Abstract
A novel technique is described to functionalize gold nanorods (GNRs) allowing for in-vivo targeting of breast cancer tumors grown in athymic nude mice. GNRs were functionalized by covalent attachment of Herceptin (HER), a monoclonal antibody that enables molecular recognition of breast cancer cells expressing highly specific tumor associated antigens, and poly(ethylene glycol) (PEG) which obscures particles against the reticuloendothelial system in the body. The stability and functionality of fabricated particles (Her-PEG GNRs) were demonstrated in-vitro in the presence of blood and then in-vivo in nude mice model for breast cancer. The results demonstrate successful tumor accumulation of functionalized gold nanorods within HER2/neu over-expressing breast tumors in tumor-bearing nude mice and support the notions that GNRs can be used for molecular imaging of tumor.
Keywords: Gold nanorods, nanoparticles, Herceptin, Poly(ethylene glycol), covalent, functionalized, in-vivo, stability, animal model, HER2
Molecular targeting of cancer cells using gold nanoparticles is a rapidly growing field of research (1). Gold nanoparticles are available in different shapes and sizes, appear to be well tolerated by cells (2–6) and are functionalized by conjugating them with a wide range of biochemical compounds such as DNA (7), drugs (8), antibodies (9), and other compounds (10). In addition, gold nanoparticles can be detected in biological samples and tissues using a variety of methods such as electron microscopy (11), dark field microscopy (12), two-photon luminescence laser scanning microscopy (13), optical coherence tomography (14), Raman spectroscopy (15), optoacoustic imaging (16–18) and X-ray imaging (19,20). Gold nanorods (GNR) are especially promising for optical-based imaging (21,22) because: (1) gold nanorods possess highest optical absorption per unit volume among all known nanoparticles, and their absorption spectrum can be tuned to any desirable wavelength in the near-infrared range, (2) by converting absorbed optical energy into heat gold nanorods possess dual-use utility as an imaging contrast agent for optoacoustic tomography and as a contrast agent for laser thermal therapy, (3) gold is inert metal nontoxic to living cells, and (4) nonspherical nanoparticles such as nanorods are shown to be internalized more than do spherical particles (23).
Successful targeting of GNRs to cancer cells in-vivo requires engineering efforts to make them (a) biocompatible, (b) stable within in-vivo microenvironment, (c) able to circulate in the blood long enough to find their target, (d) able to recognize and bind to cancer cells (1). To enhance their circulation time, GNRs should be protected against the reticuloendothelial system (24,25). Poly(ethylene glycol) has been shown to efficiently enhance the circulation time of injected nanoparticles (25). Spherical gold nanoparticles have been successfully functionalized and used in-vivo for tumor targeting (26); however, utilizing gold nanorods for in-vivo targeting has been a challenge due to their distinct physicochemical properties: gold nanorods are commonly produced and stabilized using a toxic material such as Cetyl trimethylammonium bromide (CTAB) (2). In addition, the surface crystal structure of GNRs is different from that of gold nanospheres, which means that their chemical behavior is also different (27–29). As a result, new chemical protocols are required for conjugating GNRs to biological molecules.
Covalent attachment of antibodies to GNRs without utilizing PEG has already been reported in the literature (30); however, the performance of these constructs within in-vivo microenvironment has not been studies yet. In this report, a novel protocol that allows for the engineering of hetero-functional GNRs is reported to covalently conjugate GNRs with both monoclonal antibody (i.e Herceptin) and poly(ethelene) glycol (PEG) and their stability and performance were confirmed within in-vitro and in-vivo microenvironment.
