Abstract
Despite advances in surgery, chemotherapy and radiotherapy, the outcomes of patients with GBM have not significantly improved. Tumor recurrence in the resection margins occurs in more than 80 % of cases indicating aggressive treatment modalities, such as gene therapy are warranted. We have examined photochemical internalization (PCI) as a method for the non-viral transfection of the cytosine deaminase (CD) suicide gene into glioma cells. The CD gene encodes an enzyme that can convert the nontoxic antifungal agent, 5-fluorocytosine, into the chemotherapeutic drug, 5-fluorouracil. Multicell tumor spheroids derived from established rat and human glioma cell lines were used as in vitro tumor models. Plasmids containing either the CD gene alone or together with the uracil phosphoribosyl transferase (UPRT) gene combined with the gene carrier protamine sulfate were employed in all experiments. PCI was performed with the photosensitizer AlPcS2a and 670 nm laser irradiance. Protamine sulfate/CD DNA polyplexes proved nontoxic but inefficient transfection agents due to endosomal entrapment. In contrast, PCI mediated CD gene transfection resulted in a significant inhibition of spheroid growth in the presence of, but not in the absence of, 5-FC. Repetitive PCI induced transfection was more efficient at low CD plasmid concentration than single treatment. The results clearly indicate that AlPcS2a-mediated PCI can be used to enhance transfection of a tumor suicide gene such as CD, in malignant glioma cells and cells transfected with both the CD and UPRT genes had a pronounced bystander effect.
Keywords: Glioma, Gene Therapy, PDT, PCI, Cytosine deaminase, 5-FC
Introduction
Despite substantial improvements in imaging and treatment consisting of surgery, radiation therapy and chemotherapy, the survival of patients diagnosed with glioblastoma multiforma (GBM) has not improved significantly over the past four decades, remaining at approximately 12–14 months [1, 2]. Even with MRI-defined gross surgical resection of the main tumor mass, tumor recurrence in the resection margins occurs in more than 80 % of cases [3]. Additionally, gliomas almost never metastasize to other regions outside the CNS. More aggressive intracavity post-operative treatment modalities, such as gene therapy therefore seems warranted. Among several cancer gene therapy approaches currently being developed, are insertion of wild type genes to correct primary genetic defects, and suicide gene therapy. The latter involves transfection into tumor cells of nonmammalian genes encoding enzymes that convert nontoxic pro-drugs into toxic metabolites capable of inhibiting nucleic acid synthesis [4–6]. In particular, suicide gene therapy utilizing the induction of cytosine deaminase (CD), an enzyme which converts the antifungal agent 5-fluorocytosine (5-FC) into its antimetabolite 5-fluorouracil (5-FU) has been previously studied for glioma [7–10]. Gene-directed enzyme prodrug therapy activating strategies are of interest since a high concentration of toxic drug is produced mainly at the tumor site significantly reducing the side effects caused by systemic drug administration. In addition the bystander effect, where activated drug is exported from the transfected cancer cells into the tumor microenvironment, plays an important role by inhibiting growth of adjacent tumor cells.
In order for DNA to enter cells, some form of gene carrier either viral or nonviral is required. Although recombinant viral vectors are more efficient than their nonviral counterparts, they also present some considerable disadvantages such as undesired immune responses particularly upon repeated administrations, safety concerns and issues relating to bulk production and quality control. Additionally viral vectors have varying transfection efficiency dependent on target cell type which might make their effects in patients difficult to predict [11, 12].
Nonviral vectors are therefore an interesting alternative. Polyamine gene carriers, such as protamine sulfate (PrS), have low toxcicity, form stable PrS/DNA polyplexes, and are readily taken up by cells via endocytosis. Unfortunately they usually demonstrate low transfection efficiency due to limited endosomal escape [13, 14].
The problem of endosomal entrapment can be circumvented by the technique of photochemical internalization (PCI) [15–17]. PCI is a technique which utilizes the photochemical properties of photodynamic therapy (PDT) for the enhanced delivery of macromolecules like plasmid DNA and its associated gene carrier into the cell cytoplasm. Macromolecules that are internalized into cells via endocytosis end up inside and trapped in the intracellular endosomes and lysosomes. The concept of PCI is based on using photo sensitizers which localize in the cell membrane. Since endosome membranes are formed from the cell membrane they will also contain photosensitizer which will be transported into the cell during the endocytotic event. The macromolecule is therefore inside the endosome lumen while the photosensitizer is in the membrane. When light is applied, the photo sensitizer will react with oxygen causing endocytic membrane rupture releasing the trapped macromolecules into the cell cytosol, avoiding lysosomal degradation. This is illustrated in Fig. 1a. The released gene can therefore exert its full biological activity, in contrast to being degraded by lysosomal hydrolases (Fig. 1b). PCI has been demonstrated with nonviral gene carriers to increase gene transfection of wild type suppressor genes such as P53 and PTEN into cells containing mutated or absent genes [18–20]. PCI has also demonstrated an enhanced transfection rate employing the herpes simplex virus thymidine kinase gene, increasing the cytotoxicity of ganciclovir to glioma cells [21]. To our knowledge use of this targeting technique has not been used to enhance the insertion of the CD gene into tumor cells. The aim of the present research was designed to evaluate the effects of 5-FC following PCI mediated PrS-CD gene transfection on multicell glioma spheroids from two different glioma cell lines. Tumor spheroids mimic in vivo tumors in their micro-environment in terms of gene expression, oxygen gradient characteristic and the biological behavior of the cells and are well suited to this type of in vitro study since they represent a bridge between monolayer cultures and in vivo animal experiments. [22].
