Abstract
Leptomeningeal metastasis is a cause of morbidity and mortality in medulloblastoma, but the understanding of molecular mechanisms driving this process is nascent. In this study, we examined the secretory chemokine profile of medulloblastoma cells (DAOY) and a meningothelial cell line (BMEN1). Conditioned media (CM) of meningothelial cells increased adhesion, spreading and migration of medulloblastoma. VEGFA was identified at elevated levels in the CM from BMEN1 cells (as compared to DAOY CM); however, recombinant VEGFA alone was insufficient to enhance medulloblastoma cell migration. In addition, bevacizumab, the VEGFA scavenging monoclonal antibody, did not block the migratory phenotype induced by the CM. These results reveal that paracrine factors secreted by meningothelial cells can influence migration and adherence of medulloblastoma tumor cells, but VEGFA may not be a specific target for therapeutic intervention in this context.
Keywords: Medulloblastoma, VEGFA, Leptomeningeal Metastasis
Introduction
Medulloblastoma is the most common malignant primary brain tumor in children [1,2]. Despite overall effectiveness of therapy, metastasis to the leptomeninges represents a major treatment challenge [3,4]. Thus, an improved biological basis for therapy is needed to overcome this mortality and morbidity. The specialized microenvironment of the brain, well-isolated from the systemic circulation, presents a challenging yet interesting opportunity to identify the microenvironment-specific cues such as cytokines and growth factors stimulating active or passive movement of tumor cells away from the primary site and subsequent tumor cell adhesion to the leptomeninges [5,6].
Vascular endothelial growth factor (VEGF) is known to play a pivotal role in embryonic hematopoiesis and vasculogenesis, proliferation, migration and adhesion of endothelial cells [7] and is a mediator of tumor angiogenesis and metastasis in many malignancies [8,9]. The best characterized VEGF ligand is VEGFA that activates intracellular signaling via high affinity binding to cognate receptors VEGFR1 and VEGFR2. The FDA has approved several anti-angiogenic agents for use in combination with chemotherapy to treat multiple adult solid tumors. These agents have shown efficacy/equivocal efficacy for many adult cancers [10,11]; however, the use of such drugs are still investigational for childhood tumors. Medulloblastoma cells have been reported to express VEGF ligand and cognate receptors, VEGFR1 and 2 [12]. Furthermore elevated levels of VEGF in the cerebrospinal fluid (CSF) of medulloblastoma patients whose tumors exhibit leptomeningeal metastasis (LM) [13], as well as other brain tumors [14] and extra-axial tumors with leptomeningeal trophism [15] has been reported.
Due to the high propensity of medulloblastoma tumors for leptomeningeal dissemination, we tested the hypothesis that meningothelial cells play an active role in the migration and attachment of medulloblastoma cells. We investigated the extent to which medulloblastoma cells respond to autocrine and paracrine chemokine signals that may facilitate migration and attachment to meningothelial cells of the leptomeninges. Results demonstrate that chemokines and growth factors secreted by meningothelial cells robustly promote chemotactic migration and adhesion of the TP53-mutated and SHH-pathway activated DAOY medulloblastoma cells more than that induced by autocrine factors from DAOY cells [16]. Although VEGF was secreted at elevated level from the meningothelial cells, recombinant VEGFA did not induce migration in medulloblastoma cells; furthermore, the VEGFA neutralizing antibody, bevacizumab, did not inhibit tumor cell migration in vitro.
Materials and methods
Cell culture and conditioned media preparation
Human DAOY medulloblastoma cells harboring a C242F TP53 mutation was obtained from American Type Culture Collection (Manassas, VA) were cultured in MEM media containing 10% fetal bovine serum (FBS). Immortalized human benign meningothelial meningioma cells (BMEN1) [17] used here as a model of normal meningothelial cells were obtained from DSMZ (Braunschweig, Germany) were cultured in DMEM containing 20% FBS. To prepare the conditioned media (CM), 5 × 105 cells were grown until 75% confluent in a 10 cm dish and after washing with phosphate buffer saline (PBS), further incubated in 10 ml of serum free media. At 96 hours, the culture supernatant was collected and centrifuged to clear cellular debris and stored at −80°C until further use.
Immunohistochemistry
VEGFR2 (Flk-1) immunohistochemistry was detected by VEGF Receptor 2 mAb (#2479 Cell Signaling Technology, MA) on medulloblastoma tissue microarrays (block series 30000-30-P8156) from the Pediatric Cooperative Human Tissue Network/Children’s Oncology Group Biorepository (Columbus, OH). VEGFR1 (Flt-1) staining was performed with a rabbit mAb (#1303-1, Epitomics, CA). P14ARF staining was performed with a rabbit polyclonal antibody (#NB100-57549, Novus Biologics, CO). TP53 immunohistochemistry was performed on 4μm thick paraffin-embedded sections using a Discovery XT (Ventana Medical Systems, AZ) automated instrument with a biotin-free, multimer technology detection kit and conjugate (ChromoMap DAB Kit (760-159)/OmniMap anti-Ms HRP (760-4310), Ventana). For antigen retrieval, CC1 (950-124, Ventana) was used. The antibody was incubated for 1 hour at room temperature. The primary TP53 antibody was from Dako (clone DO-7, dilution 1:20). TP53 expression was scored based on percentage of positive lesional nuclei: 0 = 0%, 1+ = 1–25%, 2+ = 26–50%, and 3+ = >50%. Only nuclear staining was scored as positive.
