Abstract
Recent evidence has suggested that disseminated intravascular coagulation (DIC) plays an integral role in death at the LD50 dose of either gamma or solar particle event (SPE)-like proton radiation in ferrets. In these studies, Yucatan minipigs were evaluated to determine whether they were susceptible to the development of radiation induced DIC. Yucatan minipigs were exposed to a dose of 2.5 Gray (Gy) with x-rays and monitored over the course of 30 days. Evidence of DIC was evaluated by way of thromboelastometry parameters, platelet counts, fibrinogen concentration, and the d-dimer assay. Pigs exposed to x-rays developed signs of DIC within 2 days post-irradiation. The development of DIC was exacerbated over the course of the studies, and one of the pigs died at day 14 and another had to be euthanized on day 16 post-irradiation. For both of these pigs, DIC was evident at the time of death. The following observations were indicated or were suggestive of DIC: whole blood clotting was impaired (as evidenced by thromboelastometry alterations), there were decreased platelet counts, elevated d-dimer concentrations in the blood, and/or hemorrhaging and the presence of fibrin in tissues observed during post-mortem examination. The extrapolation of data from these studies, in combination with other published data, have led to the hypothesis that there could be a correlation between the propensity to develop DIC, as indicated by hemorrhaging at death at relatively low doses of radiation, and the LD50 for a particular species. Our data suggest that the development of DIC may contribute to death at the LD50 dose in large mammals.
Introduction
Since the atomic bombings of Hiroshima and Nagasaki (1), the consequences of radiation exposure have been a major concern for national security. Some relatively recent human radiation exposures of great significance have occurred as well; examples of these include the Chernobyl power plant meltdown of 1985 (2), the Goiania accident in 1987 (3), and the Fukishima-Daiichi meltdown of 2011 (4). It is important for the national security of the United States that the effects of radiation exposure are known and methods are developed to mitigate any significant adverse biological effects of radiation exposure from a potential nuclear detonation or radiation accident (5, 6). Several well-known non-terrestrial sources of radiation originate in the space environment, including solar particle event (SPE) radiation (7) and galactic cosmic radiation (8), which are of particular concern for astronaut health. An SPE involves the release of highly energetic charged particles with energies greater than 10 MeV/nucleon; protons that originate from the sun are the primary type of SPE radiation. For the planned exploration class missions, such as a trip to Mars, the length of time in space will be considerably longer than the time periods involved in previous space missions and this will result in considerably higher astronaut radiation doses. As part of space radiation research, there is currently much effort focused on ensuring astronaut safety on long-term space missions.
The Acute Radiation Syndrome (ARS) involves many different types of adverse health effects resulting from acute exposure to ionizing radiation (9, 10). ARS is categorized into four areas: prodromal syndrome, gastrointestinal syndrome, cerebrovascular syndrome, and hematopoietic syndrome. The hematopoietic syndrome is observed after exposure to relatively low doses of radiation (1 – 5 Gy) and is thought to be the primary mechanism of radiation-induced death at the dose which kills half of the exposed population, which is known as the LD50; death can be due to infection, presumably from the loss of white blood cells, and/or hemorrhaging, presumably due to the loss of platelets (10).
In our previous studies, we have concluded that a coagulopathy, known as disseminated intravascular coagulation (DIC), occurred in irradiated ferrets at doses near the LD50 (11). In these studies, significant alterations in blood clotting parameters were observed in ferrets irradiated with SPE-like proton or gamma radiation, and the results were comparable for both types of radiation in terms of the onset and death from DIC (11, 12). Other studies revealed the activation of the coagulation cascade in response to radiation exposure, which is thought to have occurred through the increased presence of the biomarker fibrin (13). We have presented evidence (such as an increased soluble fibrin concentration in the blood, the presence of fibrin clots in tissues, and the inability to generate a stable clot) that doses near the LD50 can result in the development of DIC in ferrets (11). There is essentially no published evidence that radiation induced DIC occurs in other large mammals. Therefore, the current study was performed to determine whether there was evidence for the development of DIC in Yucatan minipigs exposed to ionizing radiation at a dose near the expected LD50 level, and a dose that astronauts could conceivably receive (e.g., during a worst case scenario such as that associated with the Carrington flare (14)). The LD50 of Yucatan minipigs has yet been reported. The average LD50 for other strains of pigs is 2.57 Gy (15), but LD50 values have been reported as low as 1.8 Gy in the Gottingen pig (16). In this study, Yucatan minipigs were exposed to x-rays at a dose of 2.5 Gy.
Materials & Methods
Animals and Blood Collection
Three Yucatan minipigs aged 8-14 weeks were purchased from Sinclair Bio Resources (Auxvasse, MO) and acclimated to the University of Pennsylvania Animal Facility. The Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania approved all animal procedures used in these studies. Approximately 1 week prior to radiation exposure, all animals were placed under anesthesia (isoflurane inhalant) and blood was collected from the superior vena cava and placed in either 3.8 % sodium citrate or ethylenediaminetetraacetic acid (EDTA) - containing vacutainer tubes for analysis. Blood samples were also collected on days 2, 7, 10, 13, 16, and 30 post-irradiation.
Radiation Procedures
Three pigs were irradiated with 6 MV x-rays generated by the iX linear accelerator from Varian (Palo Alto, California, USA) with an effective dose rate of 30 cGy per minute, at an extended source to surface distance of 370 cm, with collimator opening of 40 × 40 cm. 6 MV x-rays have a Bremsstrahlung spectrum with a peak energy of 6 MeV and an average energy below 3 MeV, delivering a close to exponential decay depth dose with initial buildup in the first 1.5 cm proximal depth, specified by percent depth dose at 10 cm depth as 67% for 100 cm source to surface to distance setup. In this configuration, 1450 machine monitor units (MU) were used to deliver 1.25 Gy to each side of the pigs using parallel opposed beams obtained by rotating the pig enclosures between irradiations. The output (cGy/MU) was determined using RadCalc® (Lifeline Software Inc. Austin, TA) dose calculation software. Calculation parameters were a maximal phantom depth of 10 cm (with variation from 5 to 10 cm), collimator opening of 40 × 40 cm, and a lateral phantom dimension of 55 × 50 cm, to approximate the irradiation geometry of the pigs. The calculation was verified with an ionization chamber measurement at a depth of 10 cm using 40 × 40 cm slabs of solid water. This measurement agreed within 3% of the expected calculation after accounting for the difference between the phantom dimensions used in the experiment and those used in the calculation. The measurement and calculations were performed at a phantom depth of 10 cm, so that the specified dose from the parallel opposed beams is defined at the center of a 20 cm thick phantom. A pig's head can have a diameter closer to 10 cm, instead of 20 cm; therefore, the head could get a dose 5% higher than the prescribed 2.5 Gy dose. The pigs' body radii vary between 5 and 10 cm. The dose was prescribed to an average radius (midline depth) of 8.5 cm to minimize the variation of dose received by different body parts. A head compensator was not used in this experiment for the sake of simplicity.
Thromboelastometry
Within one hour of blood collection, whole blood clotting was analyzed using a rotational thromboelastogram system (ROTEM, Munich, Germany), as previously described by Krigsfeld et al. (11). Briefly, blood clotting was measured over the course of 90 min and data on the following parameters were recorded: coagulation time, clot formation time, a-angle, and maximum clot firmness.