Fig. 1.
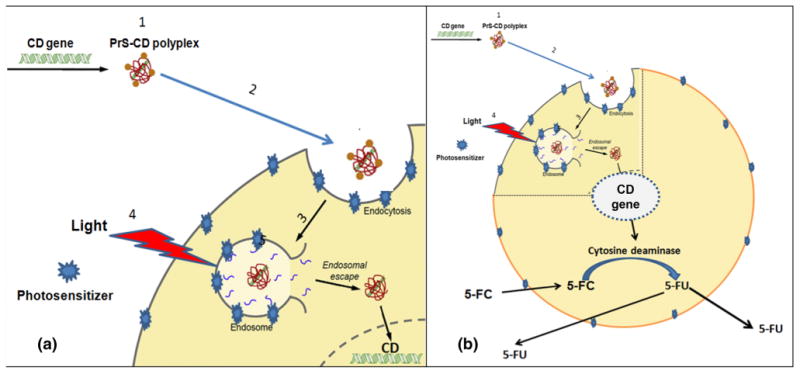
Cartoon representation of PCI mediated CD gene therapy. a Endosomal escape of hydrophilic DNA polyplex by photochemical internalization. Cell membranes are first loaded with an amphiphilic photosensitizer. (1) CD/DNA-PrS polyplex are formed. (2) Polyplexes bind to the plasma membrane and is taken into the cell together with the photosensitizer by endocytosis. (3) The photosensitizer and the polyplexs co-localize in the endosomes with the photosensitizer localized in the membrane of the endosome, while the polyplexs will localize in the lumen. (4) Light exposure leads to photo-induced rupture of the endosome with (5) subsequent release of the sequestered polyplex into the cytosol followed by incorporation in the nucleus. b Generation of intracellular 5-FU. Once the CD gene is expressed, the gene product cytosine deaminase converts exogenous prodrug 5-FC to 5-FU, killing the cell and exporting 5-FU. This results in damage to bystander non-transfected cancer cells
Materials and methods
Cell lines and plasmids
Cell lines
The human U87 and rat F98 glioma cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). The tumor cells were grown as monolayers in Advanced DMEM medium (Invitrogen Corp., Carlsbad CA., Cat. # 35101) with 2 % heat-inactivated fetal bovine serum (FBS), at 37 °C and 5 % CO2. The plasmid coding for the CD gene, used in the PCI mediated transfection experiments, was purchased from Add gene (Cambridge, MA). The plasmids were transformed in E. coli strain of DH5alpha and isolated with QIAGEN according to the manufacture's instruction. Plasmid DNA concentrations were measured with a spectrophotometer (Nanodrop 1000 Therma Scientific).
Polyamine gene carrier, protamine sulfate (PrS)
Protamine sulfate/DNA polyplexes were formed as previously described [20]. An N/P ratio of the PS/DNA polyplexes of 10:1 was used in all experiments.
Toxcicity of 5-FC and 5-FU
The direct effect of the prodrug 5-FC and the drug 5-FU on glioma cell viability was first assayed in monolayers of the cell lines. For this, 8 wells (in 96-well flat bottomed plates) for each concentration of the drugs used were seeded with F98 or U87 cells at a density of 5,000 cells per well and incubated for 24 h prior to experimentation. Following exposure to 5-FC or 5-FU, incubation was continued for 48 h, at which point the culture medium was replaced with fresh clear buffer containing MTS reagents (Promega, Madison, WI) and incubated for a further 2 h. The optical density was read using an ELx800uv Universal Microplate Reader (BIO-TEK Instruments, Inc).
PCI mediated gene transfection
Both cell monolayers and multi tumor cell spheroids were transfected in a similar manner. Spheroids were formed by a modification of the centrifugation method [23, 24]. Briefly, 2.5 × 103 U87 or F98 cells in 200 μl of culture medium were alloquated into the wells of ultra-low attachment surface 96-well round-bottomed plates (Corning Inc., NY). The plates were centrifuged at 1,000 rpm for 30 min. Spheroids were formed in every fourth column of 8 wells in the plate. 1 μg/ml of the photosensitizer AlPcS2a and the PrS/CD DNA polyplexes were added to the cell cultures and incubated for 18 h. Following a triple wash the monolayers or spheroids were incubated for 4 h in fresh medium to allow some of the photosensitizer to leach from the cell membrane in order to reduce the PDT toxic effects on the cells. Light treatment at various radiant exposures at an irradiance of 5 mW/cm2 was administered with λ = 670 nm light from a diode laser (Intense; New Jersey USA). Typically, 8–16 spheroids were followed in 3 individual trials for up to twenty-one days of incubation. Culture medium in the wells was exchanged every third day. Determination of spheroid size was carried out by averaging two measured perpendicular diameters of each spheroid using a microscope with a calibrated eyepiece micrometer and their volume calculated assuming a perfect sphere.