Migration assay
DAOY cells, pre-stained with 5μM Calcein-AM, were suspended in serum free media and 2 × 104 cells added to the upper chamber of HTS FluoroBlok® transwell permeable inserts (Becton Dickinson, NJ). DAOY CM, BMEN1 CM and serum free media (SFM) or where indicated recombinant human VEGFA ligand (Human Vascular Endothelial Growth Factor-165 (hVEGF165, #8065, Cell Signaling Technology, MA) were used in the lower wells as chemoattractant. For blocking antibody experiments, bevacizumab or control humanized IgG were added to the BMEN1 CM at indicated concentrations and incubated at 37°C for 15 minutes prior to the transwell migration assay. After 18 hours, the migrated cells were imaged using EVOS™fl inverted fluorescence microscope (Advanced Microscopy Group, Bothell, WA). Twenty fields of view were randomly imaged from each transwell using 10x objective magnification and the number of migrated cells were counted using ImageJ software (NIH).
DAOY spreading assay
Glass coverslips were coated overnight with the SFM, BMEN1 or DAOY CM. Calcein-AM-labeled DAOY cells were harvested, washed and resuspended in SFM and 2 × 104 cells were seeded on the coverslips for 30 min at 37°C. The unattached cells were removed by aspiration and after washing with PBS, adhered cells were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton-X-100. Polymerized actin filaments were stained with Rhodamine-Phalloidin (1:1000). Stained cells were mounted on microscope slides with Prolong Gold® antifade reagent containing DAPI (Life Technologies, CA) and imaged using EVOS™fl microscope (Advanced Microscopy Group).
RAC1 Activation Assay
The GTP-RAC1 assay was performed as previously described [18] using a GST fusion protein of the CRIB domain of PAK1 (GST-PAK-CRIB) as bait to enrich activated GTP-bound RAC1. RAC1 was detected by immunoblotting with a monoclonal anti-RAC1 antibody (BD Transduction lab, CA).
Angiogenesis array and ELISA for VEGFA
The expression profiling of angiogenesis-related proteins in the BMEN1 CM and DAOY CM, using 1 ml of CM, was performed according to the manufacturer’s protocol (Human Angiogenesis Array Kit, R&D Systems, MN). Chemiluminescence was detected using IVIS Lumina II imaging system (Caliper Life Sciences, Hopkinton, MA). The level of VEGFA in BMEN1 CM or DAOY CM was quantitatively determined using a VEGFA ELISA kit from Thermo-Fisher (#EH2VEGF).
Western blotting
Immunoblotting was performed as previously described [19]. The following antibodies were used to detect VEGF receptors in human medulloblastoma cell lysates: VEGF Receptor 2 (Flk-1) (#2479, Cell Signaling Technology, MA); VEGFR1 (Flt-1) (#1303-1, Epitomics, CA); Neuropilin-2 (AP11578b, Abgent, CA).
Statistical Analysis
Where indicated non-parametric t-test or one-way ANOVA analysis (Microsoft Excel or GraphPad Prism) was used to determine the statistical significance and p ≤ 0.05 was deemed significant. Asterisks in figures and the description of asterisks in figure legends indicate level of statistical significance.
Results
Secretory proteins from the benign meningioma and DAOY cells increase migration of medulloblastoma cells
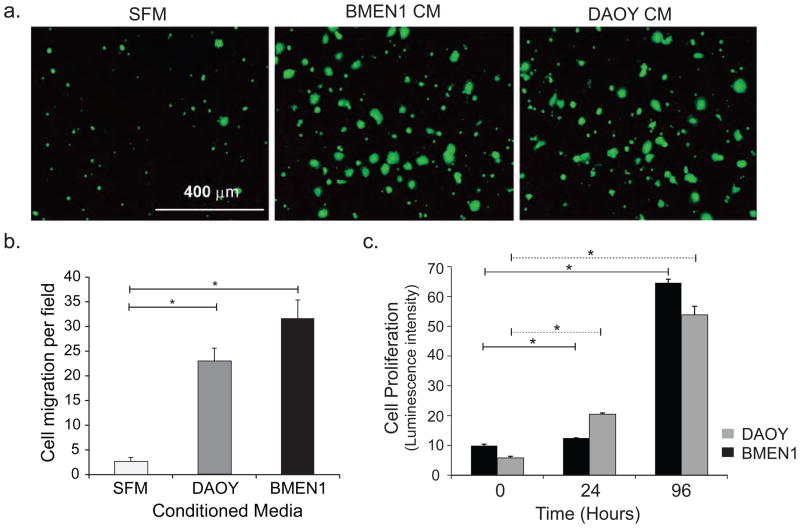
Tumor macro- and microenvironment can play important role in regulating multiple aspects of tumor progression [20]. Using conditioned media (CM) from the BMEN1 and DAOY cells, we observed that the migration of DAOY cells significantly increased (8–12 fold) when compared with basal migration in serum-free medium (SFM) (Fig. 1A, B). Notably, the secreted chemokines in the BMEN1 CM stimulated an additional four-fold increase in cell migration compared to the autocrine signals from DAOY CM. However, no difference was observed in the growth rates of BMEN1 or DAOY cells eliminating any influence of the CM on cell proliferation (Fig. 1C).
