Abstract
An overall multiscale simulation strategy for large scale coarse-grain simulations of membrane protein systems is presented. The protein is modeled as a heterogeneous elastic network, while the lipids are modeled using the hybrid analytic-systematic (HAS) methodology, where in both cases atomistic level information obtained from molecular dynamics simulation is used to parameterize the model. A feature of this approach is that from the outset liposome length scales are employed in the simulation (i.e., on the order of ½ a million lipids plus protein). A route to develop highly coarse-grained models from molecular-scale information is proposed and results for N-BAR domain protein remodeling of a liposome are presented.
Introduction
One of the most significant challenges facing the field of biomolecular simulation is the need to access the scales and complexity inherent in real biological systems, which has clearly spurred a rapid growth in the development of coarse-grained (CG) and other multiscale computational methods for biomolecular systems (see, e.g., ref. 1 and contributions therein). Within the context of membrane-protein systems, the ability to accurately simulate systems of proteins and lipids at the length and time-scales where these systems manifest their biological function presents a number of challenges. Real membranes are highly inhomogenous and include multiple lipids, cholesterol, and numerous proteins. The emerging picture of membranes, enhanced from the original fluid mosaic model,2 is one that emphasizes membrane crowding, varying membrane thickness, lipid spatial organization and even oligomerized proteins.3, 4 It is now appreciated that up to 20% or even higher of the membrane surface area is proteins.5
Protein mediated membrane remodeling is an example of a case whereby protein modules (e.g., the BAR (Bin/amphiphysin/Rvs) domain) can remodel entire liposomes having initial diameters of around 200 nm into thin tubulated structures with diameters on the order of 20 to 50 nm6–8 over time-scales significantly longer than microseconds.9 The BAR protein domain is a crescent-shaped dimer with a positively charged concave surface.6–18 The addition of an N-terminal amphipathic helix to this protein module results in an N-BAR domain and these have been observed to strongly tubulate liposomes in vitro.6–8 The N-terminal amphipathic helices “burrow” into the headgroup region of the upper membrane leaflet, resulting in a curvature generating “wedge” mechanism for membrane remodeling.8, 12, 13 The wedge mechanism of N-BAR mediated liposome remodeling has been supported by recent theoretical studies,19 and by large scale molecular dynamics (MD) simulations.13 Other remodeling mechanisms, including a “scaffolding”6, 11, 16, 17 and electrostatic sequestering4, 20 mechanism, have also been proposed. In all of these remodeling mechanisms, there is a strong degree of localized, atomistic level behavior that is collectively and ultimately responsible for the long length and time-scale process of liposome remodeling.
Large-scale atomistic MD simulation has proven invaluable in examining single N-BAR remodeling;12, 13 however, this methodology alone cannot reach the physiological length and time-scales (µm and µs) examined experimentally.6 CG simulation can potentially examine membrane-protein systems at very large length and timescales with a degree of quantitative predictability as long as the CG interactions can faithfully represent the averaged underlying atomistic-level interactions. To date, CG-MD has been employed to model both proteins and lipid bilayers (see ref. 1,21–25 for examples and reviews) including so-called “solvent-free” membranes,26–31 where the effects of the surrounding solvent are somehow incorporated into the CG lipid interactions. A previous CG simulation of N-BAR domain induced membrane remodeling18 has employed both high-resolution,32–34 and low-resolution shape-based CG schemes35 (resolved at about 150 atoms per CG site). These CG simulations were able to examine up to six N-BAR domains on a small slab of bilayer in different spatial arrangements. A generic highly coarse-grained model of protein mediated membrane remodeling has also been used to study vesiculation. 36 Still, despite the various CG schemes employed, the system sizes simulated have still been well below the liposome length scales from experiments.6
The 200 nm diameter liposomes employed in N-BAR remodeling experiments6 contain upwards of half a million lipids with possibly up to 2000 N-BAR domains bound to the surface. However, current CG simulations28, 30, 37–41 have modeled at best small vesicles with diameters in the range of 40 to 60 nm39 having typically less than 5000 lipids.28, 30, 37, 38 Current CG lipid models typically employ 10 to 15 CG sites per lipid,32, 42–45 resulting in about a 10 to 1 atom to CG site ratio. Thus, if CG methods are going to simulate N-BAR domain remodeling at experimental liposome length scales, some sort of alternative and aggressive coarse-graining scheme, combined with highly parallel computers, are needed. However, even as the degree of coarse-graining is increased, the systematic multiscale nature of the methodology must somehow be retained in order for the molecular level interactions to be bridged to the CG scale.
We have recently developed highly coarse-grained multiscale methods for lipids46 and proteins.47 The former is the hybrid analytic-systematic (HAS) approach that employs the multiscale coarse-graining methodology (MS-CG)45, 48–57 to augment and systematically parameterize key aspects of an otherwise generic analytic lipid model. The HAS approach thereby decomposes the total CG interaction into a systematic and an analytic component. The systematic part of the CG interaction is based on the MS-CG algorithm and is employed for those configurations that correspond to well sampled atomistic configurations as generated by the original MD simulation used to parameterize the MS-CG model. The analytic component describes the other configurations based on the global physical behavior.
The partitioning between the analytic and systematic components of the HAS CG interaction is tunable, and for very large systems the CG model can be initially biased towards the analytic component in order to determine the computational feasibility of a particular choice of CG scheme (e.g., is the number and rough location of the CG sites reasonable?). Then, the systematic MS-CG component can be introduced gradually in a sequence of iterations, until the model is biased towards as many interactions as possible obtained from underlying atomistic MD simulations. This approach helps to ensure computational feasibility throughout the process. It can also give important insights into the specific roles of the systematically obtained interactions as they are sequentially introduced. The tunability of the HAS approach will play a key role in the development of the full protein-membrane model to be discussed soon.
