Abstract
This research is the first to quantify complex PAH mixtures in NIST SRMs using comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC/ToF-MS), with and without extract cleanup, and reports previously unidentified PAH isomers in the NIST SRMs. We tested a novel, high orthogonality GC column combination (LC-50×NSP-35), as well as with a commonly used column combination (Rtx-5ms×Rxi-17) for the quantification of a complex mixture of 85 different PAHs, including parent (PAHs), alkyl- (MPAHs), nitro- (NPAHs), oxy- (OPAHs), thio- (SPAHs), bromo- (BrPAHs), and chloro-PAHs (ClPAHs) in extracts from two standard reference materials: NIST SRM1650b (diesel particulate matter), with cleanup and NIST SRM1975 (diesel particulate extract), with and without extract cleanup. The LC-50×NSP-35 column combination resulted in an average absolute percent difference of 33.8%, 62.2% and 30.8% compared to the NIST certified PAH concentrations for NIST SRM1650b, NIST SRM1975 with cleanup and NIST SRM1975 without cleanup, while the Rtx-5ms×Rxi-17 resulted in an absolute percent difference of 38.6%, 67.2% and 79.6% for NIST SRM1650b, NIST SRM1975 with cleanup and NIST SRM1975 without cleanup, respectively. This GC×GC/ToF-MS method increases the number of PAHs detected and quantified in complex environmental extracts using a single chromatographic run. Without clean-up, 7 additional compounds were detected and quantified in NIST SRM1975 using the LC-50×NSP-35 column combination. These results suggest that the use of the LC-50×NSP-35 column combination in GC×GC/ToF-MS not only results in better chromatographic resolution and greater orthogonality for the separation of complex PAH mixtures, but can also be used for the accurate quantification of complex PAH mixtures in environmental extracts without cleanup.
Keywords: PAHs, Comprehensive Two-dimensional Gas Chromatography, ToF-MS, Complex Environmental Samples, Quantitation of POPs
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental contaminants that constitute a large and diverse class of organic molecules. PAHs are of concern due to their potential persistence, bioaccumulation and toxic effects [1,2,3,4]. Some PAH derivatives are more carcinogenic and mutagenic than their parent compounds [5,6].
The extracts from complex environmental samples may contain a variety of PAHs with different molecular sizes and structures including: parent PAHs (PPAHs), alkylated-PAHs (MPAHs), nitro-PAHs (NPAHs), oxy-PAHs (OPAHs), thio-PAHs (SPAHs), chlorinated (ClPAHs) and brominated-PAHs (BrPAHs). The most prominent source of PPAHs and MPAHs is the incomplete combustion of organic material [7,8] in either natural processes, such as forest fires, volcanic eruptions and hydrothermal processes [9,10,11,12], or anthropogenic processes, such as the combustion of fossil fuel and biomass [13,14,15]. Heterocyclic analogs of PAHs, in which one or more carbon atoms are replaced by nitrogen, sulfur, or oxygen, have also been measured as environmental contaminants. NPAHs are formed during the pyrolysis of nitrogen-containing organic materials and significant concentrations are found in industrial and urban atmospheres, tobacco smoke, engine exhaust, coal tar and coal gasification residues [8,16]. SPAHs are emitted from most of the same combustion sources as PPAHs and NPAHs [8]. Chemical oxidation and photochemical alteration represent significant sources of OPAH derivatives to the environment [17,18,19]. Waste incinerators, water chlorination facilities and automobile and diesel exhaust have been shown to form ClPAHs and BrPAHs [4,17,18,19], in addition to PPAHs, MPAHs, NPAHs, OPAHs, and SPAHs.
The analysis of environmental extracts containing PAHs is often complex and requires cleanup steps and multiple liquid or gas chromatographic methods. Currently, the analysis and quantification of complex PAH mixtures in environmental extracts requires three different one-dimensional GC/MS methods with a total run time of 141.6 min per sample: NPAHs, SPAHs and OPAHs method (45.7 min) [16], PPAH and MPAHs method (46 min)[16], and Cl and Br-PAH method (49.9 min) [20], in addition to the time required for sample cleanup that often includes adsorption, solid phase extraction (SPE) and gel permeation chromatography (GPC).
In order to reduce the analysis time of PAHs contained in a complex environmental mixture, a technique with higher chromatographic peak capacity is needed. Comprehensive two-dimensional gas chromatography (GC×GC) enhances the gas chromatographic separation of complex organic mixtures [21] using two different GC columns, with different retention mechanisms, for the separation of analytes. Theoretically, the peak capacity generated by GC×GC is equal to the product of the individual peak capacities of each column used [22,23]. However, in practice, the peak capacity is equal to the product of individual peak capacities, minus the cross information [24]. Therefore, a GC×GC method with high orthogonality, and low correlation of retention times between dimensions, is preferred.
Quantification in GC×GC/ToF-MS is a more complex process than in one-dimensional GC/MS, where in the latter case a single retention time and peak response are associated with each analyte in the extract. In GC×GC/ToF-MS, a series of modulated peaks (sub-peaks) are generated and detected, and the retention time and response are represented by a distribution of values generated by this process [25,26]. Quantification in GC×GC/ToF-MS is an extension of one-dimensional GC/MS in that these individual sub-peak areas are added together [27]. With both GC/MS and GC×GC/ToF-MS, an increase in quantification error occurs because of inaccurate determination of the peak baseline and incorrect identification of peak start and end times, as well as tailing, fronting and overloading of each modulated peak [28]. However, these errors are compounded in GC/MS because of its decreased chromatographic resolution compared to GC×GC/ToF-MS. Peak tailing, fronting and overloading are especially important with GC×GC/ToF-MS because of the shorter and narrower second dimension column. In addition, small variations in integration parameters for the modulated peaks produce variable quantification results with a GC×GC system [29].