Fig. 1. Secretory proteins from BMEN1 meningothelial and DAOY medulloblastoma cells increase migration of DAOY cells.
(A) Representative images showing Calcein-AM-labeled migrated DAOY cells in response to BMEN1 and DAOY CM and SFM used as chemoattractant. N=4. (B) Number of migrated cells from twenty non-overlapping fields quantified using Image J software. Bars show average ± SEM. N=4, *p <0.05 (C) The proliferation of BMEN1 and DAOY cells was assessed with Cell-Titer-Glo® reagent. Solid line - BMEN1, dashed line - DAOY cells. Bars show average ± SEM. N=3, *p <0.05.
Conditioned media increases adhesion of medulloblastoma cells
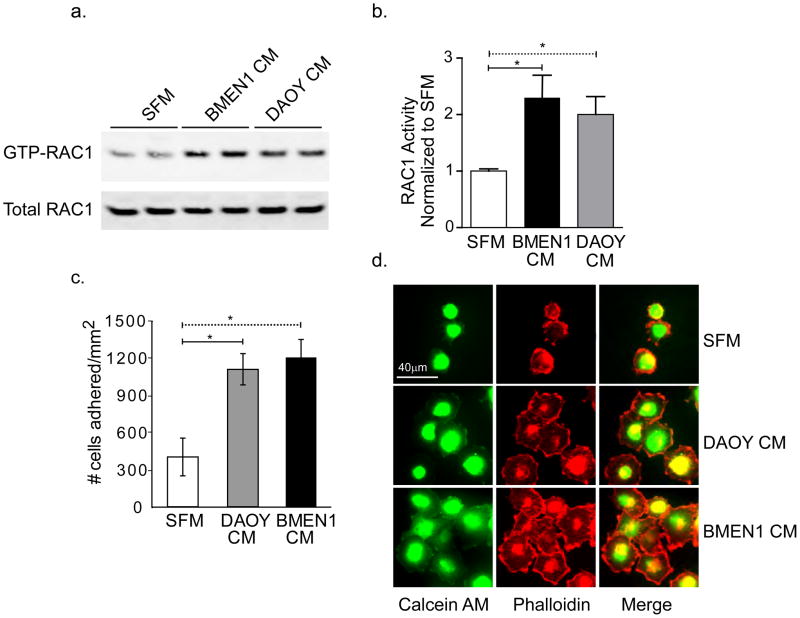
The RHO-family GTPases are small G-proteins that are essential modulators of cellular cytoskeletal networks. The overexpression of RAC1, a member of the RHO protein super-family, has been previously shown in DAOY cells and in medulloblastoma tumors and RAC1 activity is known to functionally regulate DAOY cell migration [21,22]. Our result shows significant activation of RAC1 when DAOY cells are stimulated with BMEN1 CM and to a lesser degree with DAOY CM (Fig. 2A &B).
Fig. 2. Conditioned media from BMEN1 cells enhances DAOY cell adhesion.
(A) Representative immunoblot showing RAC1 immunoreactivity. Activated RAC1 (GTP-RAC1) and the total RAC1 iare shown in the top and bottom blots respectively. (B) Quantification of relative RAC1 activity in DAOY cells after stimulation with BMEN1 CM and DAOY CM. The ratio of GTP-RAC1 and total RAC1 immunoreactivity from cells treated with SFM was set to 1. The remaining data were normalized to SFM. N=4, *p <0.05; Bars show average ± SEM. (C) Quantification of DAOY cell adhesion is described in Results and Methods. N=4, *p <0.05; Bars show average ± SEM. (D) Representative images of Calcein-AM labeled-DAOY cells stained with Alexa Fluor® 546 Phalloidin to label the polymerized actin cytoskeleton (63X magnification). Scale bar = 40 μm.
