Visualizing and tracking drusen is important in managing age-related macular degeneration (AMD). Color fundus photography (CFP) is common in clinical practice, and but quantitative evaluation of drusen in CFPs is difficult because of the varying background of macula, retinal pigment epithelium (RPE), and choroid.1
Spectral-domain optical coherence tomography (SD-OCT) provides high-resolution visualization of drusen, and 2D projections resembling CFPs can be produced from SD-OCT using summed-voxel projection (SVP).2 Though SVP is a useful method for visualizing the fundus, most drusen are not visible this way.2 Anything that produces high reflections along axial lines will produce bright areas in the SVP image and obscure drusen (Figures 1A and 1B, yellow circles) (available at http://aaojournal.org). Thus, drusen that are visible on B-scans may not be visible on the SVP image (Figure 1B and 1C, red circles) (available at http://aaojournal.org). Furthermore, drusen produce distortions in the RPE layer, but do not substantially change the intensity sums in the image columns traversing the drusen and remain invisible on SVP.
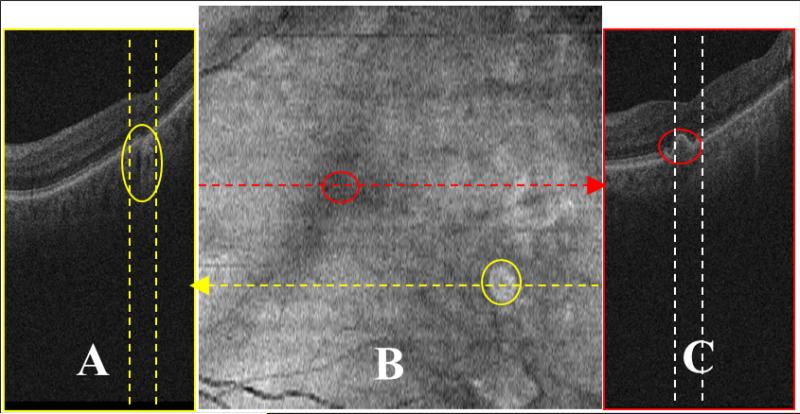
Figure 1.
Limitations in drusen visualization in summed-voxel projection (SVP) fundus images. SVP fundus image (B) was derived from a 3D optical coherence tomography (OCT) dataset from a patient with non-exudative age-related macular degeneration. Two spectral-domain OCT B-scans (A and C) obtained in planes corresponding to the yellow and red lines of the fundus image (B), respectively. The bright region marked with a yellow circle in B is the projection of the narrow region between the two dashed yellow lines in A. This region is an area of geographic atrophy (marked with a yellow oval in the left image), not drusen. The drusen marked with a red circle in C is not visible in the expected region (red circle in B).
The “slab SVP” in a commercial SD-OCT system (Carl Zeiss Meditch, Inc., unpublished) and a similar methods3, 4 have improved the SVP; however, user interaction is required to localize the RPE, and the slab SVP provides no information about drusen thickness, which could be useful for characterizing drusen.
We have developed a novel, fully automated approach, the restricted summed-voxel projection (RSVP), to generate improved visualizations of the retinal surface that incorporates image processing to enhance drusen visualization. The RSVP has two steps: (1) restrict the projection to the sub-volume of OCT data in close proximity to the RPE, and (2) supplement the projection with image processing methods that enhance the brightness of drusen. In the first step, the method detects and interpolates two contours in the RPE: (1) the abnormal contour of the upper boundary of the RPE which is distorted by drusen, and (2) the expected normal (undistorted) lower contour that would be expected if drusen were absent. The posterior boundary of the normal RPE layer is taken as the baseline of the projection region used for RSVP generation, while the top boundary of the projection region is determined by displacing the normal RPE layer anteriorly the same distance as the largest drusen peak in the cube. All other structures in the retina that could interfere with visualization of drusen are excluded (Figure 2) (available at http://aaojournal.org).
