100% confluence (monolayer) was reached, the cells were wounded by a scratch with a 100 μl pipette tip and then cultured in the presence or absence of sorafenib (20 μmol/L), nutlin-3 (20 μmol/L), or their combination for 24 h. The wounds were photographed (10× objective) at 24 h, after being washed, fixed with 90% methanol, and stained with crystal violet. The rate of cell migration was determined by comparing the sizes of scratch area using NIH Image J software (http://rsbweb.nih.gov/ij/).
F-actin staining and microscopy
The caki-1 and caki-2 cells (1×105) were plated on glass cover slip in 12 well plates. After 48 h when cells became confluent, they were cultured in the presence or absence of sorafenib (20 μmol/L), nutlin-3 (20 μmol/L), or their combination for 24 h. Following treatment, the cells were washed with PBS and fixed for 30 min at 4°C in PBS containing 4% paraformaldehyde. The fixative was removed and remaining formaldehyde was quenched with 0.05% glycine, pH 8.0 for 10 min. The fixed cells were permeabilized with 0.05% Triton ×-100 in PBS for 15 min and blocked with 3% goat serum in PBS for 1 h at room temperature. Permeabilized cells were stained with rhodamine conjugated phalloidin for 60 min at room temperature to detect F-actin. The stained cells were imaged using LSM510 Meta confocal system equipped with Axio Observer Z1 microscope (Zeiss, Germany)
Statistical analysis
Each experiment was repeated at least twice to ensure reproducibility of the results. The statistical significance of differences between control and treatment groups was determined by ANOVA followed by multiple comparison tests. Differences were considered statistically significant when the P value was less than 0.05. Synergism, additive effects, or antagonism were assessed by the Chou-Talalay method. In this method, CI was used to express synergism (CI less than 1), an additive effect (CI equal to 1) or antagonism (CI greater than 1) [30].
Results and Discussion
Anti-proliferative effects of sorafenib and nutlin-3
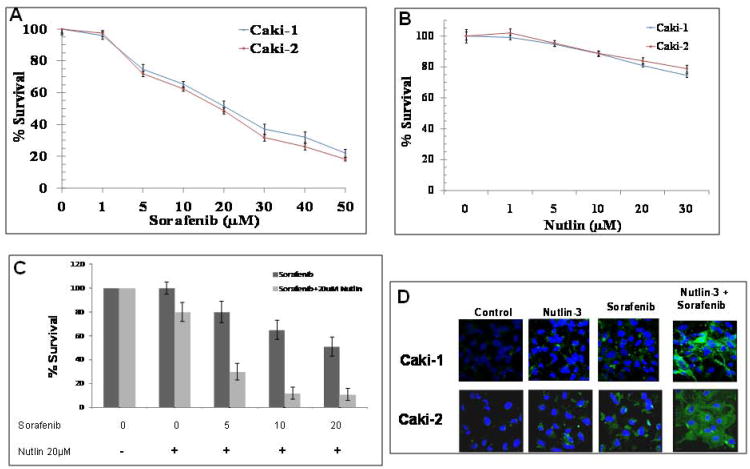
To assess the effect of co-treatment of sorafenib and nutlin-3, caki-1 and caki-2 RCC cells were treated with either of the drugs alone (Fig 1A and B) or in combination, followed by MTT assay to determine cytotoxicity (Fig 1C) and Annexin V staining to assess apoptosis (Fig 1D). Sorafenib alone, at a concentration of 50 μM, inhibited the proliferation of caki-1 and caki-2 RCC up to 80% in vitro studies. The IC50 of sorafenib for caki-1 and caki-2 RCC was 20.8 μM and 19.2 μM, respectively (Fig 1A). Nutlin-3 alone was not very effective in either of the cell lines. Nutlin-3 at a concentration of 30 μM inhibited the cell growth of caki-1 and caki-2 RCC only up to 26% and 22%, respectively (Fig 1B). To gain further insight into the possible combinatorial anti-cancer effects of sorafenib and nutlin-3, dose-response studies were conducted. There was a dose-dependent increase in the degree of apoptosis caused by the combination of sorafenib and nutlin-3 in caki-2 RCC (Fig 1C). Similar effects were observed in caki-1 RCC which indicated that the synergistic anti-cancer effect of nutlin-3 and sorafenib is not cell specific (supplemental Fig 1). For further statistical analyses of the combinatorial anti-cancer effects of sorafenib and nutlin-3, RCC cells were treated with different concentrations of sorafenib and nutlin-3, and the combination index (CI) values on apoptosis induction were determined using the Chou and Talalay test (Table 1) [30] . As shown in Fig. 1C and Table 1, the CI values were <1, indicating a synergistic anti-proliferative effects of sorafenib and nutlin-3 in RCC.
