Abstract
The inappropriate programming of the reproductive system by developmental exposure to excess steroid hormones is of concern. Sheep are well suited for investigating developmental origin of reproductive and metabolic disorders. The developmental time line of female sheep (~5 mo gestation and ~7 mo to puberty) is ideal for conducting sequential studies of the progression of metabolic and (or) reproductive disruption from the developmental insult to manifestation of adult consequences. Major benefits of using sheep include knowledge of established critical periods to target adult defects, a rich understanding of reproductive neuroendocrine regulation, availability of non-invasive approaches to monitor follicular dynamics, established surgical approaches to obtain hypophyseal portal blood for measurement of hypothalamic hormones, and the ability to perform studies in natural setting keeping behavioral interactions intact. Of importance is the ability to chronically instrument fetus and mother for determining early endocrine perturbations. Prenatal exposure of the female to excess testosterone (T) leads to an array of adult reproductive disorders that include LH excess, functional hyperandrogenism, neuroendocrine defects, multifollicular ovarian morphology, and corpus luteum dysfunction culminating in early reproductive failure. At the neuroendocrine level all three feedback systems are compromised. At the pituitary level, gonadotrope (LH secretion) sensitivity to GnRH is increased. Multifollicular ovarian morphology stems from persistence of follicles, as well as enhanced follicular recruitment. These defects culminate in progressive loss of cyclicity and reduced fecundity. Prenatal T excess also leads to fetal growth retardation, an early marker of adult reproductive/metabolic diseases, insulin resistance, hypertension and behavioral deficits. Collectively, the reproductive and metabolic deficits of prenatal T-treated sheep provide proof of concept for the developmental origin of fertility and metabolic disorders. Studies with the environmental endocrine disruptor, bisphenol-A (BPA), show that reproductive disruptions found in prenatal BPA-treated sheep are similar to those seen in prenatal T-treated sheep. The ubiquitous exposure to endocrine disrupting compounds (EDC) with steroidogenic potential via the environment and food sources, calls for studies addressing the impact of developmental exposure to environmental steroid mimics on reproductive function.
Keywords: androgens, developmental programming, estrogens, infertility
INTRODUCTION
The hormonal, nutritional, and metabolic environment to which the developing fetus is exposed during gestation can permanently program many aspects of its development. These programmed events are expressed during adulthood as altered physiology or pathology. The developing fetus has the capability to develop compensatory strategies to overcome programming via changes in the in utero environment. Such compensations could be adaptive, if they support survival, or disruptive, if they compromise postnatal survival (Godfrey et al., 2010). The ability of the developing fetus to change structure and / or function in response to physiological cues from the mother is known as developmental plasticity and underlies the concept of the developmental origin of disease theory or Barker hypothesis (Barker, 1994). In the last decades, concerns are mounting relative to exposure to environmental pollutants that include pesticides, fertilizers, and industrial byproducts, due to their ability to interfere with normal homeostasis. For example, these imposters can reach pregnant ruminants via grazing on polluted pastures, delivered via processed commercial food, modify the maternal environment, adversely influence developmental trajectory of organ differentiation, and culminate in reproductive and / or metabolic dysfunctions. It is also becoming apparent that there is significant interaction between diet and endocrine disruptors with diet playing a modulatory role in ameliorating or amplifying the impact of these endocrine disrupting chemicals (Baldi and Mantovani, 2008). This review will provide proof of principle to the concept of developmental origin of dysfunction by addressing the reproductive and metabolic defects in female sheep arising from prenatal exposure to excess native steroids or endocrine disrupting chemicals that have the ability to signal through steroid receptors during critical developmental windows with specific emphasis on those that target estrogen and androgen signaling pathways.
DEVELOPMENTAL PROGRAMMING OF REPRODUCTIVE AND METABOLIC DYSFUNCTION WITH NATIVE STEROIDS
The fetus is extremely sensitive to steroid hormone exposure during its early development. Steroids play a critical role in regulating the trajectory of tissues and organ differentiation during development. Endocrine disrupting compounds (EDC) that can signal via steroid-mediated pathways during critical periods of development can lead to long term consequences, which may be detrimental to animal and human health. For instance, it is known since the 1970s that gestational exposure to excess testosterone (T) from early to mid-gestation can induce phenotypic virilization in females. The masculinization of brain with this treatment is also evident at the behavioral level, with female offspring manifesting male typical behavior (Jost et al., 1973; Gorski, 1986; Wood and Foster, 1998). The degree of penetration of the virilization trait depends on the dose and the window of exposure in sheep (Wood and Foster, 1998). Similar outcomes at reproductive and metabolic levels have been documented in other species as well (monkey: Abbott et al., 2002, rat: Manneras et al., 2007, and mouse: Sullivan and Moenter, 2004).
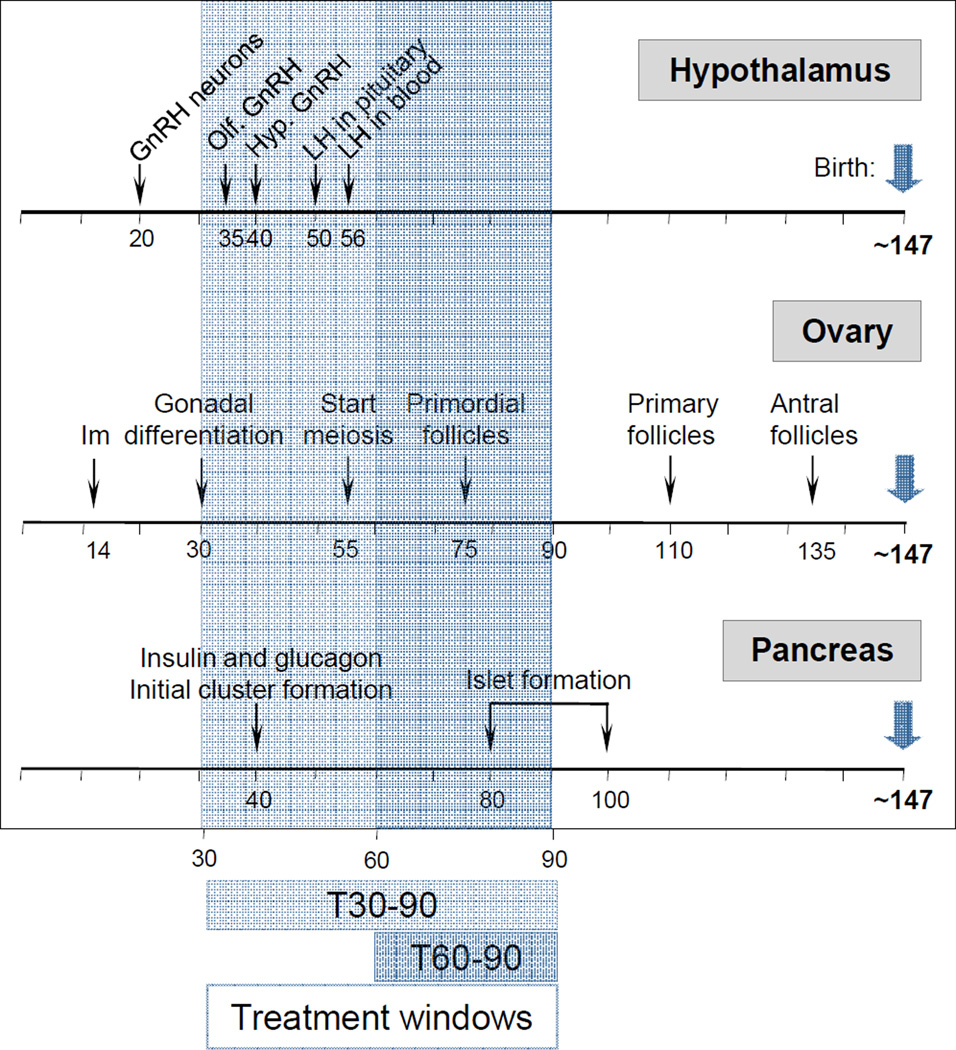
Sheep are exceptional animal models to study fetal physiology (Harding and Bloomfield, 2004). Their large size permits performance of detailed and repetitive hormonal profiling, noninvasive sequential monitoring of ovarian follicular dynamics via ultrasound, and multiple neurotransmitter measures at the brain level. In addition, because they are a domesticated species, animals can be kept in a natural setting free from stress associated with caging. Their duration of gestation (~147 d) and timing of puberty (~28 wk in females) makes them an exceptional animal model to study developmental programming of adult reproductive disorders from insult to outcome within a reasonable time frame. As a precocial species, similar to humans, most of the organs / systems differentiation occurs prior to birth. The developmental timeline for ovarian, hypothalamic, and pancreatic differentiation in sheep is shown in Figure 1. Importantly, they are amenable for collecting fetal blood samples enabling assessment of fetal endocrine milieu.
Figure 1.
Developmental trajectory of hypothalamic, ovarian, and pancreatic differentiation in sheep, where establishment of hypophyseal portal vasculature to pituitary, appearance of LH and FSH in circulation, complete ovarian follicular differentiation, and pancreatic islet formation occurs prenatally. Abbreviations used: Hypothalamus [GnRH neurons: appearance of first GnRH immunoreactive neurons, Olf. GnRH: GnRH neurons visible in olfactory bulb, and Hyp. GnRH: appearance of GnRH neurons in the hypothalamus]; Ovary [Im: implantation]. Modified from Padmanabhan and Veiga-Lopez, 2013a.