GNRs with a peak absorption wavelength at 760 nm were prepared using a seed-mediated method (31). Briefly, one mL of synthesized GNRs in CTAB was centrifuged twice in a 1.5 mL eppendorf tube at 10000 RPM and resuspended in one mL deionized water. Then, 10 μL of Nanothinks acid16 solution (#662216, 5mM in ethanol, Sigma-Aldrich, St. Louis, MO) was added to GNR solution and the solution was sonicated for 15 minutes at 50°C to prevent aggregation. The temperature was then adjusted to 30°C while sonication was continued for another 120 minutes. Next, the solution was centrifuged at 10,000 RPM for 10 minutes, supernatant removed and pellet resuspended in PBS. EDC (1-ethyl-3-[3-imethylaminopropyl]carbodiimide hydrochloride (#77149, Pierce, Rockford, IL) and sulfo-NHS (#24520, Pierce) was added at a final concentration of 4 mM and 1 mM, respectively, and the mixture was sonicated for 25 minutes at 4°C to produce activated GNRs (i.e GNRs that are capable of binding to the amine side chain of proteins). Monoclonal antibody Herceptin was a kind donation from Genentech, San Francisco, CA. Purified Herceptin was then added to a final concentration of 100 μg/mL to 1 mL of activated GNRs. The mixture was sonicated at room temperature for two hours. Following the removal of excess Herceptin, 10 μL of PEG-Thiol (10 mM) with a molecular weight of 5000 Da (mPEG-Thiol-5000, Laysan Bio Inc., Arab, AL) was added to 1 mL of Herceptin-conjugated GNRs and the mixture was incubated at room temperature for 2 hours. The final solution was diluted by adding PBS to achieve an optical density of 1.0 at 760 nm as measured by Cary50 spectrophotometer. Figure 1 demonstrates the schematics of the final product (Her-PEG).
Figure 1.
Schematics of Her-PEG GNRs. The particles are attached covalently to Herceptin through Nanothinks Acid. PEG-Thiol molecules are attached to gold nanorods through their thiol functional group.
Alternatively, the initial steps involving monoclonal antibody coating were skipped in the protocol and CTAB-coated GNRs were reacted with PEG-Thiol directly to produce PEG GNRs. Also, the steps regarding the addition of PEG-Thiol were skipped to produce Her GNRs. Again, the optical density of the final solution was adjusted to 1.0 at 760 nm.
The toxicity of fabricated GNRs was evaluated in-vitro on BT474 and SKBR3 (Breast cancer cell lines that over-expresses HER2), and Hep G2 cells (Hepatocellular carcinoma cell line, which does not express HER2) using Trypan-blue staining method (32,33). All cell lines that are used in this project were obtained from ATCC (Manassas, VA) and were grown on 24-well culture plates in RPMI-1640 culture media supplemented with 10% fetal bovine. Cell plates were rinsed with PBS to remove unattached cells and one mL fresh culture media was added to each well. Her-PEG and PEG (Control) GNRs were added to each well at a final concentration of 200 μL/mL. After 24 hours incubation, cells were suspended using Trypsin 0.25% and live/dead cells were counted under a bright-field microscope by using trypan blue staining. The dead cells stained with Trypan blue while live cells remained unstained (Figure 2). Interestingly, incubating cells with PEG GNRs or Her-PEG GNRs did not increase the rate of dead cells. This means that while CTAB-coated GNRs are toxic to cells even in short incubation periods in-vitro, PEG and Her-PEG GNRS do not induce cell death under similar conditions.
Figure 2.
Three types of GNRS were incubated with three cell lines in-vitro for 24 hours. The number of dead cells in each treatment group was counted after staining cells with Trypan Blue. CTAB-coated GNRs increased the number of dead cells in-vitro while PEG and Her-PEG GNRs did not increase the number of dead cells under similar conditions. CTL reflects cells that were not treated with gold nanoparticles (Control). Bars indicate standard deviation.
To assess if Her-PEG GNRs selectively bind to cancer cell in-vitro, five human cell lines were used: lung fibroblasts, non-cancerous breast cell line (MCF-10A), and three breast cancer cell lines: MCF-7, SK-BR3 and BT474. Lung fibroblasts, MCF-10A, and MCF-7 cells do not express HER2/neu, an extracellular membrane receptor (33) while SK-BR3 and BT-474 cell lines over-express HER2/neu. These cells were plated on glass cover-slips in 12-well culture plate and then rinsed with RPMI medium to remove unattached cells. Then, 1.6 mL RPMI-1640 culture media was mixed with 0.4 mL of Her-PEG GNRs or PEG GNRs and the mixture was added to each well. Samples were incubated at room temperature for 45 minutes to allow nanoparticles to attach to the HER2/neu receptors. Then, cell plates were rinsed with PBS twice to remove unattached nanoparticles. The samples were finally stained using silver enhancement staining kit (Artisan Grocott’s Methenamine Silver Kit; catalog # AR176; Dako; Carpenteria, CA) to visualize GNRs using an Olympus Model IX70 microscope equipped with 40X and 100X objective lenses and a Spot RT Slider digital camera (Diagnostics Instruments, Inc). Figure 3 shows the results of this experiment in which selective binding of functionalized gold nanorods to cancer cells were confirmed by visualizing gold nanorods as dark spots around SK-BR3 (C) and BT-474 cells (D) that over express HER2/neu receptor but not MCF-7 (A), fibroblast (B), or MCF-10A (E) cells that were used as negative controls. The number of dark spots in each acquired image was determined manually using cell counter plugins and Image J software that is available at NIH website (34)
Figure 3.
