Abstract
Preterm birth is the major cause of neonatal mortality and morbidity, and bacterial infections that ascend from the lower female reproductive tract (FRT) are the most common route of uterine infection leading to preterm birth. The uterus and growing fetus are protected from ascending infection by the cervix, which controls and limits microbial access by the production of mucus, cytokines and anti-microbial peptides (AMPs). If this barrier is compromised, bacteria may enter the uterine cavity leading to preterm birth. Using a mouse model, we demonstrate, for the first time, that viral infection of the cervix, during pregnancy, reduces the capacity of the FRT to prevent bacterial infection of the uterus. This is due to differences in susceptibility of the cervix to infection by virus during pregnancy and the associated changes in TLR and AMP expression and function. We suggest that preterm labor is a polymicrobial disease, which requires a multifactorial approach for its prevention and treatment.
Introduction
Preterm birth is the major cause of neonatal mortality and morbidity worldwide, yet the underlying etiologies remain poorly understood (1, 2). Preterm labor is a syndrome diagnosed in the presence of increased uterine contractility, cervical ripening, and/or membrane rupture or activation, which may occur in response to multiple pathological processes(1, 2). Neonates born with a fetal inflammatory response are more likely to develop short- and long-term complications above those expected for the gestational age at birth (3–5).
Numerous studies suggest that intrauterine infection is an important mechanism leading to preterm labor and may account for 40% of preterm births (6–8). However, this number may be higher because many infections are likely to be subclinical and the pathogenesis is not detected due to the lack of sensitivity of conventional culture techniques(9) (10). Furthermore, our understanding of the normal flora of the female reproductive tract (FRT) is limited, and we have limited knowledge of the mechanisms controlling pathogenic bacteria and their relationship with intrauterine infection and preterm labor (11, 12).
All women have microorganisms in the lower genital tract (vulva, vagina and cervix), however most studies indicate that amniotic fluid is normally sterile and does not contain microbial products such as endotoxin. During pregnancy, intrauterine infections begin in the decidua, extend to the amnion and chorion and finally reach the amniotic cavity and the fetus (13). Bacteria gain access to gestational tissues through one of three major routes: by ascending into the uterus from the lower tract, descending into the uterus from the peritoneal cavity or via the maternal circulation (14) (6). Bacterial infections that ascend from the lower FRT are the most common route of uterine infection, and it is not known why some women suffer such infections that threaten pregnancies and fetal survival (15). In a healthy pregnancy, the uterus and growing fetus are protected from ascending infection by the cervix (12, 16) (17). The cervix has a unique role in the FRT in that it actively controls and limits microbial access by the production of mucus, inflammatory cytokines and anti-microbial peptides (AMP)(18) (17). In non-pregnant women, the cervical mucus is a viscous fluid in the endocervical canal; however after conception the endocervical canal develops a structure called the cervical mucus plug, which is an anatomical and immunological barrier against ascending infection(19). Indeed, analysis of the composition and antimicrobial activity of the cervical mucus plug revealed the presence of AMPs with potent anti-microbial activity (20). Also expressed in the cervical epithelia are the pattern recognition receptors such as Toll like receptors (TLRs) capable of sensing the presence of microorganisms and eliciting an innate immune response characterized by the production of cytokines and AMPs (21, 22) (23, 24). Collectively, the cervix plays a key role in the protection against ascending intraamniotic infection. If the mucus plug is expelled or cervical length is short, the risk of ascending uterine infection increases.
Herpesviruses are the most common cause of viral-related perinatal neurologic injury in the USA (25). However, herpes simplex viruses (HSV-1 and HSV-2) and cytomegalovirus (CMV) (26) are among the eight known human herpesviruses reported to induce adverse pregnancy and neonatal outcomes and have been found in the amnion, placenta and even the lower reproductive tract of pregnant women(27). Murine gammaherpesvirus 68 (Murid herpesvirus 4 (NC_001826.2); MHV-68) is a gammaherpesvirus of rodents that shares significant genomic co-linearity with two human pathogens, Epstein-Barr virus (EBV) and Kaposi’s sarcoma-associated herpesvirus (KSHV) (28).
Viral survival depends on their capacity to disable host defenses, especially the innate immune system, establish latency and secure mechanism for reactivation. One way in which viruses could undermine host immunity is through the manipulation of innate immune receptors such as TLRs. We have previously reported the use of MHV68 as a murine model to determine whether a subclinical viral infection sensitized the mother to other microorganisms and induced preterm birth (29). Our published data suggested that bacteria or virus alone were not enough to evoke preterm labor, but the combination was a threat. HSV and CMV have latency periods and can be reactivated via TLR signaling (30), therefore it can be postulated that they could affect pregnant women the way MHV68 affects pregnant mice in our model.
We have now tested the hypothesis that a viral infection reduces the ability of the pregnant cervix to protect against ascending bacterial infection. Here we show a dramatic difference in the capacity of the cervix to prevent ascending intrauterine bacterial infection in non-pregnant and pregnant mice and furthermore, we demonstrate that viral infection compromises the nature of the innate immune response of the pregnant cervix predisposing to ascending intrauterine infection. Our results may explain the differential sensitivity observed in pregnant women to ascending bacterial infections.
Materials and Methods
Animals and treatments
C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME); adult mice (8–12 wks of age) with vaginal plugs were infected i.p. at embryonic day 8.5 post-conception with either 1×106 PFU MHV68 in 200 ul or DMEM (vehicle). To develop the ascending bacteria model, E. coli (BL21) expressing RFP induced by IPTG and arabinose (pZS2, Addgene plasmid 26598, Michael Elowitz) were collected after reaching an O.D.=.6, and resuspended gently in 40 uL of PBS. This was delivered in the vagina of mice sedated with isoflourane using a 200 uL gel tip and imaging was performed after 24hr using the Carestream In Vivo Imaging System FX PRO (Bruker Coorporation). Lymphoid aggregates were dissected from the stratum basalis after gently removing the implantation site. For experiments determining gene expression and MHV68 titers in cervix without hormone treatments, mice were sacrificed 7d post-infection (dpi) and organs were removed and stored at −80°C. In experiments comparing non-pregnant and pregnant mice, non-pregnant mice in diestrus were used. This was determined by morphology of the reproductive tract at time of sacrifice. For experiments determining the effect of systemic hormones, mice were ovariectomized, and after 21d treated with progesterone (500ug) and estrogen (500ng) or vehicle, s.c. for 3d. Both treatment groups then received injections of MHV68 and continued to get hormone or vehicle every 2d for 7d. Cervix and spleen were then collected and MHV68 infection was determined by qPCR. All animals were maintained in the Yale University School of Medicine Animal Facility under specific pathogen-free conditions and all procedures reported in this manuscript were approved by the Yale University Institutional Animal Care and Use Committee. All in vivo experiments used between 3 and 6 mice in at least two separate independent experiments.
Mycoplasma
Ureaplasma urealyticum was purchased (27618) and reconstituted in ATCC media #2616, special modified formulation, as indicated by ATCC. Serial dilutions of bacteria were made and incubated at 37C until medium changed color, indicating growth. At time of color change, individual tubes were stored at 4C until remaining dilutions exhibited growth. After 12h, bacteria were gently pelleted and resuspended in growth medium. A small aliquot was then used to determine colony-forming units (1×104/mL), while the remaining bacteria were injected intravaginally into mice at pregnancy E16.5 with or without MHV68 infection (MHV68 at d8.5).