Blood Cell Counts
For the whole blood collected in EDTA-containing tubes, a complete blood cell (CBC) count with differential analysis was performed within 24 hours of collection by an external laboratory using a Bayer Advia 120 Hematology Analyzer (Antech Diagnostics, Lake Success, NY, USA). Cell counts are referred to as the average absolute count ± standard deviation (SD).
D-dimer and Fibrinogen ELISA
Plasma was isolated by centrifugation at 3000 × G for 15 min at 4°C; isolated plasma was stored at -80°C. Thawed samples were used in the Porcine D-dimer ELISA assay (MyBioSource, San Diego, CA, USA) and the Porcine fibrinogen (Innovative Research Inc, Novi, MI, USA) ELISA assay following the vendors' protocols.
Statistical Analysis
The thromboelastometry and CBC data were analyzed by the paired Student's t-test to determine whether there were statistically significant differences between the pre and post-radiation time points using PRISM 5.0 (Graphpad, La Jolla, CA, USA) statistical and graphing software.
Results
Survival study and evidence of DIC
The radiation exposure resulted in death or euthanasia in 2 of the 3 irradiated pigs (Figure 1). In this study, the pigs were monitored two-three times per day post-irradiation for any signs of distress. There was evidence of petechiae and ecchymosis in all three irradiated pigs at various time points. During this observation period, one pig was found dead at day 14 post-exposure, while a second animal exhibited signs of hemorrhaging, such as petechiae and purpura, beginning on day 14 post-exposure. After several days of worsening signs, which included severe ecchymosis, significant petechiae in the oral mucosa and skin, positive fecal occult blood test, inability to generate a stable blood clot, a very high D-dimer concentration in the blood (the highest concentration [145 ng/mL] was observed at day 1.5) and moribund condition, this pig needed to be euthanized on day 16. At necropsy, there was evidence of extensive internal hemorrhaging.
Figure 1.
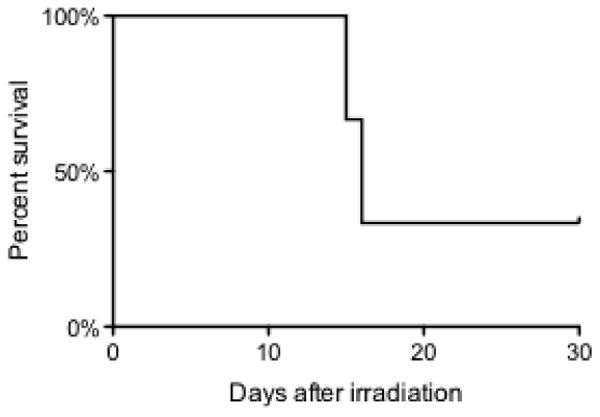
Survival curve for Yucatan mini pigs exposed to 2.5 Gy of x-irradiation.
Upon post-mortem analysis of the animal that was found dead at day 14 postexposure, the following observations were made: significant petechiae and ecchymoses were apparent and there was evidence of bleeding throughout the oral cavity, skin, abdominal musculature, mesenteric lymph nodes, stomach serosa and mucosa, small and large intestines, diaphragm, heart (transmural in right ventricle), lungs, dura and leptomeninges of the brain, within the brain, and testes. Slides were prepared for histopathological examination and reviewed by a board certified veterinary pathologist, who confirmed evidence of DIC, which included extensive hemorrhaging observed in the skeletal muscle, heart, kidney, lymph node, testes, stomach, and small intestine. A significant amount of fibrin was observed in alveoli of the lung, as well as in the heart; these observations corresponded with the diagnosis of DIC for this pig by the pathologist.
Complete blood cell counts
A statistically significant decrease in blood cell counts was observed post-irradiation in all 3 pigs (Figure 2). White blood cell (WBC) counts were decreased, in a statistically significant manner, at the earliest time point evaluated, at 1.5 days post-irradiation (Figure 2a); these blood cell counts did not recover or return to baseline values over the course of the study. Starting at day 16 post-irradiation, statistical analyses were not performed on the data, as there was an n < 3 (due to death/euthanasia). The lymphocyte count nadir was observed on day 1.5 post-irradiation, with an average count of 1,130 ± 319 cells/μL, which was approximately 13% of the control baseline value (Figure 2b). Similar to the observed decrease in total WBC count, the lymphocyte counts never returned to the average pre-irradiation values over the course of the study. The neutrophil counts decreased from 11,120 ± 1,425 cells/μL (average count at pre-irradiation) to 6,477 ± 646 cells/μL at 1.5 days post-irradiation (p < 0.1; Figure 2c). At day 7 post-irradiation, the neutrophil counts decreased further, to an average of 2135 ± 552 cells/μL; the differences between the neutrophil counts in the irradiated pigs, compared to the pre-irradiation values (11,120 ± 1425 cells/μL), were statistically significant (p < 0.01). The neutrophil counts in the irradiated pigs at days 10 and 13 post-irradiation were still lower when compared to their pre-irradiation values (p < 0.01). The decreases in the monocyte counts in the pig post-irradiation blood samples, compared to their baseline values, were of borderline statistical significance (p < 0.1; Figure 2d).
Figure 2.
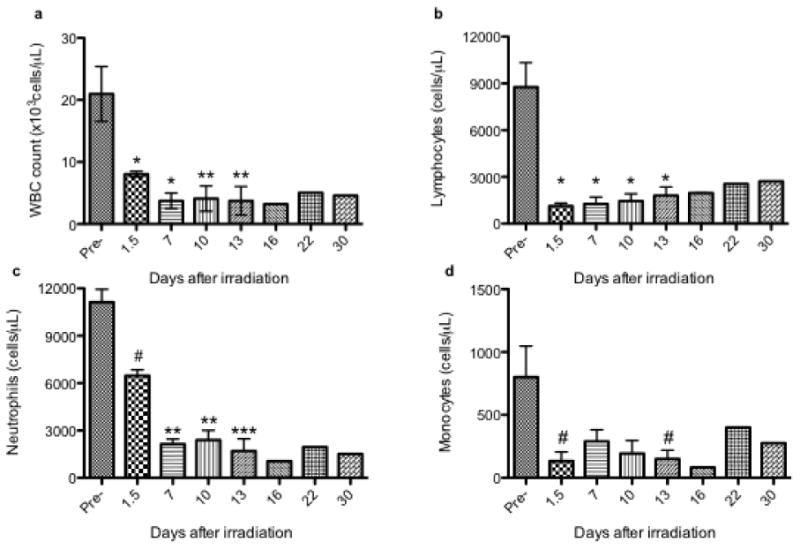
Radiation exposure significantly reduces blood cell counts. Pigs were exposed to 2.5 Gy from x-rays. Complete blood cell counts with differential were determined in peripheral blood collected pre-irradiation and at several time points post-irradiation (1.5 – 30 days). Statistically significant differences between treatments were determined with a paired Student's t-test; they are indicated in the figure by # (p < 0.1), * (p < 0.05), ** (p < 0.05), or *** (p < 0.001). Blood cell counts are reported for (a) WBCs, (b) lymphocytes, (c) neutrophils, and (d) monocytes; the blood cells counts are represented as the average absolute count ± SD (n=3, except for days 16-30, at which points n < 3 and there were no statistical analyses performed).