Bright field images of four cell lines that were incubated with Her-PEG GNRs in-vitro. Slides were stained using a sliver staining kit to visualize gold nanorods as dark spots under conventional bright field microscopy. A: MCF-7 (Breast cancer but no HER2/neu expression→ no staining), B: Fibroblast (no HER2/neu expression → no staining), C: SK-BR3 (over expression of HER2/neu), D: BT-474 (over expression of HER2/neu), E: MCF-10A (normal breast, no HER2/neu → no staining). Dark counts were determined manually using cell count plugins and Image J software.
In a similar experiment, SK-BR3 and BT-474 cells were incubated with both PEG GNRs versus Her-PEG GNRs in 24-well culture plates as described before and stained with silver staining method. Figure 4 depict a typical set of images that were obtained during this experiment. Figure 4A demonstrates minimal non-specific binding of PEG GNRs to SK-BR3 cells; however, the presence of Herceptin on GNRs remarkably enhanced the binding of Her-PEG GNRs to SK-BR3 cells (figure 4B). This provides the mean for the molecular recognition of HER2/neu over expressing cancer cells.
Figure 4.
Bright field microscopic images of SK-BR3 cells that were incubated with PEG GNRs (A) or Her-PEG GNRs (B), Her GNRs (C), or no GNRs (D) in-vitro. Samples were silver-stained to reveal GNR as dark spots. The total number of dark spots, that indicate GNRs, is higher when Her-PEG GNRs or Her GNRs were used as compared to the groups were PEG GNRs or no GNRs were used.
It is suggested that the in-vivo microenvironment of the body interferes with the stability and functionality of gold nanoparticles (35,36). For instance, blood contains a variety of ions such as hydrogen, Na, Cl, Ca, etc. as well as proteins, lipids, hydrocarbons and multiple cellular components that may affect nanoparticle stability and functionality. Studying the stability and functionality of injected GNRs within in-vivo microenvironment is technically challenging. However, it has been shown that most of the chemical and cellular components of blood such as pH, oxygen, ions, etc would remain unchanged for at least 12 hours in-vitro when blood is heparinized and preserved at room temperature (37,38). Thus, an experiment was performed to assess if Her-PEG GNRs remain stable and functional when incubated with blood in-vitro. To do this, blood was obtained from mice and heparinized to prevent coagulation (10 units heparin/mL). To each mL of heparinized mouse blood 100 μL of either CTAB-coated GNRs, PEG GNRs, or Her-PEG GNRs was added. After four hours of incubation, the samples were centrifuged in 1.5 mL eppendorf tubes at 2000 RPM for 15 minutes. Supernatant solution was collected and its absorption spectrum was determined using a Cary50 spectrophotometer. Figure 5 depicts the light absorption of these three samples as a function of wavelength. Interestingly, CTAB-Coated GNRs aggregated and thus their peak absorption at 760 nm was lost while PEG and Her-PEG GNRs remained stable as their absorption peak around 760 nm was preserved. This is a very important finding which confirms that our conjugated nanoparticles remain stable and did not aggregate while exposed to blood for 4 hours.
Figure 5.
Absorption spectra of supernatant solution after incubating three different types of GNRs for three hours in mouse heparinized blood. The disappearance of the peak absorption around 760 nm in the CTAB-Coated GNRs following incubation in blood indicated the aggregation of CTAB-coated GNRs in the blood while the presence of a preserved peak absorption at 760 nm for particles functionalized with the PEG GNRs and Her-PEG GNRs following incubation in blood confirm the stability of these particles within blood microenvironment.
The next group of experiments evaluated whether Her-PEG GNRs remain functional and still selectively bind to cancer cells after they are incubated in blood. To do this, Her-PEG GNRs were incubated with mouse blood as described before for four hours and then were incubated with BT-474 cells as well as fibroblasts for 45 minutes at 37 °C. The cell plates were rinsed with PBS to remove unattached GNRs and plates were stained using silver enhancement kit as described before. Figure 6 demonstrates the results in which GNRs are visualized as dark spots on BT-474 cells but not fibroblasts. This shows that engineered GNRs preserve their function in terms of selective binding to cancer cells that over express HER2/neu receptor (i.e. BT 474) even after incubating with mouse blood.