Cell culture
Immortalized human ectocervical cells (ECT1, ATCC, CRL-2614) were cultured in keratinocyte serum free medium (17005-042, Gibco, Grand Island, NY) with bovine pituitary extract and hEGF supplementation as recommended by ATCC under 5% CO2 at 37°C. In blocking studies, 500 ug of Fibronectin (Gibco) was added to ECT1 cells for 30 min and removed. Cells were then infected with MHV68 for 30 min, washed and maintained in medium for 24 hours. To block integrin alpha 3, the same protocol was used but with blocking antibodies for alpha 3 (P1B5, Millipore; all in vitro experiments were repeated 3 times.
MHV68 production and quantification
MHV68 expressing GFP was passaged in NIH 3T3 cells with DMEM plus 10% FBS. After lysis, supernatants were harvested, filtered (0.45 uM pore) and titered by 2-fold serial dilutions. To detect viral titers in mice, tissues were homogenized and approximately 25 mg of tissue from the reproductive tract or 10 mg of spleen were cut into small pieces and added to lysis buffer supplemented with proteinase K. Samples were incubated at 56C with shaking for 4–6 hours as recommended for the DNeasy blood and tissue kit (Qiagen, Valencia, CA). Cells from culture were lysed with the same buffer and vortexed at room temperature. All samples were then processed according to the DNeasy protocol. DNA concentration and purity was assessed using spectrophotometric analyses of 260/280 and 260/230 ratios. 100ng total DNA was then assayed using primers directed against MHV68 ORF53 and compared to a standard curve created using serial dilutions of purified virus. Results are reported as copies per 100ng DNA.
RNA synthesis, cDNA synthesis and qPCR
RNA was extracted using the Trizol method (Invitrogen, Carlsbad, CA). RNA concentration and purity was assessed using spectrophotometric analyses of 260/280 ratios and only samples with values of 1.7 or higher were used for PCR analysis. For quantitative analysis of mRNA, 1 ug of RNA was reverse transcribed for each sample using oligo (d)T priming and Verso cDNA kit which includes a DNAse enzyme (Invitrogen). Syber green master mix (KAPA Biosystems) and gene specific primers were added to the RT reactions that were diluted 1:10 with nuclease free water and run on the CFX96, C1000 system qPCR machine (Biorad). No RT controls were used to confirm that values did not represent amplification of genomic DNA, and no template controls were used to confirm lack of contamination by any reagents. Values represented were normalized to beta actin and were calculated using the delta delta Ct method: delta delta Ct= delta ct treated- delta Ct control; results expressed as fold differences are 2ˆ-(average delta delta Ct) for negative delta delta Ct values or – (2ˆ[average delta delta Ct]) for positive delta delta Ct values.
Cytokines
Cytokine concentrations were determined using cytokine multiplex assays from Bio-Rad. Briefly, wells were either loaded with 50 ul of prepared standard or 50 ul cell-free supernatant and incubated on an orbital shaker at 500 rpm for 2h in the dark at RT. Wells were washed 3x with Bio-Rad wash reagent and samples were then incubated with 25 ul biotinylated detection Ab for 30 min, washed, and incubated with 50 ul streptavidin-PE for 10 min. After final wash, samples were resuspended in assay buffer and measured using LUMINEX 200 (LUMINEX, Austin, TX). Cytokines included in this assay are as follows: IL-Ib, IL-10, GM-CSF, IFNg, TNFa, IL-1a, IL-6, IL-12p40, IL-12p70, G-CSF, KC, MIP-1a, RANTES, MCP-1 and MIP-1b.
Western blot analysis
Tissues were homogenized in phospho-cell lysis buffer (Cell Signaling, Danver, MA), and total protein concentrations were quantified using bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). Twenty micrograms of total proteins were dissolved in 1X sample buffer, boiled for 5 minutes and separated on a 12% SDS-PAGE gel with 6% stacking gel in 1X electrode buffer at a constant current of 70 mA for approximately 2 hr. The proteins were transferred to nitrocellulose membranes (Protran, 0.2 μM, Schleicher & Schuell, Keene, NH) in a Mini-Protean II Cell apparatus (Bio-Rad Laboratories, Hercules, CA) at a constant 70 V for 90 min with an ice pack. Non-fat milk (5%) was used as a blocker (Fisher Scientific, Pittsburgh, PA), and immunoblotting was performed with a 1:500 dilution of primary antibodies in 2% NFM at 4°C overnight. Antibodies were Cell Signaling CS4749S (beta 1 integrin) and Millipore AB1920 (alpha 3 integrin). Membranes were washed and a 1:10,000 dilution of goat anti-rabbit or goat anti-mouse IgG-horseradish peroxidase conjugate (Pierce) was added as appropriate. Membranes were washed and incubated with Western Lighting Plus (PerkinElmer, Waltham, MA) to detect immunoreactive proteins.
Statistics
Differences between means (3 groups or more) were determined using analysis of variance and differences between two groups were analyzed using independent t-test functions of Graph pad inSTAT statistical software (La Jolla, CA). A p-value of ≤0.05 was considered significant and data is presented as mean ± standard error of the mean (SEM).
Results
Development of a murine model of ascending bacterial infection
Our first objective was to determine whether a non-pathogenic form of E-coli could ascend through the FRT in pregnant and non-pregnant mice. Consequently, we inoculated genetically engineered bacteria, E. coli expressing RFP (RFP-E. coli) into the vagina of healthy non-pregnant and pregnant mice and monitored their location using an imaging system. In non-pregnant animals, bacteria were clearly detected in the cervix and endometrial cavity within 24 hours (Fig. 1A). In contrast, bacteria were not detectable in the cervix or uterine cavity of pregnant animals (Fig. 1B), indicating a dramatic resistance to microbial invasion of the uterine cavity during pregnancy. Bacteria were not observed at earlier time points in pregnant animals, suggesting there was no access, and not that the infection was there but rapidly eliminated (data not shown).
Figure 1. Murine model of ascending bacterial infection.
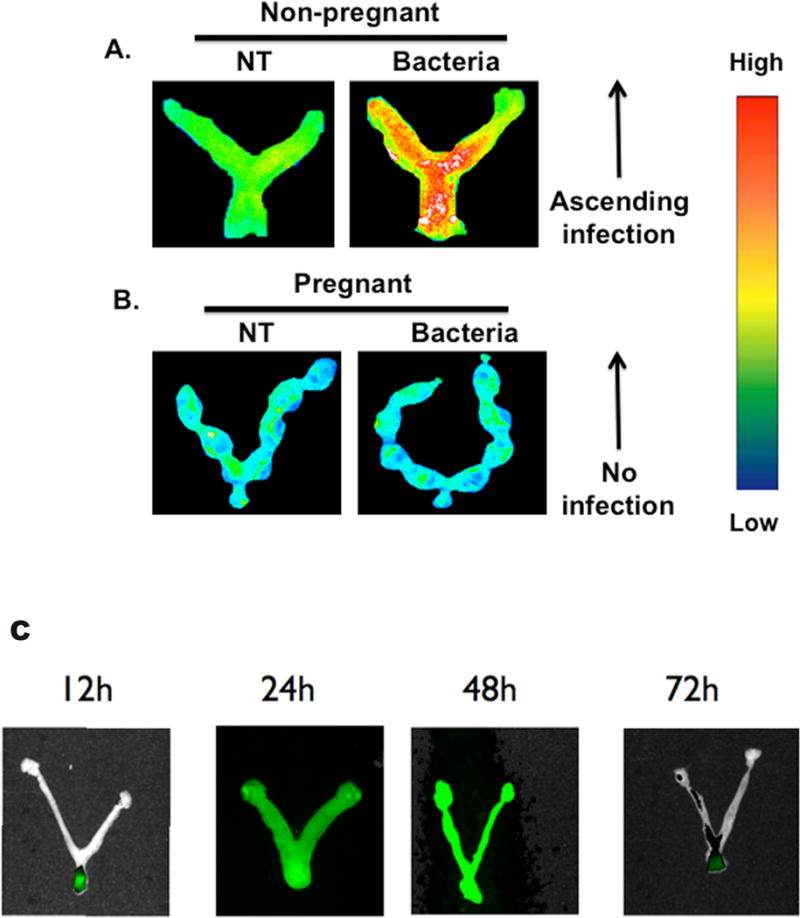
Non-pregnant (A) and pregnant (B) mice were visualized 24 hours after receiving intravaginal injections of E. coli labeled with RFP. Legend indicates that red color in non-pregnant animal has ascending bacterial infection in utero. NT=no bacterial injection, Bacteria=intravaginal bacterial injection; n=6. (C) Time course of murine model of ascending bacterial infection. Non-pregnant mice were visualized 12, 24, 48 and 72 hours after receiving intravaginal injections of E. coli labeled with GFP. Bacteria are visualized in the vagina and cervix at 12h, found throughout the reproductive tract at 24 and 48 hr, and are diminishing 72 hr post-infection.