The HAS analytic component is based on the Gay-Berne ellipsoid (GB) particle model,58, 59 and was selected as it requires only a single CG site at the center of the ellipsoid (denoted the GB CG site), along with a unit vector, to fully specify the GB ellipsoid’s location and orientation. However, the HAS approach is quite general and other analytic models can instead be employed. Small modifications to the analytic component of the interaction can give the model the symmetry of a lipid, i.e., a “head” and a “tail”.46 The result is a low resolution CG lipid model46 resolved at about a 100 to 1 atom to CG site ratio, but also a CG model that has many of its interactions systematically obtained from actual molecular-scale forces.
The present CG protein model47 employs a variant of the fluctuation matching method60 to define a heterogeneous elastic network model (HeteroENM), in this case for an N-BAR protein using our essential dynamics coarse-graining (ED-CG)61 approach to define the protein CG sites. The level of coarse-graining in this case is also approximately 100 atoms to 1 CG site.
The focus of the remainder of this paper will be to describe our multiscale strategy for large length scale membrane protein systems. The previously introduced HAS CG membrane (which will be referred to simply as the HAS membrane) along with the ED-CG HeteroENM N-BAR domain model (which will be denoted as the ED-BAR model) will be combined to give a unified multiscale CG membrane-protein model capable of operating at liposome length scales. Simulations of the early stages of N-BAR domain induced remodeling of a liposome will be used as a demonstration of the overall methodology.
A “divide and conquer” approach to systematic multiscale simulation is employed. From the outset, a fully realized ED-BAR liposome system is considered. The flexible and tunable HAS approach is applied to all CG interactions in the system, where initially the CG simulation is biased towards the analytic component. The underlying analytic interactions are then sequentially replaced with more fully realized systematic interactions as more atomistically detailed information is made available. The overall process is evolutionary in that aspect and, as will be discussed, there are still components of the interaction that will undergo further refinement in the future.
Simulation
Simulations were composed of 406 092 HAS CG lipids and 52 800 ED-BAR CG sites, the latter corresponding to 2400 ED-BAR domains. The liposome by itself was initially equilibrated, and then the ED-BARs were arranged in a disordered array just beyond the liposome surface. A total of 100 ns of CG simulation was then performed over 512 processors using our in-house CG code TANTALUS, which employs a dual spatial decomposition capable of easily scaling over 1000 processors.
Results and discussion
The ED-BAR and HAS lipid model are graphically depicted in Fig. 1a and 1b, respectively. The algorithmic details and exact functional forms of the interactions for both the protein and lipid model can be found elsewhere.46, 47 Here, aspects pertaining to the hierarchical coarse-graining strategy will be discussed. Two main components of the multiscale CG methodology have been previously developed, these are the ED-BAR protein and the HAS membrane. More details on these models are given below. In order to construct the full ED-BAR HAS membrane system, new cross interactions must be specified, and these will also be presented.
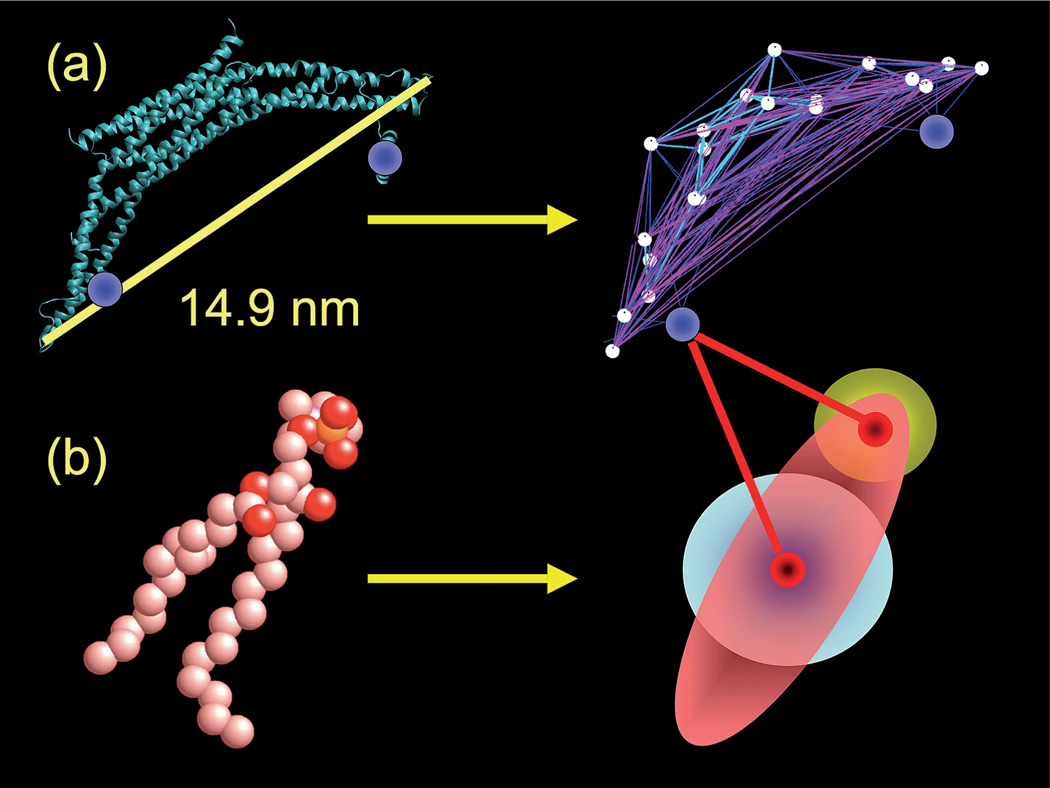
Fig. 1.