Figure 6.
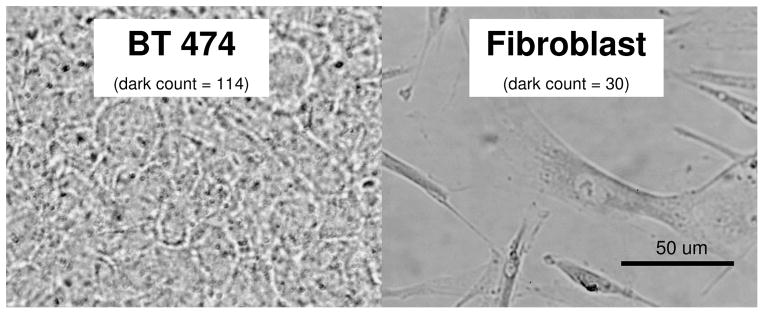
Bright field images of BT-474 and Fibroblast cells incubated with Her-PEG GNRs that were premixed with mouse blood for four hours. Samples were stained with silver enhancement kit and examined under bright field microscopy to reveal targeted GNRs. Dark counts were determined on each image manually using image J software.
The final experiment was designed to evaluate if Her-PEG GNRs accumulate within tumors that over express HER2/neu receptor in animal models. To do this, 2 × 106 BT474 cells were injected subcutaneously in the flank area of nude mice. All animal protocols were approved by the institutional animal care and use committee (IACUC) at the University of Texas Medical Branch. To enhance tumor growth, a 0.72 mg pellet of 17-beta estradiol (60-day release, Innovative Research of America, Sarasota, FL) was implanted under the skin in the shoulder area of these mice one day prior to the tumor cell injection. A total of ten nude mice were enrolled in this experiment of which eight developed a tumor with a diameter of 2–6 mm after three weeks. Six animals that had tumor were injected with 100 μL of Her-PEG GNRs with was injected intravenously into nude mice through their tail vein. Three animals with a tumor were injected with 100 μL of PEG GNRs. One mouse was used as a control and thus was injected with 100 μL of PBS through tail vein. 24 hours post tail vein injection, mice were sacrificed and samples of tumor, spleen, and kidney were fixed in formalin 4% and stained using the silver-stain method to reveal GNRs under bright field microscopy. Figure 7 shows a typical set of the result of this experiment in which Her-PEG GNRs were found to accumulate within tumor, spleen, and the liver of animals.
Figure 7.
Typical bright field images of mouse tissue slices following intravenous injection of GNRs to ten nude mice. 100 μL of PBS, PEG GNRs, or Her-PEG GNRs was injected intravenously into the tail vein and animals were sacrificed 24 hours post injection. Samples of tumor, liver, and spleen were obtained and silver stained to reveal gold nanorods. The images in the two bottom rows exhibit darker spots caused by enhanced accumulation of GNRs.
Discussion
The stability of injected gold nanorods within blood microenvironment is a key issue for successful targeting of GNRs to tumor cells in-vivo. Although non-covalent approaches has been widely used to conjugate gold nanoparticles to monoclonal antibodies for in-vitro applications (12), a non-covalent approach to bind monoclonal antibodies to gold nanoparticles should be avoided for in-vivo applications because blood contains a relatively high concentration of proteins that can compete with antibodies to bind to gold nanoparticles. This may accelerate the dissociation of antibodies from gold nanoparticles once nanoparticles are injected intravenously (39) and may result in non-specific binding. To avoid inactivation and instability of nanoparticles in-vivo, a covalent conjugation between nanoparticle and functional molecules is required (39,40).
Another important parameter is the circulation time of injected nanoparticles within body. Based on the size and surface characteristics of nanoparticles, the reticuloendothelial system of the body filters injected nanoparticles from blood circulation (25). Rapid clearance of nanoparticles from blood limits tumor targeting capabilities. To avoid this problem, nanoparticles can be coated with PEG to hide them from the reticuloendothelial system of the body. The beneficiary effects of PEGylation on the circulating time of injected nanoparticles have been demonstrated (41).