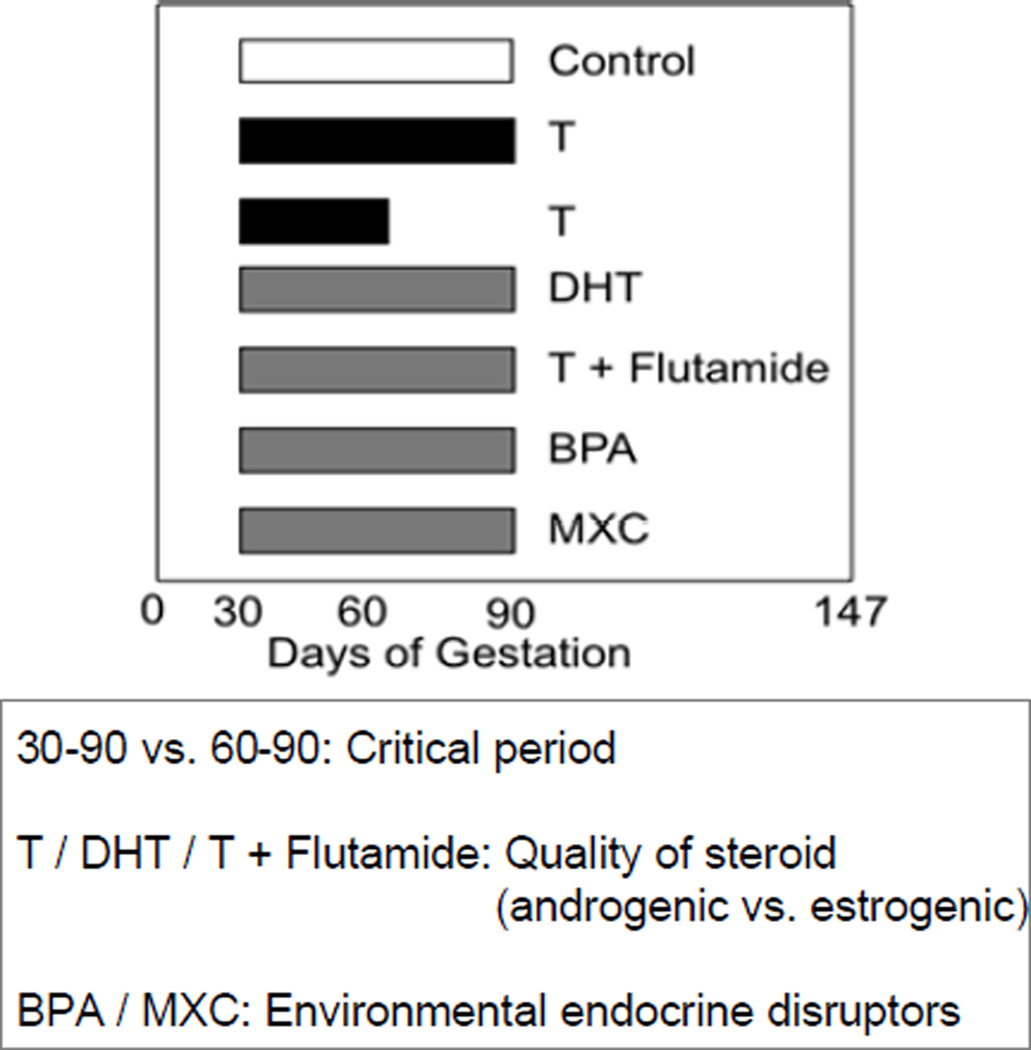
Extensive investigations have been carried out to address the impact of T programming in sheep (Padmanabhan and Veiga-Lopez, 2013a,b). Two windows of prenatal T treatment have been studied; an early and long exposure period (from d 30 to 90 of gestation; referred from now on as T30–90) and a late and short exposure period (from d 60 to 90 of gestation; referred from now on as T60–90) (Figure 2). Because T is an aromatizable androgen, some of its actions may be facilitated via estrogenic programming. In support of this premise, fetal investigations with sheep following gestational T treatment have indeed demonstrated that gestational T treatment leads to fetuses being exposed not only to increased T levels, but also increased estradiol levels (Veiga-Lopez et al., 2011). To validate this premise, in initial studies, outcomes resulting from gestational exposure to T were compared with outcomes from gestational exposure to a non-aromatizable androgen dihydrotestosterone (DHT; Figure 2). The effects were assumed to be androgen mediated when both treatments produced the same effect. Effects of gestational T that were not mimicked by DHT were assumed to be estrogen mediated. Since DHT can be metabolized to 3 beta-diol and exert its effect via estrogen receptor beta (ESR2) (Handa et al., 2008) in subsequent studies, to confirm androgen-mediated effects, androgen action was negated by co-treating with an androgen antagonist, flutamide (Figure 2). The experimental unit for all data reported in this review are female fetuses or female offspring of mothers treated during gestation with either native steroids (T and / or DHT) or endocrine disruptors.
Figure 2.
Schematic showing timing and duration of the various steroid or endocrine disrupting chemical treatments used in studies discussed in this review. Abbreviations used: T: testosterone, DHT: dihydrotestosterone, BPA: bisphenol A, and MCX: methoxychlor.
Reproductive Cyclicity and Periovulatory Disruptions
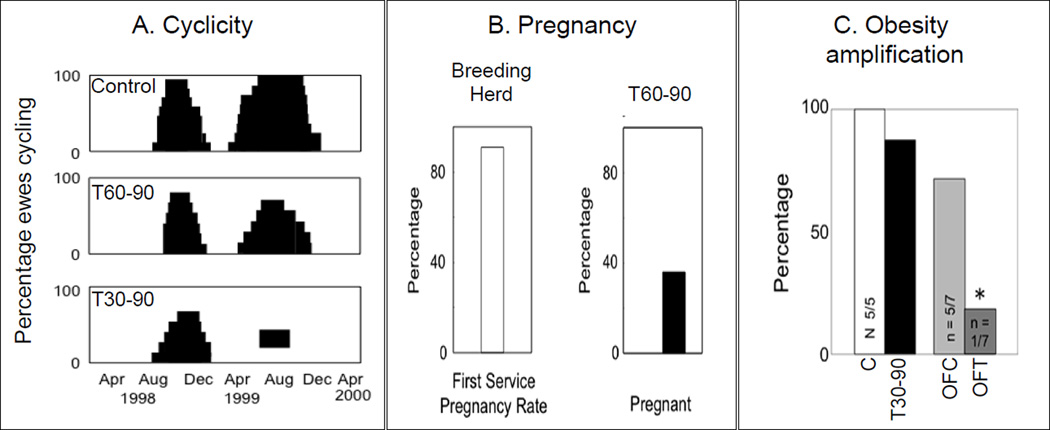
Earlier studies found T30–90 Dorsett breed females showed progressive deterioration of cyclicity culminating in anovulation during the second breeding season (Figure 3A) (Birch et al., 2003). Studies with other breeds of sheep have also reported effects on cyclicity (Clarke et al., 1977; Manikkam et al., 2006) with varying degree of severity. The differences in severity of defects found between these breeds are supportive of contribution from genetic predisposition relative to how they respond to insults. The fact that most of the T60–90 females cycled during the second breeding season as opposed to T30–90 animals becoming anovulatory (Birch et al., 2003) is supportive of critical windows of susceptibility. This may be a function of exposure relative to when the neuroendocrine feedback systems and ovarian differentiation are getting established. For instance considering that the ovary begins to differentiate by day 30 of gestation (Figure 1), treatment beginning at day 30 may be more disruptive to the ovary than treatment beginning at day 60.
Figure 3.
Panel A: reproductive cyclicity in sheep during the first and second breeding seasons (assessed via twice weekly progesterone measures) in control, T60–90, and T30–90 females (modified from Birch et al., 2003). Panel B: percentage of T60–90 females becoming pregnant (modified from Steckler et al., 2007b). Panel C: percent of control (C), over-fed control (OFC), T30–90, and overfed T30–90 (OFT) females that showed a luteal progesterone increase after estrus synchronization with 2 injections of PGF2α, 11 d apart. Note the sub- and short luteal phases in T-treated females and anovulatory nature of nearly all overfed T30–90 females (modified from Steckler et al., 2009). Abbreviations used: T30–90: females prenatally treated with testosterone (T) from d 30 to 90 of gestation, T60–90: females prenatally treated with testosterone (T) from d 60 to 90 of gestation, and PGF2α: prostaglandin F2α.
Investigations comparing the periovulatory changes in T30–90 and prenatal DHT-treated from d 30 to 90 of gestation (DHT30–90) during a synchronized estrous cycle, found T30–90, but not DHT30–90-treated females had increased preovulatory levels of estradiol, as well as delayed and severely dampened LH surges (Veiga-Lopez et al., 2009), suggestive of estrogenic programming of the defects that characterize the periovulatory phase. Investigations elucidating the critical window of exposure for the hypergonadotropic defect (LH excess) in prenatal T-treated females found that both windows of exposure (T30–90 and T60–90) lead to increased LH release (Manikkam et al., 2008; Savabieasfahani et al., 2005).