Previously, we reported greater separation of complex PAH mixtures in GC×GC/ToF-MS using a liquid crystal column (LC-50) in the first dimension and a nano-stationary phase column (NSP-35) in the second dimension due to its higher orthogonality than the commonly used combination (Rtx-5ms×Rxi-17) [30]. The objective of this research was to determine if this novel, high orthogonality column combination (LC-50×NSP-35), as well as the traditional column combination (Rtx-5ms×Rxi-17), resulted in reliable and reproducible quantification of a complex mixture of 85 different PAHs, including PPAHs, MPAHs, NPAHs, SPAHs, OPAHs, BrPAHs and ClPAHs, in two National Institute of Standards and Technology (NIST) standard reference materials (SRM), with and without cleanup. PAHs were quantified in NIST SRM1650b (diesel particulate matter) with silica gel solid phase extraction (SPE) cleanup and in NIST SRM1975 (diesel particulate extract) with and without silica gel SPE cleanup, using both column combinations. The ratio of the summation of the three most intense modulated peaks for each target PAH to the three most intense modulated peak of its corresponding surrogate perdeuterated PAH was used to overcome the quantification problems in atmospheric extracts (PM2.5) described above [25,26]. This research is the first to quantify complex PAH mixtures in NIST SRMs using GC×GC/ToF-MS, with and without extract cleanup, and reports previously unidentified PAH congeners in the NIST SRMs.
2. Materials and Methods
2.1. Chemicals and Reference Materials
The standard reference materials, SRM1975 and SRM1650b, were purchased from NIST (NIST, Gaithersburg, MD) [31]. Standard solutions of 18 PPAHs were purchased from ChemService (West Chester, PA, USA), standard solutions of 9 MPAHs, 18 NPAHs and 2 SPAHs were purchased from AccuStandard (New Haven, CT, USA), and neat standards of 17 OPAHs were purchased from Sigma-Aldrich (St. Louis, MO, USA). Standard solutions of 15 ClPAHs and 6 BrPAHs were synthesized by Dr. Takeshi Ohura from the University of Shizuoka in Shizuoka, Japan, using published procedures [20,32,33]. The entire list of PAH analytes can be found in Table S-1. Isotopically labeled PAHs, OPAHs, and NPAHs were purchased from CDN Isotopes (Point-Clare, Quebec, Canada) and Cambridge Isotopes Laboratories (Andover, MA) and included d6-1,4-naphthaquinone, d4-1,4-benzoquinone, d10-fluorene, d7-1-nitronaphthalene, d10-phenanthrene, d8-anthraquinone, d9-5-nitroacenaphthene, d10-pyrene, d9-9-nitroanthracene, d12-triphenylene, d9-3-nitrofluoranthene, d9-1-nitropyrene, d12-benzo[a]pyrene, d11-6-nitrochrysene, d12-benzo[ghi]perylene as surrogates and d10-acenaphthene, d8-9-fluorenone, d10-fluoranthene, d12-benzo[k]fluoranthene, d9-2-nitrobiphenyl, d9-2-nitrofluorene as internal standards.
2.2. Sample Preparation
Three aliquots of NIST SRM1650b and NIST SRM1975 were spiked with known amounts of labeled PAH, OPAH and NPAH surrogates prior to sample preparation. NIST SRM1650b was extracted using a method based on pressurized liquid extraction (PLE) with dichloromethane (DCM) that has been previously described [34,35,36]. The resulting NIST SRM1650b extracts and the NIST SRM1975 aliquots were cleaned up using 20 g silica gel columns (Mega BE-SI, Agilent Technologies, New Castle, DE) and eluted in three fractions, with 100 % hexane (non-polar fraction), 100 % DCM (fraction containing PAHs) and 100 % ethyl acetate (polar fraction). The DCM fraction was then concentrated to 300 µL under a gentle stream of N2 using a Turbovap II (Caliper Life Sciences, MA), solvent exchanged to ethyl acetate and spiked with known amounts of internal standards prior to analysis. An aliquot of NIST SRM1975, without cleanup, was also spiked with surrogates and internal standards prior to analysis.
2.3. GC×GC/ToF-MS Quantification
A GC×GC/ToF-MS Pegasus 4D (Leco, St. Joseph, MI, USA) was used for this study. The instrument consisted of an Agilent 6890 gas chromatograph (Palo Alto, CA, USA) with a secondary oven, a split/splitless injector, and a non-moving quad-jet dual stage modulator. The two GC columns in the system were connected using an Agilent CPM union (part no. 188–5361) for 0.1–0.25 mm I.D. columns. Two GC column combinations were used. Column combination “A” was a low-polarity Rtx-5ms column (35 m × 0.25 mm × 0.25 µm) (Restek, Bellefonte, PA, USA) with a 5 m guard column, followed by a mid-polarity Rxi-17 column (1.2 m × 0.10 mm × 0.10 µm) (Restek, Bellefonte, PA, USA). Column combination “B” was a liquid crystal LC-50 column (10 m × 0.15 mm × 0.10 µm) (J&K Scientific, Edwardsville, Nova Scotia, Canada), followed by a nano-stationary phase NSP-35 column (1.2 m × 0.10 mm × 0.10 µm) (J&K Scientific, Edwardsville, Nova Scotia, Canada). The data processing was performed using ChromaTOF version 4.33. The optimization of both column combinations has been previously described [30]. However, the modulation time was changed from 5 to 7 s in order to increase peak height and instrument sensitivity for some of the PAHs measured.
Five-point calibration curves, ranging from 5–1000 pg/µL, were used for quantification, with concentration ranges varying slightly among the different PAHs. A complete description of the concentrations in the calibration curves can be found in Table S-2. An internal standard concentration of 250 pg/µL was used for all calibration standard solutions.