Figure 1. Apoptotic effects of sorafenib and nutlin-3 in RCC.
Caki-1 and caki-2 cells were incubated with 0-50 μM of sorafenib (panel A) and 0-30 μM of nutlin-3 (panel B) for 72 h and MTT assay was performed to asses cell death. Caki-2 cells were incubated with 0-20 μM of sorafenib alone or in combination with 20 μM of nutlin-3 for 72 h to evaluate synergistic effect (panel C). Examination of phosphatidyl serine (PS) exposure at the cell surface by confocal microscopy with Alexa Fluor 488-conjugated annexin V. Caki-1 and caki-2 (1 × 105) were treated with 20 μM sorafenib and 20 μM nutlin-3, alone or in combination for 4 h to differentiate apoptosis from necrosis. After drug treatment, cells were washed with wash buffer followed by incubation with Alexa Fluor 488-conjugated annexin V at room temperature for 15 min. Cells were again washed with wash buffer and slides were mounted with Vectashield DAPI mounting medium and observed under a fluorescence microscope (Olympus) using the standard filter sets for DAPI and Alexa Fluor 488 (panel D).
Table 1.
Synergistic anti-proliferative effect of Nutlin-3 and Sorafenib in RCC.
| Nutlin-3 (μM) | Sorafenib (μM) | Combination Index (CI) |
|---|---|---|
| 20 | 5 | 0.62 |
| 20 | 10 | 0.68 |
| 20 | 20 | 0.77 |
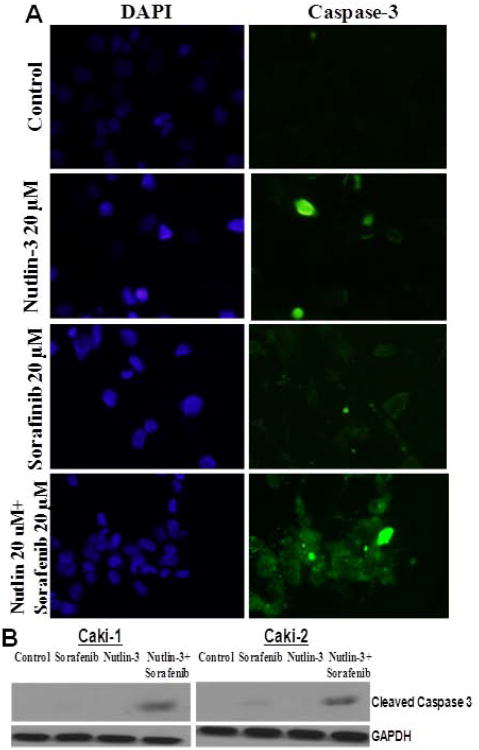
To confirm the enhanced induction of apoptosis due to co-treatment of sorafenib and nutlin-3, we first analyzed the Annexin V binding as a biological marker of apoptosis. In normal live cells, phosphatidyl serine (PS) is located on the cytoplasmic surface of the cell membrane whereas in apoptotic cells, PS translocates from inner to the outer leaflet of the plasma membrane. Annexin V, having high affinity towards PS, binds strongly with PS which is exposed in apoptotic cells and thus differentiates apoptotic cells from necrotic or live cells. Annexin V binding was substantially increased in cells treated with the combination of sorafenib and nutlin-3 as compared to either of the drugs alone (Fig 1D). To further examine whether combination of sorafenib and nutlin-3 induced toxicity involves the onset of apoptosis, the treated cells were analyzed for caspase cleavage by Western blot followed by immunofluorescence analysis for caspase release and activation using CaspACE FITC-VAD-FMK in situ marker. Again, sorafenib and nutlin-3 co-treatment enhanced apoptosis as evident by increased release of cytochrome c and subsequent activation of caspases, compared to either of the single drugs alone (Fig 2). Following the initial studies, we next investigated the mechanisms of action of sorafenib and nutlin-3 combination in RCC.