Fertility tests conducted with T60–90-treated females found mating success was 100% when T animals were separated from controls and bred. In contrast pregnancy rate in T60–90 females was only 40% as opposed to control flock that had a 90% pregnancy rate (Steckler et al., 2007b; Figure 3B). Unfortunately, fertility testing is only possible in the T60–90 females, because T30–90 females are phenotypically virilized and natural mating is not possible (Wood and Foster, 1998). Studies carried out to determine the impact of adiposity in amplifying the reproductive disruptions observed in T30–90 females (Figure 3C) (Steckler et al., 2009) found excess postnatal weight gain further deteriorates the luteal response to a synchronized cycle. These findings are supportive of a two-hit hypothesis (Tang et al., 2008); the first insult (gestational T exposure) causing organizational changes (programming) and second (excess weight gain), contributing to the severity of the adult phenotype.
Neuroendocrine Programming
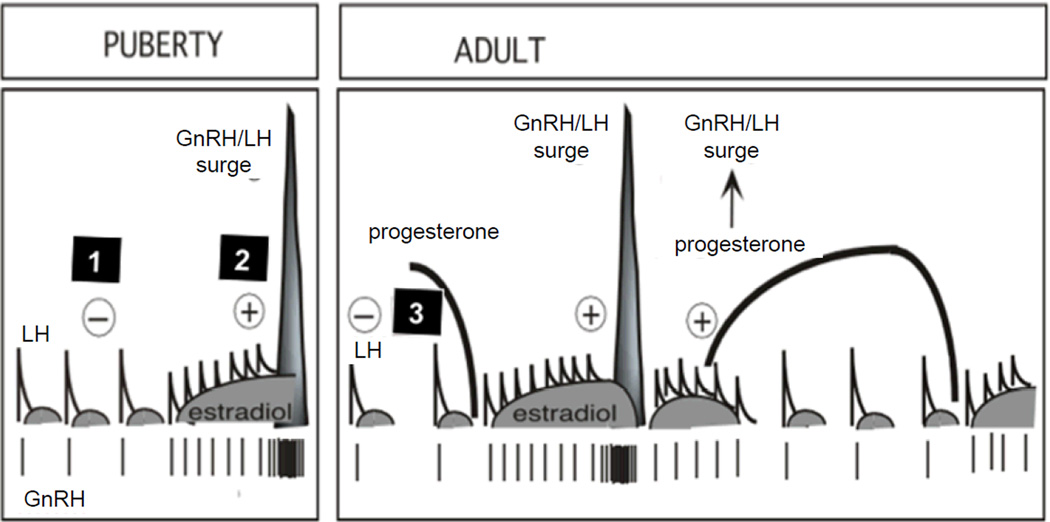
Studies with Suffolk, Poll Dorset, and Finnish-Landrace x Dorset Horn sheep breeds have shown that prenatal T30–90 treatment disrupts the sensitivity to all three major steroid feedback mechanisms involved in the control of cyclic changes in GnRH / gonadotropin secretion, namely, estradiol negative feedback (Wood and Foster, 1998; Sarma et al., 2005), estradiol positive feedback (Wood and Foster, 1998; Sharma et al., 2002, Unsworth et al., 2005) and progesterone negative feedback (Robinson et al., 1999; Veiga-Lopez et al., 2009) (Figure 4). The decreased sensitivity to estradiol and progesterone negative feedbacks contributes at least in part to the LH excess seen in the prenatal T30–90-treated sheep (Sarma et al., 2005, Robinson et al., 1999).
Figure 4.
Schematic of neuroendocrine feedback systems involved in the control of GnRH and LH secretion. Feedback 1: GnRH and LH release is under the control of negative feedback action of estradiol, which is predominant during the prepubertal and anestrus period. Feedback 2: positive feedback actions of estradiol responsible for generation of the preovulatory LH surge and onset of cyclicity. After puberty (adulthood, right panel), a third feedback mechanism comes into play (Feedback 3), namely negative feedback action of progesterone, operational during the luteal phase (modified from Foster et al., 2007).
Further research targeted to dissect out the androgenic vs. estrogenic contribution to the neuroendocrine dysfunctions has demonstrated that the estradiol negative feedback disruption is being programmed by androgenic action of T. This is substantiated by the finding that sensitivity to estradiol negative feedback is reduced by gestational exposure to T30–90 or DHT30–90 but reversed by co-treatment of T with flutamide, the androgen antagonist (Wood and Foster, 1998, Veiga-Lopez et al., 2009, Jackson et al., 2008). Sensitivity to estradiol positive feedback was found to be reduced in prenatal T30–90 females, but not in DHT30–90 females suggesting that this disruption is likely programmed via estrogenic actions of prenatal T (Wood and Foster, 1998, Veiga-Lopez et al., 2009). Paradoxically, females co-treated with T plus the androgen antagonist, flutamide, (this will block androgenic programming of T), found partial reversal of LH surge amplitude to the estradiol positive feedback challenge in the absence of any effect on timing, suggestive of dual organizational effects; with surge timing programmed likely via estrogenic pathways and androgenic pathways contributing at least in part to the LH surge magnitude (Abi-Salloum et al., 2012a). Studies testing pituitary sensitivity to GnRH found enhanced sensitivity to GnRH in both T30–90 and DHT30–90 females suggesting that altered pituitary sensitivity is programmed likely via androgenic actions of T (Manikkam et al., 2008). Altered pituitary sensitivity to GnRH is also a contributing factor to the LH hypersecretion seen in prenatal T-treated animals.
Ovarian Disruptions
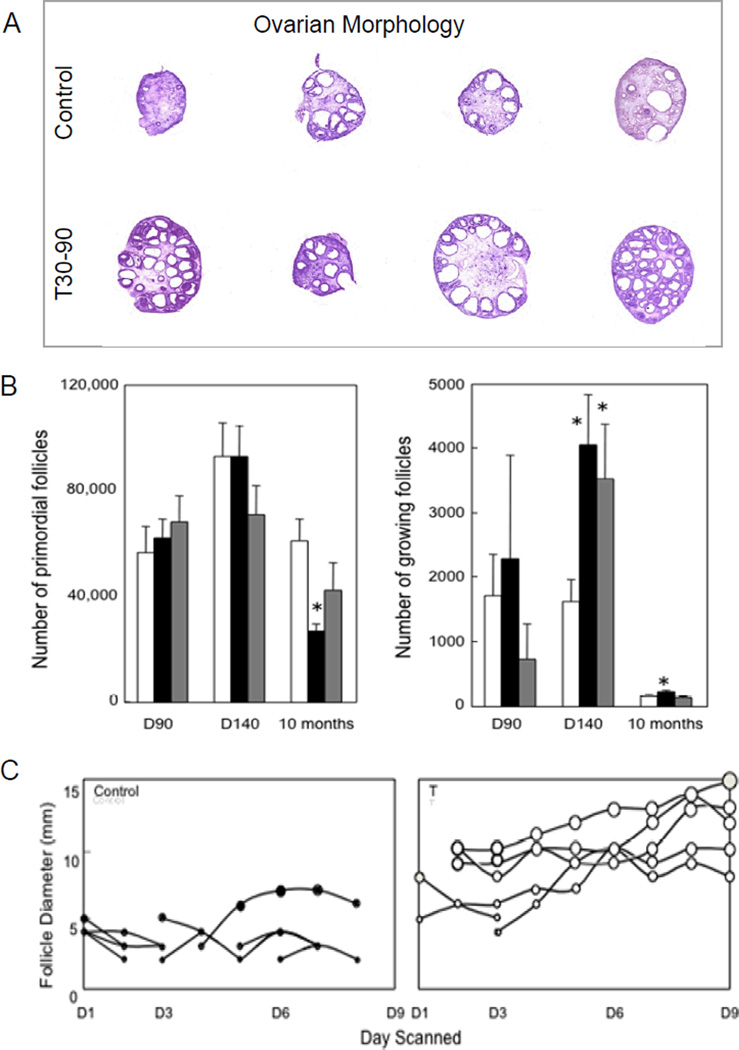
Another hallmark of the prenatal T treatment is the presence of multifollicular ovaries (accumulation of antral follicles) (Figure 5A) (West et al., 2001). These effects appear not to be facilitated by the androgenic actions of T since similar phenotype was not evident in prenatal DHT treatment animals (West et al., 2001; Steckler et al., 2007a). Potential causes for the presence of multifollicular ovaries include increased follicular recruitment or a lack of follicular atresia leading to increased follicular persistence. Both phenomena have been demonstrated in prenatal T30–90 females. Ovarian morphometric analyses found both prenatal T30–90 and DHT30–90 treatment enhanced follicular recruitment initially with ovarian follicular reserve being reduced to ~50% by the end of the first breeding season only in the prenatal T30–90, but not the DHT30–90 treated group (Smith et al., 2009) (Figure 5B). Similarly, detailed ultrasonographic evaluation found that follicles persist longer in prenatal T-treated females (Manikkam et al., 2006) (Figure 5C). Both attributes appear to be programmed via different steroidogenic pathways, with follicular recruitment programmed via androgenic actions of T (Smith et al., 2009) and follicular persistence programmed via estrogenic actions of T (Steckler et al., 2007a).