Selected modulated peaks were used for PAH quantification rather than full sub-peak summation, to reduce quantitation time [26,28]. We calculated the ratio of the peak area for the three most intense modulated sub-peaks of each PAH to the peak area for the three most intense modulated sub-peaks of the respective deuterated PAH surrogate to reduce errors associated with the partial peak area [25]. Three modulated sub-peaks were used for both in-phase and out-of-phase peaks [28]. The GC×GC/ToF-MS operating conditions can be found in Table S-3.
Each SRM was analyzed and quantified in triplicate (n = 3) and the 95% confidence interval of the concentration calculated. All PAH concentrations reported correspond to S/N ratio greater than 10 times the standard deviation of the detected noise and the limits of quantitation ranged from 3 pg/µL for PPAH to 18 pg/µL for NPAH.
3. Results and Discussion
3.1. Quantification of NIST SRM1650b (Diesel Particulate Matter) with Cleanup
Figure S-1 shows the total ion chromatogram (TIC) for the analysis of the NIST SRM1650b extract, after silica gel SPE cleanup, using column combinations “A” (Rtx-5ms×Rxi-17) (Figure S-1A) and “B” (LC-50×NSP-35) (Figure S-1F). The separation patterns observed for both column combinations were consistent to what was reported previously, showing a predictable elution pattern due to the strong correlation between the separation mechanisms in combination “A”, and a more random elution pattern in combination “B” due to its higher orthogonality [30].
Co-elutions among PAHs, and with the unresolved complex mixture (UCM), were evident when column combination “A” was used (Figures S-1C, S-1D and S-1E). With column combination “B”, the UCM eluted at earlier retention times in the first dimension and was distributed throughout the second dimension retention times compared to column combination “A”. This resulted in better separation of the PAHs from the UCM, especially for the later eluting PAHs (Figures S-1H, S-1I and S-1J) [30].
Figure 1 shows the scatter plots of the NIST certified and reference PAH concentrations for NIST SRM1650b [31] versus the PAH concentrations determined by GC×GC/ToF-MS using column combinations “A” (Figure 1A) and “B” (Figure 1B). The 1:1 line is shown in each plot, along with the 95% confidence interval (CI) (shown with error bars), for triplicate measurements (n = 3).
Figure 1.
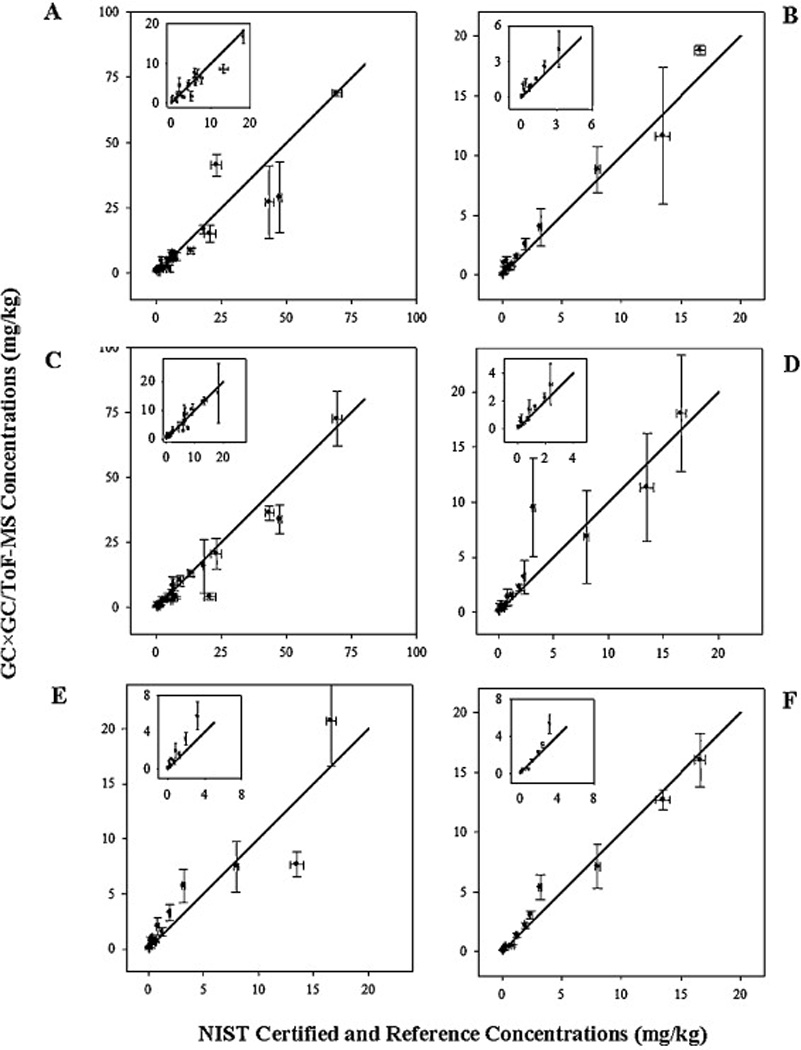
NIST certified and reference PAH concentrations compared to concentrations determined by GC×GC/ToF-MS in: (A) NIST SRM1650b with column combination “A”, (B) NIST SRM1650b with column combination “B”, (C) cleaned NIST SRM1975 with column combination “A”, (D) cleaned NIST SRM1975 with column combination “B”, (E) NIST SRM1975 without cleanup with column combination “A”, (F) NIST SRM1975 without cleanup with column combination “B”. The lines in the plots represent a slope=1 and y-intercept=0. The inner boxes show the low concentration PAH regions (see axes).
Table S-4 shows the average measured PAH concentrations for NIST SRM1650b using column combinations “A” and “B” with GC×GC/ToF-MS, the relative standard deviation (RSD), and the 95% confidence interval (CI) for the triplicate measurements (n = 3), as well as the NIST certified concentrations. The RSDs for the measured PAH concentrations for NIST SRM1650b were, on average, 11.2% for column combination “A” and 11.9% for column combination “B”.