The CG model used in the ED-BAR-liposome simulation. Part (a) shows the hetero-ENM model of the ED-BAR domain as derived from the fully atomistic representation. The two amphipathic helices (highlighted by blue spheres in the left panel) are modeled by two additional sites, as shown in the right panel image. Part (b) depicts the HAS CG lipid model. The fully atomistic lipid is modeled as a single site GB ellipsoid of revolution, augmented with a radially symmetric MS-CG interaction. See ref. 46 for more details. An additional head-group site interacts with the ED-BAR domain, but does not alter the total lipid–lipid interaction.
Heterogeneous elastic network model of an N-BAR domain
Elastic network models (ENMs) for proteins typically build an elastic network between pairs of α carbons that are within a fixed cut-off distance62 with a uniform spring constant.63, 64 These ENMs have been employed to examine a number of proteins and macromolecular complexes.65–68 A more accurate heterogeneous elastic network can instead be constructed by fitting the various spring constants to thermal fluctuations of coarse-grained distances as calculated from atomistic MD.47 The inclusion of detailed atomistic level information allows for the systematic parameterization of different elastic models of the same protein in different environments, e.g., an N-BAR in solution or bound to a membrane.
The optimal locations of the 20 CG sites of the ED-BAR were determined using the ED-CG method,61 where the locations of coarse-grained sites were selected such that the essential low frequency dynamics observed in the atomistic simulation are reproduced. An N-BAR bound to a lipid bilayer was employed for the parameterization. 12 Thus, both the location, as well at the interactions, of the ED-BAR CG sites were found from atomistic MD simulation. The N-BAR domain at the atomistic level, as shown in the left panel of Fig. 1a, is thus mapped into an ED-BAR model. For this work, additional CG sites corresponding to the N-terminal amphipathic helices were also included on the ED-BAR; however, at this level of coarse-graining the entire N-terminal helix was reduced to a single CG site. Future work will resolve these particular sites at higher levels of CG resolution, since they account for only about 2% of the total CG sites of the system.
Hybrid analytic-systematic (HAS) lipid bilayer model
The membrane in the present work employs the HAS model as was previously done.46 The original lipid, as shown in the left panel of Fig. 1b, is mapped into a single ellipsoid of revolution with a different head and tail interaction as shown in the right panel of Fig. 1b. The additional analytic component of the interaction (shown as the white sphere in the center of the ellipsoid) modulates the generic GB interaction to give the desired membrane material properties (i.e., the bending modulus, area compressibility, and diffusion) that are representative of a lipid bilayer in the liquid crystal phase. In fact, without the systematic component of the HAS interaction the membrane generally freezes into a solid.46 Previous work parameterized the model for a pure dimyristoylphosphatidylcholine (DMPC) membrane, which generally is not what is used in experiments, which typically employ mixtures of dioleoylphosphatidylserine (DOPS) and dioleoylphosphatidylcholine (DOPC).69, 70 We are currently developing a CG mixture formulation for DOPS and DOPC using MS-CG71 which can be incorporated into the HAS approach. For the present study the membrane can be considered as a single site lipid model with (in part) atomistically determined interactions that result in reasonable material properties for the system under consideration. This HAS lipid model is taken to be a good starting point for the subsequent CG simulation of N-BAR domain induced membrane remodeling.
The HAS approach for protein–lipid cross interactions
With the ED-BAR and HAS membrane in hand, the remaining task is to develop a hierarchical approach whereby systematically obtained cross interactions can be sequentially incorporated into the overall ED-BAR/HAS membrane model. The initial starting point is thus to define a set of reasonable starting analytic interactions between the various CG components (i.e., the ED-BAR/ED-BAR and ED-BAR/ HAS lipid interactions).
Each CG site on one ED-BAR (a total of 20 sites plus 2 for the N-termini) interacts with each site on another ED-BAR. Each ED-BAR CG site also interacts with the headgroup and GB CG sites on the HAS lipid (as shown in Fig. 1). Spherical ED-BAR CG sites interact with the GB ellipsoid with a GB sphere-ellipsoid interaction, employing the 3 : 1 aspect ratio of the HAS lipid.46 This additional analytic component models the excluded volume of the lipid tails and the BAR protein. The interaction strengths for the analytic components were initially varied, and the resulting behavior of the system, in terms of ED-BAR binding and membrane remodeling, was observed. Such preliminary insight into how sensitive are particular initial analytic interactions to the overall process of ED-BAR binding and membrane remodeling can prove quite valuable when the systematic modeling components are introduced later.
The analytic interaction between ED-BARs employs a standard Lennard–Jones (LJ) inverse-power law interaction, expressed as u(r) = 4ε [(σ/r)12 − (σ/r)6] where r is the distance between two CG sites, σ gives the length scale of the interaction, and ε is the interaction strength. Different interaction strengths with ε = 0.2 to 1 kcal mol−1 were initially examined in order to determine a physically meaningful interaction strength such that the ED-BARs did not coalesce and condense into an agglomerated phase. The final analytic interaction was chosen to be purely repulsive, obtained with ε = 0.24 kcal mol−1 and truncating u(r) at a distance of 21/6 σ, with σ = 2 nm. The behavior of the ED-BAR/HAS membrane system, in terms of ED-BAR binding and remodeling, was found to be relatively insensitive to the ED-BAR/ED-BAR interaction strength. Employing the repulsive interaction at this point greatly reduced the cutoff distance of the interaction, resulting in a measurable computational speed-up. A more rigorous multiscale parameterization would require extensive multi-N-BAR atomistic-level simulations in order to extract a potential of mean force (PMF) between ED-BAR CG sites. This work is currently underway.