Figure 5.
Panel A: follicular morphology of ovary from control and T30–90 females. Note the accumulation of antral follicles in the T30–90 sheep (from West et al., 2001). Panel B: mean (± SEM) number of primordial and growing follicles on fetal d 90 and 140 and at 10 mo of age in control, T30–90 and DHT30–90 ovaries (from Smith et al., 2009). D90 = fetal day 90; D140 = fetal day 140. Panel C: ovarian follicular dynamics determined by ovarian ultrasonography for 8 d in control and T30–90 sheep during the first breeding season (from Manikkam et al., 2006). Each line depicts one follicle. Note the increase in maximum size and duration of the largest follicles on the ovary in T30–90 sheep compared to control females. Abbreviations used: T30–90: females prenatally treated with testosterone (T) from d 30 to 90 of gestation and DHT30–90: females prenatally treated with dihydrotestosterone (DHT) from d 30 to 90 of gestation. D1 = day 1; D3 = day 3, D6 = day 6; D9 = day 9.
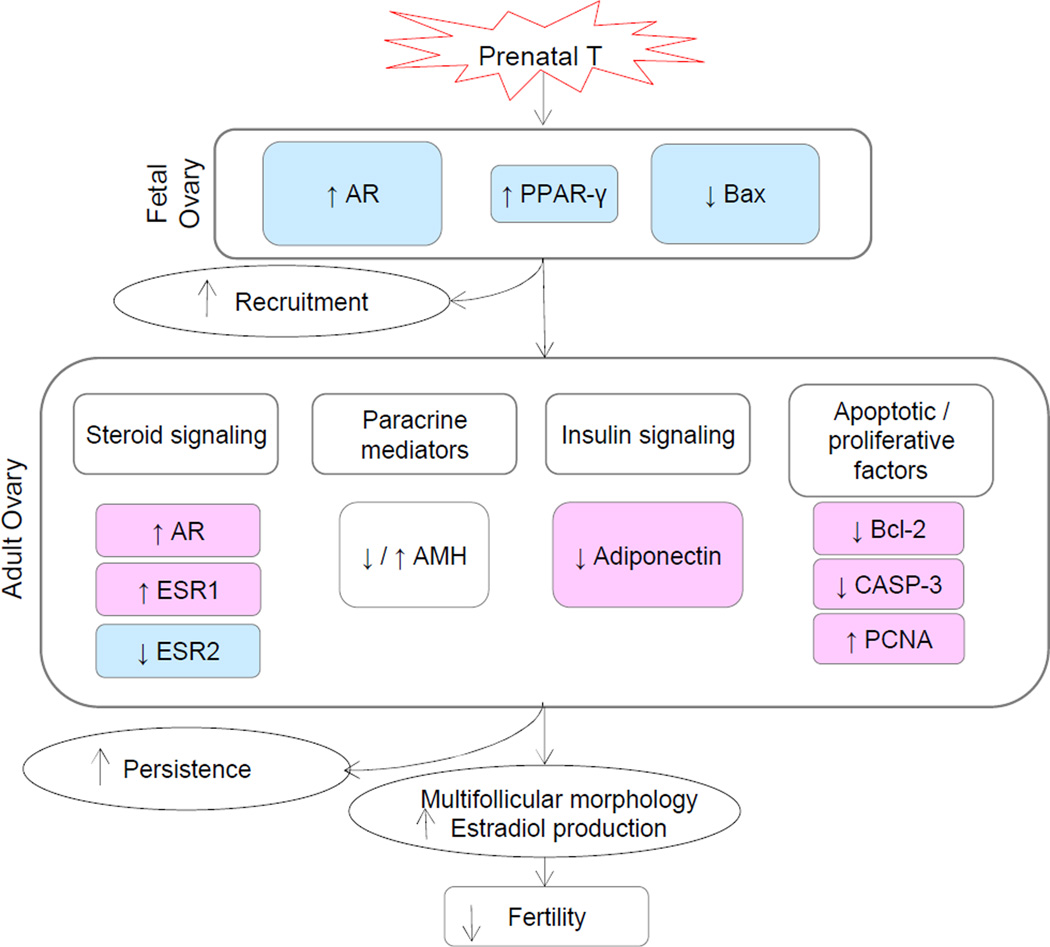
Extensive immunohistochemical studies carried out found that prenatal T treatment disrupts steroid receptor balance. Specifically, androgen receptor expression was found to be increased in the stroma and granulosa cells of fetal ovaries and granulosa cells of antral follicles in adult females with this trait likely programmed via androgenic actions; both prenatal T and DHT treatment increased androgen receptor expression during fetal life (Ortega et al., 2009). These findings are supportive of functional hyperandrogenism in prenatal T-treated females. At the ovarian level, other proteins (receptors, key follicular regulators) found to be disrupted by prenatal T30–90 include estrogen receptors 1 and 2, antimullerian hormone - a marker of follicular recruitment (Veiga-Lopez et al., 2012), peroxisome proliferator-activated receptor gamma (PPARγ), adiponectin – a metabolic mediator (Ortega et al., 2010), as well as B-cell lymphoma 2 protein (Bcl-2), Bcl-2-associated X protein (BAX), and caspase-3 (CASP-3)– factors involved in apoptosis (Salvetti et al., 2012) (Figure 6). In concert, these findings implicate involvement of steroidogenic, metabolic, and apoptotic pathways in the development of multifollicular phenotype.
Figure 6.
Schematic showing changes in ovarian protein expression in control and T30–90 females during fetal life [androgen receptor (AR), peroxisome proliferator-activated receptor γ (PPAR-γ), Bcl-2-associated X protein (Bax)] and adult life [(estrogen receptors 1 and 2 (ESR1 and ESR2), antimullerian hormone (AMH), adiponectin, B-cell lymphoma 2 protein (Bcl-2), caspase-3 (CASP-3), proliferating cell nuclear antigen (PCNA)]. Changes observed in the fetal ovary support the morphological changes reflecting increased follicular recruitment seen at gestational d 90. Changes seen with steroid and insulin signaling, paracrine mediators, and apoptotic factors, reflect morphological changes observed in adult ovaries, such as multifollicular morphology and follicular persistence. Abbreviation used: T30–90: females prenatally treated with testosterone (T) from d 30 to 90 of gestation.
Metabolic Dysfunctions
Besides the reproductive defects, prenatal T treatment leads to intrauterine growth restricted females (Steckler et al., 2005), postnatal catch-up growth and low birth weight offspring (Manikkam et al., 2004), features that are risk factors for adult wellbeing (Boney et al., 2005; Dulloo, 2008). T30–90 females are also characterized by insulin resistance (DeHaan et al., 1990; Hansen et al., 1995; Recabarren et al., 2005; Padmanabhan et al., 2010) and hypertension (assessed by radiotelemetric studies) (King et al., 2007). These metabolic outcomes are likely of translational relevance to women with polycystic ovarian syndrome (PCOS) the most prevalent (5 to 10%) reproductive disorder in reproductive-aged women; up to 70% of women with PCOS manifest insulin resistance (Diamanti-Kandarakis and Dunaif, 2012). Many believe PCOS may be a developmental disorder stemming from in utero exposure to excess androgens (Abbott et al., 2005). Interestingly gestational T treatment was found to also induce hyperinsulinemia in the mothers (Abi-Salloum et al., 2012b), suggesting that some of the programmed outcomes in prenatal T-treated females may be mediated via altered maternal insulin homeostasis.
Human and Agricultural Relevance
The reproductive and metabolic characteristics of T30–90 sheep mimic the phenotype of women with PCOS (Table 1). Women with PCOS are characterized by oligo- / anovulation, hyperandrogenism, polycystic ovaries, LH hypersecretion and reduced fecundity with most manifesting insulin resistance (Diamanti-Kandarakis and Dunaif, 2012). That steroid excess early in life may lead to manifestation of PCOS phenotype in adulthood (Apter et al., 1995) is supported by findings of such phenotype in conditions such as classical 21-hydroxylase-deficiency in which the fetus has been exposed to high amounts of sex steroids before birth (Barnes et al., 1994). Interestingly, studies using cord blood samples have found that 40% of human female fetuses are exposed to elevated levels of T, comparable to that seen in male fetuses at 19 to 25 wk of gestation (Beck-Peccoz et al., 1991). Considering that only 5–10% of reproductive aged women exhibit PCOS, genetic susceptibly of the individual to high testosterone exposure likely plays a role in the development of the phenotype. The shared reproductive and metabolic phenotype of prenatal T30–90-treated sheep to women with PCOS makes them a valuable resource for addressing the mechanisms underlying the etiology of development of PCOS phenotype. The constellation of metabolic outcomes including reduced insulin sensitivity and hypertension found in T30–90 females suggest that these animals may also be suitable for understanding the developmental origin of other metabolic diseases such as the metabolic syndrome (Mikhail, 2009).
Table 1.