To confirm the specificity of the signal and time course of ascending microbial invasion of the endometrial cavity, mice were inoculated in the vagina with the same E. coli but expressing GFP as the reporter gene. Animals were euthanized after 12, 24, 48 and 72 hours. Imaging of the genital tract was performed and as observed with RFP-E. coli, GFP- E. coli ascended through the uterus of the non-pregnant mice; furthermore, we observed an increase in fluorescent signal as a function of time (Fig 1C). That was not the case in the FRT of pregnant mice were there was no signal at any time (data not shown). These observations indicate a dramatic difference in the permissiveness of the non-pregnant and pregnant uterus to ascending infection from the lower genital tract.
Movement of Pathogenic Bacteria through the mouse female reproductive tract
Since we found that the pregnant FRT is capable of preventing an ascending E-coli bacterial infection, we investigated whether this protection is operative with the bacteria most frequently found in the amniotic fluid of pregnant women with preterm labor, U. urealyticum (31, 32) (33). U. urealyticum was inoculated intravaginally on E15.5 and after 24 hours decidua and lymphoid aggregates were harvested to determine if U. urealyticum was present in these tissues. Similar as observed with E-coli, the pregnant FRT was able to prevent the migration of U. urealyticum towards the endometrial cavity (Fig 2B).
Figure 2. Ureaplasma urealyticum ascends into the uterus in MHV68 infected animals.
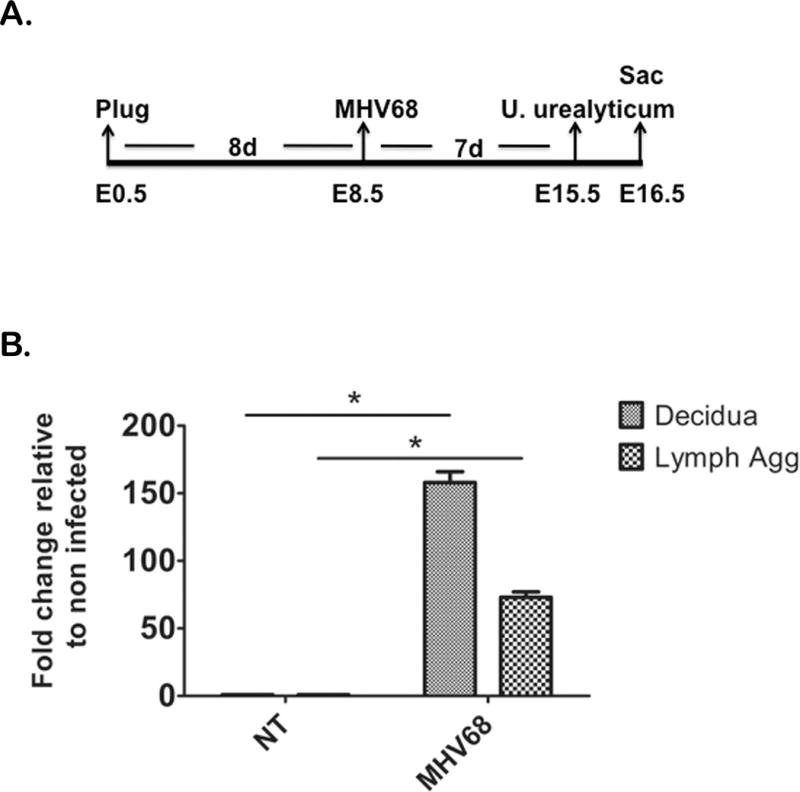
Mice were infected with MHV68 or DMEM (control) at E8.5 of pregnancy, received an intravaginal injection of pathogenic U. urealyticum at E15.5, and were sacrificed (Sac) at E16.5 (A). NT= mice with no MHV68, MHV68= virus treated animals. Both groups received U. urealyticum; decidua and lymphoid aggregates are represented as fold difference from the same tissue from mice with no viral infection. Mice with MHV68 had higher concentrations of bacteria in the decidua and lymphoid aggregates (Lymph Agg) compared to mice without viral infection (B). n=5
Since we observed that the normal FRT, during pregnancy, is highly protective of bacterial infection, we evaluated potential factors that would alter this protection and consequently would lead to an ascending bacterial infection as observed in pregnancy complications. Having previously established a mouse model of viral infection during pregnancy using a murine gammaherpes virus 68 (MHV68) which causes mice to be more susceptible to bacterial products (bacterial endotoxin or LPS),(15) we tested whether a systemic viral infection in pregnant animals could alter the resistance to microbial invasion of the uterine cavity. MHV68 was injected intra-peritoneal on E8.5 of pregnancy and U. urealyticum was inoculated intravaginally on E15.5 (Fig 2A). Twenty-four hours after bacterial inoculation we harvested decidua and lymphoid aggregates to determine if U. urealyticum bacteria was present in these tissues as determined using a specific PCR assay for U. urealyticum. U. urealyticum signal was significantly higher in the decidua and lymphoid aggregates in MHV68 infected mice compared to mice who had not been infected with MHV68 virus (Fig 2B) or a control group of pregnant animals not exposed to bacteria or virus (Data not shown). This data suggests that a viral infection during pregnancy alters the capacity of the FRT to control ascending bacterial infection.
Expression of antimicrobial peptides and TLR by the uterine cervix after viral infection with MHV68
The cervix has a unique role functioning as an interface between the upper and lower FRT, providing protection to the uterine cavity against ascending intrauterine infection from the lower genital tract. During pregnancy epithelial cells of the cervix are responsible for the formation of the mucus plug that provides a mechanical and biochemical barrier to ascending infection (20). Since we observed that a viral infection resulted in dramatic susceptibility to microbial invasion of the uterine cavity in pregnant animals, we explored whether this could be due to a change in the capacity of the cervix to control microorganisms due to alteration in the expression and function of pattern recognition receptors (TLRs), responsible for sensing microorganisms. The uterine cervix from non-pregnant and pregnant mice was analyzed for changes in TLR mRNA expression in the presence or absence of a systemic MHV68 infection. Systemic MHV68 viral infection, in the non-pregnant mice, either did not change or increased the expression of specific TLRs (Fig. 3A). In contrast, the cervix of pregnant mice that had been exposed to MHV68 showed a substantial decrease in the expression of specific TLRs (Fig 3A–B).