PCI transfection efficiency of the CD gene by fluorescent labeled antibody
5 × 104 F98 or U87 cells were plated out in 35 mm imaging dishes and incubated for 24 h. PCI mediated CD gene transfection was performed as previously described. Forty-eight hours later the monolayers were given a triple wash with HBSS (+) and Methanol fixation. The monolayers were incubated overnight at 4 °C with sheep polyclonal anti CD antibody (Abcam, Cambridge, MA. Cat. # ab35251). The dishes were washed twice and incubated for 1 h with Alexa Fluor 488 Donkey Anti Sheep antibody (Life Technology, Grand Island, NY, Cat. # A11015). Detection was done using an inverted Zeiss laser-scanning microscope (LSM 410, Carl Zeiss, Jena, Germany). The percent of transfected cells was calculated by taking the ratio of fluorescent cells to total cell numbers in multiple fields of view.
Generation of stable CD gene positive cell lines
Astable F98 cell line expressing both the CD and uracil phosphoribosyl transferase (UPRT) genes was cloned (designated CD1), using a fusion plasmid pSELECT-zeo-Fcy::fur, (InvivoGenSan Diego, CA. Cat # psrtz-fcyfur). This plasmid has combined the gene encoding uracil phosphoribosyl transferase (UPRT) to the CD gene [25, 26]. Cells positive for the CD gene were selected following iterative Zeocin treatment. Parental F98 cells gradually were eliminated over a 3 weeks period. Confirmation of the CD gene was done by fluorescent antibody staining and sensitivity to 5-FC (MTS).
Statistics
Microsoft Excel was employed for the calculation of the arithmetic mean, standard deviation, and standard error. Experimental data were analyzed using one-way analysis of variance (ANOVA) at the significance level of p < 0.05 and presented as mean with standard error unless otherwise noted.
Results
Direct toxicity on monolayers
The effects of 5-FC and 5-FU were examined on mono-layers of F98 and U87 cells in the MTS cytotoxicity assay. As shown in Fig. 2a, both cell lines were highly sensitive to 5-FU, with less than 20 % of the cells surviving at a concentration of as low as 5 μM. In contrast both cell lines were very insensitive to 5-FC. Incubation in 200 μM 5-FC for 48 h did not inhibit their growth. The effects of 5-FC and 5-FU were also examined on spheroids of F98 and U87 cells, shown in Fig. 2b. F98 multi cell spheroids were particularly susceptible to 5-FU with the growth of both cell line spheroids completely inhibited at a 5-FU concentration of 10 μM. As was the case for the monolayer cultures, the spheroids were not effected by 5-FC even at concentrations 40 times greater than those for 5-FU.
Fig. 2.
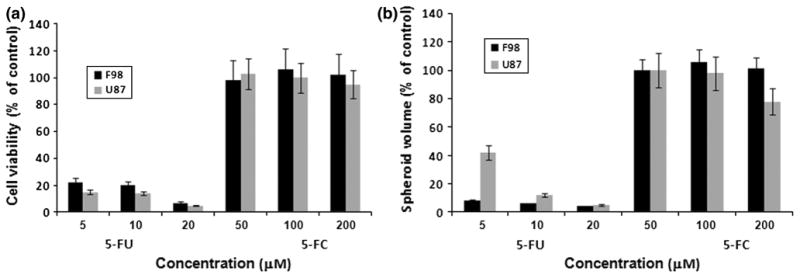
Sensitivity of glioma cells to increasing concentrations of 5-FC and 5-FU. a Cell viability in monolayer cultures incubated 48-h with 5-FU or 5-FC at the indicated concentrations, and then assayed by MTS. b Multicell glioma spheroid volume assayed following 21 days in culture. Each bar represents the results of 24 replicate cultures from 3 independent experiments and is shown as averaged percentages of control cultures ±SE
The effects of transduction of the CD gene
The presence of the CD gene product produced by normal or PCI mediated CD gene transfected F98 cells can be seen in Fig. 3a. A comparison between non-treated and PCI-CD transfected cells demonstrated a significant increase in the number of CD positive cells. The effects of PCI induced CD gene transfection for both F98 and U87 is shown in Fig. 3b. PCI had a dramatic effect on transfection efficiency for both cell lines tested, increasing from 3–4 to 30–40 % following PCI treatment. The % of CD positive transfected cells was dependent on the CD DNA concentration employed. There were no significant differences between the F98 or U87 cell monolayers in the number of CD positive cells following PCI transfection (p > 0.1).
Fig. 3.