Attributes of women with PCOS and prenatal T30–90-treated sheep. Abbreviation used: T30–90: females prenatally treated with testosterone (T) from d 30 to 90 of gestation.
| Attributes | Women with PCOS | Prenatal T-treated sheep |
|---|---|---|
| Anovulation | Yes | Yes |
| Hyperandrogenism | Yes (functional) | Yes |
| LH excess | Yes | Yes |
| Reduced sensitivity to steroids |
Yes | Yes |
| Multifollicular ovaries | Yes | Yes |
| Increased follicular recruitment / persistence |
Yes | Yes |
| Insulin resistance | Yes | Yes |
| Fetal growth retardation | Yes A | Yes |
| Altered behavior | Yes | Yes |
| Hypertension | YesB | Yes |
| Obesity amplification | Yes | Yes |
1Based on a study conducted with a Spanish cohort (Ibáñez et al., 1998). 2Risk factor in PCOS (Essah and Nestler, 2006).
Induction of similar traits by exposure to industrial chemicals with steroidogenic potential would help explain the increase in infertility and metabolic disorders in humans and likely applicable to agricultural practices, where improved fecundity is desired. Recent findings of effects of treated human waste, such as sewage sludge, producing multisystemic effects, from reproductive to metabolic disruptions in animals grazing in these pastures (Bellingham et al., 2012; Rhind et al., 2011) emphasize the importance of focusing future research endeavors towards potential effects of EDCs in agricultural practices.
ENDOCRINE DISRUPTING CHEMICALS
EDCs are hormonally active, synthetic or natural compounds that have been shown to interfere with the normal functioning of the endocrine system (Damstra et al., 2002), most notably the reproductive endocrine axis (Colborn et al. 1993; Guillette and Gunderson, 2001; Bergman et al., 2012). Of particular concern is the contamination of our environment and our food sources with synthetic EDC, some of which are persistent and are found at concentrations that are readily recognized by endocrine tissues.
These EDCs can interfere with hormone signaling by acting as agonists or antagonists (Damstra et al., 2002; Hotchkiss et al., 2008). Those EDCs that have either androgenic or estrogenic activity have the potential to interfere with biological processes involving these signaling pathways. Compounds suspected of interfering with normal binding of estrogen to its receptor provide the most direct and clear link to disrupted endocrine function by environmental chemicals. There are two classes of environmental estrogens. First class namely phytoestrogens have been shown to interfere with estrogen signaling (Murkies et al., 1998). Great Lakes fish that live downstream from paper and pulp mill industries, where the wood-derived compound β-sitosterol is found at significantly higher levels, or goldfish exposed to such pollutants in the laboratory exhibit reduced gonadal size and weight, reduced fertility, fecundity, and hatching success (Van Der Kraak et al., 1992). Another example of such deleterious effects of phytoestrogens is clover disease in sheep. The outbreak of this disease was discovered in Western Australia in 1940 when fertility in an entire flock of sheep was reduced by 80 % following grazing on a type of clover rich in phytoestrogens (Adams, 1990).
Of even more concern to human and animal reproductive health is the second class of EDCs, those of man-made origin- xenoestrogens. Compounds such as organochlorine pesticides, PCBs, phenolics, and phthalate esters are highly persistent in the environment and exhibit estrogenic activity (Schafer and Kegley, 2002). Steroidogenic pollutants, such as methoxychlor (MXC) (Gray et al., 1985; Cummings, 1997) and bisphenol A (BPA) (Rubin et al., 2001) are of concern because, when administered to rodents, they masculanize female brains, advance puberty, and disrupt reproductive cyclicity in adulthood. Exposure to estrogen mimetics [i.e., polychlorinated biphenyls (PCB)] also disrupts sexual differentiation in turtles (Crews et al., 1995) and birds (Fry and Toone, 1981). More recently, concerns over the possible additive or synergistic effects of environmental chemicals have grown.
The following section will focus on the impact of prenatal exposure of sheep to BPA, a widely used industrial chemical and MXC, a pesticide, two EDCs with steroidogenic potential. BPA is widely used in the manufacture of epoxy resins and polycarbonate plastics. Recent studies have documented ubiquitous presence of BPA in water, air, and dust, as well as in most human fluids including maternal circulation and placental fluids (Vandenberg et al., 2009; Ranjit et al., 2010). This is of concern, since BPA has been found to have not only estrogenic, but also anti-androgenic activity (Quesada et al., 2002; Sohoni and Sumpter 1998). MXC, with estrogenic and anti-androgenic properties (Staub et al., 2002) was used to control pests in agricultural, dairy, and domestic settings and found to persist in the environment (National Research Council, 1999), even though its use has been banned in the United States.
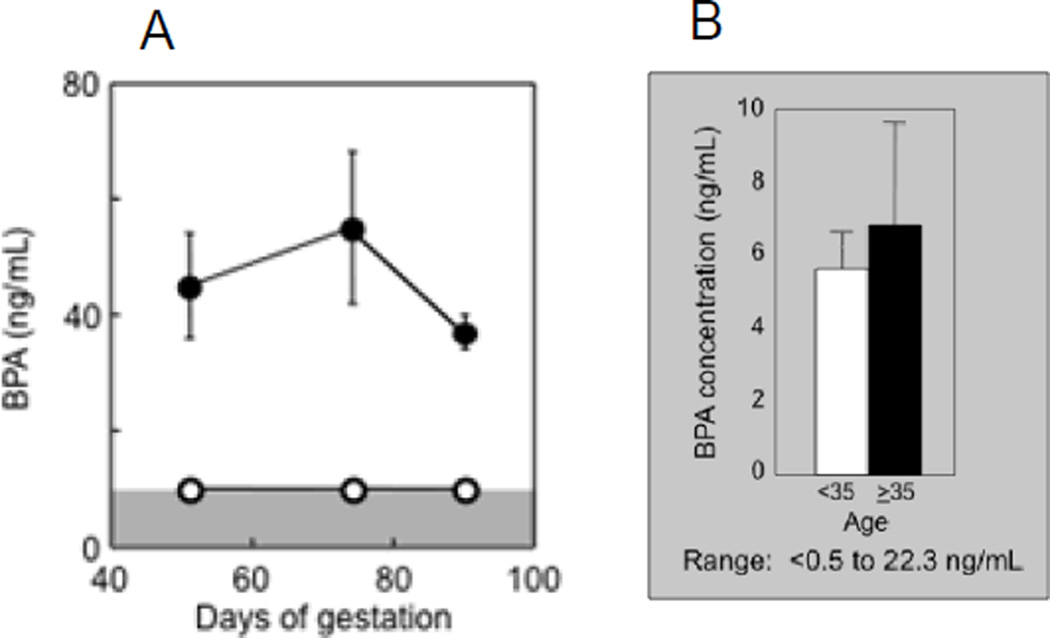
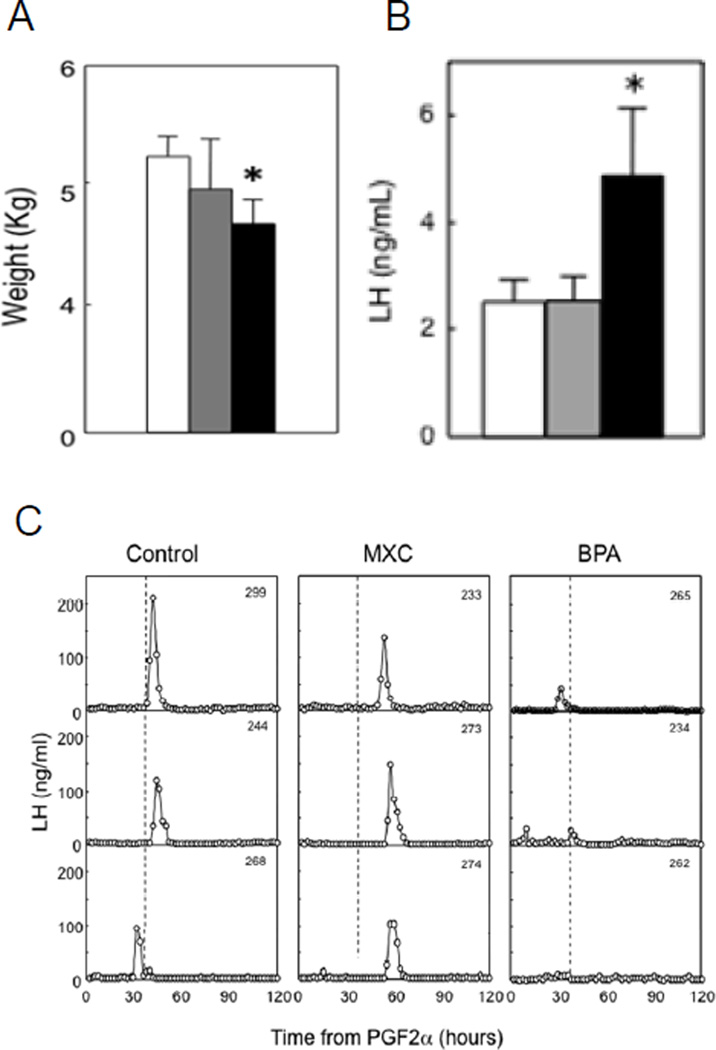
Our studies in sheep found that prenatal BPA and MXC (5 mg/kg BW per day for both treatments) treatment from d 30 to 90 of gestation (same window used in above studies with native steroids) induce reproductive disruptions (Savabieasfahani et al., 2006) albeit at different levels in the reproductive axis. The internal BPA dose achieved in maternal circulation following administration of 5 mg/kg BW of BPA (Figure 7 A) was 2 fold greater than the greatest levels observed in maternal circulation of US women (Figure 7B) (range: 0.5 – 22.3 ng/ml; Padmanabhan et al., 2008 and range: below level of detection - 52.26 ng/ml; Gerona et al., 2013) and other industrialized countries (Vandenberg et al., 2009, Schonfelder et al., 2002). This dose of BPA resulted in several disruptions including low birth weight female offspring, LH excess, and severely dampened or absent preovulatory LH surges (Figure 8) (Savabieasfahani et al., 2006). In contrast, MXC had no effect on somatic growth (Figure 8), but delayed the onset of LH surges (Figure 8). MXC has been found in the circulation of men and women (Botella et al., 2004; Carreño et al., 2007) and adipose tissue of women (Botella et al., 2004). Although it was banned in the European Union in 2002 (European Commission, 2002) and U.S. in 2003 (EPA, 2004), being a persistent chemical (Howard, 1991) is still exists in the environment (Bempah and Donkor, 2011). The similarities between prenatal T- and BPA- and MXC-treated female offspring are shown in Table 2. Prenatal BPA treatment, similar to T treatment, leads to low birth weight offspring, LH excess, dampening preovulatory LH surges, and cycle disruptions. The similarity between prenatal T and MXC treatment was restricted to delayed onset of LH surge.