Figure 2. In situ analysis of activation of caspase-3 by sorafenib and nutlin-3.
Caki-1 and caki-2 (1 × 105) RCC cells were treated with 20 μM sorafenib and 20 μM nutlin-3, alone or in combination for 12 h. The activation of caspase-3 in these cells was examined by staining with 5 μM CaspACE™ FITC-VAD-FMK in situ marker according to the manufacturer's instructions. The slides were mounted with Vectashield DAPI mounting medium and observed under a f LSM510 Meta confocal system equipped with Axio Observer Z1 microscope (Zeiss, Germany) using the standard filter sets for DAPI and FITC (panel A), and cleaved caspase was demonstrated by Western blot analysis. GAPDH was used as a loading control (panel B).
Effects of sorafenib and nutlin-3 on p53 mediated apoptotic pathway
To examine the mechanisms of synergistic effect of sorafenib and nutlin-3as observed in vitro cytotoxicity studies on RCC, we determined the effect of nutlin-3 and sorafenib on the p53 expression, phosphorylation and its downstream targets in caki-2 RCC cells. The results revealed that nutlin-3 (20 μM) and sorafenib (20 μM) increase the expression of p53 approximately up to 4 fold and 2 fold, respectively, whereas the expression of phospho-p53 was increased approximately up to 8 fold and 4 fold in nutlin-3 and sorafenib treated cells, respectively. Co-treatment of nutlin-3 (20 μM) and sorafenib (20 μM) synergistically increased the expression of both p53 and phospho-p53 as compared to either of the single drugs (Fig 3A). Sorafenib is a known multi-targeted tyrosine kinase inhibitor, but p53 as one of its downstream target has not been studied in RCC. In order to further study the mechanism of action of sorafenib in regulating p53, we incubated sorafenib with different concentrations ranging from 10 μM to 30 μM and observed the expression of p53 and its phosphorylated form for a period of 24 h. Our results demonstrated that both phospho-p53 and p53 expression increased in concentration dependent manner following sorafenib treatment (Fig 3B). Since activation and phosphorylation of p53 has been associated with apoptosis through the induction of its target genes including p21, Bcl-2 and Bax, we examined these downstream targets after the co-treatment with nutlin-3 (20 μM) and sorafenib (20 μM). As consistent with several other studies [31, 32], we observed remarkable increase (approximately up to 10 fold) in the expression of p21 upon nutlin-3 treatment, but this enhanced p21 expression was down-regulated upon co- treatment with sorafenib. Nutlin-3 has been shown to induce synergistic apoptotic effect with MEK inhibitors, to some extent by inhibiting the p53 dependent p21 induction [33]. Since sorafenib is reported to down regulate p21 expression in gastrointestinal stromal tumor [34], we believe that sorafenib contributes similarly to reduction of nutlin-3 induced up-regulation of p21in RCC. The reduction in nutlin-3 induced p21 levels, consequent to co-treatment with sorafenib, indicated that other p53 targets play a vital role in mediating the synergistic anti-cancer effects of sorafenib and nutlin-3 in RCC. Hence, we further looked for changes in the expression of p53 inducible gene, p53 up-regulated modulator of apoptosis (PUMA), which is a pro-apoptotic member of the Bcl-2 family of proteins and is known to be involved in p53-mediated apoptosis. We observed that both sorafenib and nutlin-3 increased the expression PUMA, but their combination was more effective in increasing PUMA expression as compared to either of the drugs alone. The p53 protein is known to be an upstream regulator of the Bax [35]. The p53 protein binds to the Bax promoter and directly transactivates the transcription of pro-apoptotic Bax. Several studies have also shown that p53 also down regulates Bcl-2 [36-38]. The cellular levels of expression of pro-apoptotic Bax and anti-apoptotic Bcl2 play a vital role in regulating the survival or cell death responses to apoptotic signals. The co-treatment of sorafenib and nutlin-3 significantly decreased the anti-apoptotic Bcl-2 levels whereas the expression of pro-apoptotic Bax was significantly increased. Since anti-apoptotic function of the Bcl-2 protein is modulated by its ability to heterodimerize with other members of the gene family, predominantly Bax, the changes in the levels of expression of p53, PUMA, Bax and Bcl-2 describe a highly significant mechanistic basis for the sorafenib and nutlin-3 induced synergistic cytotoxicity in RCC.