Covalent conjugation of gold nanorods to monoclonal antibodies without PEGylation has been reported in the literature (30). In the current work, hetero-functional gold nanorods were engineered by covalent conjugation of both monoclonal antibodies and PEG to gold nanorods. In-vitro toxicity study of engineered GNRs revealed that engineered nanoparticles were not toxic to cells. In addition, the stability and functionality of engineered particles in blood was investigated by incubating conjugated gold nanorods with mouse blood and then exposing them to cancer cells. Finally, engineered particles were injected intravenously into nude mice carrying breast cancer tumors demonstrating successful uptake of Her-PEG GNRs into tumors.
In a previous report, it was demonstrated that laser optoacoustic imaging system (LOIS) as an imaging modality can detect gold nanorods at a low concentration in-vivo (17). The current work supports the notion that functionalized gold nanoparticles covalently linked to Herceptin results in targeted accumulation of Herceptin allowing for in-vivo targeting and molecular imaging of tumor.
CONCLUSION
The current results indicate that Her-PEG GNRs are tolerated by cells, do not aggregate when exposed to blood microenvironment in-vitro, and preserve their selective binding properties in blood. In-vivo targeting of breast tumors that over express HER2/neu receptor in animal models was also achieved using Her-PEG GNRs. Considering the unique properties of GNRs to function as molecular imaging agent, applications of GNRs may lead to the development of highly promising imaging technique for early detection of cancer.
Acknowledgments
This project was funded by the National Cancer Institute (R44CA110137), National Science Foundation (Grant # 0632281) and NASA. Authors greatly appreciate technical support from Brandy Edenfield, Mayo Clinic, who performed the fixation of tumor tissues and silver enhanced staining.
Reference List
- 1.Ferrari M. Nature Reviews Cancer. 2005;5(3):161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 2.Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y, Niidome Y. Journal of Controlled Release. 2006;114(3):343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Small. 2005;1(3):325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 4.Ditrich H, Splechtna H. Tissue & Cell. 1988;20(6):891–898. doi: 10.1016/0040-8166(88)90030-4. [DOI] [PubMed] [Google Scholar]
- 5.O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Cancer Letters. 2004;209(2):171–176. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Chithrani BD, Ghazani AA, Chan WCW. Nano Letters. 2006;6(4):662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 7.Moses S, Brewer SH, Lowe LB, Lappi SE, Gilvey LBG, Sauthier M, Tenent RC, Feldheim DL, Franzen S. Langmuir. 2004;20(25):11134–11140. doi: 10.1021/la0492815. [DOI] [PubMed] [Google Scholar]
- 8.Cuenca AG, Jiang HB, Hochwald SN, Delano M, Cance WG, Grobmyer SR. Cancer. 2006;107(3):459–466. doi: 10.1002/cncr.22035. [DOI] [PubMed] [Google Scholar]
- 9.El Sayed IH, Huang XH, El Sayed MA. Cancer Letters. 2006;239(1):129–135. doi: 10.1016/j.canlet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 10.Gole A, Murphy CJ. Langmuir. 2005;21(23):10756–10762. doi: 10.1021/la0512704. [DOI] [PubMed] [Google Scholar]
- 11.Sosinsky GE, Giepmans BN, Deerinck TJ, Gaietta GM, Ellisman MH. Methods Cell Biol. 2007;79:575–591. doi: 10.1016/S0091-679X(06)79023-9. [DOI] [PubMed] [Google Scholar]
- 12.Huang XH, El Sayed IH, Qian W, El Sayed MA. Journal of the American Chemical Society. 2006;128(6):2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 13.Nagesha D, Laevsky GS, Lampton P, Banyal R, Warner C, DiMarzio C, Sridhar S. Int J Nanomedicine. 2007;2(4):813–819. [PMC free article] [PubMed] [Google Scholar]
- 14.Adler DC, Huang SW, Huber R, Fujimoto JG. Opt Express. 2008;16(7):4376–4393. doi: 10.1364/oe.16.004376. [DOI] [PubMed] [Google Scholar]