Figure 7.
Panel A: levels of circulating bisphenol A (BPA) achieved in control (open circles) and BPA treated (closed circles) pregnant sheep on d 50, 70, and 90 of gestation (d 20, 40, and 60 of treatment, respectively) following daily administration of 5 mg/kg of BPA, subcutaneous (Savabieasfahani et al., 2006). Panel B: maternal levels of BPA (mean ± SEM) in Southeastern Michigan relative to maternal age (Padmanabhan et al., 2008).
Figure 8.
Panel A: birth weight of control, prenatal MXC, and bisphenol-A (BPA)-treated female offspring (Savabieasfahani et al., 2006). Panel B: mean circulating levels of LH in prepubertal control, prenatal MXC, and BPA-treated females (Savabieasfahani et al., 2006). Panel C: circulating patterns of LH from 3 control, 3 prenatal MXC- and 3 BPA-treated females taken at 2-h intervals for 120 h, after induction of luteolysis with 2 injections of PGF2α 11 d apart (Savabieasfahani et al., 2006).
Table 2.
Attributes of prenatal T30–90-treated, bisphenol A (BPA)-treated, and methoxychlor (MCX)-treated sheep. Abbreviation used: T30–90: females prenatally treated with testosterone (T) from d 30 to 90 of gestation.
| Attributes | Prenatal T-treated |
Prenatal BPA-treated |
Prenatal MXC-treated |
|---|---|---|---|
| LH excess | Yes | Yes | No |
| Cycle disruption | Yes | Yes | Yes |
| Dampened LH surge | Yes | Yes | No |
| Increased amplitude of E2 | Yes | Yes | No |
| Delayed LH surge onset | Yes | No | Yes |
| Fetal growth retardation | Yes | Yes | No |
Thus far, most studies addressing impact of environmental toxicants have focused on human exposure and risks (Vandenberg et al., 2010). Similar attention has not been extended to the agricultural side. This is because EDCs have not been considered a risk to farm animal health or productivity. Considering farm animals are part of the food chain, one cannot overlook the possibility that livestock have the potential to be exposed to EDCs via drinking water, air, sewage dumps, and pesticides. For instance BPA, has been found in natural drinking water (Bennie et al., 1997), water delivery systems that use PVC pipes, air, and dust (vom Saal and Hughes, 2005). There are also several reports of elevated exposure to several EDCs following the application of sewage sludge to pasture (Rhind, 2005).
As such, while very little is known regarding levels of exposure of farm animals to EDCs and effects are yet to be seen in agricultural practices, the few farm animal studies conducted thus far along with epidemiologic and laboratory animal studies raise awareness to the potential for future risks to farm animal health. Low birth weight, such as evidenced with in utero BPA treated females, if translatable to agricultural practices, could be of potential economic significance (Savabieasfahani et al., 2006). Low birth weight increases the feed costs to bring an animal to market weight and may impair meat tenderness (Shavlakadze and Grounds, 2006). Since insulin and IGF-1 play critical roles in muscle development, including the deposition of intramuscular adipose that produces marbling (Shavlakadze and Grounds, 2006), disruption of these signaling pathways may well have adverse effects on meat quality (Gondret et al., 2006). More importantly, since livestock is a food source for humans, if EDCs bioaccumulate in tissues, it would pose risk in terms of human health. Thus, a better understanding of the effects of in utero exposure to EDCs on the reproductive and metabolic axis of livestock will help lay the foundation for assessment of adverse effects on efficient production and development of methods to overcome them.
SUMMARY AND CONCLUSIONS
Studies discussed in this review provide support for developmental origin of adult dysfunctions by documenting that gestational exposure to excess T perturbs reproductive and metabolic systems in sheep leading to infertility and metabolic dysfunctions. The finding that severity of perturbations differs with different breeds of sheep supports the premise that the adult phenotype is the result of interactions between genetic susceptibility and the environment, and as such, consequences of developmental insults on adult pathologies differ from individual to individual. Thus far, very little is known regarding the potential interactions between genetic susceptibility of the mother to the insult during gestation and the genetic susceptibility of the progeny to maternal alternations. Exposure of the mother to stress, improper nutrition, or industrial byproducts would impact maternal milieu and fetal development leading to alterations in organ differentiation. Considering that programming of fetal organ systems are mediated in part via changes in maternal milieu, studies addressing impact of insults during gestation should dissect out effects directed at the fetal level (direct effects) vs. those that are mediated via changes in maternal milieu (indirect effects). Only then, appropriate intervention strategies can be developed.
While the focus of this review is on the female offspring, it is important to recognize that male fetuses are equally susceptible to such insults. Studies focusing on male offspring found that gestational T treatment increases Sertoli cells in the seminiferous tubules (Rojas-Garcia et al., 2010), reduces number of germ cells, culminating in low sperm count and motility (Recabarren et al., 2008). Studies in humans also indicate a trend for low and declining semen quality, increased frequency of undescended testis, and hypospadias with recent studies implicating adverse influences from endocrine disrupting chemicals as potential contributors (Skakkebaek et al., 2001)
It is imperative to recognize that exposure to such ubiquitously present EDCs in the environment has the potential to jeopardize animal production efficiency, lower reproductive outcomes, and pose risks to human health via consumption of such food sources exposed to EDCs. Unfortunately, while large number of studies is being carried out in agricultural species relative to impact of maternal diet (Langley-Evans, 2009; Oliver et al., 2007; Nathanielsz et al., 2013; Gardner et al., 2008; Ford and Long, 2011; Bertram and Hanson, 2001; Harding et al., 2011) and stress (Gräbner et al., 2009; Schwab et al., 2012; Braun et al., 2013), the impact of exposure to endocrine disrupting chemicals on reproductive and metabolic health has not received the needed attention. Large animal models, such as ruminants, are of critical importance in biomedical research, especially in perinatal medicine to develop interventions targeted at the fetal level (Rudolph, 2008). The long gestational period in ruminants allow chronic feto-placental adaptations (Morrison, 2008). Capitalizing on principles of developmental programming that have been carefully worked out using dietary interventions (Nathanielsz, 2006), future studies should extend studies carried out in rodent models to determine risks posed by environmental factors in ruminants. This will facilitate a better understanding of the underlying mechanisms, as well as help in developing interventions to overcome developmental pathologies in agricultural species, as well as humans.
Footnotes
Based on a presentation at the Reproduction Symposium titled “External Influences on Reproductive Neuroendocrinology” at the Joint Annual Meeting, July 8–12, 2013, Indianapolis, IN.
Grant Support: P01HD044232 and R01ES016541 to V. Padmanabhan.