Figure 3. MHV68 infection decreases TLR expression in the pregnant cervix.

NT= no MHV68, MHV68= mice infected with virus. TLR mRNA expression was higher or did not change in the cervix of non pregnant MHV68 infected animals relative to non infected controls (A) but specific TLR mRNA was significantly decreased in the cervix of MHV68-infected pregnant animals (B). Significance as denoted by bars is a p <.05. n=5
TLRs play a central role in maintaining the control of microorganisms in the cervix by sensing microbial products and eliciting an innate immune response. To determine if these changes in TLRs gene expression resulted in functional differences in the capacity of the cervix to respond to bacterial infection, pregnant mice were either exposed to MHV68 or vehicle at pregnancy E8.5, followed by an intravaginal challenge of E. coli bacteria at E15.5 of pregnancy. Since TLR ligation induces changes in cytokine/chemokine expression and AMP secretion, we characterized the cervical cytokine/chemokine profile and AMP mRNA expression. We observed a robust pro-inflammatory cytokine response to bacteria in the cervix of pregnant mice not exposed to MHV68 infections characterized by high tissue concentration of IL-1β, IL-6, KC, MCP-1, MIP-1α/β, IL12 and RANTES (Fig 4A–C). This cytokine/chemokine profile is stereotypic for a strong pro-inflammatory anti-bacterial response. A dramatically different profile was observed in MHV68 infected pregnant animals. The presence of bacteria in the lower genital tract in MHV68 infected mice was not able to elicit a similar inflammatory response as the one observed in mice, which received the same type of bacteria (Fig 4A–C). Indeed, the cervical protein concentration of IL-12, MIP-1α and β, RANTES and IL-6 of mice with MHV68 viral infection and bacteria were similar to those of the control group (not exposed to either bacteria or virus).
Figure 4. MHV68 infection reduces cytokine response to bacteria in the cervix.
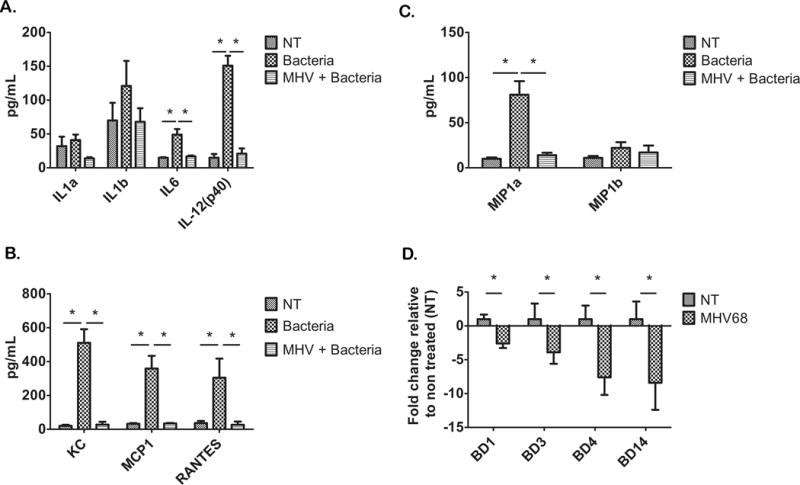
Cervical cytokines were measured using luminex technology; “NT” are cytokines from the cervix from animals that did not receive MHV68 or bacteria, “Bacteria” are cytokines from the cervix of animals with an intravaginal bacteria injection, but no MHV68, and “MHV + Bacteria” are cytokines from the cervix of animals that received an intravaginal injection of bacteria while having an MHV68 infection. A number of cytokines and chemokines (A–C) were upregulated in the cervices of animals with artificial intravaginal bacterial infection, while this response was significantly reduced in animals with MHV68 infection. Beta defensin 1 (BD1), beta defensin 3 (BD3), beta defensin 4 (BD4) and beta defensin 14 (BD14) mRNA expression was also reduced in cervices from MHV68 infected animals (D). Significance is denoted by bars and is p <.05. n=5
In addition, AMP mRNA expression was lower in the pregnant cervix of MHV68 infected mice than in that of pregnant animals not exposed to the virus (Fig 4D). These observations suggest that the decreased TLR gene expression in the cervix of MHV68 infected mice is associated with a lack of response to bacteria as demonstrated by the absence of pro-inflammatory cytokines and decreased expression of AMPs. These changes in the MHV68 infected pregnant mice may be functionally linked to the increased susceptibility to ascending bacterial infection.
Systemic administration of MHV68 results in viral infection of the cervix of pregnant mice
The differences in the cervical response to viral and bacterial infections of pregnant and non-pregnant mice could be attributed to variations in the systemic response to the virus or changes in cervical viral infection. To test this postulate, we sought to determine if the virus was directly infecting the cervix. Pregnant and non-pregnant mice received i.p. injections with MHV68 as described above; cervix and spleen samples were collected 7 days after injection and analyzed for MHV68 infection using qPCR for MHV68 ORF53. MHV68 viral infection was observed at similar concentrations in the spleen of pregnant and non-pregnant mice (Fig 5A), but surprisingly, MHV68 viral infection was observed in the cervix of pregnant mice but was absent in the cervix of non-pregnant mice (Fig 5B). These results indicate pregnancy renders the uterine cervix susceptible to a systemic viral infection.
Figure 5. MHV68 infects the cervix of pregnant mice.
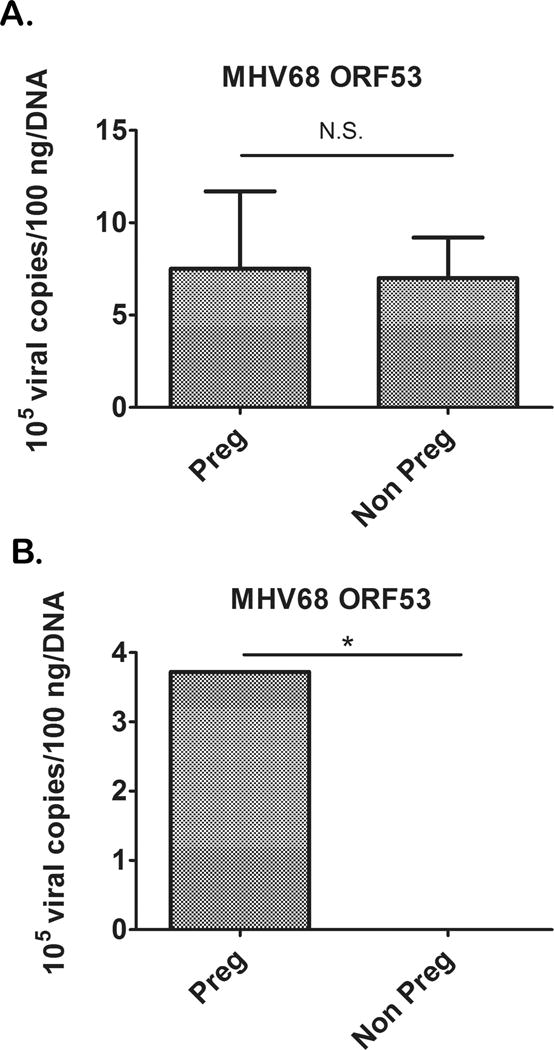
Both pregnant and non-pregnant mice had similar systemic infections as determined by MHV68 ORF53 in the spleen (A), but pregnant mice also had high MHV68 titers in the cervix while non pregnant mice had no cervical infection (B). n=4, p<.001
Sex hormones increase the susceptibility of the cervix to viral infection
Estrogen and progesterone are major factors associated with morphologic and functional changes of the cervix, during pregnancy(19). Since we found that virus was only infecting the cervix of pregnant mice, we hypothesized high hormone concentrations during pregnancy induced modifications in the epithelium of the cervix that increase its susceptibility to viral infection. To test this premise, we ovariectomized mice and they received hormone treatment [estradiol (500ng) and progesterone (500ug)] or placebo (vehicle) 21d post-surgery for 3d (Fig 6A) mimicking the hormonal status during pregnancy. Both treatment groups then received injections of MHV68 i.p. and continued to get hormone therapy or vehicle every 2d for 7d (Fig 6A). Afterwards, the cervix was collected and it was determined whether sex hormones made the cervix more susceptible to MHV68 infection. As suspected, MHV68 was detected in the cervix of animals receiving hormone therapy but not in ovariectomized mice receiving only vehicle (Fig 6B). These data suggest that hormonal changes associated with pregnancy induced modifications of the cervix leading to increased susceptibility to viral infection, and this could in turn affect the role of the uterine cervix in protecting against ascending bacterial infection.