The percent difference between PAH concentrations determined by GC×GC/ToF-MS and the NIST certified PAH concentrations (shown in Table S-4 and Table S-5) was calculated using equation (1):
| (1) |
Where Δ [PAHs] is the percent change of PAHs relative to the NIST certified concentrations, [PAHs]exp is the PAH concentrations determined by GC×GC/ToF-MS and [PAHs]NIST is the NIST certified PAH concentrations. For NIST SRM1650b, Δ [PAHs] ranged from −65.1% for naphthalene to 340.5% for dibenzo[a,h]anthracene, with an average |Δ[PAHs]| of 38.6%, when column combination “A” was used (Table S-4).Δ[PAHs] ranged from −50.8% for anthracene to 259.5% for dibenzo[a,h]anthracene, with an average |Δ[PAHs]| of 33.8%, when column combination “B” was used (Table S-4, NIST SRM1650b). Combination “B” resulted in PAH concentrations that were slightly closer to the NIST certified PAH concentrations for NIST SRM1650b (33.8% average absolute percent difference) compared to combination “A” (38.6% average absolute percent difference). Dibenz[a,h]anthracene (DahA) had the highest |Δ[PAHs]| in both column combinations due to its partial co-elution with indeno[1, 2,3-c,d]pyrene in column combination “A”, peak broadening in column combination “B”, and its relatively low concentration in NIST SRM1650b.
A two-sample student t-test was used to determine if the average measured PAH concentrations for NIST SRM1650b were statistically different from the NIST certified PAH concentrations. Out of the 20 PAHs that were measured in NIST SRM1650b and had certified values, 11 of the 20 PAHs had measured concentrations that were statistically different (p < 0.05) from the NIST certified concentration when column combination “A” was used and 6 of the 20 PAHs had measured concentrations that were statistically different (p < 0.05) when column combination “B” was used (Table S-4). This suggests that column combination “B” can be used for the quantitation of complex PAH mixtures with greater accuracy than combination “A”.
Naphthalene (NAP), 1-methylnaphthalene (1 met NAP) and 2-methylnaphthalene (2 met NAP) showed weak interaction with the liquid crystal column used in column combination “B”, eluting with the solvent peak in less than 5 minutes, and are reported as not detected (n.d.) in Table S-4 and Table S-1. In addition, peak tailing was observed in sub-peaks of polar PAHs, including some OPAHs and SPAHs with both column combinations. A similar behavior has been previously reported in the literature and was described as an effect coming from excessive cold jet flow modulation; where the hot jet was not able to efficiently launch all of the analyte mass that was trapped in the modulator into the second dimension column, increasing the number of sub-peaks generated for each compound and decreasing the S/N ratio [37]. In our experiment, this behavior represented a source of error in both column combinations for the quantification of PAHs such as dibenzothiopene (Dibenzth), 9,10-anthraquinone (9,10 ANTq), 9-fluorenone (9 Fluo), 9-chlorophenanthrene (9 Cl PHE) and phenanthrene-1,4-dione (1,4 PHEq).
Eighty-six PAHs (32 PPAHs, 31 MPAHs, 22 NPAHs and 1 SPAHs) had NIST certified or reference concentrations for NIST SRM1650b based on their measurement using one-dimensional GC/MS with three different stationary phases [31]. Of the 85 different PAHs in our compound list (Table S-1), 47 PAHs (17 PPAHs, 8 MPAHs, 8 NPAHs, 1 SPAH, 10 OPAHs and 3 ClPAHs) were measured by GC×GC/ToF-MS using column combination “A” and 49 PAHs (17 PPAHs, 7 MPAHs, 10 NPAHs, 2 SPAHs, 10 OPAHs, 1 BrPAH, 2 ClPAHs) using column combination “B”. The PAH concentration profiles measured in NIST SRM1650b are shown in Figures 2A and 2B for column combinations “A” and “B”, respectively. These profiles include PAHs that were not previously reported by NIST, including 1,3-dimethylnaphthalene, 2,6-dimethylnaphthalene, retene, 2-nitroanthracene, 2-nitropyrene, 3-nitrodibenzofuran, 4-nitrobiphenyl, 2-nitrodibenzothiopene, 1,4-anthraquinone, 1,4-naphthaquinone, 2-methyl-9,10-anthraquinone, 5,12-naphthacenequinone, 9,10-anthraquinone, 9,10-phenanthrenequinone, 9-fluorenone, benz[a]antracene-7-12-dione, benzanthrone, benzo[a]fluorenone, benzo[c]phenanthrene-[1,4]-quinone, phenanthrene-1,4-dione, 2-bromofluorene, 1-chloropyrene, 2-chloroanthracene, 9-chloroanthracene and 9-chlorophenanthrene (Table S-1). The measurement of this NIST SRM by GC×GC/ToF-MS results in a more comprehensive determination of its complex PAH mixture, without significant increase in analysis time, compared to one-dimensional GC/MS.
Figure 2.