LITERATURE CITED
- Abbott DH, Eisner JR, Colman RJ, Kemnitz J, Dumesic DA. Prenatal androgen excess programs for PCOS in female rhesus monkeys. In: Chang RJ, Dunaif A, Heindel J, editors. Polycystic ovary syndrome. New York, NY: Marcel Dekker, Inc.; 2002. pp. 119–133. [Google Scholar]
- Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum. Reprod. Update. 2005;11:357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- Apter D, Butzow T, Laughlin GA, Yen SS. Metabolic features of polycystic ovary syndrome are found in adolescent girls with hyperandrogenism. J. Clin. Endocrinol. Metab. 1995;80:2966–2973. doi: 10.1210/jcem.80.10.7559882. [DOI] [PubMed] [Google Scholar]
- Abi Salloum B, Herkimer C, Lee JS, Veiga-Lopez A, Padmanabhan V. Developmental programming: prenatal and postnatal contribution of androgens and insulin in the reprogramming of estradiol positive feedback disruptions in prenatal testosterone-treated sheep. Endocrinology. 2012a;153:2813–2822. doi: 10.1210/en.2011-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi Salloum B, Veiga-Lopez A, Abbott DH, Padmanabhan V. Developmental Programming: Exposure to excess testosterone during gestation by its androgenic action disrupts maternal steroidal and metabolic environment in sheep. 94th Annual Meeting of the Endocrine Society, Houston, USA. Endocr. Rev. 2012b;33 MON-15. [Google Scholar]
- Adams NR. Permanent infertility in ewes exposed to plant oestrogens. Aust. Vet. J. 1990;67:197–201. doi: 10.1111/j.1751-0813.1990.tb07758.x. [DOI] [PubMed] [Google Scholar]
- Baldi F, Mantovani A. A new database for food safety: EDID (Endocrine disrupting chemicals - Diet Interaction Database) Ann. Ist. Super. Sanita. 2008;44:57–63. doi: 10.1016/j.reprotox.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Mothers, babies, and disease in later life. BMJ Publishing Group. London: United Kingdom; 1994. Programming the baby; pp. 14–36. [Google Scholar]
- Barnes RB, Rosenfield RL, Ehrmann DA, Cara JF, Cuttler L, Levitsky LL, Rosenthal IM. Ovarian hyperandrogenism as a result of congenital adrenal virilizing disorders: evidence for prenatal masculanization of neuroendocrine function in women. J. Clin. Endocrinol. Metab. 1994;79:1328–1333. doi: 10.1210/jcem.79.5.7962325. [DOI] [PubMed] [Google Scholar]
- Beck-Peccoz P, Padmanabhan V, Baggiani AM, Cortelazzi D, Buscaglia M, Medri G, Marconi AM, Pardi G, Beitins IZ. Maturation of hypothalamic-pituitary-gonadal function in normal human fetuses: circulating levels of gonadotropins, their common alpha-subunit and free testosterone, and discrepancy between immunological and biological activities of circulating follicle-stimulating hormone. J. Clin. Endocrinol. Metab. 1991;73:525–532. doi: 10.1210/jcem-73-3-525. [DOI] [PubMed] [Google Scholar]
- Bellingham M, McKinnell C, Fowler PA, Amezaga MR, Zhang Z, Rhind SM, Cotinot C, Mandon-Pepin B, Evans NP, Sharpe RM. Foetal and post-natal exposure of sheep to sewage sludge chemicals disrupts sperm production in adulthood in a subset of animals. Int. J. Androl. 2012;35:317–329. doi: 10.1111/j.1365-2605.2011.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennie D, Sullivan C, Lee H, Peart T, Maguire R. Occurrence of alkylphenols and alkylphenol mono- and diethoxylates in natural waters of the Laurentian Great Lakes and basin and the upper St. Lawrence River. Sci. Total. Environ. 1997;193:263–275. [Google Scholar]
- Bempah CK, Donkor AK. Pesticide residues in fruits at the market level in Accra Metropolis, Ghana, a preliminary study. Environ. Monit. Assess. 2011;175:551–561. doi: 10.1007/s10661-010-1550-0. [DOI] [PubMed] [Google Scholar]
- Bergman A, Heindel JJ, Jobling S, Kidd KA, Zoeller RT. WHO/UNEP. State of the science of endocrine disrupting chemicals –2012. Geneva, Switzerland: United National Environment Programme World Health Organization; 2012. [Google Scholar]
- Bertram CE, Hanson MA. Animal models and programming of the metabolic syndrome. Br. Med. Bull. 2001;60:103–121. doi: 10.1093/bmb/60.1.103. [DOI] [PubMed] [Google Scholar]
- Birch RA, Padmanabhan V, Foster DL, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144:1426–1434. doi: 10.1210/en.2002-220965. [DOI] [PubMed] [Google Scholar]
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- Botella B, Crespo J, Rivas A, Cerrillo I, Olea-Serrano MF, Olea N. Exposure of women to organochlorine pesticides in Southern Spain. Environ. Res. 2004;96:34–40. doi: 10.1016/j.envres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Braun T, Challis JR, Newnham JP, Sloboda DM. Early life glucocorticoid exposure: The hypothalamic pituitary adrenal axis, placental function and long term disease risk. Endocr Rev. 2013 Aug 22; doi: 10.1210/er.2013-1012. [Epub ahead of print] PMID 23970762. [DOI] [PubMed] [Google Scholar]
- Carreño J, Rivas A, Granada A, Lopez-Espinosa J, Mariscal M, Olea N, Olea-Serrano F. Exposure of young men to organochlorine pesticides in Southern Spain. Environ. Res. 2007;103:55–61. doi: 10.1016/j.envres.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Scaramuzzi RJ, Short RV. Ovulation in prenatally androgenized ewes. J. Endocrinol. 1977;73:385–389. doi: 10.1677/joe.0.0730385. [DOI] [PubMed] [Google Scholar]
- Colborn T, Saal FSvom, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health. Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Bergeron JM, McLachlan JA. The role of estrogen in turtle sex determination and the effect of PCBs. Environ. Health Perspect. 1995;103:73–77. doi: 10.1289/ehp.95103s773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings AM. Methoxychlor as a model for environmental estrogens. Crit. Rev. Toxicol. 1997;27:367–379. doi: 10.3109/10408449709089899. [DOI] [PubMed] [Google Scholar]
- Damstra T, Barlow S, Bergman A, Kavlock R, Van der Kraak G. International Programme on Chemical Safety (IPCS) Geneva, Switzerland: World Health Organization; 2002. Global assessment of the state-of-the-science of endocrine disruptors. [Google Scholar]
- DeHaan KC, Berger LL, Bechtel PJ, Kesler DJ, McKeith FK, Thomas DL. Effect of prenatal testosterone treatment on nitrogen utilization and endocrine status of ewe lambs. J. Anim. Sci. 1990;68:4100–4108. doi: 10.2527/1990.68124100x. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulloo AG. Thrifty energy metabolism in catch-up growth trajectories to insulin and leptin resistance. Best Pract. Res. Clin. Endocrinol. Metab. 2008;22:155–171. doi: 10.1016/j.beem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- European Commission. [Accessed April 8 2014];E.U. pesticides database. 2002 Regulation (EC) no. 2076/2002. http://ec.europa.eu/food/plant/plant_protection_products/pesticides_database/
- Foster DL, Jackson LM, Padmanabhan V. Novel concepts about normal sexual differentiation of reproductive neuroendocrine function and the developmental origins of female reproductive dysfunction: the sheep model. Soc. Reprod. Fertil. 2007;64(Suppl):83–107. doi: 10.5661/rdr-vi-83. [DOI] [PubMed] [Google Scholar]
- Ford SP, Long NM. Evidence for similar changes in offspring phenotype following either maternal undernutrition or overnutrition: potential impact on fetal epigenetic mechanisms. Reprod. Fertil. Dev. 2011;24:105–111. doi: 10.1071/RD11911. [DOI] [PubMed] [Google Scholar]
- Fry DM, Toone TK. DDT-induced feminization of gull embryos. Science. 1981;213:922–924. doi: 10.1126/science.7256288. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Lea RG, Sinclair KD. Developmental programming of reproduction and fertility: what is the evidence? Animal. 2008;2:1128–1134. doi: 10.1017/S1751731108002607. [DOI] [PubMed] [Google Scholar]
- Gerona RR, Woodruff TJ, Dickenson CA, Pan J, Schwartz JM, Sen S, Friesen MW, Fujimoto VY, Hunt PA. Bisphenol-A (BPA), BPA glucuronide, and BPA sulfate in midgestation umbilical cord serum in a northern and central California population. Environ. Sci, Technol. 2013;47:12477–12485. doi: 10.1021/es402764d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends. Endocrinol. Metab. 2010;21:199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Gondret F, Lefaucheur L, Juin H, Louveau I, Lebret B. Low birth weight is associated with enlarged muscle fiber area and impaired meat tenderness of the longissimus muscle in pigs. J. Anim. Sci. 2006;84:93–103. doi: 10.2527/2006.84193x. [DOI] [PubMed] [Google Scholar]
- Gorski RA. Sexual differentiation of the brain: A model for drug-induced alterations of the reproductive system. Environ. Health Perspect. 1986;70:163–175. doi: 10.1289/ehp.8670163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LEJr, Ferrell JM, Ostby JS. Alteration of behavioral sex differentiation by exposure to estrogenic compounds during a critical neonatal period: effects of zearalenone, methoxychlor, and estradiol in hamsters. Toxicol. Appl. Pharmacol. 1985;80:127–136. doi: 10.1016/0041-008x(85)90107-3. [DOI] [PubMed] [Google Scholar]
- Gräbner M, Kanitz E, Otten W. Prenatal stress in farm animals: a survey. Berl. Munch. Tierarztl. Wochenschr. 2009;122:73–81. [PubMed] [Google Scholar]