Next, we observed that increased number of DAOY cells adhered to coverslips coated with BMEN1 or DAOY CM as compared to serum free medium (Fig. 2C & Supplementary Fig. A). Consistent with the migration assay, a modest increase in cell adhesion was present with BMEN1 CM than DAOY CM. Further; DAOY cells that were plated on glass surface coated with BMEN1 and DAOY CM exhibited highly prominent lamellipodial extensions that are enriched in stable polymerized actin filaments. In contrast, the number of adhered DAOY cells, the extent of cell spreading and lamellipodial extensions were minimal in SFM (Fig. 2D).
Comparative screening of growth factors secreted from DAOY or BMEN1 cells
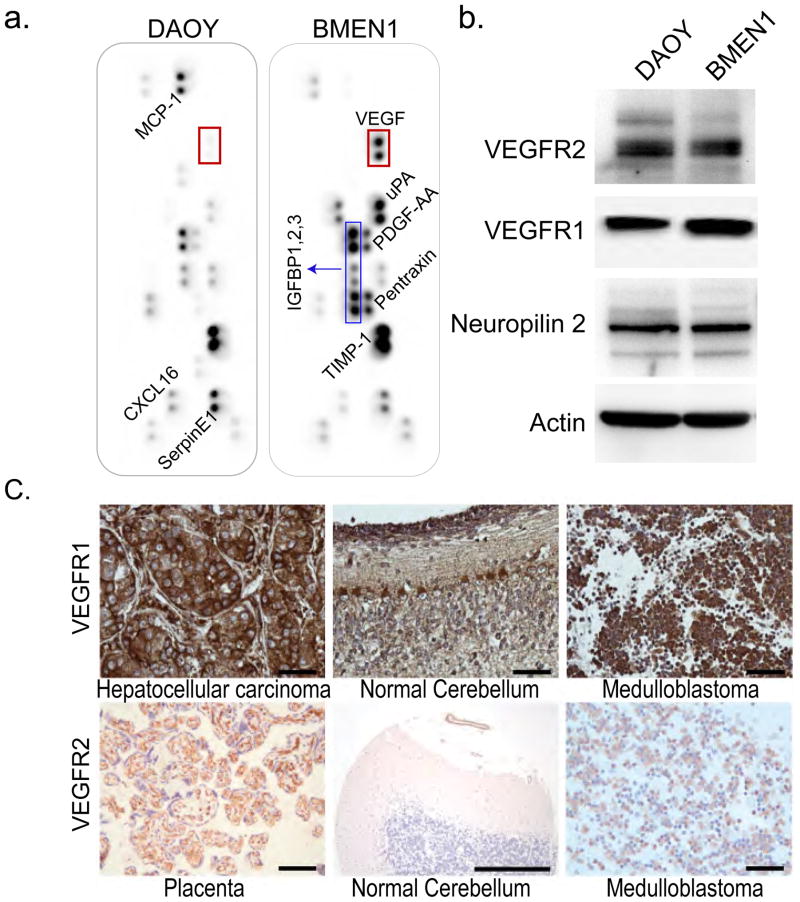
To determine the identity of the secreted factors in the BMEN1 and DAOY CM, we examined the relative level of 55 angiogenesis-related proteins using hybridization array panels. As shown in Figure 3a, although the level of selective proteins such as insulin-like growth factor binding proteins -1, -2, and -3, TIMP-1 and SerpinE1 is elevated in both BMEN1 and DAOY CM, a striking increase in VEGFA was found in BMEN1 CM compared to DAOY CM. Using an ELISA assay, we quantitatively assessed the level of VEGFA in both BMEN1 CM and DAOY CM as compared to 10% FBS containing growth medium and SFM. In accordance with the qualitative data from the angiogenesis array, we found the levels of VEGFA to be higher in BMEN1 cells (875.8 ± 97.1 pg/ml) as compared to DAOY CM (28 pg/ml). Growth media (with 10% FBS) and SFM had no detectable VEGFA (assay sensitivity range of 32.5 to 2000 pg/ml). The role of VEGF in the proliferation and angiogenesis of many tumor cell types has been well studied; however, the role of VEGF as a chemoattractant in the leptomeningeal metastasis of medulloblastoma is not known. Therefore, we focused on the role of VEGF in chemotaxis and adhesion of medulloblastoma cells. We examined the expression of VEGFR2, VEGFR1 or the VEGFR co-receptor Neuropilin2, receptors for the ligand VEGF, in DAOY and BMEN1 cells (Fig. 3B). The DAOY as well as BMEN1 cells expressed both receptors for the VEGFA ligand, VEGFR1 and VEGFR2 as well as the VEGFR co-receptor, neuropillin2. To determine the clinical relevance of VEGFR expression, we next examined the prevalence of VEGFR2 and VEGFR1 expression in human medulloblastoma by immunohistochemistry of tissue microarrays. VEGFR1 was more strongly expressed in the human medulloblastoma tumor samples with 41 out of 45 medulloblastoma sections staining strongly for VEGFR1 but only 3 cases staining weakly for VEGFR2 with no apparent relationship to p14ARF/P53 status (Fig. 3C, Supplementary Fig. D, and Supplementary Table 1).