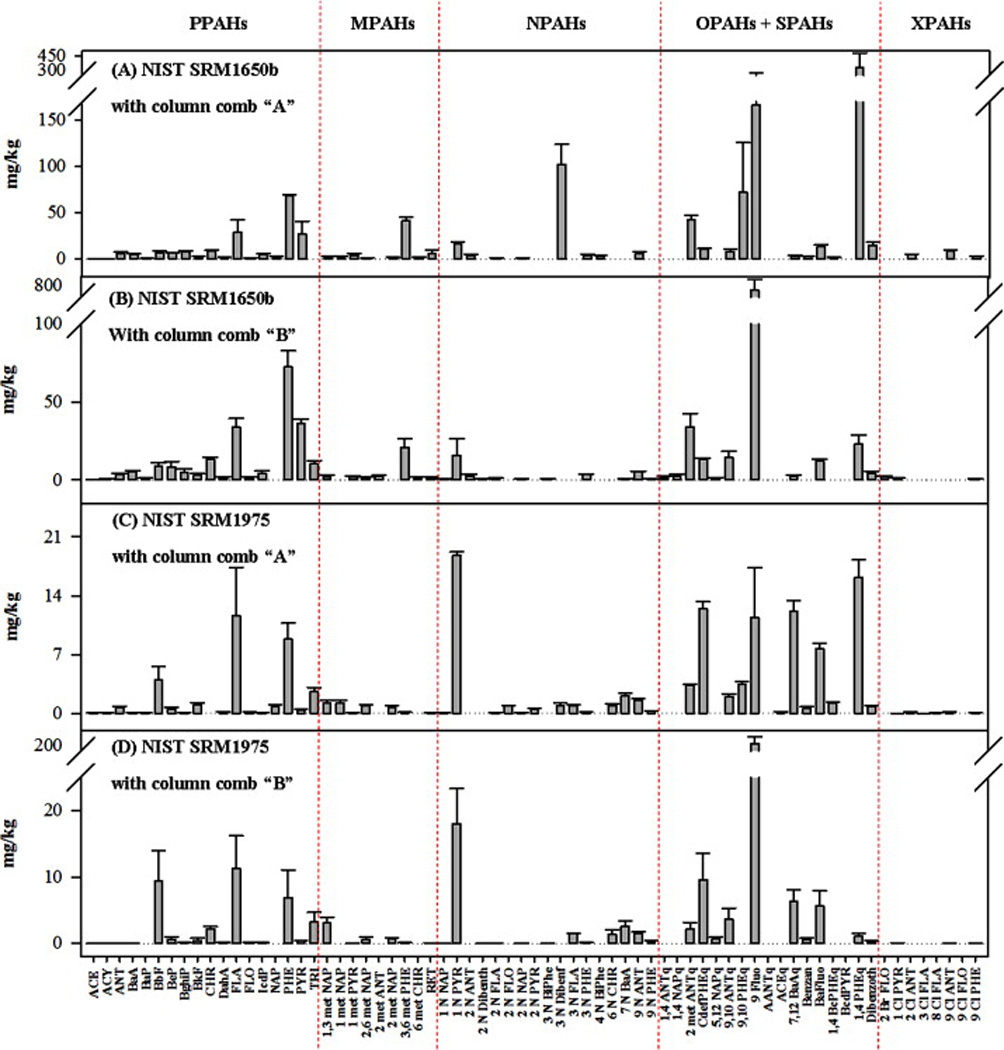
Average concentrations of PAHs determined by GC×GC/ToF-MS in: (A) NIST SRM1650b (diesel particulate matter) with column combination “A”, (B) NIST SRM1650b (diesel particulate matter) with column combination “B”, (C) NIST SRM1975 (diesel particulate extract) with column combination “A”, (D) NIST SRM1975 (diesel particulate extract) with column combination “B”. The error bars represent the 95% CI for each triplicated measurement (n=3)
3.2. Quantification of PAHs in NIST SRM1975 (Diesel Particulate Extract) with Cleanup
Figure S-2 shows the TIC for the analysis of the NIST SRM1975 extract, after silica gel SPE cleanup, using column combinations “A” (Figure S-2A) and “B” (Figure S-2F). The elution pattern observed was also consistent with what was previously reported [30]. Co-elutions among the PAHs and the UCM were evident with column combination “A” in Figures S-2C, S-2D and S-2E. When column combination “B” was used (Figure S-2F), the PAHs eluted in a more random pattern compared to column combination “A”, with better resolution and separation from the UCM, especially for the late eluting PAHs (Figures S-2H, S-2I and S-2J).
Figure 1 shows the scatter plots for the NIST certified and reference PAH concentrations for NIST SRM1975 versus the concentrations determined by GC×GC/ToF-MS using column combinations “A” (Figure 1C) and “B” (Figure 1D). For the NIST SRM1975 extract after cleanup, column combination “B” resulted in PAH concentrations that were slightly closer to the NIST certified PAH concentrations (62.2% absolute percent difference) compared to column combination “A” (67.2% absolute percent difference).
Table S-5 shows the average measured PAH concentrations for NIST SRM1975 using column combinations “A” and “B” with GC×GC/ToF-MS, as well as the NIST certified PAH concentrations. The RSDs for the measured PAH concentrations for NIST SRM1975 after cleanup were, on average, 9.28% for column combination “A” and 16.6% for column combination “B” (n = 3).
Equation 1 was used to evaluate the percent difference between PAH concentrations determined by GC×GC/ToF-MS and the NIST certified PAH concentrations. For NIST SRM1975 after cleanup, Δ[PAHs] ranged from −14.1% for fluoranthene to 494.1% for benzo[k]fluoranthene, with an average |Δ[PAHs]| of 67.2% when column combination “A” was used (Table S-5). Δ[PAHs] ranged from −16.3% for fluoranthene to 196.2% for benzo[b]fluoranthene, with an average |Δ[PAHs]| of 62.2%, when column combination “B” was used (Table S-5). Out of the 11 PAHs that were measured in NIST SRM1975 and had certified values, 5 of the PAHs had measured concentrations that were statistically different (p < 0.05) from the NIST certified concentrations when column combination “A” was used and 6 of the PAHs had measured concentrations that were statistically different (p < 0.05) when column combination “B” was used (Table S-5).