The platelet counts were also decreased after radiation exposure (Table I). The average platelet count prior to radiation exposure was 556 × 103 cells/μL; the platelet count remained in a normal range (> 200 × 103 cells/μL) until day 10 and was decreased at days 10 and 13 (p < 0.05). For the surviving pig, the platelet count returned to the normal range by day 30 (225 × 103 cells/μL). The estimated platelet counts are also reported at various time points post-irradiation in Table 1, as the presence of platelet clumps prevents an accurate platelet count; the presence of platelet clumps in the irradiated pigs was presumably due to the onset of DIC. The use of estimated platelet counts and platelet clumping was previously established in ferret studies focused on the development of DIC, as described by Krigsfeld et al. (11). In this study, decreases in the platelet estimates were first observed at 10 days post-irradiation, at which time two pigs exhibited thrombocytopenia, with platelet counts < 200 × 103 cells/μL. By day 13, all 3 of the irradiated pigs had thrombocytopenia. Platelet clumping, which reflects the activation of platelets, corresponds with the observed decrease in platelet counts, as indicated in Table I. Platelet clumping was evident at day 10 post-irradiation, with blood smear reviews indicating platelet clumping in 100% (3/3) of the irradiated animals. At day 13 post-irradiation, blood smears indicated that 1/3 of the animals exhibited platelet clumping, while the other two pigs exhibited thrombocytopenia, with platelet counts < 20 × 103 cells/μL.
Table I.
Radiation exposure severely impacts platelet function. Platelet counts, estimates, and clumping values are shown for the irradiated pigs. The platelet estimates are reported in terms of normal and low platelet counts for the pigs in this study. Platelet clumping data were determined from blood smears that indicated platelet clumps. A paired Student's t-test was used to determine statistical significance, as indicated by * (p < 0.05).
| Days Post-Radiation | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | 1.5 | 7 | 10 | 13 | 16 | 22 | 30 | |||||||||
| Platelet Count (103 cells/μL) | 556 (± 75) | 211 (± 117) | 321 (± 28) | 49* (± 2) | 23* (± 15) | 14.5 | 56 | 225 | ||||||||
| Platelet Estimate (N, L) | N | L | N | L | N | L | N | L | N | L | N | L | N | L | N | L |
| 3 | 0 | 3 | 0 | 3 | 0 | 1 | 2 | 0 | 3 | 0 | 2 | 0 | 1 | 1 | 0 | |
| Pigs with Platelet Clumping | 0/3 | 2/3 | 0/3 | 3/3 | 1/3 | 2/2 | 1/1 | 0/1 | ||||||||
Platelet Estimates:
N = Normal, > 200 × 103 cells/μL
L=Low, < 200 × 103 cells/μL
Thromboelastography
In order to characterize blood clotting and coagulation parameters as part of the diagnosis of DIC, whole blood clotting was evaluated using a thromboelastogram (TEG). The irradiated pigs exhibited significant blood clotting aberrations. Four parameters were evaluated, as indicated in Figure 3: coagulation time, clot formation time, alpha angle, and maximum clot firmness. In the irradiated animals, there was no observed change in coagulation time (time to generate a 2 mm clot, sometimes referred to as the reaction time) for any of the time points investigated (post-exposure vs. pre-irradiation exposure; Figure 3a). The time to generate a stable clot is measured as the clot formation time (or the time to generate a 20 mm clot); the data related to the clot formation time are shown in Figure 3b. Prior to the radiation exposure, the average clot formation time was 49 ± 7 sec. Radiation exposure resulted in a statistically significant increase in the clot formation time, which was initially observed at day 7 post-irradiation, with a clot formation time of 158 ± 50 sec. Severe impairment in blood clotting in the pigs was observed throughout the remainder of the study, with a statistically significant increase in clot formation time of 21-fold (1,056 ± 828 sec) by day 13 post-exposure. At day 16 postexposure, the two surviving animals had a clot formation time of ∼ 4800 and 400 sec. The a-angle parameter assesses the rate of clot formation through measurement of the angle of the slope of the line generated between the coagulation time and clot formation time. The angle represents the acceleration (kinetics) of fibrin buildup and cross-linking. Prior to the radiation exposure, the average α-angle was 81° (Figure 3c). By day 13 post-exposure, the average α-angle was 54° (p < 0.05, compared to the pre-radiation exposure average). The maximum clot firmness represents the estimated maximum amplitude of the clot generated for the particular blood sample, representing the strength of the fibrin clot. Prior to the radiation exposure, the average maximum clot firmness was 76 ± 9 mm (Figure 3d). The clot firmness remained relatively unchanged until day 10 post-exposure, when it began to decrease. The clot firmness continued to decrease, and at day 13 post-exposure, the differences in the clot firmness values post-irradiation were statistically significant (p < 0.05), compared to the pre-irradiation values. Together, the information obtained from 3/4 thromboelastography parameters evaluated indicated severe blood clotting impairment by day 10 post-irradiation.
Figure 3.
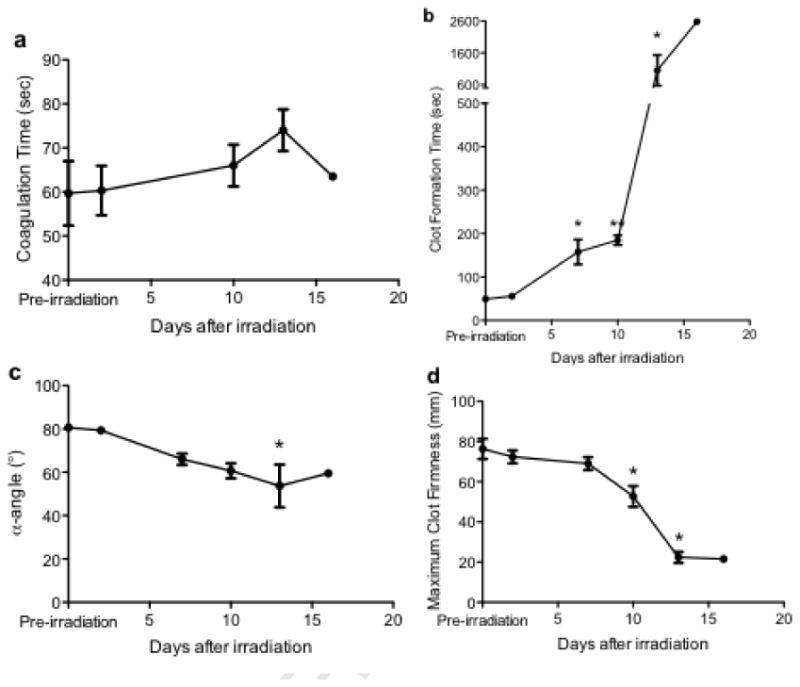
Radiation exposure severely impairs whole blood clotting. Thromboelastometry was utilized to determine the effects of radiation exposure on whole blood clotting parameters: (a) coagulation time, (b) clot formation time, (c) a-angle, and (d) maximum clot firmness. A paired Student's t-test was used to determine statistical significance, as indicated by * (p < 0.05) or ** (p < 0.05) (n=3, except for days 16-30, at which points n < 3 and no statistical analyses were performed.)