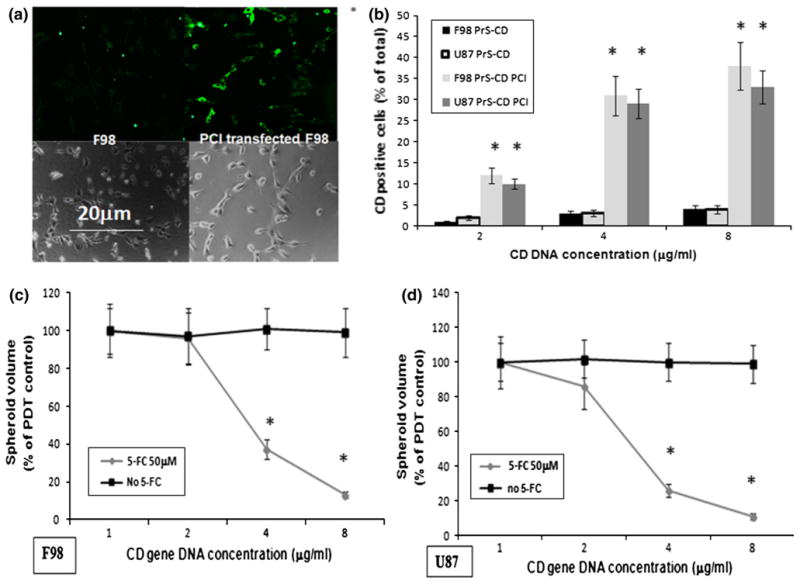
Effects of PCI-CD on gene transfection and on sensitivity of F98 and U87 spheroids to 5-FC. a Fluorescence microscopy images of F98 monolayer with and without PCI transfected CD gene cells. Cell monolayers incubated with anti-CD antibody and a second tagged antibody with green fluorescence; left panel F98 fluorescent and phase contrast, respectively; right panel transfected cells, fluorescent and phase contrast, respectively; green CD gene product labeled with fluorescent antibody. b % of CD positive F98 and U87 cells as determined from fluorescence microscopy images compared to identical regions on phase contrast images. c Effects of 5-FC on the growth and development of F98 and U87 spheroids following PCI-CD gene transfection. Spheroids incubated 18-h with PrS-CD polyplexs at CD DNA concentrations shown in the figure and AlPcS2a. Light treatment, 1.5 J/cm2 @ 5 mw/cm2. 50 μM 5-FC added to groups as shown in figure. Each experimental point represents the results of 24 replicate cultures from 3 independent experiments and is shown as averaged percentages of PDT control cultures ± SE. Asterisks represents significance compared to control values at p <0.05
Spheroid growth inhibition by 5-FC following PCI-CD gene insertion
The effects 5-FC on the growth and development of spheroids following PCI CD gene transfection is shown in Fig. 3c. F98 or U87 glioma spheroids were incubated for 18 h with the PrS-CD polyplexs (DNA concentration 0, 2, 4 or 8 μg/ml) together with AlPcS2a, and following 4 h incubation in fresh medium, treated with 1.5 J/cm2 of laser light. 50 μM 5-FC was added to the cultures in the experimental group. Radiant exposures representing 70-80 % cell survival (1.5 J/cm2) were chosen in order to optimize the PCI effect. Figure 3c shows the average spheroid volume as a percentage of PDT controls, measured after a 3-week period. Three identical experiments were performed with 16 spheroids in each group per experiment. As can be seen from Fig. 3c, PCI transfection of the CD gene in the absence of 5-FC had only a slight effect on spheroid growth, with no significant differences between the PDT control and the cultures containing the PrS-CD polyplexes at all the CD DNA concentrations tested. In contrast the addition of 5-FC for both F98 and U87 cells caused a significant growth inhibition (p < 0.05), that was dependent on the CD gene concentration employed for gene transfection. The differences between the two cell lines was not significant (p > 0.1) at all of the CD-DNA concentrations tested.
Growth inhibition by repetitive treatment
Since the effects of PCI-CD gene transfection were not pronounced at low CD DNA concentrations (1–2 μg/ml) repetitive treatment protocols were examined. The spheroids were transfected with either 1 or 2 complete PCI cycles 2 and 5 days following spheroid generation. 50 μM of 5-FC was added after light treatment in each case. Spheroid growth was followed in all for a period of 3 weeks. As seen in the low power micrograph taken after 2 weeks in culture (Fig. 4a), a second PCI mediated CD gene transfection and 5-FC dose significantly inhibited spheroid growth compared to single treatment. The kinetics of the growth of CD transfected spheroids following single and double treatment is shown in Fig. 4b and c for F98 and U87 respectively. Single treatment with 2 μg/ml CD DNA induced a growth delay but by week 3 spheroid volume was comparable to controls. In contrast, repetitive treatment for both cell types, especially at CD DNA concentration of 4 μg/ml, had a significant inhibitory effect (p < 0.05) compared to PDT controls.
Fig. 4.
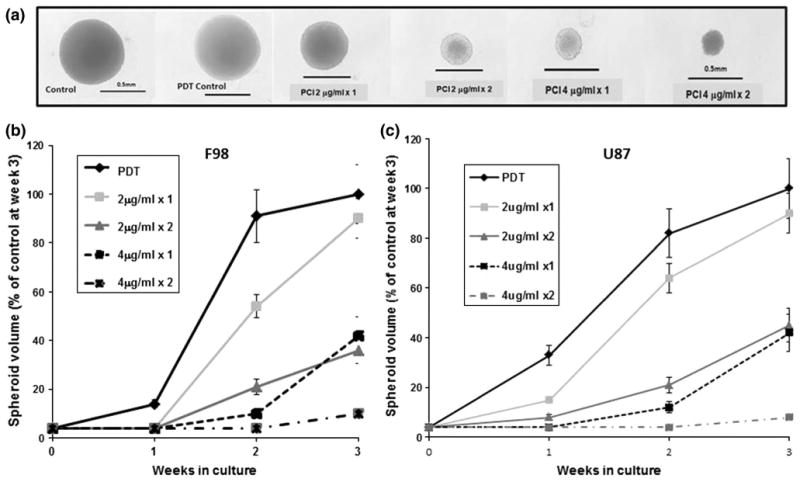
Effects of repetitive PCI PrS-CD gene transfection. F98 or U87 spheroids were transfected with 1 or 2 complete PCI cycles. 50 μM of 5-FC was added after light treatment per session. Spheroid growth was observed for a maximum period of 21 days. a Low power micrograph after 14 days for F98 cell spheroids. b growth kinetics of CD transfected F98 and U87 spheroids following single (91) or double treatment (×2). CD 2 and 4 in the figure refers to μg/ml of CD DNA plasmid used. The results are shown as a percentage of PDT controls ± SE. Each bar represents the average of 24 replicate cultures from 3 independent experiments
Analysis of the bystander effect
The CD1 cell line could be show by fluorescent labeled antibody to be 100 % CD positive (Fig. 5a). To determine the by stander effect, hybrid spheroids were generated containing varying ratios of CD positive (CD1) and CD negative native F98 cells. Each spheroid initially containing a total of 5 × 103 cells per well. 50 μM of 5-FC was added 24 h following spheroid generation to the experimental wells. 5-FC had a dramatic effect on the CD1 as well as the hybrid spheroids at all the F98:CD1 ratios tested. As shown in the photomicrographs (Fig. 5b), by week 3 of culture, spheroids containing only CD1 cells were completely disrupted and contained no viable cells. This was also the case for spheroids containing F98:CD1 ratios of 1:1, 2:1 and 4:1 where, by week 3, the spheroids had disintegrated (data not shown). Spheroids containing F98:CD1 ratios of 10:1 maintained their original (day 1) size and shape but did not disintegrate. Since by week 3 many of the spheroids had completely lost their initial shape the spheroid volume was assayed following only 2 weeks in culture. At all of the F98:CD1 ratios examined the spheroids showed no growth but remained at their initial volume (Fig. 5c). By week 3 many of the spheroids had disintegrated, in a similar pattern as seen in Fig. 5b, for CD1 spheroids.