Fifty-seven PAHs (18 PPAHs, 20 MPAHs and 19 NPAHs) had NIST certified or reference concentrations for NIST SRM1975 based on their measurement using one-dimensional GC/MS with two different stationary phases [31]. Of the 85 different PAHs in our compound list (Table S-1), 55 PAHs (17 PPAHs, 7 MPAHs, 13 NPAHs, 1 SPAH, 11 OPAHs and 6 ClPAHs) were measured by GC×GC/ToF-MS using column combination “A” and 46 PAHs (16 PPAHs, 5 MPAHs, 11 NPAHs, 2 SPAHs, 9 OPAHs and 3 ClPAHs) using column combination “B”. The PAH concentration profiles measured in NIST SRM1975, including those not reported by NIST (acenaphthene, acenaphthylene, anthracene, benzo[a]pyrene, 1,3-dimethylnaphthalene, 1-methylpyrene, 2,6-dimethylnaphthalene, retene, 1-nitronaphthalene, 2-nitrofluoranthene, 2-nitrofluorene, 2-nitronaphthalene, 2-nitropyrene, 3-nitrobiphenyl, 3-nitrofluoranthene, 3-nitrophenanthrene, 7-nitrobenz[a]anthracene, 9-nitrophenanthrene, 2-nitrodibenzothiopene, dibenzothiopene, 2-methyl-9,10-anthraquinone, 4H-cyclopenta[def]phenanthrene-4-one, 5,12-naphthacenequinone, 9,10-phenanthrenequinone, 9-fluorenone, acenaphthenequinone, benz[a]anthracene-7,12-dione, benzanthrone, benzo[a]fluorenone, benzo[c]phenanthrene-1,4-quinone, phenanthrene-1,4-dione, 1-chloropyrene, 2-chloroanthracene, 3-chlorofluoranthene, 8-chlorofluoranthene, 9-chloroanthracene, 9-chlorophenanthrene) are shown in Figures 2C and 2D for column combinations “A” and “B”, respectively.
3.3. Quantification of PAHs in NIST SRM1975 (Diesel Particulate Extract) without Cleanup
To further test the use of GC×GC/ToF-MS to measure complex mixtures of PAHs in complex environmental matrices, a 225 µL aliquot of the NIST SRM1975 extract, without cleanup, was spiked with 75 µL of both internal standard and surrogate solutions. This extract was analyzed in triplicate using GC×GC/ToF-MS with column combinations “A” and “B”.
Figures 3A and 3F show the TICs for the analysis of the NIST SRM1975 extract without cleanup using column combinations “A” and “B”, respectively. The PAHs eluted in a similar pattern to the cleaned extract (Figures S-2A and S-2F). However, Figures 3C, 3D and 3E show that the UCM co-eluted and interfered with some of the PAHs when column combination “A” was used, while Figure 3F shows that the majority of the UCM eluted in the first 15 minutes and did not co-elute with the PAHs when column combination “B” was used (Figures 3I and 3J).
Figure 3.
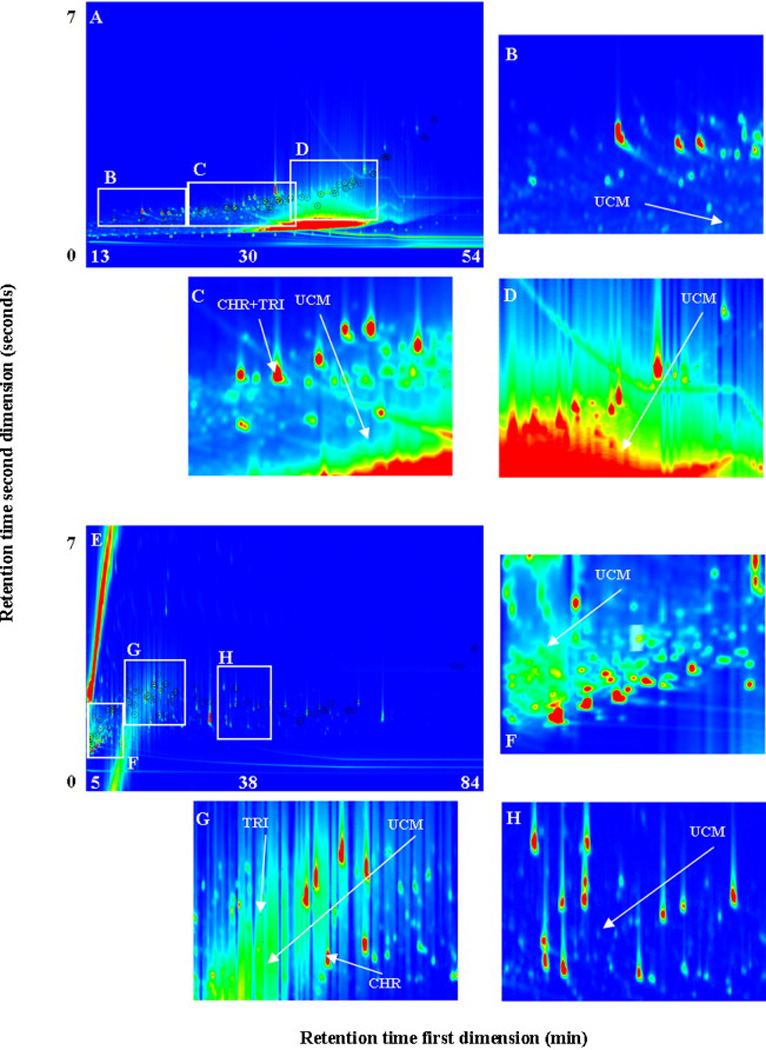
GC×GC/ToF-MS TICs for NIST SRM1975 (diesel particulate extract) without cleanup (A) using column combination “A”, (B) black and white version of A, (C), (D), (E) portions of chromatogram shown in A, (F) using column combination “B”, (G) black and white version of F, and (H), (I), (J) portions of chromatogram shown in F.
