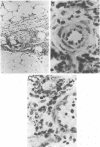
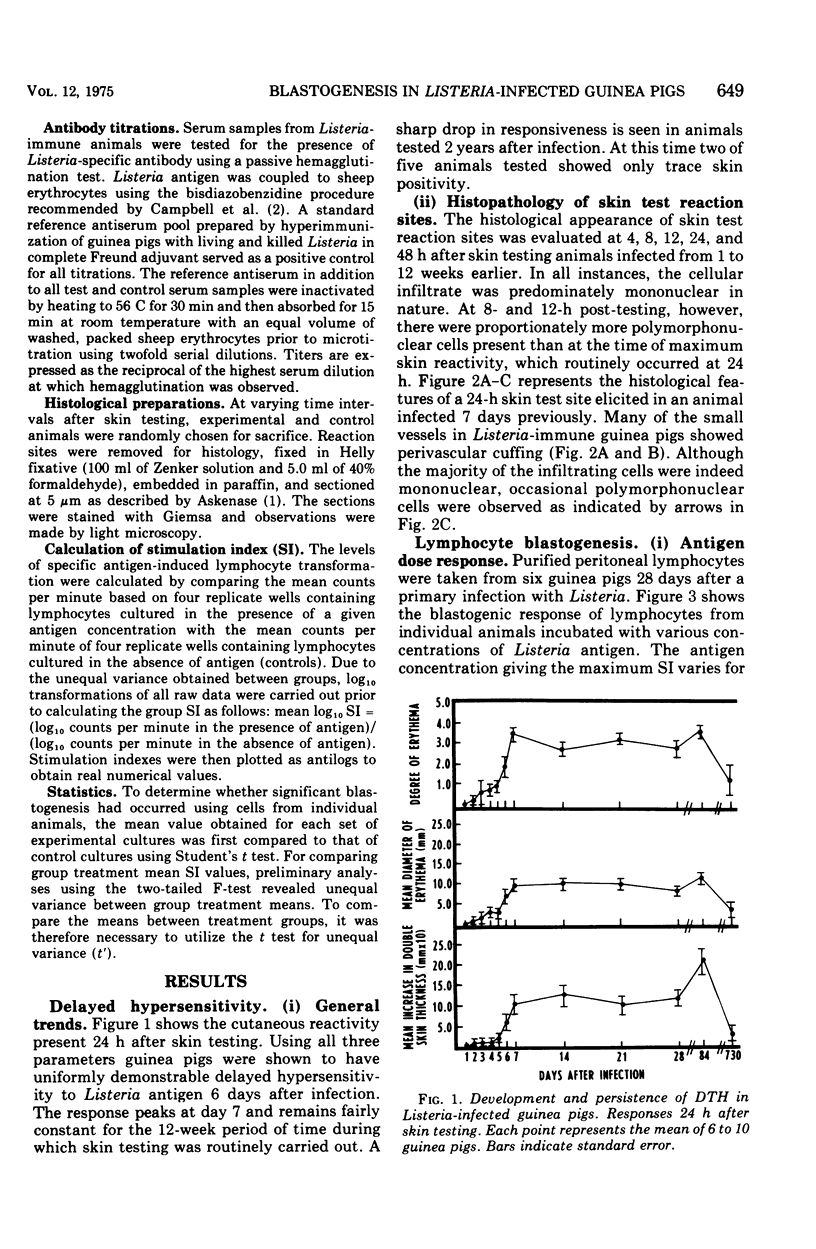
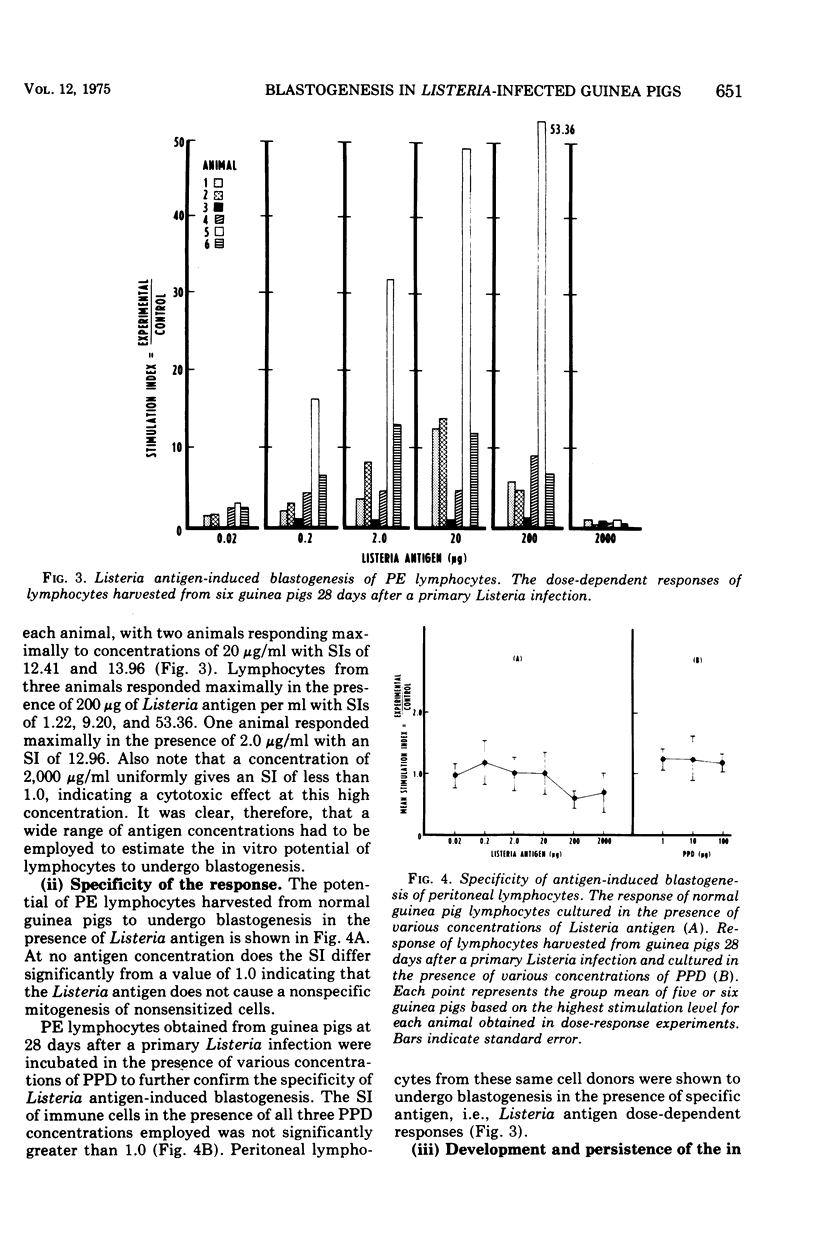
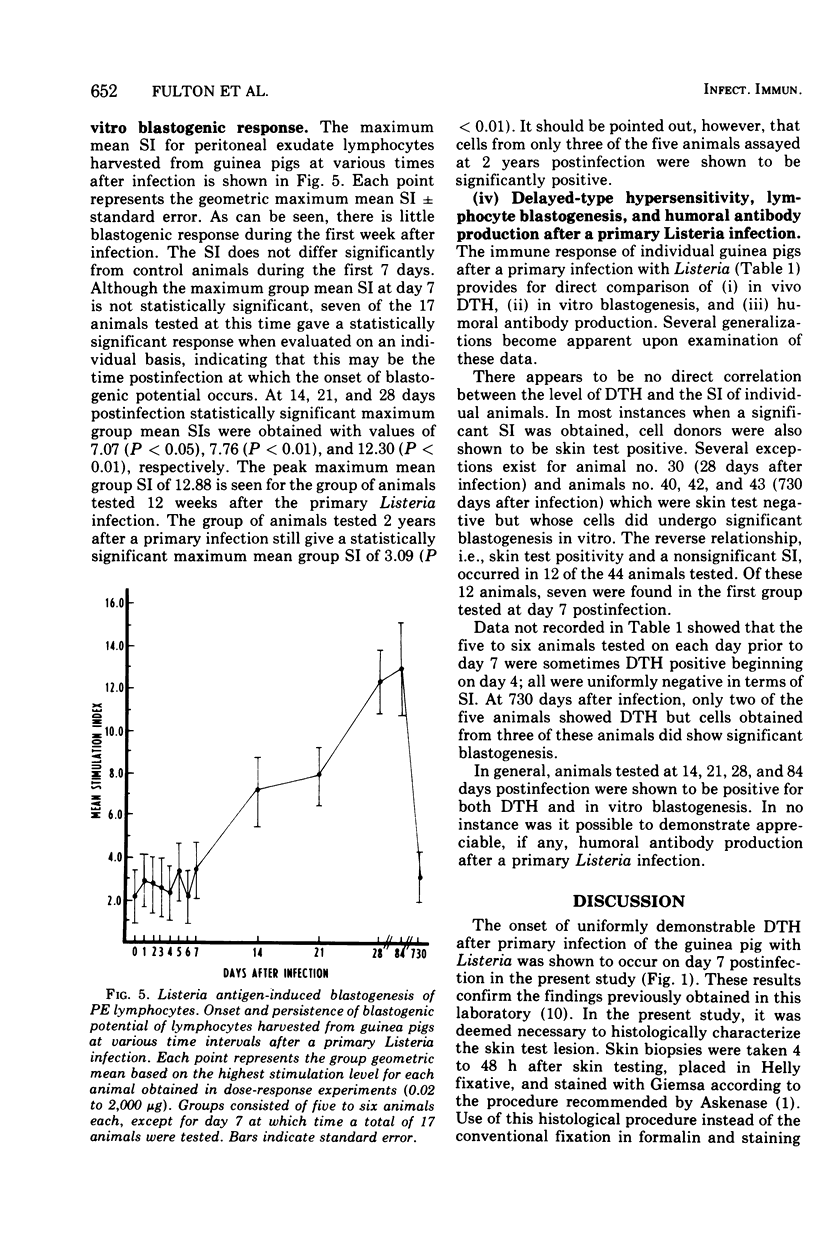
Abstract
Randomly bred guinea pigs of both sexes were injected intracardially with one-half a 50% lethal dose of Listeria monocytogenes. When these animals were skin tested with 30 mug of a water-soluble extract of sonically disrupted Listeria, animals had uniformly detectable levels of delayed-type hypersensitivity (DTH) 6 days after infection. Histological examination of skin test reaction sites, after fixation in Helly fixative and Giemsa staining, revealed a classical tuberculin-type infiltrate consisting primarily of mononuclear cells with few polymorphonuclear cells. Many of the small vessels showed perivascular cuffing. When purified peritoneal exudate lymphocytes from these animals were cultured in vitro in the presence of various concentrations of Listeria antigen, it was fount that the optimal antigenic dose for specific antigen-induced incorporation of [3H]thymidine varied for individual animals. In contrast to the early onset of uniformly detectable levels of in vivo DTH, in vitro lymphocyte blastogenesis was not uniformly demonstrable until 14 days postinfection and remained highly significant