D-dimer and fibrinogen concentrations
To confirm the onset of DIC, the activation of hemostasis was determined by the measurement of the fibrin degradation-related markers, d-dimer and fibrinogen. The concentration of d-dimer in plasma for the pigs was measured pre- and post-irradiation, and the average d-dimer concentrations for the pigs are shown in Figure 4. In the pre-irradiation blood samples, the d-dimer concentration was below the threshold of the assay and was therefore arbitrarily reported in Figure 4 as 1 ng/mL. At 1.5 days post-irradiation, the pigs (n=3) exhibited an approximate 100-fold increase in d-dimer concentrations, with an average d-dimer plasma concentration of 106 ng/mL. The levels of plasma d-dimer remained increased over the course of this study. Plasma fibrinogen concentrations remained unchanged with radiation exposure (data not shown). There were no statistically significant changes in fibrinogen levels after radiation exposure, when compared to pre-irradiation levels, regardless of the time point evaluated.
Figure 4.
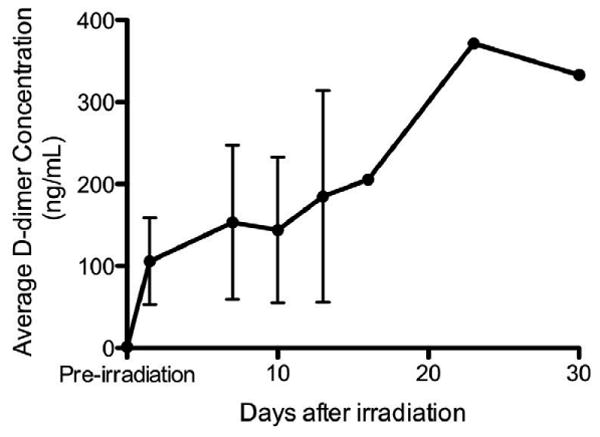
Radiation exposure significantly increases the average d-dimer concentrations in the blood. The concentration of plasma d-dimers was determined in each animal prior to and at several time points after radiation exposure, and averages ± standard errors from the pigs are shown in the figure; error bars are not given for time-points with n < 3 (day 16+) An increase in average d-dimer concentration was observed in the pigs as early as 1.5 days post-irradiation.
DIC scoring system
To diagnose DIC in irradiated pigs, the human International Society of Thrombosis & Hemostasis (ISTH) scoring system was adapted and modified for this study (17). The human scoring system utilizes the following parameters to generate a DIC score: clotting times (specifically, prothrombin [PT] and activated partial thromboplastin [aPTT] times), platelet counts, markers of the formation of fibrin strands (typically reported through fibrin degradation products, specifically d-dimer), and plasma fibrinogen concentrations. It has been previously determined that TEG data correlate with a diagnosis of DIC in patients when there are significant changes in at least two of four parameters: coagulation time, clot formation time, α-angle, and maximum clot firmness (18). TEG data were collected in the current study instead of PT and aPTT times; therefore, the existing scoring system was modified by replacing the PT/aPTT values with TEG data (Table II). Platelet estimates were used in the current scoring system (as opposed to actual platelet counts) as well as the d-dimer and fibrinogen concentrations.
Table II.
The pig DIC scoring system is adapted from the ISTH scoring system, which has been modified for irradiated pigs.
| Category | Criterion | Score |
|---|---|---|
| Significant Changes in 4 TEG Parameters | < 2 changes | 0 |
| 2 changes | 1 | |
| ≥ 3 changes | 2 | |
| Platelet Estimate | High | 0 |
| Medium | 1 | |
| Low | 2 | |
| D-dimer Increase | None | 0 |
| Moderate | 2 | |
| Strong | 3 | |
| Fibrinogen Concentration | > 1g/L | 0 |
| < 1g/L | 1 | |
| Total DIC Score | (sum) |
DIC Score ≥ 5: overt DIC
> 2.5 and < 5: non-overt DIC
Based on the scoring system established in Table II, each pig was given an individual score for the four parameters (changes in TEG parameters, platelet counts, d-dimer, and fibrinogen); after a score was given for each category, the total DIC score was compiled and the average DIC score for the irradiated pigs is indicated in Figure 5. For example, the pig that was euthanized at day 16 has a score of 7 (2 from TEG, 2 from platelet counts, 3 for d-dimer change, and 0 from fibrinogen). In the clinic, a score > 5 is indicative of overt DIC, while a score of < 5 is suggestive of DIC (17). In these studies in pigs, our results have led to the conclusion that a score of < 5 and > 2.5 is suggestive of DIC and a score of ≥ 5 is indicative of DIC (shown as dashed lines in Fig. 5). The results from pigs were suggestive of DIC at 1.5 days post-irradiation, with an average DIC score of 2.7. By day 10 post-irradiation, the average DIC score was 5.3, indicating overt DIC. As described above, 1 animal died, 1 animal was euthanized, and the last pig survived the 30-day experimental period. The surviving pig had DIC scores that were in the overt range (i.e., >5) from days 10-23. At day 30, the pig had a non-overt DIC score of 4.
Figure 5.
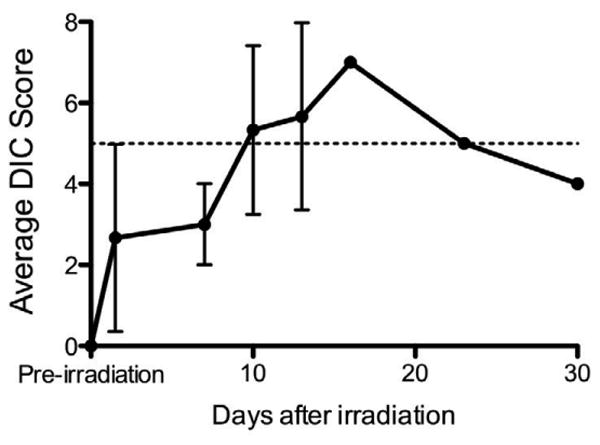
Radiation exposure results in non-overt and overt DIC in pigs. The DIC score was determined for each individual pig and the average DIC score (± standard errors) for all pigs surviving at a given time point is indicated for the time points investigated during the course of the study. The dashed line indicates a score of 5; a score ≥ 5 indicates overt DIC and < 5 and >2.5 is suggestive of DIC. The average DIC score increased over the first 16 days of the study, and then began to decline toward values that were not suggestive of DIC due to the recovery of one of the pigs irradiated at 2.5 Gy.
Discussion
We have recently reported that DIC was the most likely cause of death in ferrets exposed to doses near the LD50 level (19). One of the major drawbacks of the ferret model, as well as most other animal model systems, for studies of DIC is the lack of availability of assays specific to this species to monitor the progression of DIC in a living animal. Therefore, much of the diagnosis of DIC in animal models is performed post-mortem, with histochemical analysis to confirm activation of the coagulation cascade. The human scoring system established by the ISTH utilizes assays and tests that are verified using human blood components (17), and therefore the guidelines established by the ISTH can serve as a predictive tool for use in human patients in the clinic. Therefore, it was necessary to develop a DIC scoring system primarily as a research tool in order to monitor, diagnose, and treat DIC in research animals; the guidelines for monitoring DIC progression suggested here may be useful to other researchers attempting to evaluate the development of DIC in large animal model systems. In the scoring system presented here (shown in Table II), we are proposing that a DIC score of ≥ 5 refers to overt DIC, while a score of < 5 and > 2.5 refers to non-overt DIC.