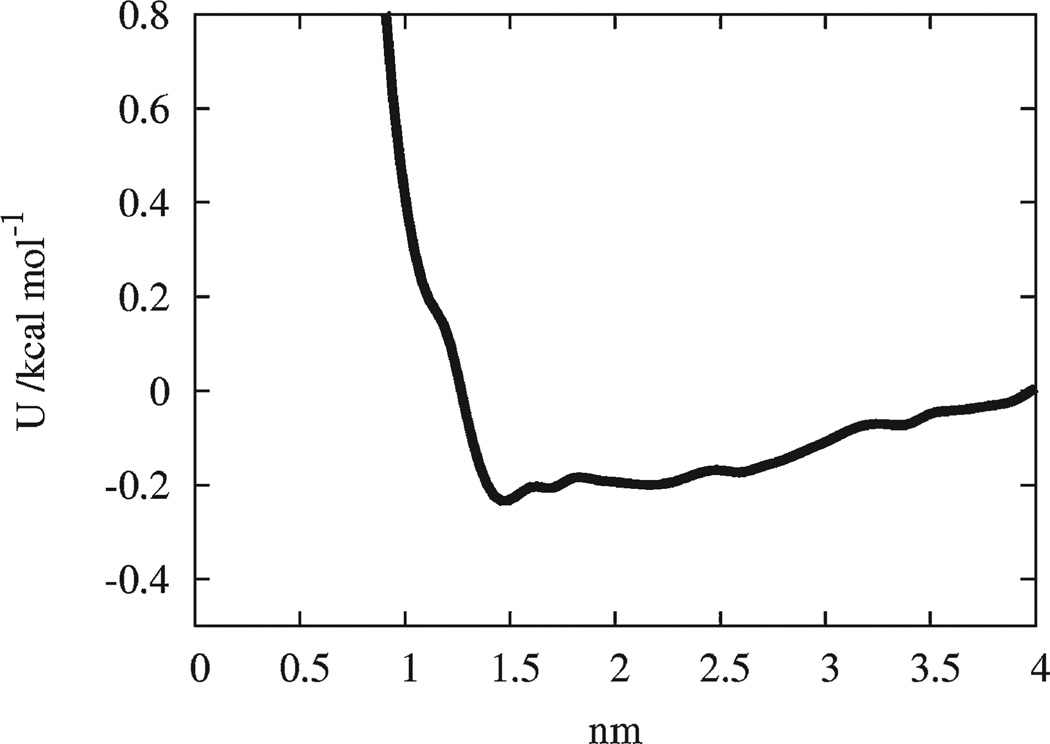
The initial analytic cross interactions between the CG sites of an ED-BAR (excluding the N-terminal sites) and the headgroup site of the HAS lipid (see Fig. 1) were also taken to be of a LJ form. The systematic multiscale component was incorporated at this point from an atomistically obtained averaged radial distribution function between the phosphorus atom in the lipid headgroups (as shown in Fig. 1) and the centers of mass of the positively charged residues underneath the arch of the N-BAR. As shown in Fig. 2, a PMF was then constructed, and the resulting well depth of the interaction was employed to parameterize the LJ cross interaction. The well depth was found to be around 0.2 kcal mol−1, and the repulsive wall of the interaction was at about 1.5 nm. The range of the attractive component of the interaction was found to be on the order of 3 to 4 nm. This interaction is not strong enough alone for the individual ED-BARS to strongly bind onto the membrane, and at least at this level of analysis suggests that electrostatic binding of the BAR domain “arch” to the lipid headgroups may not be the dominant binding interaction for N-BAR domains on membranes. A more advanced form of this interaction would not average over all the residues on the N-BAR arch, and it could instead employ a tabulated potential interaction obtained from the MS-CG method.45, 48–56 This approach requires additional MD simulations in order to obtain statistically reasonable sampling for these interactions.
Fig. 2.
The potential of mean force (PMF) obtained from the distribution function for the distance between the phosphorus atom in the lipid headgroups to the center of mass of the positively charged residues underneath the BAR domain.
The N-terminal amphipathic helix CG sites of the ED-BAR were given a LJ interaction such that they can bury themselves and bind into the upper leaflet of the membrane. This is depicted in Fig. 1b where the lines connecting the N-terminal amphipathic CG site on the ED-BAR with the two sites of the HAS lipid designate the interaction between sites. Given that the entire elongated helix is mapped into a single spherical site at this point, it proved difficult to accurately map an atomistically determined PMF onto the single CG interaction site. The approximate “volume” of the helix was thus employed to reproduce the size of the ED-BAR helix, and interaction strengths ranging from ε = 5 to 15 kcal mol−1 were tested. The large value of the interaction strength draws from the observation from MD simulation that the N-terminal helices generally remain tightly bound in the upper leaflet of the bilayer.12 Over this range, the interaction resulted in ED-BARs burying their N-terminal sites into the upper leaflet of the membrane without penetrating through to the other side, but also not consistently floating off. The next level of refinement of this model would be to better resolve the CG N-terminal helix with possibly two or three sites as determined from the ED-CG analysis such that an atomistically obtained PMF can be constructed. This work is currently under way.
Simulation results
The overall behavior of the final ED-BAR HAS membrane model is such that the ED-BARs can bind and unbind to the liposome surface. The N-terminal sites can embed into, and dislodge from, the upper leaflet. The interaction between the ED-BARs and membrane is not so strong that the ED-BARs automatically condense on the membrane surface, yet they are not so weak that they remain unbound.