Figure 6. Sex hormones play a role in MHV68 infection of cervix tissue.

Mice were overiectomized (OVX) and after 3w, given either P4+E2 or vehicle for 3d; they were then injected with MHV68, and remained on either hormone treatments or vehicle for 7d (A). Seven days after infection, MHV68 was detected in the cervices of mice treated with hormones, but absent in vehicle treated controls (B). n=4, p< .05
Integrins are increased in the cervix of pregnant mice and have a role in viral entry
Having determined that the high levels of sex hormones found during pregnancy might increase the susceptibility of the cervix to viral infection, we hypothesized that this was due to increased expression of a protein or proteins on cervical cells that may act as a receptor for viral entry. Although the specific receptor for MHV68 entry is unknown, integrins are common entry receptors for related viruses (34, 35). To test the possibility that integrins are responsible for MHV68 infection of cervical cells we established an in vitro model using human ectocervical cells clone ECT1 that are permissive to MHV68 infection. Fibronectin is a high molecular weight glycoprotein present in the extracellular matrix that binds to integrins (35), therefore, we postulated that fibronectin would bind to integrins present in cervical cells and block viral entry. To test this premise, we incubated human cervical cells with fibronectin (500 ug/mL) followed by MHV68 infection. We observed a significant decrease in MHV68 infection in cells pre-treated with fibronectin compared to the non-fibronectin treated control, suggesting an integrin might be involved in viral entry into cervical cells (Fig 7A). We then examined integrins in the cervix of non-pregnant and pregnant mice to determine if their expression was correlated with MHV68 infectivity. Our data showed integrin alpha 3 and beta 1 expression was positively correlated with MHV68 infection in the cervix of infected pregnant (high expression) and non-pregnant (low expression) mice (Fig 7B). This was not the case for integrin alpha 4, which was equally expressed in non-pregnant and pregnant cervices (Fig 7B). Finally, to determine if integrin alpha 3, specifically, had a role in the susceptibility of MHV68 infection in the cervix, we evaluated whether specific blocking antibodies for this integrin could prevent MHV68 viral infection, in vitro. As shown in figure 7C, we found that there was a significant decrease in the MHV68 titers in human cervical cells pre-treated with alpha 3 blocking antibody (Fig 7C) but not with the control IgG. Collectively, these data suggest a possible mechanism whereby pregnancy can increase the susceptibility of the cervix to viral infection with MHV68.
Figure 7. Integrins play a role in MHV68 entry into cervical cells.
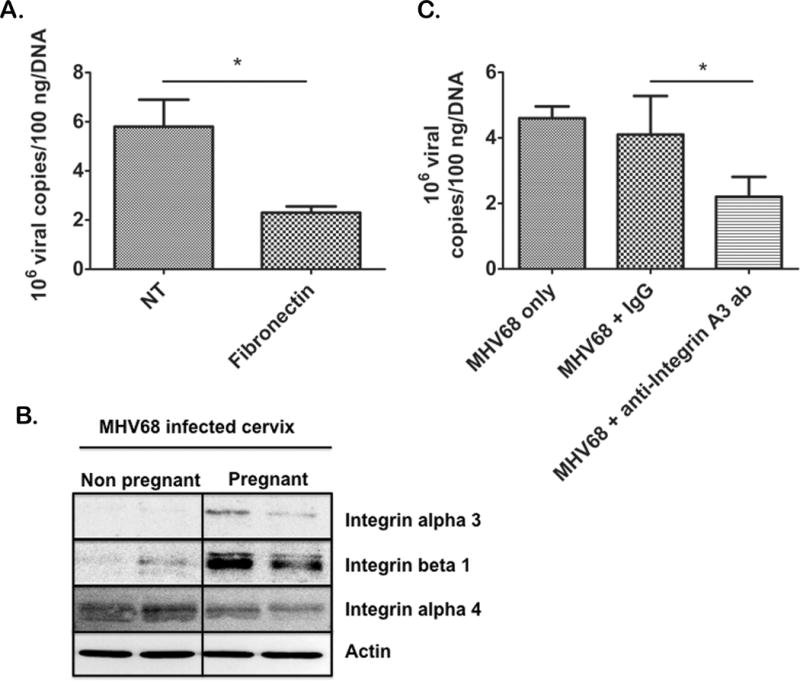
Human ectocervical cells (ECT1) were pretreated with fibronectin to block integrin-ligand interactions and then infected with MHV68; 24h after infection, MHV68 was found to be partially blocked from cells treated with fibronectin (Fibronectin) compared to cells treated with MHV68 but no fibronectin (NT)(3 independent experiments) (A). Western blot analysis showed integrin alpha 3 and beta 1 were upregulated in the pregnant cervix of infected mice (B) n=3. Antibodies blocking integrin alpha 3 (MHV68 + anti-integrin a3 ab) partially blocked MHV68 infection, while treatment with non specific IgG (MHV68 + IgG) did not block MHV68 infection as compared to cells only treated with MHV68 (MHV68 only) (Representative of 3 independent experiments with a minimum of 3 animals per group) (C). Significance is denoted by bars and is p <.05.
Discussion
In the present study we demonstrate, for the first time, that a viral infection of the cervix, during pregnancy, reduces the capacity of the lower reproductive tract to prevent bacterial infection of the pregnant uterus. Furthermore, we report that pregnancy increases the sensitivity of the cervix to viral infection leading to changes in the expression and function of TLRs and antimicrobial products. This sensitivity is partially caused by hormonal regulation of proteins in the cervix that cause it to be permissive to virus.
Approximately 30% of all neonatal death is directly caused by preterm birth and 50% of premature births are idiopathic (1, 36, 37). Since preterm birth represents a major health problem it is, therefore, of the greatest importance to determine its causes, which then will allow us to develop novel ways for prevention and treatment. A strong body of evidence suggests that a majority of intrauterine bacterial infections during pregnancy result from bacteria ascending from the lower reproductive tract (2, 6). The amniotic cavity is normally sterile and a major transition during life is the emergence from a sterile to a non-sterile environment at the time of birth. Microbial invasion of the amniotic cavity can lead to preterm labor with intact membranes, preterm premature rupture of membranes, cervical insufficiency, a short cervix, fetal sepsis, and preterm delivery. The presence of organisms in the amniotic cavity elicits an intense inflammatory response, which may evolve into a fetal inflammatory response syndrome. The latter predisposes preterm neonates to short- and long-term consequences such as neonatal sepsis, bronchopulmonary dysplasia, and cerebral palsy. It has been estimated that one of every three preterm neonates is born to a mother with intraamniotic infection, identified with either cultivation or molecular microbiologic techniques (38).