It is noteworthy that the diagnosis of DIC in humans does not rely upon a score from a single parameter contributing to the diagnosis of DIC, but instead relies upon scores for a number of different endpoints considered to be of importance. At necropsy or autopsy, the presences of bleeding and fibrin deposition together are the strongest histopathological evidence for terminal DIC. For the diagnosis of DIC in the clinic, the lack of normal blood clotting function and evidence of fibrin in the blood are strong evidence that DIC has occurred, and the scoring system is focused on these endpoints. To get to an overt DIC score in the clinic, more than one of the biological endpoints scored must be at an elevated level, as a single high score by itself is insufficient to result in a diagnosis of DIC. While the evidence of a high d-dimer score is highly suggestive of DIC when it is observed along with other biomarkers related to the development of DIC, it can not lead to a diagnosis of DIC by itself. There are other conditions involving the activation of the coagulation cascade that lead to a high d-dimer concentration in the blood (20); examples include deep vein thrombosis (DVT) and pulmonary embolism (PE) (21). It is also known that false negatives for the d-dimer test may occur due to a number of reasons (22-24); some examples include the presence of an anti-coagulant in the blood, improper blood collection/assay performance, and when the measurements are evaluated after clot formation (25). In this study, the blood levels of d-dimer were sufficiently high to be suggestive of DIC in two of the three irradiated pigs, but they were not substantially elevated in the pig that died. This pig did, however, have clear evidence of DIC upon histopathological analysis of tissues, as there was evidence of hemorrhage and fibrin deposition in tissues; thus, these results have led us to conclude that the results of the d-dimer test represented a false-negative result in this pig. In the two pigs having elevated blood d-dimer levels, the levels were over 100 fold higher than the normally undetectable d-dimer levels in pig blood. In normal humans, the blood levels of d-dimer are also normally undetectable utilizing the assay systems that exist (26), and diagnoses of DIC occur with blood levels containing high d-dimer concentrations (27). In a study in dogs with and without DIC, it was reported that the normal average d-dimer concentration was 0.13 ug/mL and dogs with DIC had a mean d-dimer blood concentration of 0.73 ug/mL (28). We report that Yucatan minipigs exposed to a radiation dose expected to induce death in approximately 50% of the population results in DIC, using a comprehensive DIC scoring system established from the results of studies reported here. The onset and progression of DIC induced potentially fatal conditions in the irradiated animals, with signs of coagulopathies present well into the later time points of this study. The first signs of radiation-induced coagulopathies (i.e., petechiae and ecchymosis) were observed around day 11 post-irradiation, and these signs were followed by a rapid decline in health in 2/3 pigs. One pig died at day 14 post-irradiation and was diagnosed by a pathologist with “DIC secondary to radiation exposure”. Porcine ELISA assays were adapted and verified for the purposes of monitoring DIC in these studies. Porcine d-dimer concentrations were elevated at the earliest time point of 1.5 days post-irradiation in two of the three pigs. These concentrations remained elevated in these pigs throughout the course of this study. Abnormal increases in d-dimer concentration in humans are indicative of the activation of the coagulation cascade, which is commonly observed and considered as part of the DIC diagnosis.
LD50 values vary widely in different mammalian species, from 1.4 Gy in cows (29) to 13.4 Gy in certain strains of mice (15). Prior to our publication utilizing the ferret model (11), there were no studies indicating the development or occurrence of DIC as a potential or contributing cause of radiation induced death in the literature. There is, however, a considerable amount of data in the literature addressing the occurrence of radiation induced hemorrhage occurring at death. Hemorrhage is a hallmark of DIC in mammals; thus, we propose the use of hemorrhage around the time of mammalian death as a biomarker of DIC. Using published data, we compared the percentage of animals exhibiting hemorrhage at death (from radiation doses near the LD50 level) as a function of the LD50 of the species (Figure 6; data from the literature in the figure are from (11, 15, 30-32)). We observed an excellent correlation between the percent of animals exhibiting hemorrhage at death or euthanasia (at doses near the LD50) and the LD50 value for the species/strain. From our data in ferrets (11) and pigs, along with the data shown in Fig. 6, we hypothesize that the LD50 correlates with the propensity to exhibit hemorrhage at death. Based on the fact that hemorrhage is a hallmark of DIC, it is further hypothesized that the propensity to develop radiation-induced DIC in mammalian species may be correlated with the LD50 values of the mammalian species or strain. At this point, radiation biologists do not have a reasonable explanation for the great variability in mammalian LD50 values, and the propensity to develop DIC is one reasonable and novel hypothesis.
Figure 6.
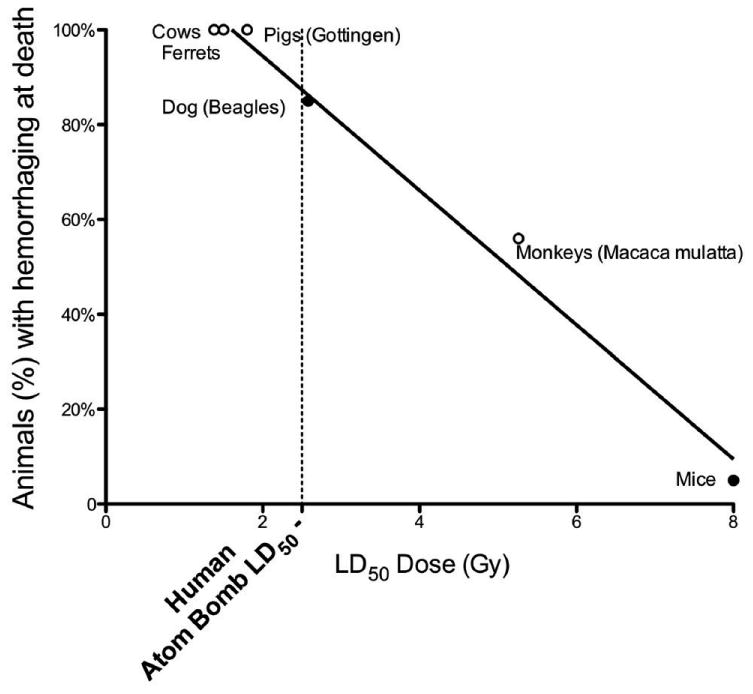
The percentage of animals exhibiting hemorrhage at death (at levels of radiation near the LD50 value for the species) is plotted against the LD50 value for the species or strain of mammal. LD50 values – with open circles (0) represent exact values from ferrets (11), cattle (29), Gottingen pigs (30) and monkeys (31). LD50 values – with closed circles (•) are estimated for dogs (32), in which it is claimed that the majority of the dogs exhibit hemorrhaging at death), and for mice, represent an average value (15). Human data (dotted line): a) LD50 ∼ 2.5 Gy – Atom Bomb Casualties (33, 34); b) Percentage dying with hemorrhage (35). At the LD50 for mice, mice die from infection and do not exhibit hemorrhaging (47, 48). Thus, it is assumed that ∼0% of the mice die from hemorrhaging.