In Fig. 3, snapshots of the ED-BAR liposome after 100 ns of CG simulation time are shown. After a simulation of this relatively short duration (keeping in mind that CG time has no relation to “real” time without additional modifications72 and that we have made no attempt to re-scale time as is often done in CG modeling), only the early stages of liposome remodeling are examined here. The original system shows the cloud of ED-BARs over the liposome surface, with some bound, and some not. It should be noted that the starting configuration set the ED-BARs at a close proximity to the liposome surface, but they were not embedded in the bilayer from the outset. During the course of the simulation, the ED-BAR cloud remained intact, although it can be seen that some ED-BARs float off into the surrounding medium.
Fig. 3.
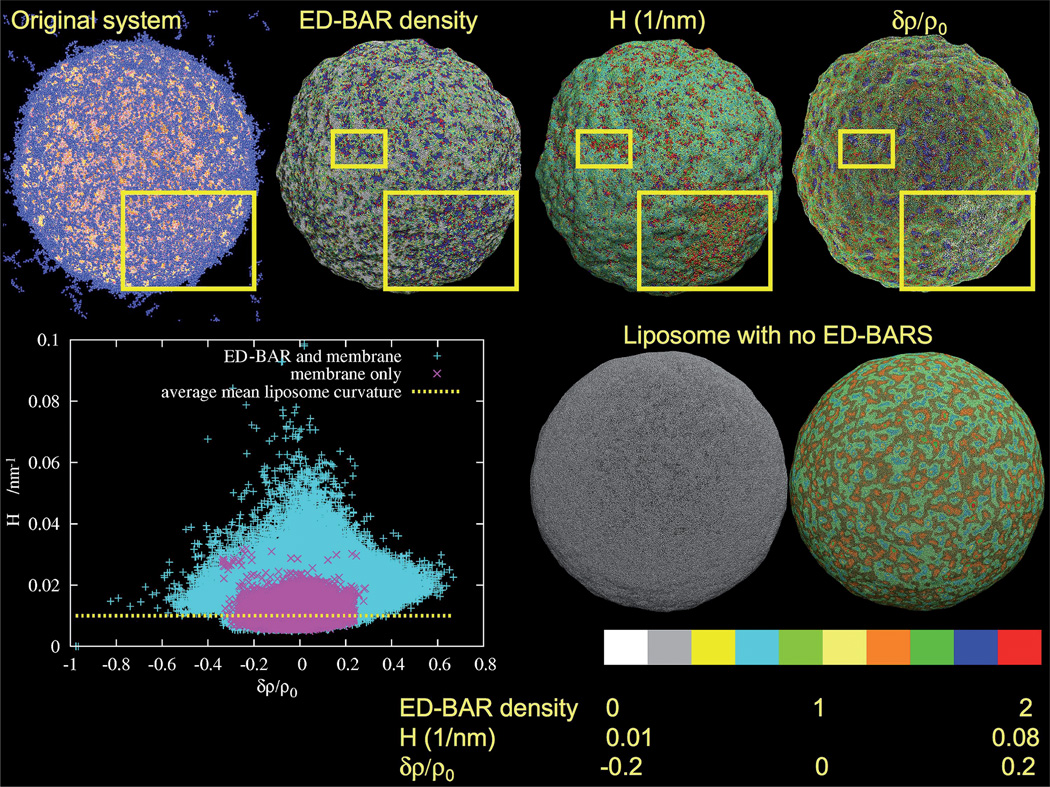
Analysis of the ED-BAR density, mean curvature, H, and relative membrane density change, δρ/ρ0, in the remodeled and isolated liposomes. The original system is a snapshot of the ED-BAR coated liposome. The local ED-BAR density shows local ED-BAR enhancements and depletions that are correlated with both the mean curvature and membrane density. The yellow squares highlight regions where the correlations are most clearly visible. The lower panel on the right shows corresponding plots for the isolated liposome. The scale bar at the bottom gives the reference for each color in the upper panels. The inset at the lower left plots the mean curvature versus liposome density for the two systems. Purple is for the isolated liposome while cyan shows how the local mean curvature is enhanced and distorted because of the ED-BAR membrane remodeling. The upper values of the mean induced curvature correspond to those observed from MD simulations (i.e., about half that observed from MD simulation of a single N-BAR domain,12 since the mean curvature is the average over two principle curvatures and the single N-BAR domain induces curvature in only one direction).
It is possible to measure the average N-BAR density, the membrane curvature, and the local membrane density using the density field discretization procedure employed in smooth particle applied mechanics.73 The color bar at the bottom of Fig. 3 gives an indication of the behavior of various quantities over the liposome surface. The local ED-BAR density gives the density of ED-BAR CG sites in close proximity to the membrane surface. Clearly, the ED-BAR density is far from homogenous and it shows enhancements and depletions over the surface. The regions highlighted in the yellow squares were selected as they visibly show a local ED-BAR density enhancement. When correlated with the mean curvature, H, it suggests that these regions also have an enhanced curvature. Furthermore, the relative membrane density change, δρ/ρ0, in this (and similar) regions exhibits depletion. Putting all three together, it suggests that the N-terminal sites have penetrated into the upper leaflet of the membrane, resulting in an induced curvature and subsequently a lower membrane density.
When compared to a liposome with no ED-BARs (cf. Fig. 3, lower right), it is clear how significant the initial stages of the membrane remodeling are. Both simulations were performed for the same duration. However, the isolated liposome with no ED-BARs does not exhibit any large curvature deviations and only has small local density fluctuations. It may in fact be possible that the ED-BARs are seeking out regions of the liposome surface with a slightly lower density so the amphipathic helices can be more readily inserted, rather than just seeking out regions with an enhanced curvature.