Two major events need to occur for bacteria to induce preterm labor: 1) bacteria need to breach the cervix and reach the decidua, amnion and potentially the fetus; 2) bacterial numbers must reach a threshold that will trigger an inflammatory response that in turns induces preterm labor (39). A major question is how bacteria can breach the cervical barrier and reach the maternal-fetal interface. All women have microorganisms in the lower genital tract, yet these microorganisms do not gain access to the amniotic cavity during normal pregnancy. What are the mechanisms responsible for preventing ascending intrauterine infection in most pregnant women? It is widely believed that the cervical mucus plug plays an important role in host defense against microbial invasion of the amniotic cavity(20, 40). Indeed, the cervical mucus plug has antimicrobial activity (41), contains antimicrobial peptides, as well as immunoglobins and cellular components of the innate immune system (macrophages, neutrophils, etc.) (42). Therefore, alterations of the composition or formation of the cervical plug may affect the protection against ascending bacteria.
In this study we first evaluated the regulation of bacteria in the mouse FRT. We provide novel insights into the regulation of bacterial movement through the reproductive tract and reveal major differences depending on the reproductive stage. We observed, in our murine model, that bacteria delivered in the lower FRT is able to ascend to the uterine cavity confirming new findings in women, showing that the non pregnant uterus has a normal flora (2, 43). On the other hand pregnant mice were able to prevent ascending bacteria from the lower FRT. These findings could suggest that bacteria in the upper FRT may not be a result of changes of the type of vaginal bacteria but of the capacity of the cervix to regulate its ascension(44). These differences correlate with the biochemical and physical differences between the pregnant and non-pregnant cervix (20, 44).
A puzzling question has been why some pregnant women develop an ascending intraamniotic infection and others do not. In pregnancies complicated with bacterial infection, we propose that it is due to alteration in the capacity of the cervix (lower FRT) to control bacteria and not in the type of bacteria present in the vagina. In the present study we demonstrate that under normal conditions, Ureaplasma urealyticum, the most common microorganism isolated in tissues from the maternal-fetal interface (6, 45) (31) is protected from the upper FRT by the cervix; however, if the cervix is infected with virus, the protection is decreased and U. urealyticum is able to reach the pregnant uterus. We propose that this reduced protection is due to the decrease in TLRs, antimicrobials and inflammatory cytokines in the cervix infected with virus.
Pregnant women are more susceptible to the effects of viral infection than non-pregnant women. The mortality rate of pregnant women infected by influenza in the 1918 pandemic and in the most recent H1N1 epidemic was significantly greater than that of non-pregnant women (46). This has been attributed to either an exaggerated inflammatory response (cytokine storm caused by the prime state of activation of the innate immune system during normal pregnancy) or secondary bacterial infections (47). The latter has been a subject of interest for many years and Dr. Luis Cruveilhier is cited to have stated that “flu condemns, an additional infection executes” (48). Compelling evidence suggests that more than one microorganism causes many infectious diseases of humans and animals (49). Polymicrobial diseases are defined as pathologies caused by the synergistic or sequential action of infectious agents from either the same or different kingdoms (49, 50). Among the best known examples is the relationship between influenza and bacterial pneumonia. It is noteworthy that viral infections of the lower genital tract are relatively common (e.g. papilloma virus, herpes virus, HIV, etc.). Yet, there is a paucity of knowledge about the effect of such localized infections on mucosal immunity of the lower genital tract during pregnancy (51). In this study we show for the first time that a viral infection could interfere in the normal anti-microbial function of the cervix during pregnancy.
In previous studies we used a murine gamma herpesvirus, MHV68 (29). This is a DNA virus of the same family as CMV and the HHVs, which have been found in the amnion, placenta and even the lower reproductive tract of pregnant women. These viruses all have latency periods, produce immunosuppressive factors and many can be reactivated by regulating TLRs function and expression. Naturally, MHV68 infects the nasopharyngeal tissue, leading to lytic infection and expansion in lung epithelial cells, followed by infection of B-cells, macrophages and dendritic cells, and viral latency (52). Interestingly, in our study, we found that it also infected the cervical cells of the pregnant mice. In humans, the most common viral infection of the cervix is human papillomavirus (HPV), and a handful of studies have shown that HPV infection of the placenta is associated with adverse pregnancy outcomes (53, 54). Even more recently, HPV infection of the cervix was related to placental abnormalities and preterm birth (55).
To understand why the cervix protection was compromised we first characterized the molecular response to viral infection. One of the main characteristics of the pregnant cervix is the formation of the cervical plug, which is normally enriched with natural antimicrobial peptides such as alpha and beta defensins that can kill bacteria by damaging their outer barriers (20) and recruit immune cells to the site of bacterial invasion. The epithelial cells further help to protect against pathogens by the appropriate expression of TLRs, secretion of cytokines and antimicrobial peptides (56, 57). We demonstrate that MHV68 infection of the cervix decreases TLR expression and consequently dampens the anti-microbial and inflammatory response to bacteria. This effect could be part of the viral mechanism of evading host immune recognition. In contrast, TLR3, TLR9 and TLR2 are upregulated in the cervix of non-pregnant animals, potentially for heightened protection against viral infection. These results show that systemic infection regulates specific TLRs in mucosal epithelia quite differently, as compared to direct infection.
The pregnant state requires dramatic remodeling of the uterus. Sex steroid hormones and, in particular, estrogen and progesterone, have a powerful effect in remodeling the uterine cervix and the myometrium. Since only the pregnant cervix was infected by MHV68, this suggested that the changes of the cervix are associated with the hormonal profile characteristic of pregnancy. This was confirmed with the observation that ovariectomized mice receiving estrogen and progesterone were more susceptible to viral infection of the cervix, suggesting that a systemic endocrine milieu can create conditions favoring viral invasion and multiplication. We were able to establish that integrin alpha 3 plays a role in the permissiveness of the cervix to MHV68 infection, although additional factors may also be involved since the blocking studies did not completely diminish MHV68 infection.
Based on these findings we propose the following model: commensal bacteria are located in the lower reproductive tract, and during healthy pregnancy, the uterine cervix provides protection against bacteria ascending into the upper reproductive tract (Fig 8A). If the protection provided by the uterine cervix is jeopardized, bacteria can ascend from the lower tract, to the decidua and amnion, leading to inflammation and pregnancy complications such as preterm birth (Fig 8B). In summary, these experiments are the first to describe a viral infection during pregnancy that alters the physiologic protection of the cervix against intrauterine infection. These studies also provide evidence that such changes are potentially mediated by altered components of the innate immune response. Together our results suggest that preterm labor is a polymicrobial disease, which requires a multifactorial approach for its prevention and treatment.
Figure 8. Model of polymicrobial disease during pregnancy.