Fig. 5.
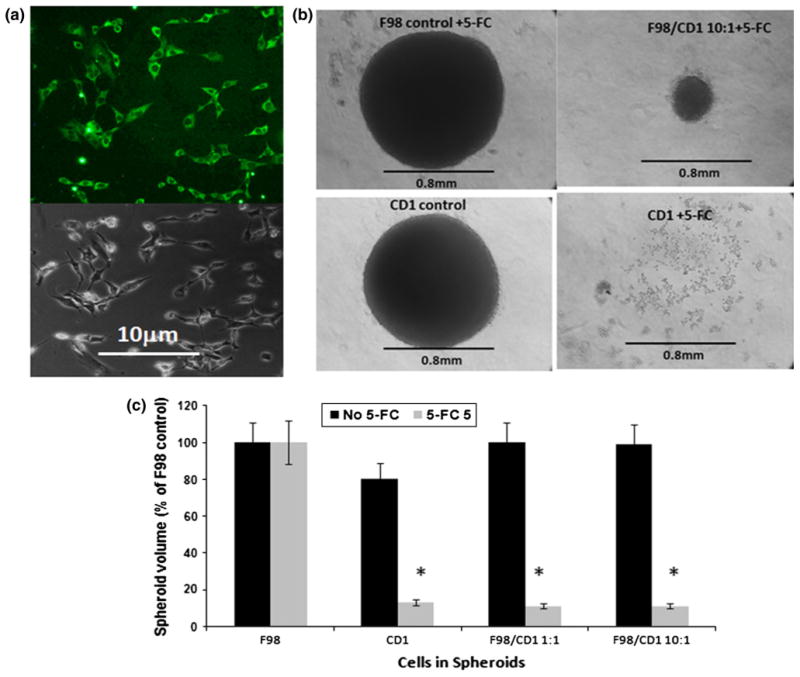
Effects of 5-FC on F98 + CD1 hybrid spheroids. a The CD1 cell line monolayer, show by fluorescent labeled antibody (green cells) to be 100 % CD positive. b Photomicrograph of CD1 and hybrid spheroid; F98:CD1 ratio, 10:1, exposed to 50 μM of 5-FC and assayed after 21-days in culture. Spheroids containing only CD1 cells were completely disrupted and contained no viable cells. This was also the case for spheroids containing F98:CD1 ratios of 1:1, 2:1 and 4:1, where by week 3, the spheroids had disintegrated (not shown in the figure). c CD1 and hybrid spheroids generated at 1:1 and 10:1 ratios of F98:CD1 cells. 50 μM of 5-FC added 24-h following spheroid generations, growth volume was assayed following 14-days in culture. The results are shown as averaged percentages of F98 control volume at day 14 ± SE. Each bar represents the the average of 24 replicate cultures from 3 independent experiments.* represents significance compared to no 5-FC control values at p < 0.05
Discussion
The ability to enhance the nonviral insertion of functioning prodrug activating genes into glioma cells by PCI formed the basis of the study presented here. The aim of PCI, in a more general context, would be the site-specific transfection of tumor cells limited to the nests of tumor cells sequestered in the margins of the resection cavity following surgical removal of bulk tumor, while minimizing a more wide spread damage to surrounding brain tissue. The rapid attenuation of light in the brain limits the efficacy of PCI to the light irradiated area.
Among the most studied suicide gene therapy approaches, activation of ganciclovir (GCV) by herpes simplex virusthymidine kinase (HSV-TK) and activation of 5-FC by the CD gene have been and are now presently being tested in clinical trials for glioma treatment [27].
For treatment of brain tumors it is important that the pro-drug can pass the blood brain barrier. 5-FC is a small molecule with relatively low protein binding and penetrates into cerebrospinal fluid (CSF). CSF concentrations of 5-FC have been shown to obtain about 75 % of serum concentration [28]. 5-FC has been used routinely for many years and has a high oral bio-availability of 80 % [29] compared to GCV's 6 % [30]. Additionally, the penetration of GCV into CSF and the brain is very low [31]. Moreover, serious side effects are not encountered when serum concentrations of 5-FC do not exceed 770 μM [32].