on days 21, 28, and 84 postinfection. At 7 days postinfection, lymphocytes from 7 of 17 animals were capable of undergoing sifnificant blastogenesis. The Listeria antigen preparation was not mitogenic for peritoneal exudate lymphocytes from normal animals. It was found that no direct correlation exists between the in vivo levels of DTH and in vitro blastogenesis. Cell donors showing significant in vitro blastogenesis nevertheless were also skin test positive for most animals tested. Humoral antibody was found to play no significant role in the immune response of guinea pigs to a primary infection with Listeria monocytogenes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askenase P. W. Cutaneous basophil hypersensitivity in contact-sensitized guinea pigs. I. Transfer with immune serum. J Exp Med. 1973 Nov 1;138(5):1144–1155. doi: 10.1084/jem.138.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparas S. D., Sheagren J. N., DeMeo A., Hedrick S. Correlation of human skin reactivity with lymphocyte transformation induced by mycobacterial antigens and histoplasmin. Am Rev Respir Dis. 1970 Jan;101(1):67–73. doi: 10.1164/arrd.1970.101.1.67. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Rodriguez G. E., Kind P. D., Campbell P. A. Listeria cell wall fraction: a B cell mitogen. J Immunol. 1975 Mar;114(3):1132–1134. [PubMed] [Google Scholar]