Figure 1 shows the scatter plots of the NIST certified and reference PAH concentrations in NIST SRM1975 versus the concentrations determined by GC×GC/ToF-MS in NIST SRM1975 without cleanup using column combinations “A” (Figure 1E) and “B” (Figure 1F). For the NIST SRM1975 extract without cleanup, column combination “B” resulted in PAH concentrations that were closer to the NIST certified PAH concentrations for NIST SRM1975 (30.8% absolute percent difference) compared to combination “A” (79.6% absolute percent difference).
Table S-5 shows the average measured PAH concentrations for NIST SRM1975 (without cleanup) using column combinations “A” and “B” with GC×GC/ToF-MS, as well as the NIST certified PAH concentrations. The RSDs for the measured PAH concentrations for NIST SRM1975 without cleanup were, on average, 27.1% for column combination “A” (higher compared to the NIST SRM1975 extract with cleanup using column combination “A”) and 16.6% for column combination “B” (comparable to the NIST SRM1975 extract with cleanup using column combination “B”) (n = 3).
For NIST SRM1975 without cleanup, Δ[PAHs] ranged from −43.3% for fluoranthene to 335.3% for benzo[k]fluoranthene, with an average |Δ[PAHs]| of 79.6%, when column combination “A” was used (Table S-5). Δ[PAHs] ranged from −40.0% for 6-nitrochrysene to 70.42% for benzo[e]pyrene, with an average |Δ[PAHs]| of 30.8%, when column combination “B” was used (Table S-5). Out of the 11 PAHs that were measured in the NIST SRM1975 extract without cleanup and had NIST certified values, 6 of the PAHs had measured concentrations that were statistically different (p < 0.05) from the NIST certified values when column combination “A” was used and 4 of the PAHs had measured concentrations that were statistically different (p < 0.05) when column combination “B” was used (Table S-5). This suggests that column combination “B” can be used for an accurate quantitation of complex PAH mixtures in samples with reduced or no cleanup.
Fifty-seven PAHs (18 PPAHs, 20 MPAHs and 19 NPAHs) had NIST certified or reference concentrations for NIST SRM1975 based on their measurement using one-dimensional GC/MS with two different stationary phases [31]. Of the 85 different PAHs in our compound list (Table S-1), 50 PAHs (17 PPAHs, 5 MPAHs, 11 NPAHs, 1 SPAH, 14 OPAHs and 2 ClPAHs) were measured by GC×GC/ToF-MS using column combination “A” (less than the 55 PAHs identified in the NIST SRM1975 cleaned extract) and 53 PAHs (17 PPAHs, 6 MPAHs, 13 NPAHs, 2 SPAHs, 12 OPAHs and 3 ClPAHs) were measured using column combination “B” (more than the 46 PAHs identified in the NIST SRM1975 cleaned extract). Some of the compounds detected in extracts without cleanup, that were not detected in the cleaned extracts, included relatively polar compounds compared to the other PAHs detected (3-dibenzofuran, 4-nitrbiphenyl, 1,4-anthraquinone, 1,4-naphthaquinone, acenaphthenequinone, aceanthrenequinone and benzo[cd]pyrenone). This suggests that these more polar PAHs may be lost during the sample cleanup process.
4. Conclusions
The analyses of NIST SRMs by GC×GC/ToF-MS resulted in a more comprehensive determination of its complex PAH composition, without a significant increase in analysis time compared to the multiple one-dimensional GC/MS methods that would be needed to target a similar number of PAHs. The quantitation results suggest that the use of column combination “B” (LC-50×NSP-35) not only resulted in better resolution and greater orthogonality for the separation of complex PAH mixtures compared to column combination “A” (Rtx-5ms×Rxi-17) [30], but also resulted in the accurate quantification of complex PAH mixtures in environmental samples containing 50 or more PPAHs, MPAHs, OPAHs, SPAHs, NPAHs, ClPAHs and BrPAHs in a single chromatographic run, and including extracts with minimal or no extract cleanup. This research is the first to quantify complex PAH mixtures in NIST SRMs using GC×GC/ToF-MS, with and without extract cleanup, using a high orthogonality column combination (LC-50×NSP-35), and reports previously unidentified PAH congeners in both NIST SRMs, including OPAHs, ClPAHs, NPAHs and MPAHs using only one chromatographic run, with a significant reduction in analysis time compared to one dimensional methods.
Supplementary Material
Acknowledgements
Funding for this research was provided by the U.S. National Science Foundation (ATM-0841165). This publication was made possible in part by National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), grants P30 ES00210 and P42 ES016465 and the National Children’s Study Formative Research Project (HHSN267200700021C, National Institute of Child Health and Human Development). The authors thank the analytical chemistry core of OSU’s Superfund Research Program for supplying standards and Prof. Takeshi Ohura at the University of Shizouka in Shizouka, Japan for supplying ClPAH and BrPAH standards. Its contents are solely responsibility of the authors and do not necessarily represent the official view of NIEHS, NIH, National Institute of Child Health and Human Development.