The results of the experiments presented here could demonstrate that PCI mediated non viral gene transfection, using protamine as gene carrier, significantly enhanced the effects of 5-FC on multi cell glioma spheroids derived from both rat and human cell lines. In a previous study, protamine/DNA polyplexes demonstrated a dramatic increase in transfection rate for the reporter GFP gene following PCI [20]. Transfection rates of 30–40 % were obtained and this agrees well with the results shown in Fig 3b. In particular, the observation that repeated PCI CD gene transfection (Fig. 4) was more efficient than single treatment is of importance. The low toxicity of a gene carrier like PrS, compared to polyethylenimine, would allow PCI iterative therapy and would therefore constitute a considerable advantage. The option of repetitive treatment protocols are severely limited if viral transfection vectors are employed owing to undesired immune responses to the viral carrier.
One of the advantages 5-FC CD therapy has over other forms of gene therapy is the pronounced bystander effect, especially if combined with the UPRT gene, as is the case for the CD1 cell line. Co-expression of CD and UPRT has been shown to increase the toxic effect of 5-FC by 1–3 orders of magnitude over treatment with CD/5-FC alone and provides a greater bystander effect [33–35].
Even 10 % of CD1 cells incorporated into hybrid F98 spheroids could completely stop their growth (Fig. 5c). This would indicate that a transfection efficiency of only 10 %, well below the 30–40 % PCI transfection rate observed employing immune fluorescence (fig 3b), is sufficient to inhibit tumor progression. For this reason we did not titrate the F98:CD1 cell ratio beyond the 10:1 level.
Although it is difficult to translate in vitro results into clinical expectations, PCI has the potential to increase non-viral gene transfection rates to those obtained with viral vectors and in a site specific manner. From a clinical point of view, in patients undergoing cyto reductive surgery, light could be applied via an indwelling balloon applicator implanted in the resection cavity [36–38]. CD gene transfection and subsequent bystander cytotoxicity would therefore be limited to the walls of the cavity where tumor recurrences usually form. This would also allow repetitive treatment protocols. In addition to the PDT effect on the endosomal membrane (PCI effect), PDT also has a direct effect on the vasculature in the vicinity of the illuminated volume. PDT has been shown to open the blood brain barrier both to drugs and to cells carrying magnetic nano-particles [39, 40]. This effect can prove advantageous by increasing delivery of gene carrying nanoparticles that can protect the gene-carrying polyplex during circulation, allowing for systemic injection and cellular internalization. [41]. In vivo studies in a rat glioma model, employing this type of nanoparticle in combination with PCI as a non viral delivery system for the CD gene, are now in progress.
Acknowledgments
This work was supported by grants from the Norwegian Radium Hospital Research Foundation and the Chao Cancer Center. Portions of this work were made possible through access to the Laser Microbeam and Medical Program (LAMMP) and the Chao Cancer Center Optical Biology Shared Resource at the University of California, Irvine.
Footnotes
Frederick Wang and Genesis Zamora have contributed equally to this Study.
Contributor Information
Frederick Wang, Email: frederick.wang.ix@gmail.com, Beckman Laser Institute and Medical Clinic, University of California Irvine, 1002 Health Sciences Rd, Irvine, CA 92617, USA.
Genesis Zamora, Beckman Laser Institute and Medical Clinic, University of California Irvine, 1002 Health Sciences Rd, Irvine, CA 92617, USA.
Chung-Ho Sun, Beckman Laser Institute and Medical Clinic, University of California Irvine, 1002 Health Sciences Rd, Irvine, CA 92617, USA.
Anthony Trinidad, Beckman Laser Institute and Medical Clinic, University of California Irvine, 1002 Health Sciences Rd, Irvine, CA 92617, USA.
Changho Chun, Department of Chemical Engineering/Material Science, University of California Irvine, Irvine, CA, USA.
Young Jik Kwon, Department of Chemical Engineering/Material Science, University of California Irvine, Irvine, CA, USA; Department of Pharmaceutical Sciences, University of California Irvine, Irvine, CA, USA.
Kristian Berg, Department of Radiation Biology, The Norwegian Radium Hospital, Oslo University Hospital, Oslo, Norway.