Figure 3. Effects of co-treatment of sorafenib and nutlin-3 on p53 mediated apoptotic pathway in caki-2 cells.
Caki-2 cells were treated with 20 μM sorafenib and 20 μM nutlin-3, alone or in combination for 24 h at 37 °C. The protein lysates (30 μg) were analyzed by Western blotting for the protein expression of p53, p-p53, p21, PUMA, Bcl-2 and Bax. GAPDH was used as a loading control (panel A). Caki-2 cells were treated with sorafenib (0-30 μM) for 24 h at 37 °C. The protein lysates (30 μg) were analyzed by Western blotting for the protein expression of p53 and p-p53 (panel B).
Effects of sorafenib and nutlin-3 on proliferative and angiogenic pathways
To further explore the mechanisms of synergistic effect induced due to co-treatment of sorafenib and nutlin-3 in RCC, we determined the impact on angiogenic signaling mediated by VEGFR-2 (Flk1) and ERK. Given the evidence indicating a prominent role for VEGFR-2 and ERK in maintaining tumor vasculature and enhanced proliferation rates, it has been confirmed in many clinical trials focused on anti-angiogenic drugs that inhibition of the VEGFR and ERK signaling pathway might have profound effect on managing RCC by simultaneously controlling tumor proliferation and angiogenesis. Sorafenib, being a multispecific tyrosine kinase inhibitor, can inhibit VEGFR, a tyrosine kinase. Hence, we further tested the effect of nutlin-3 on sorafenib induced inhibition of VEGFR-2. Interestingly, the co-treatment of sorafenib and nutlin-3 significantly decreased the VEGFR-2 and phospho-VEGFR-2 levels (Fig 4A). Similar to cell survival and apoptotic signaling studies focused on p53 pathway, the co-treatment of sorafenib and nutlin-3 resulted in more profound VEGFR-2 inhibition compared to either of the drugs alone. The decrease in pERK after treatment with sorafenib alone was significantly evident after 6 h. Nutlin-3, which is believed to be specific for targeting p53 pathway, also inhibited pERK after 24 h of treatment. Further, after 60 min of co-treatment with sorafenib and nutlin-3, there was a greater decrease in ERK phosphorylation which was sustained till 24 h (Fig 4B). These results suggest that co-treatment of nutlin-3 with sorafenib significantly enhances and sustains the inhibitory effect of sorafenib on VEGFR-2 and ERK, which represent critical factors for the regulation of proliferation, angiogenesis, and survival of RCC.
Figure 4. Effects of co-treatment of sorafenib and nutlin-3 on VEGFR-2 and ERK in caki-2 cells.
Caki-2 cells were treated with 20 μM sorafenib and 20 μM nutlin-3, alone or in combination for 24 h at 37 °C. The protein lysates (30 μg) were analyzed by Western blotting for the protein expression of VEGFR-2, p-VEGFR-2, ERK and pERK. GAPDH was used as a loading control (panel A). Caki-2 cells were treated with 20 μM sorafenib and 20 μM nutlin-3, alone or in combination for 1, 6 and 24 h at 37 °C. The protein lysates (30 μg) were analyzed by Western blotting for the protein expression of ERK and pERK. GAPDH was used as a loading control (panel B).
Effects of sorafenib and nutlin-3 on RCC cell migration and cytoskeleton morphology
The metastatic ability of cancers is determined by the potential of the cells to migrate and in this regard, we tested the effect of sorafenib and nutlin-3, as single agents and in combination, in regulating migration of RCC cells. Sorafenib and nutlin-3 inhibited the migration of caki-1 and caki-2 RCC cells but this effect was more profound when treated with the combination of sorafenib and nutlin-3 (Fig 5A). Reorganization of the actin cytoskeleton is essential for the cell migration and invasion in cancer cells and their inhibition can be of great benefit in inducing regression of rapidly metastasizing tumors [11, 12].
Figure 5. Co-treatment of sorafenib and nutlin-3 inhibits wound healing.