Fig. 3. Profiling angiogenesis-related proteins in the conditioned media from BMEN1 and DAOY cells.
(A) The expression level of 55 secretory proteins in CM from BMEN1 and DAOY cells was examined in pairs using angiogenesis antibody arrays. The red box in both panels shows location of VEGFA signal. The image is representative of two independent experiments. (B) Representative western blot images examining expression of VEGFR1, VEGFR2, Neuropilin 2 and Tubulin from DAOY and BMEN1 meningioma cells. (C) Representative images showing immunohistochemical staining for VEGFR1 and VEGFR2 from human medulloblastoma tissue microarray. Hepatocellular carcinoma and placenta were used as positive and the normal cerebellum as positive and negative controls, respectively. Scale bar = 50 μm.
Recombinant vascular endothelial growth factor activity alone does not regulate the migration of DAOY cells
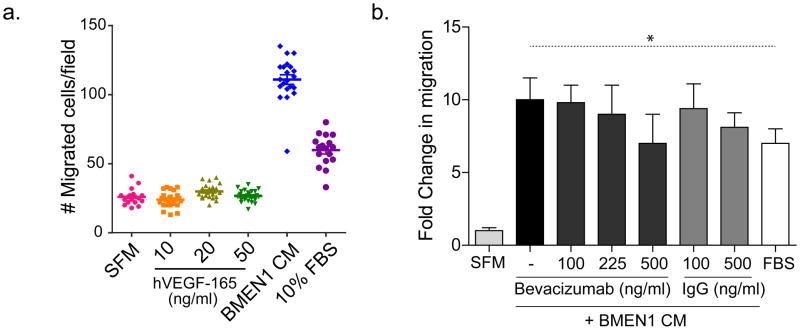
Since VEGFA ligand expression is higher in BMEN1 CM compared to DAOY CM and both VEGFR1 and VEGFR2 are expressed in DAOY cells, we postulated that VEGFA might be a functionally relevant chemoattractant for medulloblastoma cells. We tested this hypothesis by examining the relative migration of DAOY cells in response to recombinant human VEGFA (hVEGF-165) as compared with BMEN1 CM. In contrast to our hypothesis, no detectable increase in cell migration was observed when DAOY cells were incubated with increasing concentration of recombinant hVEGF-165 (Fig. 4A; Supplementary Fig. B). As observed before, DAOY cells exhibited significant cell migration when incubated with BMEN1 CM or 10% fetal bovine serum-containing growth media that serve as an internal positive control, confirming that the DAOY cells were viable and responsive to chemokines. Additionally, recombinant hVEGF-165 did not significantly increase DAOY cell proliferation as measured by mean calcein AM fluorescence intensity (Supplementary Fig. C). To further examine the role of VEGFA in inducing migration of DAOY cells in response to the BMEN1 CM, we used bevacizumab, a selective VEGFA-binding antibody, to sequester bioavailable VEGFA from the CM in the transwell migration assays. Bevacizumab doses ranging from 100 ng/ml (0.66nM) to 500ng/ml (3.3nM) had no significant inhibitory effect on the ability of BMEN1 CM to induce DAOY cells migration (Fig. 4B). Based on quantitative ELISA, the BMEN1 CM contained 1100 pg/ml VEGFA (0.026nM). The amounts of bevacizumab used (100, 225 or 500ng/ml) were 25- to 125-fold in excess of VEGFA, hence the lack of suppression of DAOY cell migration by bevacizumab is unlikely to be due to inefficient ligand-sequestration. Therefore, these data conclusively demonstrate that the ligand VEGF alone is insufficient to account for the pronounced effects on DAOY cell migration and growth that are observed when these cells are incubated with BMEN1 CM.
Fig. 4. Treatment with recombinant hVEGF-165 or VEGF-blocking antibody bevacizumab does not affect BMEN1 CM-induced DAOY cell migration.
(A) Quantification of DAOY cell migration assay as described in Methods. Each symbol in the scatter plots represents an individual field of cells. (B) Quantification of the effect of bevacizumab or humanized IgG on DAOY cell migration induced by BMEN1 CM. The graph shows fold change in migration where median migration data from test conditions are normalized to the number of cells undergoing basal migration in SFM. Bars show average ± SEM; N = 4. * indicates p < 0.05.
Discussion
Micro-metastatic seeding of medulloblastoma tumor cells on the leptomeninges significantly worsens patient prognosis and is a primary reason for the use of craniospinal radiation that frequently leaves surviving patients with life-long neurocognitive sequelae. We aimed to inform adjuvant targeted therapy selection by identifying chemokines that augment the migration and adhesion of tumor cells to the leptomeninges.