It is known that people frequently exhibit hemorrhaging at the LD50 dose. The LD50 of the atom bomb casualties has been estimated to be approximately 2.5 Gy (33, 34). The percent of those dying from atom bomb exposure was estimated from the summary of the atom bomb casualty data reviewed by Liebow et al. (35), with casualty dose estimates given in a report by the U.S. Atomic Energy Commission (36). In the Liebow et al. review (35), people exposed to radiation from the atom bomb (used in Hiroshima) were classified into several groups. In Group II (which included patients dying between weeks 3-6 or surviving severe clinical symptoms), some of the people lived and some of the people died; thus, it is assumed that the autopsy data for this group represented people dying at a dose near the LD50. The data represented one tissue section from each tissue/organ examined, and the fraction of tissues exhibiting hemorrhage is given. For many of the organs/tissues, such as the kidney, liver, etc., the percentages exhibiting hemorrhage were as high as 60%, but it is not clear what fraction of exposed individuals experienced hemorrhaging in one or more organs. For example, it is not indicated whether 60% of the livers exhibiting hemorrhage are from the same people as 60% of the kidneys exhibiting hemorrhage. Thus, if it is assumed that 60% of the people exhibiting hemorrhage is a minimum value, then the true value lies between 60-100% of the people dying in Hiroshima at a dose near the LD50 that had evidence of hemorrhage. If these human data are added to Figure 6 (the dotted line shown in the figure), it can be observed that people are approximately as sensitive as dogs to radiation induced hemorrhaging at death.
The major finding in this study is that the development of DIC has been documented in three pigs, from the following evidence: 1) the pig that died at day 14 had evidence of extensive hemorrhage and fibrin deposition in organs; for the board-certified veterinary pathologist examining the histopathologic data, these findings were clear evidence for the diagnosis of DIC. 2) The pig that was euthanized at day 16 had evidence of DIC from the following observations: severe ecchymosis, significant petechiae in the oral mucosa and skin, positive fecal occult blood test, inability to generate a stable blood clot, a very high D-dimer concentration in the blood (up to 145 ng/mL) and moribund condition, as well as evidence of extensive hemorrhaging at necropsy. High D-dimer concentrations in blood samples are directly correlated with activation of the coagulation cascade (37), and elevated D-dimer concentrations are a major sign/indicator of DIC in humans in the clinic (17). 3) The third irradiated minipig also had indications that DIC was present (specifically, increased time to generate a stable clot, decrease in maximum clot firmness, increased levels of D-dimer concentrations in the blood, and thrombocytopenia), but ultimately this pig survived up to 30 days post-irradiation. In the scoring system developed in this study, which is similar to the scoring system in the clinic, all 3 pigs had evidence of non-overt DIC at early time points and 2 pigs had evidence of overt DIC at death or euthanasia.
The fact that one of the animals with early evidence for the development of DIC, which included overt DIC scores for days 10-23 post-irradiation, but which fell to a non-overt DIC score later in the study, gives hope that prevention of terminal DIC is a real possibility. As the eventual progression to death from DIC was precluded by endogenous mechanisms in this surviving animal, these results suggest the possibility that drugs or natural supplements may be able to increase defense mechanisms and reverse the progression of DIC after the disease process has been initiated. Understanding the mechanism(s) involved in radiation-induced DIC in pigs and other species is important for the development of countermeasures. There are many ways in which radiation exposure could result in DIC, some of which are as follows. 1) Radiation could produce DIC through its ability to induce inflammation. DIC is an inflammatory disease and it is well-known that radiation induces inflammation (38). Radiation could induce inflammation through its ability to cause the rapid release of cytokines (39); the inflammatory response resulting from cytokine signaling is known to activate the coagulation cascade and induce DIC. 2) Radiation could produce DIC by its ability to cause the release of tissue factor. Tissue factor is a known trigger for coagulation (40); in response to injury, monocytes and endothelial cells release tissue factor. It is well-established that radiation can kill both monocytes and endothelial cells. 3) Radiation could cause DIC through its ability to cause breaks in the lining of the gastrointestinal (G.I.) tract, which can result in a loss of barrier function for bacteria localized in the gut and the translocation of gut bacteria and bacterial products into the bloodstream (41). The presence of a bacterial product, lipopolysaccharide (LPS), also known as endotoxin, in the bloodstream and tissues outside of the G.I. tract would have great significance for the development of DIC, as endotoxin is a known inducer of DIC (42). 4) Radiation exposure could result in DIC through its ability to produce neutrophil extracellular traps (NETs), as it is known that NETS can induce DIC (43). Radiation exposure is known to result in the death of neutrophils and NETs result from dying neutrophils. More specific knowledge about the mechanism by which radiation induces DIC is necessary before hypotheses can be developed to explain how the varying LD50 levels are related to the differing sensitivities for the development of DIC between small and large animals.
While the results reported here for this pig study used x-irradiation, it is believed that the results for parameters related to the development of DIC are comparable for x-irradiation, gamma radiation and SPE-like proton radiation, as discussed elsewhere (44). Thus, it believed that the findings reported here apply to various forms of space radiation as well as those encountered on earth.
For astronauts, the highest internal dose from SPE radiation has been estimated to be approximately 2 Gy (7, 14), which is below the internal dose received by the pigs in the studies described here. The SPE internal radiation dose estimate is based on models presented by Hu et al. (7). Employing the spectra from the Carrington Flare of 1859, doses as high as 2.81 Gy to the blood forming organs (BFO) were predicted (14). Stephens et al. (14) concluded that such doses could potentially result in death. Doses considerably lower than the dose used in these studies can result in death in other pig strains. For example, the LD50 for Gottingen pigs is 1.8 Gy (30). Thus, the response of Gottingen pigs to doses of < 2 Gy may be more relevant for assessment of the likelihood of DIC development from the predicted SPE radiation doses. Moroni et al. (30) concluded that the changes associated with ARS and radiation-induced death in Gottingen pigs are similar to those in humans.
In addition to other types of space radiations to which the astronauts will be exposed, there are also other stressors likely to be present in the space environment that could affect the development of DIC. As some examples, these other stresses include microgravity and high oxygen levels during extravehicular activities (EVAs). In ground-based analog studies, it has been observed that simulated microgravity has a major impact on the dose-response relationship for radiation induced adverse immunological effects in mice. The functional characteristics of cells of the immune system in animals exposed to SPE-like radiation along with simulated microgravity (HS) have been measured in bacterial challenge studies. It has been observed that a nontoxic (or minimally toxic) dose of bacteria (Pseudomonas aeruginosa or Klebsiella pneumoniae) in two different strains of mice (C3H/HeN and Balb/c) exposed to a 2 Gy dose of SPE radiation, with or without additional exposure to simulated microgravity (hindlimb suspension), leads to a high mortality rate (45). It is noteworthy that Pseudomonas aeruginosa is present in spacecrafts and has been previously implicated in astronaut infections and Klebsiella pneumoniae is part of the normal bacterial flora of the mouse, skin and intestines (as reviewed recently by Kennedy, 2014). The results presented by Li et al. (45) indicate that, under simulated microgravity conditions, the effects of a given dose of SPE radiation can be considerably more severe than the effects observed for the same dose of radiation in normal, control animals. These results also indicate that, in the space microgravity environment, the effects of a given dose of SPE radiation can be comparable to those observed for a significantly higher dose of radiation (44). So, for just one of the stressors present during space travel, microgravity, the dose response relationship for a biological endpoint like DIC could be quite different from that observed for radiation exposure in ground-based studies, with the result that DIC could develop in space at a considerably lower dose than expected on earth.