The correlation between the ED-BAR induced curvature and membrane density is shown in the inset in the lower left of Fig. 3. Each point on the plot corresponds to a HAS CG lipid site at some point in space r. The local mean curvature, H, for liposomes with and without ED-BARS is plotted versus relative membrane density change, δρ/ρ0, where ρ0 is the average density δρ = ρ − ρ0, and ρ is the density at r. Both the upper and lower monolayers are included in this calculation. The liposome without ED-BARS shows only a small variation of curvature that on average recovers the mean curvature of the liposome. However, the presence of the ED-BARS greatly modifies the liposome structure. The membrane density is spread out and the curvature is increased everywhere. Interestingly, the largest mean curvatures correspond to what has been predicted from MD simulations of a single N-BAR,12 suggesting that the multiscale nature of the present CG methodology is robust.
Fig. 4 gives some evidence as to how the ED-BARS locally remodel the liposome curvature. The four snapshots of the liposome were found by rotating the liposome, and selecting out regions with distinct structural motifs. The yellow box highlights specific regions for discussion. In (a), a pronounced “double hump” is observed, where the tails of the ED-BARs protrude into the “divot” between the two humps. The N-terminal sites are not right at the end of the ED-BARS and can still embed into the bilayer at the bottom of the divot. The measurement of the mean curvature in these very highly curved divots is suspect due to the fact that the CG membrane structure in these regions is so highly disrupted. This type of ripple structure appears to be evident over the surface of the liposome and seems to correspond to a local mechanism whereby ED-BARS can generate strong curvature, yet still retain the membrane structural integrity. Panel (b) shows a large remodeled section where the embedded helices in the upper leaflet are visible. The lower leaflet remains surprisingly structurally intact. Panel (c) shows just how significantly the ED-BAR induced remodeling can proceed, where the membrane has almost folded in on itself. This particular region, at much longer simulation times, may potentially become a region where tubulation is initiated. Panel (d) shows a relatively flat region of the membrane surface with a correspondingly low ED-BAR density. In this small region, the membrane curvature does not deviate greatly from the average liposome curvature.
Fig. 4.
Selected close-up snapshots of the remodeled liposome, obtained by rotating the liposome and selecting out specific regions. The square boxes highlight specific remodeled regions of interest: (a) shows a double hump, (b) a large curved region where the embedded helix sites are visible, (c) a tightly bent region with a high density of ED-BARs on the surface, and (d) a relatively flat region with a correspondingly low ED-BAR density in that region.
It is interesting how the ED-BARs are able to globally increase the local curvatures of the liposome without drastically altering its radius. The radius of the liposome without ED-BARs averaged to 96 ± 3 nm, while the liposome with ED-BARs was 88 ± 5 nm, where the enhanced curvature fluctuations arise from the ED-BAR induced membrane sculpting. The overall driving force for remodeling also appears to be related to N-terminal helix insertion. Indeed, some preliminary simulations were performed without the N-terminal helices, and in those cases either the ED-BARs did not bind to the membrane or they elected to, quite often, bind sideways or too deeply in the membrane. In either case, the pronounced and robust membrane sculpting observed in the simulations with ED-BARs containing N-terminal helices was not observed to the same degree.
The above observations seem consistent with the “wedge” mechanism of N-BAR remodeling.8, 13 In fact, N-BARs may not be curvature sensors as much as they are density sensors. The N-terminal amphipathic helices seek out regions of the liposome surface with a slightly lower density so that they can more efficiently embed themselves into the outer leaflet of the bilayer. The “arch” of the N-BAR domain, with its screened electrostatic interactions to the lipid headgroups, may exist primarily to “loosen up” the lipid headgroup density and also to scaffold the N-terminal amphipathic helices in place. The result of this is a curvature generation that, when combined with some degree of N-BAR oligomerization, can result in a spontaneous curvature along one direction and hence tubulation. The electrostatic effect gives a soft attractive binding force of the N-BAR to the liposome surface, but it is not enough to drive remodeling without the additional helix insertions into the upper membrane leaflet.
Conclusions
The paper has outlined a general multiscale simulation methodology for protein– membrane systems applicable to very large length scales where highly coarse-grained models are required, but molecular-scale information can still be employed to parameterize key aspects of the CG model. The example of N-BAR induced membrane remodeling at full liposome length scales was employed to demonstrate the features of the methodology. The hybrid analytic-systematic (HAS) CG approach, where the CG interactions contain both analytic and systematic components, provides a means whereby initial analytic components of the overall model can be replaced by systematically determined interactions obtained in a multiscale fashion from underlying MD simulations.
Future work will focus on increasing the overall systematic nature of the model. For example, a more refined lipid–lipid interaction from the MS-CG method will be employed that maps over to the actual lipid mixtures used in experiments. The N-BAR membrane interaction will also be refined based on molecular simulation. Another key component will be to model the N-BAR oligomerization interactions at a higher level of detail. This effort will draw on extensive atomistic-level MD simulations of multi-N-BAR systems, and this work is currently under way.
Acknowledgements
This research was supported by the National Institutes of Health (R01-GM063796). Computational resources were provided by the National Science Foundation through TeraGrid computing resources, specifically the Texas Advanced Computing Center. We acknowledge Professor Vinzenz Unger of Yale University for helpful discussions and insights.