- Halliburton B. L., Blazkovec A. A. Delayed hypersensitivity and acquired cellular resistance in guinea pigs infected with Listeria monocytogenes. Infect Immun. 1975 Jan;11(1):1–7. doi: 10.1128/iai.11.1.1-7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsdill R. D., Berman D. T. Antigens of Brucella abortus. I. Chemical and immunoelectrophoretic characterization. J Bacteriol. 1967 Feb;93(2):544–549. doi: 10.1128/jb.93.2.544-549.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz C. F., Jr, Daniel T. M., Baum G. L. Quantitative aspects of the stimulation of lymphocytes by tuberculin purified protein derivative. Int Arch Allergy Appl Immunol. 1970;38(2):119–129. doi: 10.1159/000230265. [DOI] [PubMed] [Google Scholar]
- Jaffer A. M., Jones G., Kasdon E. J., Schlossman S. F. Local transfer of delayed hypersensitivity by T lymphocytes. J Immunol. 1973 Oct;111(4):1268–1269. [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsaniotis N., Tsenghi C., Economou-Mavrou C., Metaxotou-Stavridaki C. Skin hypersensitivity and in vitro lymphocytic reactivity to tuberculin in childhood. J Pediatr. 1968 May;72(5):599–605. doi: 10.1016/s0022-3476(68)80001-0. [DOI] [PubMed] [Google Scholar]