The robust increase in the DAOY cell migration in response to BMEN1 CM suggests a potential mechanism wherein chemokines or growth factors secreted by meningothelial cells stimulate the migration of medulloblastoma cells in a paracrine manner whereas tumor cells can respond to secretory proteins in both autocrine and paracrine fashion to complement survival as well as migration. These data suggest that both autocrine (DAOY CM) and paracrine (BMEN1 CM) secreted factors may stabilize DAOY cells adhesion to novel attachment sites. Additionally, the rare population of medulloblastoma cells that may escape tumor niche due to intrinsic genetic propensity for metastatic dissemination will be subject to a very low concentration of autocrine secreted factors due to the substantial dilution in CSF. In contrast, the metastatic tumor cells in the CSF may encounter the meningeal microenvironment wherein the relative concentration of the cytokine or growth factor(s) secreted by the meningeal cells is likely to induce robust chemoattraction and adhesion.
Using an antibody array and quantitative ELISA assay, we identified significantly higher level of VEGFA secreted from the BMEN1 cells, a model for the meningothelial cells, than from DAOY medulloblastoma cells, which express both VEGFR1 and VEGFR2. These results suggested a role for VEGFA in facilitating a paracrine relationship between meningothelial cells and metastatic medulloblastoma cells. However, functional reconstitution as well as blocking experiments reveals that recombinant VEGFA alone does not stimulate the chemotactic migration of medulloblastoma cells as compared to BMEN1 CM. These data suggest that VEGFA is not a predominant or required growth factor regulating the migration to or stable adhesion of medulloblastoma cells to the meningeal cell layer.
In light of these observations, the use of the VEGF signaling antagonist, bevacizumab, as a clinical agent to prevent establishment or progression of leptomeningeal metastasis of Shh+ medulloblastoma might be called into question. Despite the intermittent compassionate use of bevacizumab in progressive metastatic medulloblastoma, few clinical case histories appear in the literature. In one report, however, a patient with established (M3) medulloblastoma treated with bevacizumab and irinotecan for 6 months experienced new thoracic and thecal sac LM metastases [23]. Since this patient’s disease progressed latently, it is difficult to classify this case as either a success or a failure. Similarly, the value of VEGF inhibition to decrease tumor progression once metastases are established is yet to be determined in a clinical trial setting.
Our results cannot rule out that bevacizumab may still have a role in metastatic medulloblastoma if tumors are already established on the leptomeninges. This hypothesis is based on the assumption that VEGFR2 or VEGFR1 may have roles in medulloblastoma cell growth that are independent of angiogenesis, given that VEGFR inhibition using a multikinase small molecule has been previously shown to inhibit DAOY proliferation [12].
Recent complementary studies implicate the Neuropilin-1 receptor, but not VEGFR1, as the receptor for placental growth factor (PLGF) and as a determinant of medulloblastoma tumor growth and metastasis in human and murine medulloblastoma [24]. PLGF was not present at a detectable level in our experiments. However, both studies observe robust expression of VEGFR1, and the functional role of prevalent VEGFR1 expression in medulloblastoma remains to be determined.
Supplementary Material
Immunohistochemistry of a Human Medulloblastoma Tissue Microarray. Table shows clinical and pathological data associated with each tumor sample and the relative expressed of VEGFR1, VEGFR2, p53 and p14ARF as scored by a clinical neuropathologist.
(A) Representative images of Calcein-AM labeled-DAOY cells adhered to glass coverslips that are coated with SFM, BMEN1 CM or DAOY CM. Scale bar is 30 μm. (B) Representative images of migrated Calcein-AM-labeled DAOY cells after incubation with recombinant VEGF (10, 20, 50 ng/ml), BMEN1 CM and 10% FBS media (growth media). Scale bar is 100 μm. * indicates p < 0.05 (C) Relative DAOY cell growth and viability. Fluorescent reading of Calcein AM-labeled DAOY cells measured at 18 hours post incubation in indicated media. Graph shows average ± SEM from six independent wells. (D) The representative image shows immunohistochemical staining of TP53 and P14ARF in human medulloblastoma tissues. Scale bar = 50 μm.
Highlights.
Chemokines from meningothelial cells enhance the migration of medulloblastoma cells.
Autocrine and paracrine chemokines are essential for the spreading of DAOY cells.
VEGF is secreted at high levels from BMEN1 cells but alone fails to enhance DAOY migration.
Bevacizumab, an anti-VEGF antibody, does not block migration of DAOY cells in vitro.
Targeting VEGFA alone may be insufficient to inhibit the leptomeningeal metastases.
Acknowledgments
This work was supported in part by a pilot research grant funds from the 2P30CA069533-14 and funds provided to the Knight Cancer Institute at Oregon Health Sciences University by the Schnitzer Investment Corporation. Key equipment was made possible by gifts from the Kyla McCullough Gift Fund.