Caki-1 and Caki-2 cells at confluence were injured by a scratch with a 10 μL pipette tip. Wounded cells were allowed to heal for 24 h in the presence or absence of sorafenib (20 μM), nutlin-3 (20 μM) and combination of both of sorafenib (20 μM) and nutlin-3 (20 μM). Bars represents migration distance in wound healing assay was quantitated using NIH Image J program (panel A). Caki-1 and caki-2 cells were grown in monolayers were treated with sorafenib (20 μM), nutlin-3 (20 μM) and in combination for 24 h. Phase contrast images were taken on Nikon TMS-F for cell morphology (panel B). The cells were fixed, permeabilized and stained with rhodamine-conjugated phalloidin to detect F-actin. Cells were imaged using a confocal system (panel C).
The diverse morphological dynamics of actin structures that rapidly adapt to various cellular functions play a critical role in maintaining intracellular structure and function in normal and stress conditions. Cytoskeletal structures adjoining plasma membrane determine cellular survival, invasion and migration of cells by providing requisite shape and mechanical strength. In the context of sorafenib and nutlin-3 induced anti-proliferative and pro-apoptotic effects, we next investigated the effect on cell shape by phase contrast microscopy. The sorafenib and nutlin-3 co-treatment caused enhanced cell rounding and detachment of RCC cells (Fig 5B). To further investigate the effect on actin cytoskeleton, caki-1 and caki-2 RCC cells were stained by rhodamine conjugated phalloidin to detect polymerized F-actin by immuno-fluorescence microscopy. We found that treatment of caki-1 and caki-2 RCC with sorafenib and nutlin-3 induced cell shape changes as manifested by cell elongation, aggregation, and finally cell detachment from matrix in cultures (Fig 5C). The actin filaments in the control caki-1 and caki-2 RCC were mainly observed as cell cortex layer which is a network of short, cross-linked actin filaments beneath the plasma membrane with a role in providing mechanical strength to the cell membrane. But, the treatments with either nutlin-3 or sorafenib lead to disruption of cytoskeletal architecture and formation of stress fibers. Specifically, in nutlin-3 treated cells, formation of trans-cellular actin stress fibers was observed in the cytoplasmic region whereas in sorafenib treated cells pan-cellular elongated stress fibers, from one end to the other end of the cell, were observed. Co-treatment of sorafenib and nutlin-3 resulted in greater cell shrinkage and formation of stress fibers through out the cell. Thus, the sorafenib and nutlin-3 effectively inhibited the proliferation and migration of RCC to a greater extent along with modulating the cytoskeletal dynamics in RCC which together reveal the enhanced antiproliferative and anti-migratory potential of the combination of sorafenib and nutlin-3 than as either of the single drugs.
Conclusion
The renal tumors are highly angiogenic with a characteristic refractoriness to conventional radiotherapy and chemotherapy. The multi-specific tyrosine kinase, sorafenib, is the current drug of choice in RCC. Though sorafenib has increased the progression free-survival and the overall survival in RCC, recurrent and metastatic RCC is still a major concern which has lead to investigation of many combinatorial regimens. Functional or genetic inactivation of p53 is a hallmark of many cancers including RCC. Increasing the half-life and hence function of p53 by inhibiting MDM2 is an effective strategy to restore the normal function of p53 in cancerous cells. In this regard, we investigated the effect of sorafenib and MDM2 inhibitor, nutlin-3, in RCC.
Sorafenib and nutlin-3 exerted potent synergistic effects that were confirmed by both cell survival and apoptotic assays. Sorafenib and nutlin-3 caused greater decrease in RCC survival and enhanced caspase activation along with a significant impact on p53 pathway. The increase in the p21 levels consequent to nutlin-3 treatment might explain the lack of apoptosis in nutlin-3 treated RCC cells though the p53 half-life is increased. A study by Kojima et al has implicated the attenuation of p21 as a mechanism of enhancing apoptotic effects of nutlin-3 in the presence of MAPK pathway inhibitors [33]. In this regard, our results are in accordance with previous findings and established rationale. Sorafenib is a multi-targeting tyrosine kinase inhibitor which does cause inhibition of ERK which in turn leads to decrease in p21 levels [39]. In addition, our study has characterized that sorafenib alone induces the expression and phosphorylation of p53. Also, in our study we found that the sorafenib and nutlin-3 cotreatment increases the levels of PUMA. It has been shown that over-expression of PUMA, a vital mediator apoptotic effects down-stream of p53, enhances the nuclear pro-apoptotic functions of p53 [40]. Thus, the distinct regulation of p53/PUMA pathway provides relevant mechanistic rationale for the induction of enhanced apoptosis due to nutlin-3 and sorafenib in RCC.