Footnotes
Associated Content
Supporting Information: This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Baek SO, Field RA, Goldstone ME, Kirk PW, Lester JN, Perry R. Water Air Soil Pollut. 1991;60:279–300. [Google Scholar]
- 2.Miet K, Budzinski H, Villenave E. Polycyclic Aromat. Compd. 2009;29:267–281. [Google Scholar]
- 3.Lee J, Lane DA. Atmos. Environ. 2010;44:2469–2477. [Google Scholar]
- 4.Eklund G, Stromberg B. Chemosphere. 1983;12:657–660. [Google Scholar]
- 5.Durant JL, Busby WF, Lafleur AL, Penman BW, Crespi CL. Mutat. Res. 1996;371:123–157. doi: 10.1016/s0165-1218(96)90103-2. [DOI] [PubMed] [Google Scholar]
- 6.Dipple A. Polycyclic Hydrocarbons and Carcinogenesis. American Chemical Society. 1985;283:1–17. [Google Scholar]
- 7.Lima ALC, Farrington JW, Reddy CM. Environ. Forensics. 2005;6:109–131. [Google Scholar]
- 8.Harvey RG. In: PAHs and Related Compounds- Chemistry. Nielson AH, editor. Vol. 3.1. New York: Springer; 1998. [Google Scholar]
- 9.Okuda T, Kumata H, Zakaria MP, Naraoka H, Ishiwatari R, Takada H. Atmos Environ. 2002;36:611–618. [Google Scholar]
- 10.Zolotov MY, Shock EL, Geophys J. Res. 2000;105:539–559. doi: 10.1029/1999jb900369. [DOI] [PubMed] [Google Scholar]
- 11.Yamanaka T, Mizota C, Murae T, Hashimoto J. Geochem. J. 1999;33:355–367. [Google Scholar]
- 12.Wilcke W, Amelung W, Martius C, Garcia MVB, Zech W. J. Plant Nutr. Soil Sci. 2000;163:27–30. [Google Scholar]
- 13.Schmidt W, Grimmer G, Jacob J, Dettbarn G. Toxicol. Environ. Chem. 1986;13:1–16. [Google Scholar]
- 14.Okuda T, Kumata H, Naraoka H, Takada H. Org. Geochem. 2002;33:1737–1745. [Google Scholar]
- 15.Lu H, Zhu L, Zhu N. Atmos. Environ. 2009;43:978–983. [Google Scholar]
- 16.Wang W, Jariyasopit N, Schrlau J, Jia Y, Tao S, Yu T-W, Dashwood RH, Zhang W, Wang X, Simonich SLM. Environ. Sci. Technol. 2011;45:6887–6895. doi: 10.1021/es201443z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horii Y, Ok G, Ohura T, Kannan K. Environ. Sci. Technol. 2008;42:1904–1909. doi: 10.1021/es703001f. [DOI] [PubMed] [Google Scholar]
- 18.Ma J, Horii Y, Cheng J, Wang W, Wu Q, Ohura T, Kannan K. Environ. Sci. Technol. 2009;43:643–649. doi: 10.1021/es802878w. [DOI] [PubMed] [Google Scholar]
- 19.Shiraishi H, Pilkington NH, Otsuki A, Fuwa K. Environ. Sci. Technol. 1985;19:585–590. doi: 10.1021/es00137a001. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Chen S, Tian M, Zheng X, Gonzales L, Ohura T, Mai B, Simonich SLM. Environ Sci. Technol. 2012;43:643–649. doi: 10.1021/es302272a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnelle-Kreis J, Welthagen W, Sklorz M, Zimmermann R. J. Sep. Sci. 2005;28:1648–1657. doi: 10.1002/jssc.200500120. [DOI] [PubMed] [Google Scholar]
- 22.Poster DL, Schantz MM, Sander LC, Wise SA. Anal. Bioanal. Chem. 2006;386:859–881. doi: 10.1007/s00216-006-0771-0. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton J. J. Chromatogr. Sci. 2010;48:274–282. doi: 10.1093/chromsci/48.4.274. [DOI] [PubMed] [Google Scholar]
- 24.Erni F, Frei RW. J. Chromatogr. A. 1978;149:561–569. [Google Scholar]
- 25.Amador-Munoz O, Marriott PJ. J. Chromatogr. A. 2008;1184:323–340. doi: 10.1016/j.chroma.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 26.Amador-Munoz O, Villalobos-Pietrini R, Aragon-Pina A, Tran TC, Morrison P, Marriott PJ. J. Chromatogr. A. 2008;1201:161–168. doi: 10.1016/j.chroma.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 27.Humston E, Synovec R. In: Comprehensive Chromatography in Combination with Mass Spectrometry. Mondello L, editor. John Wiley & Sons, Inc.; 2011. pp. 449–475. [Google Scholar]
- 28.Khummueng W, Harynuk J, Marriott PJ. Anal. Chem. 2006;78:4578–4587. doi: 10.1021/ac052270b. [DOI] [PubMed] [Google Scholar]
- 29.Paulina de la Mata A, Nizio KD, Harynuk JJ. J. Chrom A. 2012;1255:190–195. doi: 10.1016/j.chroma.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 30.Manzano C, Hoh E, Simonich SLM. Environ. Sci. Technol. 2012;46:7677–7684. doi: 10.1021/es301790h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NIST. Gaithersburg, MD: 2000. [Google Scholar]
- 32.Kitazawa A, Amagai T, Ohura T. Environ. Sci. Technol. 2006;40:4592–4598. doi: 10.1021/es0602703. [DOI] [PubMed] [Google Scholar]
- 33.Ohura T, Kitazawa A, Amagai T, Makino M. Environ. Sci. Technol. 2005;39:85–91. [PubMed] [Google Scholar]
- 34.Primbs T, Simonich S, Schmedding D, Wilson G, Jaffe D, Takami A, Kato S, Hatakeyama S, Kajii Y. Environ. Sci. Technol. 2007;41:3551–3558. doi: 10.1021/es062256w. [DOI] [PubMed] [Google Scholar]
- 35.Primbs T, Genualdi S, Simonich SM. Environ. Toxicol. Chem. 2008;27:1267–1272. doi: 10.1897/07-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Primbs T, Piekarz A, Wilson G, Schmedding D, Higginbotham C, Field J, Simonich SM. Environ. Sci. Technol. 2008;42:6385–6391. doi: 10.1021/es702160d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaines RB, Frysinger GS. J. Sep. Sci. 2004;27:380–388. doi: 10.1002/jssc.200301651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