Footnotes
Conflict of Interest Statement
Author CK has received honoraria for scientific presentations at Novartis, Millennium/Takeda Pharmaceutical and GlaxoSmithKline, and has non-sponsored research collaborations with Novartis, Regeneron and Vasgene Therapeutics. CK is also a paid consultant to the NCI/CTEP Pediatric Preclinical Testing Program (PPTP). The remaining authors have no other conflicts to declare related to these studies.
Author Contributions
Overall conception and design: C. Keller, M. Davare
Analysis and interpretation of experimental data: C. Keller, J. Peckham, M. Davare, S. Lal, S. Prajapati, S. Gultekin, B. Rubin
Development of experimental methodology: C. Keller, M. Davare, S. Lal
- Figure 1A (S. Lal, M. Davare) 1B–C (S. Lal)
- Figure 2A–D (M. Davare)
- Figure 3A–C (S. Lal)
- Figure 4A–B and Supplemental Figure A–C (M. Davare)
- Supplementary Figure D (B. Rubin)
- Supplementary Table S1 (S. Lal and S. Gultekin)
Writing, review, and/or revision of the manuscript: C. Keller, M. Davare, S. Lal, B. Rubin, J. Peckham Study supervision: C. Keller
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fruhwald MC, Plass C. Metastatic medulloblastoma--therapeutic success through molecular target identification? Pharmacogenomics J. 2002;2:7–10. doi: 10.1038/sj.tpj.6500077. [DOI] [PubMed] [Google Scholar]
- 2.Partap S, Curran EK, Propp JM, LGM, Sainani KL, Fisher PG. Medulloblastoma incidence has not changed over time: a CBTRUS study. J Pediatr Hematol Oncol. 2009;31:970–971. doi: 10.1097/MPH.0b013e3181bbc502. [DOI] [PubMed] [Google Scholar]
- 3.Korah MP, Esiashvili N, Mazewski CM, Hudgins RJ, Tighiouart M, Janss AJ, Schwaibold FP, Crocker IR, Curran WJ, Jr, Marcus RB., Jr Incidence, risks, and sequelae of posterior fossa syndrome in pediatric medulloblastoma. Int J Radiat Oncol Biol Phys. 2010;77:106–112. doi: 10.1016/j.ijrobp.2009.04.058. [DOI] [PubMed] [Google Scholar]
- 4.Dufour C, Beaugrand A, Pizer B, Micheli J, Aubelle MS, Fourcade A, Couanet D, Laplanche A, Kalifa C, Grill J. Metastatic Medulloblastoma in Childhood: Chang’s Classification Revisited. Int J Surg Oncol. 2012;2012:245385. doi: 10.1155/2012/245385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culig Z. Cytokine disbalance in common human cancers. Biochim Biophys Acta. 2011;1813:308–314. doi: 10.1016/j.bbamcr.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Sciume G, Santoni A, Bernardini G. Chemokines and glioma: invasion and more. J Neuroimmunol. 2010;224:8–12. doi: 10.1016/j.jneuroim.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Ng YS, Rohan R, Sunday ME, Demello DE, D’Amore PA. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn. 2001;220:112–121. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1093>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Brastianos PK, Batchelor TT. VEGF inhibitors in brain tumors. Clin Adv Hematol Oncol. 2009;7:753–760. 768. [PubMed] [Google Scholar]
- 9.Shen S, Fan J, Cai B, Lv Y, Zeng M, Hao Y, Giancotti FG, Fu BM. Vascular endothelial growth factor enhances cancer cell adhesion to microvascular endothelium in vivo. Exp Physiol. 2010;95:369–379. doi: 10.1113/expphysiol.2009.050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamberlain MC. Bevacizumab for the treatment of recurrent glioblastoma. Clin Med Insights Oncol. 2011;5:117–129. doi: 10.4137/CMO.S7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, du Bois A, Sehouli J, Kimmig R, Stahle A, Collinson F, Essapen S, Gourley C, Lortholary A, Selle F, Mirza MR, Leminen A, Plante M, Stark D, Qian W, Parmar MK, Oza AM. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 12.Slongo ML, Molena B, Brunati AM, Frasson M, Gardiman M, Carli M, Perilongo G, Rosolen A, Onisto M. Functional VEGF and VEGF receptors are expressed in human medulloblastomas. Neuro Oncol. 2007;9:384–392. doi: 10.1215/15228517-2007-032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stockhammer G, Poewe W, Burgstaller S, Deisenhammer F, Muigg A, Kiechl S, Schmutzhard E, Maier H, Felber S, Schumacher P, Gunsilius E, Gastl G. Vascular endothelial growth factor in CSF: a biological marker for carcinomatous meningitis. Neurology. 2000;54:1670–1676. doi: 10.1212/wnl.54.8.1670. [DOI] [PubMed] [Google Scholar]