In accordance with the regulation of p53 expression and activation, the co-treatment of sorafenib and nutlin-3 enhanced the levels of pro-apoptotic Bax and caused greater decrease in the levels of anti-apoptotic Bcl-2. Our results also show for the first time that sorafenib can activate p53 in a concentration dependent manner in RCC (Fig 3B). Thus, stabilizing and enhancing the half-life of p53 by using nutlin-3, a known MDM2 inhibitor, could further facilitate the activation of p53 pathway by sorafenib. Along with providing strong mechanistic evidence for the mechanisms of action of sorafenib and nutlin-3 through p53 dependent pathways, our results indicate a greater inhibition of VEGFR-2 and ERK activation, which together provide strong corroborative rationale for the enhanced pro-apoptotic, anti-angiogenic and anti-proliferative effects due to the combination of sorafenib and nutlin-3 in RCC (Fig 6). Sorafenib and nutlin-3 co-treatment also caused greater inhibition of cell migration, enhanced cell rounding and decreased the sub-plasma membrane actin network that provides mechanical strength along with changing actin cytoskeletal dynamics to reflect cell rounding and cell detachment, characteristic of decreased mobility and enhanced cytotoxicity.
Figure 6. Mechanisms of action of synergistic combination of sorafenib and nutlin-3 in RCC.
Our studies revealed that sorafenib and nutlin-3 induced synergistic inhibition of cell-survival and enhance apoptosis in RCC. For the first time, we show that sorafenib increases the phosphorylation of p53 in a concentration dependent manner which indicates that the pro-apoptotic effects of sorafenib, a known multi-specific tyrosine kinase inhibitor, are mediated by p53 in RCC. Treatment of nutlin-3 will enhance the levels of p53 in cytoplasm which in turn will lead to activation of p53 by sorafenib. The other mechanisms include known inhibition of VEGFR2 and ERK by sorafenib, which also potentiated by the presence of nutlin-3. The enhanced expression of PUMA, Bax and decrease in Bcl-2 levels leads to increased caspase-3 activation following cytochrome c release from mitochondria to initiate apoptosis.
In summary, the sorafenib and nutlin-3 co-treatment induced synergistic anti-proliferative and cytotoxic effects in RCC that were accompanied by enhanced activation of p53 pathway and inhibiton of angiogenic and proliferative signaling in RCC. In this regard, further clinical testing of sorafenib and nutlin-3 combinatorial regimens in RCC would lead to development of synergistic combinatorial therapy to target recurrent and metastatic RCC which are resistant to conventional chemotherapy and radiotherapy.
Supplementary Material
Acknowledgments
This work was supported in part by USPHS grant CA 77495, Cancer Research Foundation of North Texas, and Institute for Cancer Research & the Joe & Jessie Crump Fund for Medical Education.