References
- 1.Voth GA, editor. Coarse-graining of condensed phase and biomolecular systems. Boca Raton: CRC Press/Taylor and Francis Group; 2009. [Google Scholar]
- 2.Singer SJ, Nicolson GL. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 3.Engelman DM. Nature. 2005;438:578–580. doi: 10.1038/nature04394. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin S, Murray D. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 5.Dupuy AD, Engelman DM. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2848–2852. doi: 10.1073/pnas.0712379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJG, Evans PR, McMahon HT. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 7.Takei K, Slepnev VI, Haucke V, De Camilli P. Nat. Cell Biol. 1999;1:33–39. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- 8.Gallop JL, Jao CC, Kent HM, Butler PJG, Evans PR, Langen R, McMahon HT. EMBO J. 2006;25:2898–2910. doi: 10.1038/sj.emboj.7601174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masuda M, Takeda S, Sone M, Ohki T, Mori H, Kamioka Y, Mochizuki N. EMBO J. 2006;25:2889–2897. doi: 10.1038/sj.emboj.7601176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallop JL, McMahon HT. Biochem. Soc. Symp. 2005;72:223–231. doi: 10.1042/bss0720223. [DOI] [PubMed] [Google Scholar]
- 11.Mattila PK, Pykalainen A, Saarikangas J, Paavilainen VO, Vihinen H, Jokitalo E, Lappalainen P. J. Cell Biol. 2007;176:953–964. doi: 10.1083/jcb.200609176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blood PD, Voth GA. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15068–15072. doi: 10.1073/pnas.0603917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blood PD, Swenson RD, Voth GA. Biophys. J. 2008;95:1866–1876. doi: 10.1529/biophysj.107.121160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayton GS, Blood PD, Voth GA. Biophys. J. 2007;92:3595–3602. doi: 10.1529/biophysj.106.101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Futterer K, Machesky LM. Cell. 2007;129:655–657. doi: 10.1016/j.cell.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Shimada A, Niwa H, Tsujita K, Suetsugu S, Nitta K, Hanawa-Suetsugu K, Akasaka R, Nishino Y, Toyama M, Chen L, Liu Z-J, Wang B-C, Yamamoto M, Terada T, Miyazawa A, Tanaka A, Sugano S, Shirouzu M, Nagayama K, Takenawa T, Yokoyama S. Cell. 2007;129:761–772. doi: 10.1016/j.cell.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 17.Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman EH, De Camilli P, Unger VM. Cell. 2008;132:807–817. doi: 10.1016/j.cell.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arkhipov A, Yin Y, Schulten K. Biophys. J. 2008;95:2806–2821. doi: 10.1529/biophysj.108.132563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campelo F, McMahon HT, Kozlov MM. Biophys. J. 2008;95:2325–2339. doi: 10.1529/biophysj.108.133173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamper N, Shapiro MS. J. Physiol. 2007;582:967–975. doi: 10.1113/jphysiol.2007.132787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayton GS, Noid WG, Voth GA. Curr. Opin. Struct. Biol. 2007;17:192–198. doi: 10.1016/j.sbi.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Tozzini V. Curr. Opin. Struct. Biol. 2005;15:144–150. doi: 10.1016/j.sbi.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Lindahl E, Sansom MSP. Curr. Opin. Struct. Biol. 2008;18:425–431. doi: 10.1016/j.sbi.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Sherwood P, Brooks BR, Sansom MSP. Curr. Opin. Struct. Biol. 2008;18:630–640. doi: 10.1016/j.sbi.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liwo A, Czaplewski C, O1dziej S, Scheraga HA. Curr. Opin. Struct. Biol. 2008;18:134–139. doi: 10.1016/j.sbi.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brannigan G, H. Brown FL. J. Chem. Phys. 2004;120:1059–1071. doi: 10.1063/1.1625913. [DOI] [PubMed] [Google Scholar]
- 27.Brannigan G, L. Lin LC, Brown FLH. Eur. Biophys. J. 2006;35:104–124. doi: 10.1007/s00249-005-0013-y. [DOI] [PubMed] [Google Scholar]
- 28.Cooke IR, Deserno M. Biophys. J. 2006;91:487–495. doi: 10.1529/biophysj.105.078683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooke IR, Deserno M. J. Chem. Phys. 2005;123:224710. doi: 10.1063/1.2135785. [DOI] [PubMed] [Google Scholar]
- 30.Cooke IR, Kremer K, Deserno M. Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys. 2005;72:011506. doi: 10.1103/PhysRevE.72.011506. [DOI] [PubMed] [Google Scholar]
- 31.Farago O. J. Chem. Phys. 2003;119:596–605. [Google Scholar]
- 32.Marrink SJ, deVries AH, Mark AE. J. Phys. Chem. B. 2004;108:750–760. [Google Scholar]
- 33.Marrink SJ, Risselada HJ, Yefimov S, Tieleman DP, deVries AH. J. Phys. Chem. B. 2007;111:7812–7824. doi: 10.1021/jp071097f. [DOI] [PubMed] [Google Scholar]
- 34.Shih AY, Arkhipov A, Freddolino PL, Schulten K. J. Phys. Chem. B. 2006;110:3674–3684. doi: 10.1021/jp0550816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arkhipov A, Freddolino PL, Schulten K. Structure. 2006;14:1767–1777. doi: 10.1016/j.str.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Reynwar BJ, Illya G, Harmandaris VA, Muller MM, Kremer K, Deserno M. Nature. 2007;447:461–464. doi: 10.1038/nature05840. [DOI] [PubMed] [Google Scholar]