- 14.Herrlinger U, Wiendl H, Renninger M, Forschler H, Dichgans J, Weller M. Vascular endothelial growth factor (VEGF) in leptomeningeal metastasis: diagnostic and prognostic value. Br J Cancer. 2004;91:219–224. doi: 10.1038/sj.bjc.6601953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Langerijt B, Gijtenbeek JM, de Reus HP, Sweep FC, Geurts-Moespot A, Hendriks JC, Kappelle AC, Verbeek MM. CSF levels of growth factors and plasminogen activators in leptomeningeal metastases. Neurology. 2006;67:114–119. doi: 10.1212/01.wnl.0000223348.42106.97. [DOI] [PubMed] [Google Scholar]
- 16.Jacobsen PF, Jenkyn DJ, Papadimitriou JM. Establishment of a human medulloblastoma cell line and its heterotransplantation into nude mice. J Neuropathol Exp Neurol. 1985;44:472–485. doi: 10.1097/00005072-198509000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Puttmann S, Senner V, Braune S, Hillmann B, Exeler R, Rickert CH, Paulus W. Establishment of a benign meningioma cell line by hTERT-mediated immortalization. Lab Invest. 2005;85:1163–1171. doi: 10.1038/labinvest.3700307. [DOI] [PubMed] [Google Scholar]
- 18.Davare MA, Saneyoshi T, Soderling TR. Calmodulin-kinases regulate basal and estrogen stimulated medulloblastoma migration via Rac1. J Neurooncol. 2011;104:65–82. doi: 10.1007/s11060-010-0472-6. [DOI] [PubMed] [Google Scholar]
- 19.Ohshima-Hosoyama S, Hosoyama T, Nelon LD, Keller C. IGF-1 receptor inhibition by picropodophyllin in medulloblastoma. Biochem Biophys Res Commun. 2010;399:727–732. doi: 10.1016/j.bbrc.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Chen B, Gao Y, Jiang T, Ding J, Zeng Y, Xu R, Jiang X. Inhibition of tumor cell migration and invasion through knockdown of Rac1 expression in medulloblastoma cells. Cell Mol Neurobiol. 2011;31:251–257. doi: 10.1007/s10571-010-9615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zavarella S, Nakada M, Belverud S, Coniglio SJ, Chan A, Mittler MA, Schneider SJ, Symons M. Role of Rac1-regulated signaling in medulloblastoma invasion. Laboratory investigation. J Neurosurg Pediatr. 2009;4:97–104. doi: 10.3171/2009.4.PEDS08322. [DOI] [PubMed] [Google Scholar]
- 23.Aguilera DG, Goldman S, Fangusaro J. Bevacizumab and irinotecan in the treatment of children with recurrent/refractory medulloblastoma. Pediatr Blood Cancer. 2011;56:491–494. doi: 10.1002/pbc.22868. [DOI] [PubMed] [Google Scholar]
- 24.Snuderl M, Batista A, Kirkpatrick ND, Ruiz de Almodovar C, Riedemann L, Walsh EC, Anolik R, Huang Y, Martin JD, Kamoun W, Knevels E, Schmidt T, Farrar CT, Vakoc BJ, Mohan N, Chung E, Roberge S, Peterson T, Bais C, Zhelyazkova BH, Yip S, Hasselblatt M, Rossig C, Niemeyer E, Ferrara N, Klagsbrun M, Duda DG, Fukumura D, Xu L, Carmeliet P, Jain RK. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell. 2013;152:1065–1076. doi: 10.1016/j.cell.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, Rutka JT, Pfister S, Taylor MD. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabori U, Baskin B, Shago M, Alon N, Taylor MD, Ray PN, Bouffet E, Malkin D, Hawkins C. Universal poor survival in children with medulloblastoma harboring somatic TP53 mutations. J Clin Oncol. 2010;28:1345–1350. doi: 10.1200/JCO.2009.23.5952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunohistochemistry of a Human Medulloblastoma Tissue Microarray. Table shows clinical and pathological data associated with each tumor sample and the relative expressed of VEGFR1, VEGFR2, p53 and p14ARF as scored by a clinical neuropathologist.
(A) Representative images of Calcein-AM labeled-DAOY cells adhered to glass coverslips that are coated with SFM, BMEN1 CM or DAOY CM. Scale bar is 30 μm. (B) Representative images of migrated Calcein-AM-labeled DAOY cells after incubation with recombinant VEGF (10, 20, 50 ng/ml), BMEN1 CM and 10% FBS media (growth media). Scale bar is 100 μm. * indicates p < 0.05 (C) Relative DAOY cell growth and viability. Fluorescent reading of Calcein AM-labeled DAOY cells measured at 18 hours post incubation in indicated media. Graph shows average ± SEM from six independent wells. (D) The representative image shows immunohistochemical staining of TP53 and P14ARF in human medulloblastoma tissues. Scale bar = 50 μm.