- 15.Kneipp K, Kneipp H, Kneipp J. Acc Chem Res. 2006;39(7):443–450. doi: 10.1021/ar050107x. [DOI] [PubMed] [Google Scholar]
- 16.Copland JA, Eghtedari M, Popov VL, Kotov N, Mamedova N, Motamedi M, Oraevsky AA. Molecular Imaging and Biology. 2004;6(5):341–349. doi: 10.1016/j.mibio.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Eghtedari M, Oraevsky A, Copland JA, Kotov NA, Conjusteau A, Motamedi M. Nano Lett. 2007;7(7):1914–1918. doi: 10.1021/nl070557d. [DOI] [PubMed] [Google Scholar]
- 18.Oraevsky AA. Gold and silver nanoparticles as contrast agents for optoacoustic imaging. In: Wang L, editor. Photoacoustic imaging and spectroscopy. 1. Chapter 30 Taylor and Francis Group; New York: 2008. [Google Scholar]
- 19.Cai QY, Kim SH, Choi KS, Kim SY, Byun SJ, Kim KW, Park SH, Juhng SK, Yoon KH. Invest Radiol. 2007;42(12):797–806. doi: 10.1097/RLI.0b013e31811ecdcd. [DOI] [PubMed] [Google Scholar]
- 20.Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM. British Journal of Radiology. 2006;79(939):248–253. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- 21.El Sayed MA. Abstracts of Papers of the American Chemical Society. 2000;220:U236. [Google Scholar]
- 22.Hu M, Chen JY, Li ZY, Au L, Hartland GV, Li XD, Marquez M, Xia YN. Chemical Society Reviews. 2006;35(11):1084–1094. doi: 10.1039/b517615h. [DOI] [PubMed] [Google Scholar]
- 23.Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. Proc Natl Acad Sci USA. 2008;105(33):11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamboni WC. Oncologist. 2008;13(3):248–260. doi: 10.1634/theoncologist.2007-0180. [DOI] [PubMed] [Google Scholar]
- 25.Storm G, Belliot SO, Daemen T, Lasic DD. Advanced Drug Delivery Reviews. 1995;17(1):31–48. [Google Scholar]
- 26.Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L. Drug Delivery. 2004;11(3):169–183. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 27.Caswell KK, Wilson JN, Bunz UHF, Murphy CJ. Journal of the American Chemical Society. 2003;125(46):13914–13915. doi: 10.1021/ja037969i. [DOI] [PubMed] [Google Scholar]
- 28.Wang ZL, Mohamed MB, Link S, El Sayed MA. Surface Science. 1999;440(1–2):L809–L814. [Google Scholar]
- 29.Wang ZL, Gao RP, Nikoobakht B, El Sayed MA. Journal of Physical Chemistry B. 2000;104(23):5417–5420. [Google Scholar]
- 30.Yu C, Irudayaraj J. Biophys J. 2007;93(10):3684–3692. doi: 10.1529/biophysj.107.110064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sau TK, Murphy CJ. Langmuir. 2004;20(15):6414–6420. doi: 10.1021/la049463z. [DOI] [PubMed] [Google Scholar]
- 32.Chang CJ, Chiu JH, Tseng LM, Chang CH, Chien TM, Wu CW, Lui WY. Eur J Clin Invest. 2006;36(8):588–596. doi: 10.1111/j.1365-2362.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- 33.Le XF, Arachchige-Don AS, Mao W, Horne MC, Bast RC., Jr Mol Cancer Ther. 2007;6(11):2843–2857. doi: 10.1158/1535-7163.MCT-07-0109. [DOI] [PubMed] [Google Scholar]
- 34.Rasband W. [December 2008];National Institute of Health 2008. http://rsb.info.nih.gov/ij/ version 1.40.
- 35.El Sayed IH, Huang X, El Sayed MA. Nano Lett. 2005;5(5):829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 36.Rouhana LL, Jaber JA, Schlenoff JB. Langmuir. 2007;23(26):12799–12801. doi: 10.1021/la702151q. [DOI] [PubMed] [Google Scholar]
- 37.Gokce G, Citil M, Gunes V, Atalan G. Res Vet Sci. 2004;76(2):121–127. doi: 10.1016/j.rvsc.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Fura A, Harper TW, Zhang H, Fung L, Shyu WC. J Pharm Biomed Anal. 2003;32(3):513–522. doi: 10.1016/s0731-7085(03)00159-6. [DOI] [PubMed] [Google Scholar]
- 39.Caruso F. Advanced Materials. 2001;13(1):11–22. [Google Scholar]
- 40.Katz E, Willner I. Angewandte Chemie-International Edition. 2004;43(45):6042–6108. doi: 10.1002/anie.200400651. [DOI] [PubMed] [Google Scholar]
- 41.Romberg B, Hennink WE, Storm G. Pharm Res. 2008;25(1):55–71. doi: 10.1007/s11095-007-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]