Steen J. Madsen, Department of Health Physics and Diagnostic Sciences, University of Nevada, Las Vegas, NV, USA
Henry Hirschberg, Beckman Laser Institute and Medical Clinic, University of California Irvine, 1002 Health Sciences Rd, Irvine, CA 92617, USA; Department of Health Physics and Diagnostic Sciences, University of Nevada, Las Vegas, NV, USA.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Brandes AA, Tosoni A, Franceschi E, Reni M, Gatta G, Vecht C. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67:139–152. doi: 10.1016/j.critrevonc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Petrecca K, Guiot MC, Panet-Raymond V, Souhami L. Failure pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with Glioblastoma. J Neurooncol. 2013;111:19–23. doi: 10.1007/s11060-012-0983-4. [DOI] [PubMed] [Google Scholar]
- 4.Culver KW, Ram Z, Wallbridge S, Ishii H, Oldfield EH, Blaese RM. In vivo gene transfer with retroviral-vector-producer cells for treatment of experimental brain tumors. Science. 1992;256:1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 5.Moolton F. Tumor chemosensitivity conferred by inserted herpes thymidine-kinase genes: paradigm for a prospective cancer-control strategy. Cancer Res. 1986;46:5276–5281. [PubMed] [Google Scholar]
- 6.Mullen CA, Coale MM, Lowe R, Blase RM. Tumors expressing the cytosine-deaminase suicide gene can be eliminated in vivo with 5-fluorocytosine and induce protective immunity to wild-type tumor. Cancer Res. 1994;54:1503–1506. [PubMed] [Google Scholar]
- 7.Kai G, Lingfei X, Zhongcheng Z, Dehua X, Lanyin S, Xinyuan L. Transduction of cytosine deaminase gene makes rat gliomas cells highly sensitive to 5-fluorocytosine. Int J Cancer. 1997;71:675–679. doi: 10.1002/(sici)1097-0215(19970516)71:4<675::aid-ijc26>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Miller RC, Williams CR, Buchsbaum DJ, Gillespie GY. Intratumoral 5-fluorouracil produced by cytosine deaminase/5-fluorocytosine gene therapy is effective for experimental human glioblastomas. Cancer Res. 2002;62:773–780. [PubMed] [Google Scholar]
- 9.Ostertag D, Amundson KK, Espinoza FL, Martin B, Buckley T, da Silva G, et al. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a non lytic retroviral replicating vector. Neuro-oncology. 2012;14:145–159. doi: 10.1093/neuonc/nor199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aboody KS, Najbauer J, Metz MZ, D'Apuzzo M, Gutova M, Annala AJ, Synold TW, Couture LA, Blanchard S, Moats RA, Garcia E, Aramburo S, Valenzuela VV, Frank RT, Barish ME, Brown CE, Kim SU, Badie B, Portnow J. Neural stem cell-mediated enzyme-prodrug therapy for glioma: preclinical studies. Transl Med Sci. 2013 doi: 10.1126/scitranslmed.3005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez OD, Logg CR, Hiraoka K, et al. Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol Ther. 2012 doi: 10.1038/mt.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paar M, Schwab S, Rosenfellner D, Salmons B, Gunzburg WH, Renner M, et al. Effects of viral strain, transgene position, and target cell type on replication kinetics, genomic stability, and transgene expression of replication-competent murine leukemia virus-based vectors. J Virol. 2007;81:6973–6983. doi: 10.1128/JVI.02470-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Guo S, Li Z, Liu L, Gu J. Synthesis and characterization of stearyl protamine and investigation of their complexes with DNA for gene delivery. Colloids Surf B Biointerfaces. 2009 doi: 10.1016/j.colsurfb.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 14.Sun X, Zhang N. Cationic Polymer Optimization for Efficient Gene Delivery. Mini Rev Med Chem. 2010;10:108–125. doi: 10.2174/138955710791185109. [DOI] [PubMed] [Google Scholar]
- 15.Høgset A, Engesaeter BO, Prasmickaite L, Berg K, Fodstad O, Maelandsmo GM. Light induced adenovirus gene transfer, an efficient and specific gene delivery technology for cancer gene therapy. Cancer Gene Ther. 2002;9:365–371. doi: 10.1038/sj.cgt.7700447. [DOI] [PubMed] [Google Scholar]
- 16.Berg K, Berstad M, Prasmickaite L, Weyergang A, Selbo PK, Hedfors I, Høgset A. Photochemical internalization (PCI): a new tool for gene and oligonucleotide delivery. Top Curr Chem. 2010;296:251–281. doi: 10.1007/128_2010_63. [DOI] [PubMed] [Google Scholar]
- 17.Selbo PK, Weyergang A, Høgset A, Norum OJ, Berstad MB, Vikdal M, Berg K. Photochemical internalization provides time and space-controlled endolysosomal escape of therapeutic molecules. J Control Release. 2010;148:2–12. doi: 10.1016/j.jconrel.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Maurice-Duelli A, Ndoye A, Bouali S, Leroux A, Merlin JL. Enhanced cell growth inhibition following PTEN nonviral gene transfer using polyethylenimine and photochemical internalization in endometrial cancer cells. Technol Cancer Res Treat. 2004;3:459–465. doi: 10.1177/153303460400300507. [DOI] [PubMed] [Google Scholar]