The abbreviations used are
- RCC
Renal Cell Carcinoma
- MDM2
murine double minute 2
- VEGFR
vascular endothelial growth factor receptor
References
- 1.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 2.Brugarolas J. Renal-cell carcinoma - molecular pathways and therapies. N Engl J Med. 2007;356:185–187. doi: 10.1056/NEJMe068263. [DOI] [PubMed] [Google Scholar]
- 3.Takimoto CH, Awada A. Safety and anti-tumor activity of sorafenib (Nexavar®) in combination with other anti-cancer agents: a review of clinical trials. Cancer Chemother Pharmacol. 2008;61:535–548. doi: 10.1007/s00280-007-0639-9. [DOI] [PubMed] [Google Scholar]
- 4.Lyons JF, Wilhelm S, Hibner B, Bollag G. Discovery of a novel Raf kinase inhibitor. Endocr Relat Cancer. 2001;8:219–225. doi: 10.1677/erc.0.0080219. [DOI] [PubMed] [Google Scholar]
- 5.Wilhelm SM, Carter C, Tang L. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 6.Rausch V, Liu L, Kallifatidis G, Baumann B, Mattern J, Gladkich J, Wirth T, Schemmer P, Büchler MW, Zöller M, Salnikov AV, Herr I. Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Res. 2010;70:5004–5013. doi: 10.1158/0008-5472.CAN-10-0066. [DOI] [PubMed] [Google Scholar]
- 7.Dahut WL, Scripture C, Posadas E, Jain L, Gulley JL, Arlen PM, Wright JJ, Yu Y, Cao L, Steinberg SM, Aragon-Ching JB, Venitz J, Jones E, Chen CC, Figg WD. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clin Cancer Res. 2008;14:209–214. doi: 10.1158/1078-0432.CCR-07-1355. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM TARGET Study Group. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Ricci S, Mazzaferro V. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 11.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 12.Gridelli C, Maione P, Del Gaizo F, Colantuoni G, Guerriero C, Ferrara C, Nicolella D, Comunale D, De Vita A, Rossi A. Sorafenib and sunitinib in the treatment of advanced non-small cell lung cancer. Oncologist. 2007;12:191–200. doi: 10.1634/theoncologist.12-2-191. [DOI] [PubMed] [Google Scholar]
- 13.Dal Lago L, D'Hondt V, Awada A. Selected combination therapy with sorafenib: a review of clinical data and perspectives in advanced solid tumors. Oncologist. 2008;13:845–858. doi: 10.1634/theoncologist.2007-0233. [DOI] [PubMed] [Google Scholar]
- 14.Amagai Y, Matsumoto M, Hojo K, Iguchi M, Wada T, Tanaka H, Ide N, Kato A, Shichijo M, Abe K. Combination therapy of interleukin-2 and sorafenib improves survival benefits and prevents spontaneous pulmonary metastasis in murine renal cell carcinoma models. Jpn J Clin Oncol. 2010;40:503–507. doi: 10.1093/jjco/hyp200. [DOI] [PubMed] [Google Scholar]
- 15.Azad NS, Posadas EM, Kwitkowski VE, Steinberg SM, Jain L, Annunziata CM, Minasian L, Sarosy G, Kotz HL, Premkumar A, Cao L, McNally D, Chow C, Chen HX, Wright JJ, Figg WD, Kohn EC. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol. 2008;26:3709–714. doi: 10.1200/JCO.2007.10.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arranz JA, Climent MA, González-Larriba JL, León L, Maroto JP. Sorafenib in renal cell carcinoma. Crit Rev Oncol Hematol. 2011 doi: 10.1016/j.critrevonc.2011.01.008. In press. [DOI] [PubMed] [Google Scholar]
- 17.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 18.Michael D, Oren M. The p53-Mdm2 module and the ubiquitin system. Semin Cancer Biol. 2003;13:49–58. doi: 10.1016/s1044-579x(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 19.Leone G, Voso MT, Sica S, Morosetti R, Pagano L. Therapy related leukemias: susceptibility, prevention and treatment. Leu Lymphoma. 2001;41:255–276. doi: 10.3109/10428190109057981. [DOI] [PubMed] [Google Scholar]
- 20.Smith MA, McCaffrey RP, Karp JE. The secondary leukemias: challenges and research directions. J Natl Cancer Inst. 1996;88:407–418. doi: 10.1093/jnci/88.7.407. [DOI] [PubMed] [Google Scholar]
- 21.Kubota M, Lin YW, Hamahata K, Sawada M, Koishi S, Hirota H. Cancer chemotherapy and somatic cell mutation. Mutat Res. 2000;470:93–102. doi: 10.1016/s1383-5742(00)00043-0. [DOI] [PubMed] [Google Scholar]