Signs of the hematopoietic syndrome were observed in the irradiated pigs with decreased white blood cell and platelet counts within 2 days post-irradiation. These findings are not uncommon at doses approaching the expected LD50. The radiation-induced hematopoietic cell loss in the peripheral blood observed in this study is consistent with the results from a previous study from our group utilizing inhomogenous proton radiation exposure (mimicking solar particle event [SPE]-like radiation) in Yucatan mini pigs (46). In the previous study, animals were exposed to 5-10 Gy proton doses to the skin, but the calculated dose to the BFO was 0.42 Gy/5 Gy skin. This calculated dose applies not only to the bone marrow, thymus, and spleen, where hematopoietic cells are developing and differentiating, but also to the lymph node/lymphovascular space, where mature, circulating cells of blood origin are also located. In this study involving a homogeneous 2.5 Gy dose of x-radiation, total WBC, lymphocyte, neutrophil, and monocyte counts decreased after radiation exposure in a similar manner to that observed in the previous study. For example, the lymphocyte counts were reduced by approximately 85% by 1.5 days post-radiation in this study and approximately 75% by day 1 post-radiation (5 Gy) in the previous study (46). Interestingly, when the lymphocyte count at day 1 post-irradiation in mini pigs exposed to 10 Gy SPE-like proton radiation (or approximately an 0.84 Gy dose to the BFO) is evaluated, a 90% decrease in lymphocyte counts is observed, compared to baseline values, suggesting that the 0.84 Gy proton dose to the BFO is just as efficacious in inducing lymphocyte toxicity as is a 2.5 Gy dose of x-radiation. Lastly, in both studies, the total WBC, lymphocyte, and neutrophil counts did not return to baseline levels by 30 days post-irradiation with either proton or x-ray irradiation.
In the current study, severe blood clotting abnormalities and other indications of DIC, such as increases in d-dimer concentrations in the blood, were evident early after radiation exposure and these findings are novel in the porcine model. With the generation of a DIC scoring system in pigs (Table II), we are now able to monitor DIC progression in at least one animal model post-irradiation (Figure 5). We hypothesize that radiation-induced DIC may be a contributing factor to the differences observed in LD50 values among mammalian species. The scoring system adapted here is in agreement with the diagnostic tool used in the clinical setting for prescribing appropriate treatments, such as platelet infusions, activated protein C or anti-inflammatory drugs, and has the potential for use in monitoring and treating the progression of radiation-induced DIC in animal model systems.
Acknowledgments
The authors alone are responsible for the content and writing of the paper. The research was supported by the NSBRI Center of Acute Radiation Research (CARR) grant. The NSBRI is funded through NASA NCC 9-58.
Footnotes
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ishikawa E, Swain DL. The Committee for the Compilation of Materials on Damage Caused by the Atomic Bombs in Hiroshima and Nagasaki. Hiroshima and Nagasaki: The Physical, Medical, and Social Effects of the Atomic Bombings. New York: Basic Books; 1981. [Google Scholar]
- 2.Dallas CE. Medical lessons learned from Chernobyl relative to nuclear detonations and failed nuclear reactors. Disaster Med Public Health Prep. 2012;6:330–334. doi: 10.1001/dmp.2012.72. [DOI] [PubMed] [Google Scholar]
- 3.I.A.E. Agency, editor. IAEA. The Radiological Accident in Goiania. Vienna: 1988. [Google Scholar]
- 4.I.A.E. Agency, editor. IAEA. Report of the Japanese Government to the IAEA Ministerial Conference on Nuclear Saety - The Accident at TEPCOs Fukushima Nuclear Power Stations. 2011. [Google Scholar]
- 5.Weisdorf D, Chao N, Waselenko JK, Dainiak N, Armitage JO, McNiece I, Confer D. Acute radiation injury: contingency planning for triage, supportive care, and transplantation. Biol Blood Marrow Transplant. 2006;12:672–682. doi: 10.1016/j.bbmt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Medalia J. Nuclear Terrorism: A Brief Review of Threats and Responses. CRS Report for Congress 2004 [Google Scholar]
- 7.Hu S, Kim MHY, McClellan GE, Cucinotta FA. Modeling the acute health effects of astronauts from exposure to large solar particle events. Health Physics. 2009;96:465–476. doi: 10.1097/01.HP.0000339020.92837.61. [DOI] [PubMed] [Google Scholar]
- 8.Todd P. Space radiation health: a brief primer. Gravit Space Biol Bull. 2003;16:1–4. [PubMed] [Google Scholar]
- 9.Dorr H, Meineke V. Acute radiation syndrome caused by accidental radiation exposure - therapeutic principles. BMC Med. 2011;9:126. doi: 10.1186/1741-7015-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall EJ, Giaccia AJ. Acute Effects of Total-Body Irradiation. Radiobiology for the Radiologist. 2006:117–128. [Google Scholar]
- 11.Krigsfeld GS, Savage AR, Billings PC, Lin L, Kennedy AR. Evidence for Radiation Induced Disseminated Intravascular Coagulation as a Major Cause of Radiation Induced Death in Ferrets. International Journal of Radiation Oncology, Biology, Physics. 2014;88:940–946. doi: 10.1016/j.ijrobp.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krigsfeld GS, Sanzari JK, Kennedy AR. The effects of proton radiation on the prothrombin and partial thromboplastin times of irradiated ferrets. International Journal of Radiation Biology. 2012;88:327–334. doi: 10.3109/09553002.2012.652727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krigsfeld GS, Savage AR, Sanzari JK, Wroe AJ, Gridley DS, Kennedy AR. Mechanism of hypocoagulability in proton-irradiated ferrets. Int J Radiat Biol. 2013;89:823–831. doi: 10.3109/09553002.2013.802394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stephens DL, Jr, Townsend LW, Hoff JL. Interplanetary crew dose estimates for worst case solar particle events based on historical data for the Carrington flare of 1859. Acta Astronautica. 2005;56:969–974. doi: 10.1016/j.actaastro.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 15.Morris MD, Jones TD. A comparison of dose-response models for death from hematological depression in different species. International Journal of Radiation Biology. 1988;53:439–456. doi: 10.1080/09553008814552571. [DOI] [PubMed] [Google Scholar]
- 16.Moroni M, Coolbaugh TV, Lombardini E, Mitchell JM, Moccia KD, Shelton LJ, Nagy V, Whitnall MH. Hematopoietic radiation syndrome in the Gottingen minipig. Radiat Res. 2011;176:89–101. doi: 10.1667/rr2481.1. [DOI] [PubMed] [Google Scholar]
- 17.Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–1330. [PubMed] [Google Scholar]
- 18.Sharma P, Saxena R. A novel thromboelastographic score to identify overt disseminated intravascular coagulation resulting in a hypocoagulable state. Am J Clin Pathol. 2010;134:97–102. doi: 10.1309/AJCPPZ4J6CAFYDVM. [DOI] [PubMed] [Google Scholar]