- 19.Ndoye A, Dolivet G, Høgset A, Leroux A, Fifre A, Erbacher P, Berg K, Behr JP, Guillemin F, Merlin JL. Eradication of p53-mutated head and neck squamous cell carcinoma xenografts using nonviral p53 gene therapy and photochemical internalization. Mol Ther. 2006;13:1156–1162. doi: 10.1016/j.ymthe.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Mathews MS, Shih EC, Zamora G, Sun CH, Cho SK, Kwon YJ, Hirschberg H. Glioma cell growth inhibition following photochemical internalization enhanced non-viral PTEN gene transfection. Lasers Surg Med. 2012;44:746–754. doi: 10.1002/lsm.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasmickaite L, Høgset A, Olsen VM, Kaalhus O, Mikalsen SO, Berg K. Photochemically enhanced gene transfection increases the cytotoxicity of the herpes simplex virus thymidine kinase gene combined with ganciclovir. Cancer Gene Ther. 2004;11:514–523. doi: 10.1038/sj.cgt.7700720. [DOI] [PubMed] [Google Scholar]
- 22.Madsen SJ, Sun CH, Tromberg BJ, Cristini V, De Magalhães N, Hirschberg H. Multicell tumor spheroids in photodynamic therapy. Lasers Surg Med. 2006;38:555–564. doi: 10.1002/lsm.20350. [DOI] [PubMed] [Google Scholar]
- 23.Ivascu A, Kubbies M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J Biomol Screen. 2006;11:922–932. doi: 10.1177/1087057106292763. [DOI] [PubMed] [Google Scholar]
- 24.Mathews MS, Blickenstaff JW, Shih EC, Zamora G, Vo V, Sun CH, Hirschberg H, Madsen SJ. Photochemical internalization of bleomycin for glioma treatment. J Biomed Opt. 2012;17:058001. doi: 10.1117/1.JBO.17.5.058001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adachi Y, Tamiya T, Ichikawa T, Terada K, Ono Y, Matsumoto K, Furuta T, Hamada H, Ohmoto T. Experimental gene therapy for brain tumors using adenovirus-mediated transfer of cytosine deaminase gene and uracil phosphoribosyltransferase gene with 5-fluorocytosine. Hum Gene Ther. 2000;11:77–89. doi: 10.1089/10430340050016175. [DOI] [PubMed] [Google Scholar]
- 26.Erbs P, Regulier E, Kintz J, Leroy P, Poitevin Y, Exinger F, Jund R, Mehtali M. In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene. Cancer Res. 2000;60:3813–3822. [PubMed] [Google Scholar]
- 27.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 28.Fass RJ, Perkins RL. 5-fluorocytosine in the treatment of cryptococcal and candida mycoses. Ann Intern Med. 1971;74:535–539. doi: 10.7326/0003-4819-74-4-535. [DOI] [PubMed] [Google Scholar]
- 29.Cutler RE, Blair AD, Kelly MR. Flucytosine kinetics in subjects with normal and impaired renal function. Clin Pharmacol Ther. 1978;24:333–342. doi: 10.1002/cpt1978243333. [DOI] [PubMed] [Google Scholar]
- 30.Morse GD, Shelton MJ, O'Donnell AM. Comparative pharmacokinetics of antiviral nucleoside analogues. Clin Pharmacokinet. 1993;24:101–123. doi: 10.2165/00003088-199324020-00002. [DOI] [PubMed] [Google Scholar]
- 31.Brewester ME, Raghavan K, Pop E, Bodor N. Enhanced delivery of ganciclovir to the brain through the use of redox targeting. Antimicrob Agents Chemother. 1994;38:817–823. doi: 10.1128/aac.38.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barriere SL. Pharmacology and pharmacokinetics of traditional systemicantifungal agents. Pharmacotherapy. 1990;10:134S–140S. doi: 10.1002/j.1875-9114.1990.tb02598.x. [DOI] [PubMed] [Google Scholar]
- 33.Huber BE, Austin EA, Richards CA, Davis ST, Good SS. Metabolism of 5-fluorocytosine to 5 fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci USA. 1994;91:8302–8306. doi: 10.1073/pnas.91.17.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams CW, Buchsbaum DJ, Miller CR. Uracil phosphoribosyltransferase potentiates 5-fluorouracil and cytosine deaminase/5-fluorocytosine cytotoxicity in prostate cancer. Proc Am Assoc Cancer Res. 2001;42:455. [Google Scholar]
- 35.Johnson AJ, Ardiani A, Sanchez-Bonilla M, Black ME. Comparative analysis of enzyme and pathway engineering strategies for 5FC-mediated suicide gene therapy applications. Cancer Gene Ther. 2011;18:533–542. doi: 10.1038/cgt.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madsen SJ, Sun CH, Tromberg BJ, Hirschberg H. Development of a novel indwelling balloon applicator for optimizing light delivery in photodynamic therapy. Lasers Surg Med. 2001;29:406–412. doi: 10.1002/lsm.10005. [DOI] [PubMed] [Google Scholar]
- 37.Johannesen TB, Watne K, Lote K, Norum J, Hennig R, Tverå K, Hirschberg H. Intracavity fractionated balloon brachytherapy in glioblastoma. Acta Neurchiurica. 1999;141:127–133. doi: 10.1007/s007010050276. [DOI] [PubMed] [Google Scholar]
- 38.Eljamel MS, Goodman C, Moseley H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: a single centre Phase III randomised controlled trial. Lasers Med Sci. 2008;23:361–367. doi: 10.1007/s10103-007-0494-2. [DOI] [PubMed] [Google Scholar]
- 39.Hirschberg H, Zhang MJ, Gach HM, Uzal FA, Chighvinadze D, Sun CH, Peng Q, Madsen SJ. Targeted delivery of bleomycin to the brain using photo-chemical internalization of Clostridium perfringens epsilon prototoxin. J Neurooncol. 2009;95:317–329. doi: 10.1007/s11060-009-9930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madsen SJ, Gach HM, Hong SJ, Uzal FA, Peng Q, Hirschberg H. Increased nanoparticle-loaded macrophage migration into the brain following PDT-induced blood-brain barrier disruption. Lasers Surg Med. 2013;45:524–532. doi: 10.1002/lsm.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho SK, Kwon YJ. Polyamine/DNA polyplexes with acid-degradable polymeric shell as structurally and functionally virus-mimicking nonviral vectors. J Control Release. 2011;150:287–297. doi: 10.1016/j.jconrel.2010.12.004. [DOI] [PubMed] [Google Scholar]