- 22.Shangary S, Wang S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol. 2009;49:223–241. doi: 10.1146/annurev.pharmtox.48.113006.094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vassilev LT. MDM2 inhibitors for cancer therapy. Trends Mol Med. 2007;13:23–31. doi: 10.1016/j.molmed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Kojima K, Konopleva M, McQueen T, O'Brien S, Plunkett W, Andreeff M. Mdm2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanisms and may overcome Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia. Blood. 2006;108:993–1000. doi: 10.1182/blood-2005-12-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu L, Zhu N, Findley HW, Zhou M. MDM2 antagonist nutlin-3 is a potent inducer of apoptosis in pediatric acute lymphoblastic leukemia cells with wild-type p53 and overexpression of MDM2. Leukemia. 2008;22:730–739. doi: 10.1038/leu.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, Myklebost O, Heimbrook DC, Vassilev LT. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci. 2006;103:1888–1893. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurova KV, Hill JE, Razorenova OV, Chumakov PM, Gudkov AV. p53 pathway in renal cell carcinoma is repressed by a dominant mechanism. Cancer Res. 2004;64:1951–1958. doi: 10.1158/0008-5472.can-03-1541. [DOI] [PubMed] [Google Scholar]
- 28.Haitel A, Wiener HG, Baethge U, Marberger M, Susani M. MDM2 Expression as a prognostic indicator in clear cell renal cell carcinoma: Comparison with p53 over-expression and clinic-pathological parameters. Clin Cancer Res. 2000;6:1840–1844. [PubMed] [Google Scholar]
- 29.Nagaprashantha LD, Vatsyayan R, Singhal J, Lelsani P, Prokai L, Awasthi S, Singhal SS. 2′-Hydroxyflavanone inhibits proliferation, tumor vascularization and promotes normal differentiation in VHL-mutant renal cell carcinoma. Carcinogenesis. 2011;32:568–575. doi: 10.1093/carcin/bgr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou TC, Talalay P. Quantitative analysis of dose effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1980;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 31.Maimets T, Neganova I, Armstrong L, Lako M. Activation of p53 by nutlin leads to rapid differentiation of human embryonic stem cells. Oncogene. 2008;27:5277–5287. doi: 10.1038/onc.2008.166. [DOI] [PubMed] [Google Scholar]
- 32.Tabe Y, Sebasigari D, Jin L, Rudelius M, Davies-Hill T, Miyake K, Miida T, Pittaluga S, Raffeld M. MDM2 antagonist nutlin-3 displays antiproliferative and proapoptotic activity in mantle cell lymphoma. Clin Cancer Res. 2009;15:933–942. doi: 10.1158/1078-0432.CCR-08-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kojima K, Konopleva M, Samudio IJ, Ruvolo V, Andreeff M. Mitogen-activated protein kinase kinase inhibition enhances nuclear proapoptotic function of p53 in acute myelogenous leukemia cells. Cancer Res. 2007;67:3210–3219. doi: 10.1158/0008-5472.CAN-06-2712. [DOI] [PubMed] [Google Scholar]
- 34.Huynh H, Lee JW, Chow PK, Ngo VC, Lew GB, Lam IW, Ong HS, Chung A, Soo KC. Sorafenib induces growth suppression in mouse models of gastrointestinal stromal tumor. Mol Cancer Ther. 2009;8:152–159. doi: 10.1158/1535-7163.MCT-08-0553. [DOI] [PubMed] [Google Scholar]
- 35.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 36.Haldar S, Negrini M, Monne M, Sabbioni S, Croce CM. Down-regulation of bcl-2 by p53 in breast cancer cells. Cancer Res. 1994;54:2095–2097. [PubMed] [Google Scholar]
- 37.Harn HJ, Ho LI, Liu CA, Liu GC, Lin FG, Lin JJ, Chang JY, Lee WH. Down regulation of bcl-2 by p53 in nasopharyngeal carcinoma and lack of detection of its specific t(14;18) chromosomal translocation in fixed tissues. Histopathology. 1996;28:317–323. doi: 10.1046/j.1365-2559.1996.d01-431.x. [DOI] [PubMed] [Google Scholar]
- 38.Fuhrken PG, Apostolidis PA, Lindsey S, Miller WM, Papoutsakis ET. Tumor suppressor protein p53 regulates megakaryocytic polyploidization and apoptosis. J Biol Chem. 2008;283:15589–15600. doi: 10.1074/jbc.M801923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang D, Wu D, Hirao A, Lahti JM, Liu L, Mazza B, Kidd VJ, Mak TW, Ingram AJ. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J Biol Chem. 2002;277:12710–12717. doi: 10.1074/jbc.M111598200. [DOI] [PubMed] [Google Scholar]
- 40.Chipuk JE, Bouchier-Hayes L, Kuwana T, Newmeyer DD, Green DR. PUMA couples the nuclear and cytoplasmic pro-apoptotic function of p53. Science. 2005;309:1732–1735. doi: 10.1126/science.1114297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.