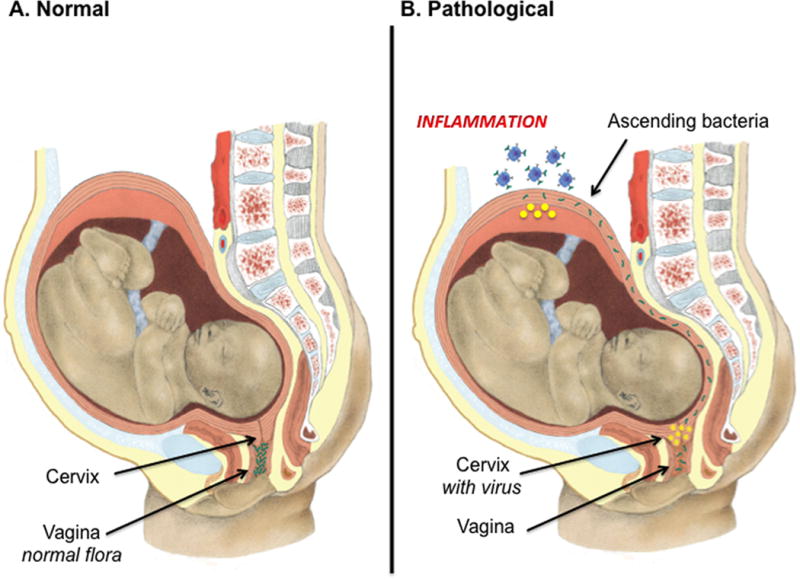
(A) Commensal bacteria are located in the lower reproductive tract, and during healthy pregnancy, the uterine cervix provides protection against bacteria ascending into the upper reproductive tract. If the protection provided by the uterine cervix is jeopardized, bacteria can ascend from the lower tract, to the decidua and amnion, leading to inflammation and pregnancy complications such as preterm birth. We propose a model of polymicrobial disease during pregnancy: in this model, pregnancy and the associated sex hormones increase the susceptibility of the cervix to viral infection. Viral infection then results in a decrease in the protection against ascending bacteria. The decrease in protection can then lead to intrauterine inflammation in response to bacteria and preterm birth (B).
Acknowledgments
We acknowledge Dr. Michael Elowitz for the generous gift of the pZS2 plasmid used in this study and Melinda Wells for the model illustration.
Grant Support This study is in part funded by grants from the National Institute of Health, NICDH P01HD054713 and 3N01 HD23342 and the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services
References
- 1.Iams JD, Romero R, Culhane JF, Goldenberg RL. Preterm birth 2 – Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371:164–175. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- 2.Espinoza J, Erez O, Romero R. Preconceptional antibiotic treatment to prevent preterm birth in women with a previous preterm. delivery Am J Obstet Gynecol. 2006;194:630–637. doi: 10.1016/j.ajog.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 3.Digiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Sanders K, Bik EM, Chaiworapongsa T, Oyarzun E, Relman DA. Prevalence and Diversity of Microbes in the Amniotic Fluid, the Fetal Inflammatory Response, and Pregnancy Outcome in Women with Preterm Pre-Labor Rupture of Membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 2006;49:506–514. doi: 10.1111/j.1365-2559.2006.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, Kim JS, Edwin SS, Gomez R, Draghici S. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol. 2010;63:73–92. doi: 10.1111/j.1600-0897.2009.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Seminars in reproductive medicine. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DC, Romero R, Kim JS, Yoo W, Lee J, Mittal P, Kusanovic JP, Hassan SS, Yoon BH, Kim CJ. Evidence for a Spatial and Temporal Regulation of Prostaglandin-Endoperoxide Synthase 2 Expression in Human Amnion in Term and Preterm Parturition. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2010-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SE, Romero R, Lee SM, Yoon BH. Amniotic fluid volume in intra-amniotic inflammation with and without culture-proven amniotic fluid infection in preterm premature rupture of membranes. J Perinat Med. 2010;38:39–44. doi: 10.1515/JPM.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Y, Tarquini F, Romero R, Kim CJ, Tarca AL, Bhatti G, Lee J, Sundell IB, Mittal P, Kusanovic JP, Hassan SS, Kim JS. Peripheral CD300a+CD8+ T lymphocytes with a distinct cytotoxic molecular signature increase in pregnant women with chronic chorioamnionitis. Am J Reprod Immunol. 2012;67:184–197. doi: 10.1111/j.1600-0897.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranjard L, Poly F, Nazaret S. Monitoring complex bacterial communities using culture-independent molecular techniques: application to soil environment. Research in microbiology. 2000;151:167–177. doi: 10.1016/s0923-2508(00)00136-4. [DOI] [PubMed] [Google Scholar]
- 11.Mor G, Cardenas I. The immune system in pregnancy: A unique complexity. Am J Reprod Immunol. 2010;63 doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel DO. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Reprod Immunol. 2010;63:544–565. doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Sanders K, Bik EM, Chaiworapongsa T, Oyarzun E, Relman DA. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woudwyk MA, Monteavaro CE, Jensen F, Soto P, Barbeito CG, Zenclussen AC. Study of the uterine local immune response in a murine model of embryonic death due to Tritrichomonas foetus. Am J Reprod Immunol. 2012;68:128–137. doi: 10.1111/j.1600-0897.2012.01159.x. [DOI] [PubMed] [Google Scholar]
- 15.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth CJ, Manzur A, Oyarzun E, Romero R, Mor G. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185:1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hickey DK, Fahey JV, Wira CR. Mouse estrous cycle regulation of vaginal versus uterine cytokines, chemokines, alpha-/beta-defensins and TLRs. Innate immunity. 2012 doi: 10.1177/1753425912454026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee DC, Hassan SS, Romero R, Tarca AL, Bhatti G, Gervasi MT, Caruso JA, Stemmer PM, Kim CJ, Hansen LK, Becher N, Uldbjerg N. Protein profiling underscores immunological functions of uterine cervical mucus plug in human pregnancy. Journal of proteomics. 2011;74:817–828. doi: 10.1016/j.jprot.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper MD, Roberts MH, Barauskas OL, Jarvis GA. Secretory leukocyte protease inhibitor binds to Neisseria gonorrhoeae outer membrane opacity protein and is bactericidal. Am J Reprod Immunol. 2012;68:116–127. doi: 10.1111/j.1600-0897.2012.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becher N, Adams Waldorf K, Hein M, Uldbjerg N. The cervical mucus plug: structured review of the literature. Acta obstetricia et gynecologica Scandinavica. 2009;88:502–513. doi: 10.1080/00016340902852898. [DOI] [PubMed] [Google Scholar]
- 20.Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol. 2002;187:137–144. doi: 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- 21.Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. 2010;63:587–600. doi: 10.1111/j.1600-0897.2010.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nasu K, Narahara H. Pattern recognition via the toll-like receptor system in the human female genital tract. Mediators Inflamm. 2010;2010:976024. doi: 10.1155/2010/976024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hertz CJ, Wu Q, Porter EM, Zhang YJ, Weismuller KH, Godowski PJ, Ganz T, Randell SH, Modlin RL. Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta. defensin-2 J Immunol. 2003;171:6820–6826. doi: 10.4049/jimmunol.171.12.6820. [DOI] [PubMed] [Google Scholar]
- 24.Homma T, Kato A, Hashimoto N, Batchelor J, Yoshikawa M, Imai S, Wakiguchi H, Saito H, Matsumoto K. Corticosteroid and cytokines synergistically enhance toll-like receptor 2 expression in respiratory epithelial cells. American journal of respiratory cell and molecular biology. 2004;31:463–469. doi: 10.1165/rcmb.2004-0161OC. [DOI] [PubMed] [Google Scholar]
- 25.Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. Jama. 2006;296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 26.Haun L, Kwan N, Hollier LM. Viral infections in pregnancy. Minerva Ginecol. 2007;59:159–174. [PubMed] [Google Scholar]
- 27.Gervasi MT, Romero R, Bracalente G, Chaiworapongsa T, Erez O, Dong Z, Hassan SS, Yeo L, Yoon BH, Mor G, Barzon L, Franchin E, Militello V, Palu G. Viral invasion of the amniotic cavity (VIAC) in the midtrimester of pregnancy. J Matern Fetal Neona. 2012 doi: 10.3109/14767058.2012.683899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olivadoti M, Toth LA, Weinberg J, Opp MR. Murine gammaherpesvirus 68: a model for the study of Epstein-Barr virus infections and related diseases. Comp Med. 2007;57:44–50. [PubMed] [Google Scholar]