- Mills J. A. The immunologic significance of antigen induced lymphocyte transformation in vitro. J Immunol. 1966 Aug;97(2):239–247. [PubMed] [Google Scholar]
- Oppenheim J. J. Relationship of in vitro lymphocyte transformation to delayed hypersensitivity in guinea pigs and man. Fed Proc. 1968 Jan-Feb;27(1):21–28. [PubMed] [Google Scholar]
- Rosenstreich D. L., Blake J. T., Rosenthal A. S. The peritoneal exudate lymphocyte. I. Differences in antigen responsiveness between peritoneal exudate and lymph node lymphocytes from immunized guinea pigs. J Exp Med. 1971 Nov 1;134(5):1170–1186. doi: 10.1084/jem.134.5.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonozaki H., Cohen S. The macrophage disappearance reaction. II. Mediation by lymphocytes which lack complement receptors. Cell Immunol. 1972 Apr;3(4):644–652. doi: 10.1016/0008-8749(72)90126-8. [DOI] [PubMed] [Google Scholar]
- Sultzer B. M., Nilsson B. S. PPD tuberculin--a B-cell mitogen. Nat New Biol. 1972 Dec 13;240(102):198–200. doi: 10.1038/newbio240198a0. [DOI] [PubMed] [Google Scholar]
- Thomas J. W., Clements D., Grzybowski S. In vitro lymphocyte responses and skin test reactivity following BCG vaccination. Clin Exp Immunol. 1971 Nov;9(5):611–623. [PMC free article] [PubMed] [Google Scholar]