- 19.Krigsfeld GS, Kennedy AR. Is disseminated intravascular coagulation the major cause of mortality from radiation at relatively low whole body doses? Radiat Res. 2013;180:231–234. doi: 10.1667/RR3321.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinegris MC, ten Cate H, ten Cate-Hoek AJ. D-dimer as a marker for cardiovascular and arterial thrombotic events in patients with peripheral arterial disease. A systematic review. Thromb Haemost. 2013;110:233–243. doi: 10.1160/TH13-01-0032. [DOI] [PubMed] [Google Scholar]
- 21.Pabinger I, Thaler J, Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood. 2013;122:2011–2018. doi: 10.1182/blood-2013-04-460147. [DOI] [PubMed] [Google Scholar]
- 22.Dentali F, Squizzato A, Marchesi C, Bonzini M, Ferro JM, Ageno W. D-dimer testing in the diagnosis of cerebral vein thrombosis: a systematic review and a meta-analysis of the literature. J Thromb Haemost. 2012;10:582–589. doi: 10.1111/j.1538-7836.2012.04637.x. [DOI] [PubMed] [Google Scholar]
- 23.Freyburger G, Labrouche S. Comparability of D-dimer assays in clinical samples. Semin Vasc Med. 2005;5:328–339. doi: 10.1055/s-2005-922478. [DOI] [PubMed] [Google Scholar]
- 24.Wahl WL, Ahrns KS, Zajkowski PJ, Brandt MM, Proctor M, Arbabi S, Greenfield LJ. Normal D-dimer levels do not exclude thrombotic complications in trauma patients. Surgery. 2003;134:529–532. doi: 10.1016/s0039-6060(03)00271-x. discussion 532-523. [DOI] [PubMed] [Google Scholar]
- 25.Kraaijenhagen RA, Wallis J, Koopman MM, de Groot MR, Piovella F, Prandoni P, Buller HR. Can causes of false-normal D-dimer test [SimpliRED] results be identified? Thromb Res. 2003;111:155–158. doi: 10.1016/j.thromres.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 26.Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood. 2009;113:2878–2887. doi: 10.1182/blood-2008-06-165845. [DOI] [PubMed] [Google Scholar]
- 27.Dempfle CE, Wurst M, Smolinski M, Lorenz S, Osika A, Olenik D, Fiedler F, Borggrefe M. Use of soluble fibrin antigen instead of D-dimer as fibrin-related marker may enhance the prognostic power of the ISTH overt DIC score. Thromb Haemost. 2004;91:812–818. doi: 10.1160/TH03-09-0577. [DOI] [PubMed] [Google Scholar]
- 28.Stokol T, Brooks MB, Erb HN, Mauldin GE. D-dimer concentrations in healthy dogs and dogs with disseminated intravascular coagulation. Am J Vet Res. 2000;61:393–398. doi: 10.2460/ajvr.2000.61.393. [DOI] [PubMed] [Google Scholar]
- 29.Brown DG, Thomas RE, Jo Nes LP, Cross FH, Sasmore DP. Lethal dose studies with cattle exposed to whole-body Co60 gamma radiation. Radiat Res. 1961;15:675–683. [PubMed] [Google Scholar]
- 30.Moroni M, Lombardini E, Salber R, Kazemzedeh M, Nagy V, Olsen C, Whitnall MH. Hematological changes as prognostic indicators of survival: similarities between Gottingen minipigs, humans, and other large animal models. PLoS One. 2011;6:e25210. doi: 10.1371/journal.pone.0025210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eldred E, Trowbridge WV. Radiation sickness in the monkey. Radiology. 1954;62:65–73. doi: 10.1148/62.1.65. [DOI] [PubMed] [Google Scholar]
- 32.Norris WP, Fritz TE, Rehfeld CE, Poole CM. The response of the beagle dog to cobalt-60 gamma radiation: determination of the LD50(30) and description of associated changes. Radiat Res. 1968;35:681–708. [PubMed] [Google Scholar]
- 33.Lushbaugh CC. Reflections on some recent progress in human radiobiology. Advances in Radiation Biology. 1969;3:277–314. [Google Scholar]
- 34.Fujita S, Kato H, Schull WJ. The LD50 associated with exposure to the atomic bombing of Hiroshima and Nagasaki. J Radiat Res. 1991;32(Suppl):154–161. doi: 10.1269/jrr.32.supplement_154. [DOI] [PubMed] [Google Scholar]
- 35.Liebow AA, Warren S, De CE. Pathology of atomic bomb casualties. Am J Pathol. 1949;25:853–1027. [PMC free article] [PubMed] [Google Scholar]
- 36.USEAC. Medical Effects of Atomic Bomb. The Report of the Joint Commission for the Investigation of the Effects of the Atomic Bomb in Japan III 1951 [Google Scholar]
- 37.Bates SM. D-dimer assays in diagnosis and management of thrombotic and bleeding disorders. Semin Thromb Hemost. 2012;38:673–682. doi: 10.1055/s-0032-1326782. [DOI] [PubMed] [Google Scholar]
- 38.Lorimore SA, Coates PJ, Scobie GE, Milne G, Wright EG. Inflammatory-type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects? Oncogene. 2001;20:7085–7095. doi: 10.1038/sj.onc.1204903. [DOI] [PubMed] [Google Scholar]
- 39.Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiat Res. 2012;178:505–523. doi: 10.1667/RR3031.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gando S, Sawamura A, Hayakawa M. Trauma, shock, and disseminated intravascular coagulation: lessons from the classical literature. Ann Surg. 2011;254:10–19. doi: 10.1097/SLA.0b013e31821221b1. [DOI] [PubMed] [Google Scholar]
- 41.Ni H, Balint K, Zhou Y, Gridley DS, Maks C, Kennedy AR, Weissman D. Effect of solar particle event radiation on gastrointestinal tract bacterial translocation and immune activation. Radiat Res. 2011;175:485–492. doi: 10.1667/RR2373.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hook KM, Abrams CS. The loss of homeostasis in hemostasis: new approaches in treating and understanding acute disseminated intravascular coagulation in critically ill patients. Clin Transl Sci. 2012;5:85–92. doi: 10.1111/j.1752-8062.2011.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kambas K, Mitroulis I, Apostolidou E, Girod A, Chrysanthopoulou A, Pneumatikos I, Skendros P, Kourtzelis I, Koffa M, Kotsianidis I, et al. Autophagy mediates the delivery of thrombogenic tissue factor to neutrophil extracellular traps in human sepsis. PLoS One. 2012;7:e45427. doi: 10.1371/journal.pone.0045427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kennedy AR. Biological effects of space radiation and development of effective countermeasures. Life Sciences in Space Research. 2014;1:10–43. doi: 10.1016/j.lssr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M, Holmes V, Zhou Y, Ni H, Sanzari JK, Kennedy AR, Weissman D. Hindlimb suspension and SPE-like radiation impairs clearance of bacterial infections. PLoS One. 2014;9:e85665. doi: 10.1371/journal.pone.0085665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanzari JK, Wan XS, Wroe AJ, Rightnar S, Cengel KA, Diffenderfer ES, Krigsfeld GS, Gridley DS, Kennedy AR. Acute hematological effects of solar particle event proton radiation in the porcine model. Radiat Res. 2013;180:7–16. doi: 10.1667/RR3027.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boone IU, Woodward KT, Harris PS. Relation between bactermia and death in mice following x-ray and thermal column exposures. Journal of Bacteriology. 1956;71:188–195. doi: 10.1128/jb.71.2.188-195.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eisele GR, West JL. Bacteriological evaluations of swine exposed to lethal levels of gamma radiation. J Anim Sci. 1973;37:27–32. doi: 10.2527/jas1973.37127x. [DOI] [PubMed] [Google Scholar]