- 29.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth C, Manzur A, Oyarzun E, Romero R, Mor G. Viral Infection of the Placenta Leads to Fetal Inflammation and Sensitization to Bacterial Products Predisposing to Preterm Labor. J Immunol. 2010;185 doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gargano LM, Forrest JC, Speck SH. Signaling through Toll-like receptors induces murine gammaherpesvirus 68 reactivation in vivo. J Virol. 2009;83:1474–1482. doi: 10.1128/JVI.01717-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38:261–268. doi: 10.1515/JPM.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon BH, Kim YA, Romero R, Kim JC, Park KH, Kim MH, Park JS. Association of oligohydramnios in women with preterm premature rupture of membranes with an inflammatory response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1999;181:784–788. doi: 10.1016/s0002-9378(99)70301-7. [DOI] [PubMed] [Google Scholar]
- 33.Cassell GH, Waites KB, Watson HL, Crouse DT, Harasawa R. Ureaplasma urealyticum intrauterine infection: role in prematurity and disease in newborns. Clinical microbiology reviews. 1993;6:69–87. doi: 10.1128/cmr.6.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heldwein EE, Krummenacher C. Entry of herpesviruses into mammalian cells. Cellular and molecular life sciences : CMLS. 2008;65:1653–1668. doi: 10.1007/s00018-008-7570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akula SM, Pramod NP, Wang FZ, Chandran B. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposis sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell. 2002;108:407–419. doi: 10.1016/s0092-8674(02)00628-1. [DOI] [PubMed] [Google Scholar]
- 36.Conde-Agudelo A, Romero R, Hassan SS, Yeo L. Transvaginal sonographic cervical length for the prediction of spontaneous preterm birth in twin pregnancies: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010 doi: 10.1016/j.ajog.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Annals of the New York Academy of Sciences. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 38.Berger A, Witt A, Haiden N, Kaider A, Klebermasz K, Fuiko R, Langgartner M, Pollak A. Intrauterine infection with Ureaplasma species is associated with adverse neuromotor outcome at 1 and 2 years adjusted age in preterm infants. J Perinat Med. 2009;37:72–78. doi: 10.1515/JPM.2009.016. [DOI] [PubMed] [Google Scholar]
- 39.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7:259–274. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- 40.Sakai M, Ishiyama A, Tabata M, Sasaki Y, Yoneda S, Shiozaki A, Saito S. Relationship between cervical mucus interleukin-8 concentrations and vaginal bacteria in pregnancy. Am J Reprod Immunol. 2004;52:106–112. doi: 10.1111/j.1600-0897.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 41.Sakai M, Ishiyama A, Tabata M, Sasaki Y, Yoneda S, Shiozaki A, Saito S. Relationship between cervical mucus interleukin-8 concentrations and vaginal bacteria in pregnancy. Am J Reprod Immunol. 2004;52:106–112. doi: 10.1111/j.1600-0897.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 42.Wira CR, Grant-Tschudy KS, Crane-Godreau MA. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immunol. 2005;53:65–76. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhou X, Brotman RM, Gajer P, Abdo Z, Schuette U, Ma S, Ravel J, Forney LJ. Recent advances in understanding the microbiology of the female reproductive tract and the causes of premature birth. Infectious diseases in obstetrics and gynecology. 2010;2010:737425. doi: 10.1155/2010/737425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hassan SS, Romero R, Pineles B, Tarca AL, Montenegro D, Erez O, Mittal P, Kusanovic JP, Mazaki-Tovi S, Espinoza J, Nhan-Chang CL, Draghici S, Kim CJ. MicroRNA expression profiling of the human uterine cervix after term labor and delivery. Am J Obstet Gynecol. 2010;202:80 e81–88. doi: 10.1016/j.ajog.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 45.Romero R, Kadar N, Hobbins JC, Duff GW. Infection and labor: the detection of endotoxin in amniotic fluid. American journal of obstetrics and gynecology. 1987;157:815–819. doi: 10.1016/s0002-9378(87)80061-3. [DOI] [PubMed] [Google Scholar]
- 46.Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, Fonseca VP, Ritger KA, Kuhles DJ, Eggers P, Bruce H, Davidson HA, Lutterloh E, Harris ML, Burke C, Cocoros N, Finelli L, MacFarlane KF, Shu B, Olsen SJ. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 47.Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, Louie J, Doyle TJ, Crockett M, Lynfield R, Moore Z, Wiedeman C, Anand M, Tabony L, Nielsen CF, Waller K, Page S, Thompson JM, Avery C, Springs CB, Jones T, Williams JL, Newsome K, Finelli L, Jamieson DJ, for the Pandemic H1N1 Influenza in Pregnancy Working Group Pandemic 2009 Influenza A(H1N1) Virus Illness Among Pregnant Women in the United States. Jama. 2010;303:1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cruveilhier L, Faguet M, Grand Jean N. Revue de la tuberculose. 1945;9:329–332. [Not Available] [PubMed] [Google Scholar]
- 49.Bakaletz LO. Developing animal models for polymicrobial diseases. Nature reviews Microbiology. 2004;2:552–568. doi: 10.1038/nrmicro928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nish S, Medzhitov R. Host defense pathways: role of redundancy and compensation in infectious disease phenotypes. Immunity. 2011;34:629–636. doi: 10.1016/j.immuni.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaushic C. HIV-1 infection in the female reproductive tract: role of interactions between HIV-1 and genital epithelial cells. Am J Reprod Immunol. 2011;65:253–260. doi: 10.1111/j.1600-0897.2010.00965.x. [DOI] [PubMed] [Google Scholar]
- 52.Taylor WR, Rasley A, Bost KL, Marriott I. Murine gammaherpesvirus-68 infects microglia and induces high levels of proinflammatory cytokine production. Journal of neuroimmunology. 2003;136:75–83. doi: 10.1016/s0165-5728(03)00011-0. [DOI] [PubMed] [Google Scholar]
- 53.You H, Liu Y, Agrawal N, Prasad CK, Edwards JL, Osborne AF, Korourian S, Lowery CL, Hermonat PL. Multiple human papillomavirus types replicate in 3A trophoblasts. Placenta. 2008;29:30–38. doi: 10.1016/j.placenta.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Gomez LM, Ma Y, Ho C, McGrath CM, Nelson DB, Parry S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Human reproduction. 2008;23:709–715. doi: 10.1093/humrep/dem404. [DOI] [PubMed] [Google Scholar]
- 55.Zuo Z, Goel S, Carter JE. Association of cervical cytology and HPV DNA status during pregnancy with placental abnormalities and preterm birth. American journal of clinical pathology. 2011;136:260–265. doi: 10.1309/AJCP93JMIUEKRPIW. [DOI] [PubMed] [Google Scholar]
- 56.Stern JE, Givan AL, Gonzalez JL, Harper DM, White HD, Wira CR. Leukocytes in the cervix: a quantitative evaluation of cervicitis. Obstet Gynecol. 1998;91:987–992. doi: 10.1016/s0029-7844(98)00086-6. [DOI] [PubMed] [Google Scholar]
- 57.Givan AL, White HD, Stern JE, Colby E, Gosselin EJ, Guyre PM, Wira CR. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350–359. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]