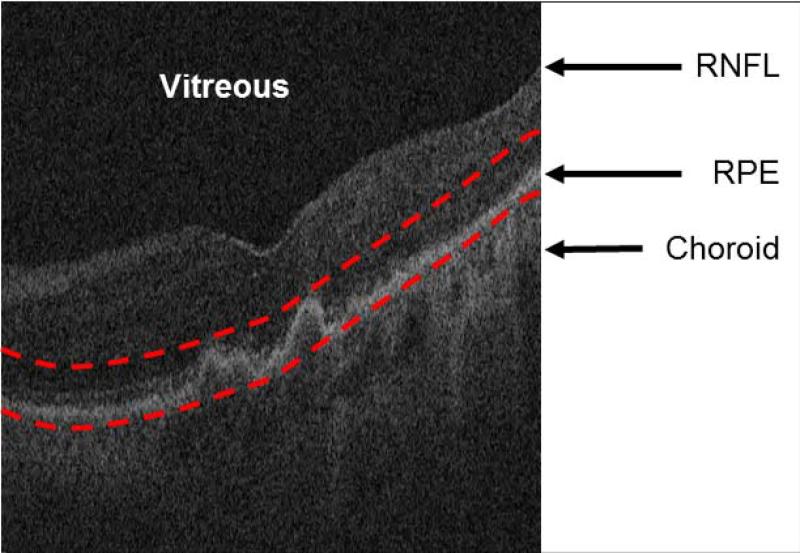
Figure 2.
Restricting the optical coherence tomography image data to the vicinity of the retinal pigment epithelium (RPE) layer for generating the restricted summed-voxel projection (RSVP). The bottom red curve is the baseline of the normal RPE layer. The top red curve is determined by the largest drusen height in all of B-scans. The RSVP thus excludes extraneous portions of the retina that may contain noise to the projection, such as those caused by the vitreous, retinal nerve fiber layer (RNFL), and choroid.
In the second step, the method replaces the values of the pixels underneath distorted RPE with the maximum intensity value in each image column of the B-scan. (Figure 3) (available at http://aaojournal.org). Since the projection is based on the sum of the pixel values in each image column, the larger the height of the drusen, the brighter the drusen in the projection; thus, in addition to enhancing drusen visualization, the method provides a visual indication of the thickness of drusen.
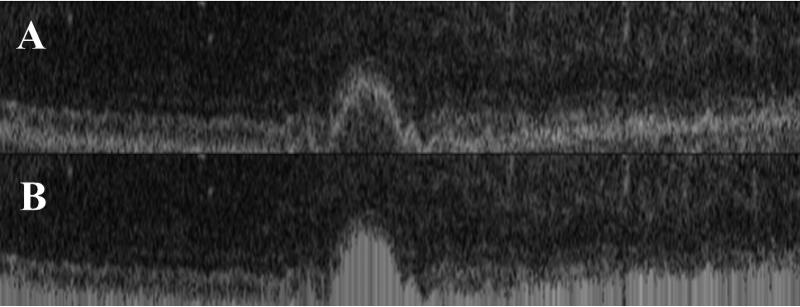
Figure 3.
Filling of the projection region. Projection region (A) and filled projection region (B).
Using 46 OCT datasets from eight patients, we found that the RSVP method was more effective than conventional SVP in displaying drusen and retinal vessels (Figure 4) (available at http://aaojournal.org), and was more accurate in detecting drusen in qualitative and quantitative evaluations. Qualitative evaluation was conducted by visual inspection of SVP and RSVP images, and comparing them with CFPs. Quantitative evaluation consisted of a comparison of drusen detected by manual segmentation of the SVP and RSVP images in four eyes from three patients. Gold standards were obtained by manual segmentation of each of the b-scans in the cubes. They were performed twice by two separate readers. The results showed that while, on average, only 2.1% of drusen could be detected from SVP images, 89.3% of drusen were detected in RSVP images.