- 37.Lyubartsev AP. Eur. Biophys. J. 2005;35:53–61. doi: 10.1007/s00249-005-0005-y. [DOI] [PubMed] [Google Scholar]
- 38.Markvoort AJ, van Santen RA, J. Hilbers PA. J. Phys. Chem. B. 2006;110:22780–22785. doi: 10.1021/jp064888a. [DOI] [PubMed] [Google Scholar]
- 39.Risselada HJ, Mark AE, Marrink SJ. J. Phys. Chem. B. 2008;112:7438–7447. doi: 10.1021/jp0758519. [DOI] [PubMed] [Google Scholar]
- 40.Markvoort AJ, Pieterse K, Steijaert MN, Spijker P, J. Hilbers PA. J. Phys. Chem. B. 2005;109:22649–22654. doi: 10.1021/jp053038c. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto S, Maruyama Y, Hyodo S. J. Chem. Phys. 2002;116:5842–5849. [Google Scholar]
- 42.Faller R, Marrink SJ. Langmuir. 2004;20:7686–7693. doi: 10.1021/la0492759. [DOI] [PubMed] [Google Scholar]
- 43.Stevens MJ. J. Chem. Phys. 2004;121:11942–11948. doi: 10.1063/1.1814058. [DOI] [PubMed] [Google Scholar]
- 44.Shelley JC, Shelley MY, Reeder RC, Bandyopadhyay S, Klein ML. J. Phys. Chem. B. 2001;105:4464–4470. [Google Scholar]
- 45.Izvekov S, Voth GA. J. Phys. Chem. B. 2005;109:2469–2473. doi: 10.1021/jp044629q. [DOI] [PubMed] [Google Scholar]
- 46.Ayton GS, Voth GA. J. Phys. Chem. B. 2009;113:4413–4424. doi: 10.1021/jp8087868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lyman E, Pfaendtner J, Voth GA. Biophys. J. 2008;95:4183–4192. doi: 10.1529/biophysj.108.139733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noid WG, Chu JW, Ayton GS, Krishna V, Izvekov S, Voth GA, Das A, Anderson HC. J. Chem. Phys. 2008;128:244114. doi: 10.1063/1.2938860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noid WG, Liu P, Wang Y, Chu J-W, Ayton GS, Izvekov S, Andersen HC, Voth GA. J. Chem. Phys. 2008;128:244115. doi: 10.1063/1.2938857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ayton GS, Noid WG, Voth GA. MRS Bull. 2007;32:929–934. [Google Scholar]
- 51.Noid WG, Chu J-W, Ayton GS, Voth GA. J. Phys. Chem. B. 2007;111:4116–4127. doi: 10.1021/jp068549t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Izvekov S, Voth GA. J. Chem. Theory Comput. 2006;2:637–648. doi: 10.1021/ct050300c. [DOI] [PubMed] [Google Scholar]
- 53.Izvekov S, Voth GA. J. Chem. Phys. 2005;123:134105. doi: 10.1063/1.2038787. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Izvekov S, Yan T, Voth GA. J. Phys. Chem. B. 2006;110:3564–3575. doi: 10.1021/jp0548220. [DOI] [PubMed] [Google Scholar]
- 55.Zhou J, Thorpe IF, Izvekov S, Voth GA. Biophys. J. 2007;92:4289–4303. doi: 10.1529/biophysj.106.094425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thorpe I, Zhou J, Voth GA. J. Phys. Chem. B. 2008;112:13079–13090. doi: 10.1021/jp8015968. [DOI] [PubMed] [Google Scholar]
- 57.Izvekov S, Voth GA. J. Phys. Chem. B. 2009;113:4443–4455. doi: 10.1021/jp810440c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gay JG, Berne BJ. J. Chem. Phys. 1981;74:3316–3319. [Google Scholar]
- 59.Brown JT, Allen MP. Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys. 1998;57:6685–6699. [Google Scholar]
- 60.Chu J-W, Voth GA. Biophys. J. 2006;90:1572–1582. doi: 10.1529/biophysj.105.073924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Z, Lu L, Noid WG, Krishna V, Pfaendtner J, Voth GA. Biophys. J. 2008;95:5073–5083. doi: 10.1529/biophysj.108.139626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haliloglu T, Bahar I, Erman B. Phys. Rev. Lett. 1997;79:3090–3093. [Google Scholar]
- 63.ben-Avraham D. Phys. Rev. B: Condens. Matter Mater. Phys. 1993;47:14559–14560. doi: 10.1103/physrevb.47.14559. [DOI] [PubMed] [Google Scholar]
- 64.Tirion MM. Phys. Rev. Lett. 1996;77:1905–1908. doi: 10.1103/PhysRevLett.77.1905. [DOI] [PubMed] [Google Scholar]
- 65.Tobi D, Bahar I. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18908–18913. doi: 10.1073/pnas.0507603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bahar I, Atilgan AR, Demirel MC, Erman B. Phys. Rev. Lett. 1998;80:2733–2736. [Google Scholar]
- 67.Wang Y, Rader AJ, Bahar I, Jernigan RL. J. Struct. Biol. 2004;147:302–314. doi: 10.1016/j.jsb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 68.Maragakis P, Karplus M. J. Mol. Biol. 2005;352:807–822. doi: 10.1016/j.jmb.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 69.Yoshida Y, Kinuta M, Abe T, Liang S, Araki K, Cremona O, Di Paolo G, Moriyama Y, Yasuda T, De Camili P, Takei K. EMBO J. 2004;23:3483–3491. doi: 10.1038/sj.emboj.7600355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindahl E, Edholm O. J. Chem. Phys. 2000;113:3882–3893. [Google Scholar]
- 71.Lu L, Voth GA. J. Phys. Chem. B. 2009;113:1501–1510. doi: 10.1021/jp809604k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Izvekov S, Voth GA. J. Chem. Phys. 2006;125:151101. doi: 10.1063/1.2360580. [DOI] [PubMed] [Google Scholar]
- 73.Ayton GS, McWhirter JL, McMurtry P, Voth GA. Biophys. J. 2005;88:3855–3869. doi: 10.1529/biophysj.105.059436. [DOI] [PMC free article] [PubMed] [Google Scholar]