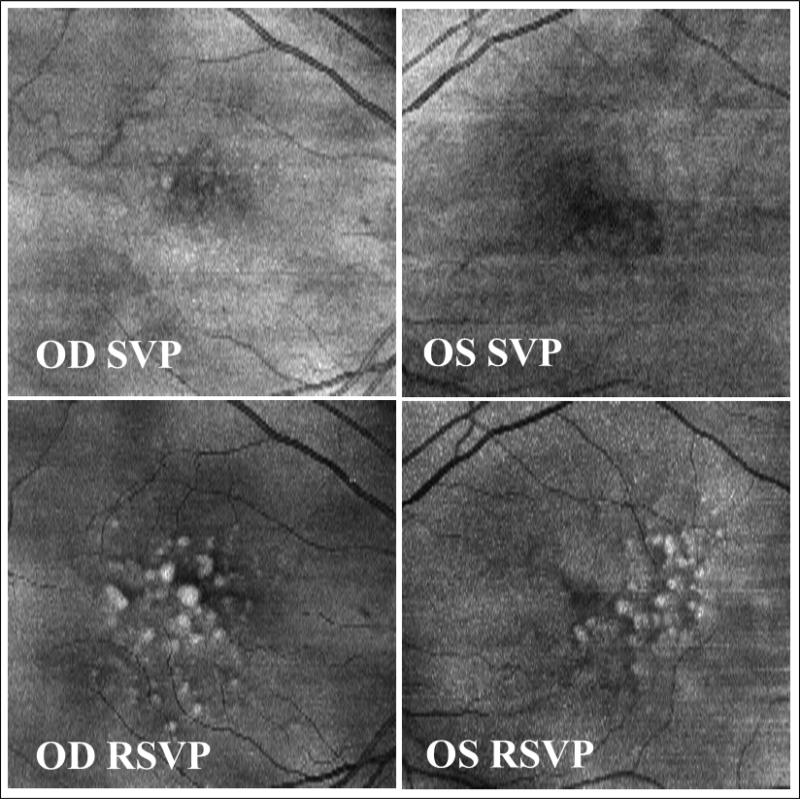
Figure 4.
Comparison of summed-voxel projection (SVP) (top) and restricted summed-voxel projection (RSVP) (bottom) in the right and left right eyes of a patient. Drusen poorly visualized or not apparent on the SVP are clearly shown in the RSVP, and the degree of brightness indicates the height of drusen.
Our method differs from prior approaches in that it is fully automated and it includes a highly restricted volume of the OCT in proximity to the RPE where drusen reside; thus, drusen visualization with a RSVP will be degraded less than current slab-like SVP methods. Furthermore, our method incorporates image processing to enhance drusen. To date, the RSVP method is substantially more effective than current SVP methods for visualizing drusen, and it may be useful to ophthalmologists in rapidly and quantitatively assessing drusen in AMD patients.
Acknowledgments
Financial support: Supported by a grant from the Bio-X Interdisciplinary Initiatives Program of Stanford University, a grant from the National Cancer Institute, National Institutes of Health, grant No. U01-CA-142555, and grants from the National Natural Science Foundations of China under Grant No. 60805003.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: no conflicting relationship exists for any author
REFERENCES
- 1.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–34. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 2.Jiao S, Knighton R, Huang X, Gregori G, Puliafito C. Simultaneous acquisition of sectional and fundus ophthalmic images with spectral-domain optical coherence tomography. Optics express. 2005;13:444–52. doi: 10.1364/opex.13.000444. [DOI] [PubMed] [Google Scholar]
- 3.Gorczynska I, Srinivasan VJ, Vuong LN, et al. Projection OCT fundus imaging for visualising outer retinal pathology in non-exudative age-related macular degeneration. The British journal of ophthalmology. 2009;93:603–9. doi: 10.1136/bjo.2007.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stopa M, Bower BA, Davies E, Izatt JA, Toth CA. Correlation of pathologic features in spectral domain optical coherence tomography with conventional retinal studies. Retina. 2008;28:298–308. doi: 10.1097/IAE.0b013e3181567798. [DOI] [PubMed] [Google Scholar]