- Guillette LJ, Jr, Gunderson MP. Alterations in development of reproductive and endocrine systems of wildlife populations exposed to endocrine-disrupting contaminants. Reproduction. 2001;122:857–864. doi: 10.1530/rep.0.1220857. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm. Behav. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LR, Drackley JK, Berger LL, Grum DE. Prenatal androgenization of lambs: I. Alterations of growth, carcass characteristics, and metabolites in blood. J. Anim. Sci. 1995;73:1694–1700. doi: 10.2527/1995.7361694x. [DOI] [PubMed] [Google Scholar]
- Harding JE, Bloomfield FH. Prenatal treatment of intrauterine growth restriction: Lessons from the sheep model. Pediatr. Endocrinol. Rev. 2004;2:182–192. [PubMed] [Google Scholar]
- Harding JE, Jaquiery AL, Hernandez CE, Oliver MH, Derraik JGB, Bloomfield FH. Animal studies of the effects of early nutrition on long-term health. In: Goudoever Hvan, Guandalini S, Kleinman R., editors. Early nutrition: impact on short and long-term health: Nestlé Institute Workshop Series, Pediatric Program. Basel, Switzerland, Karger: 2011. pp. 1–16. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE. Fifteen years after “Wingspread”-environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol. Sci. 2008;105:235–259. doi: 10.1093/toxsci/kfn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard PH. In: Handbook of environmental fate and exposure data for organic chemicals. Howard PH, editor. Vol 3. Chelsea, MI: Lewis Publishers Inc.; 1991. pp. 502–504. [Google Scholar]
- Jackson LM, Timmer KM, Foster D. Sexual differentiation of the external genitalia and the timing of puberty in the presence of an antiandrogen in sheep. Endocrinology. 2008;149:4200–4208. doi: 10.1210/en.2007-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost A, Vigier B, Prepin J, Perchellet J. Studies on sex differentiation in mammals. Recent Prog. Horm. Res. 1973;29:1–41. doi: 10.1016/b978-0-12-571129-6.50004-x. [DOI] [PubMed] [Google Scholar]
- King AJ, Olivier NB, MohanKumar PS, Lee JS, Padmanabhan V, Fink GD. CHBR: Hypertension caused by prenatal testosterone excess in female sheep. Am. J. Physiol. Endocrinol. Metab. 2007;292:E1837–E1841. doi: 10.1152/ajpendo.00668.2006. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC. Nutritional programming of disease: unravelling the mechanism. J. Anat. 2009;215:36–51. doi: 10.1111/j.1469-7580.2008.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manneras L, Cajander S, Holmang A, Seleskovic Z, Lystig T, Lönn M, Stener-Victorin EA. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148:3781–3791. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal Programming: Prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Steckler TS, Welch KB, Inskeep EK, Padmanabhan V. Fetal Programming: prenatal testosterone treatment leads to follicular persistence / luteal defects; partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology. 2006;147:1997–2007. doi: 10.1210/en.2005-1338. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Thompson RC, Herkimer C, Welch KB, Flak J, Karsch FJ, Padmanabhan V. Developmental Programming: impact of prenatal testosterone excess on pre- and postnatal gonadotropin regulation in sheep. Biol. Reprod. 2008;78:648–660. doi: 10.1095/biolreprod.107.063347. [DOI] [PubMed] [Google Scholar]
- Mikhail N. The metabolic syndrome: insulin resistance. Curr. Hypertens. Rep. 2009;11:156–158. doi: 10.1007/s11906-009-0027-4. [DOI] [PubMed] [Google Scholar]
- Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin. Exp. Pharmacol. Physiol. 2008;35:730–743. doi: 10.1111/j.1440-1681.2008.04975.x. [DOI] [PubMed] [Google Scholar]
- Murkies AL, Wilcox G, Davis SR. Clinical Review 92: Phytoestrogens. J. Clin. Endocrinol. Metab. 1998;83:297–303. doi: 10.1210/jcem.83.2.4577. [DOI] [PubMed] [Google Scholar]
- Nathanielsz PW. Animal models that elucidate basic principles of the developmental origins of adult diseases. ILAR J. 2006;47:73–82. doi: 10.1093/ilar.47.1.73. [DOI] [PubMed] [Google Scholar]
- Nathanielsz PW, Ford SP, Long NM, Vega CC, Reyes-Castro LA, Zambrano E. Interventions to prevent adverse fetal programming due to maternal obesity during pregnancy. Nutr. Rev. 2013;71(Suppl 1):S78–S87. doi: 10.1111/nure.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Hormonally active agents in the environment. National Academy Press; Washington DC, p: 1999. p. 430. [Google Scholar]
- Oliver MH, Jaquiery AL, Bloomfield FH, Harding JE. The effects of maternal nutrition around the time of conception on the health of the offspring. Soc. Reprod. Fertil. Suppl. 2007;64:397–410. doi: 10.5661/rdr-vi-397. [DOI] [PubMed] [Google Scholar]
- Ortega HH, Salvetti NR, Padmanabhan V. Developmental programming: prenatal androgen excess disrupts ovarian steroid receptor balance. Reproduction. 2009;137:865–877. doi: 10.1530/REP-08-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega HH, Rey F, Velazquez MM, Padmanabhan V. Developmental programming: effect of prenatal steroid excess on intraovarian components of insulin signaling pathway and related proteins in sheep. Biol. Reprod. 2010;82:1065–1075. doi: 10.1095/biolreprod.109.082719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Siefert K, Ransom S, Johnson T, Pinkerton J, Anderson L, Tao L, Kannan K. Maternal bisphenol-A levels at delivery: a looming problem? J. Perinatol. 2008;28:258–263. doi: 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A, Abbott DH, Recabarren SE, Herkimer C. Developmental programming: impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology. 2010;151:595–605. doi: 10.1210/en.2009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids. 2013a;78:734–740. doi: 10.1016/j.steroids.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol. Cell. Endocrinol. 2013b;373:8–20. doi: 10.1016/j.mce.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada I, Fuentes E, Viso-Leon MC, Soria B, Ripoll C, Nadal A. Low doses of the endocrine disruptor bisphenol-A and the native hormone 17beta-estradiol rapidly activate transcription factor CREB. FASEB J. 2002;16:1671–1673. doi: 10.1096/fj.02-0313fje. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J. Perinatol. 2010;30:2–9. doi: 10.1038/jp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recabarren SE, Padmanabhan V, Codner E, Lobos A, Durán C, Vidal M, Foster DL, Sir-Petermann T. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am. J. Physiol. Endocrinol. Metab. 2005;289:801–806. doi: 10.1152/ajpendo.00107.2005. [DOI] [PubMed] [Google Scholar]
- Recabarren SE, Rojas-García PP, Recabarren MP, Alfaro VH, Smith R, Padmanabhan V, Sir-Petermann T. Prenatal testosterone excess reduces sperm count and motility. Endocrinology. 2008;149:6444–6448. doi: 10.1210/en.2008-0785. [DOI] [PubMed] [Google Scholar]
- Rhind SM. Are Endocrine Disrupting Compounds a Threat to Farm Animal Health, Welfare and productivity? Reprod Dom Anim. 2005;40:282–290. doi: 10.1111/j.1439-0531.2005.00594.x. [DOI] [PubMed] [Google Scholar]
- Rhind SM, Kyle CE, Kerr C, Osprey M, Zhang ZL. Effect of duration of exposure to sewage sludge-treated pastures on liver tissue accumulation of persistent endocrine disrupting compounds (EDCs) in sheep. Sci. Total. Environ. 2011;409:3850–3856. doi: 10.1016/j.scitotenv.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Forsdike RA, Taylor JA. In utero exposure of female lambs to testosterone reduces the sensitivity of the gonadotropin-releasing hormone neuronal network to inhibition by progesterone. Endocrinology. 1999;140:5797–5580. doi: 10.1210/endo.140.12.7205. [DOI] [PubMed] [Google Scholar]
- Rojas-Garcia PP, Recabarren MP, Sarabia L, Schon J, Gabler C, Einspanier R, Maliqueo M, Sir-Petermann T, Rey R, Recabarren SE. Prenatal testosterone excess alters Sertoli and germ cell number and testicular FSH receptor expression in rams. Am. J. Physiol. Endocrinol. Metab. 2010;299:E998–E1005. doi: 10.1152/ajpendo.00032.2010. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity and plasma LH levels. Environ. Health Perspect. 2001;109:675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph AM. Significance of animal studies for perinatal research. Gynakologe. 2008;26:24–28. [PubMed] [Google Scholar]
- Salvetti NR, Ortega HH, Veiga-Lopez A, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on ovarian cell proliferation and apoptotic factors in sheep. Biol. Reprod. 2012;87:1–10. doi: 10.1095/biolreprod.112.100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma HN, Manikkam M, Herkimer C, Dell’Orco J, Foster DL, Padmanabhan V. Fetal programming: excess prenatal testosterone reduces postnatal LH, but not FSH responsiveness to estradiol negative feedback in the female. Endocrinology. 2005;146:4281–4291. doi: 10.1210/en.2005-0322. [DOI] [PubMed] [Google Scholar]
- Savabieasfahani M, Kannan K, Astapova O, Evans N, Padmanabhan V. Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology. 2006;147:5956–5966. doi: 10.1210/en.2006-0805. [DOI] [PubMed] [Google Scholar]
- Savabieasfahani M, Lee JS, Herkimer C, Sharma TP, Foster DL, Padmanabhan V. Fetal Programming: Testosterone exposure of the female sheep during mid-gestation disrupts the dynamics of its adult gonadotropin secretion during the periovulatory period. Biol. Reprod. 2005;72:221–229. doi: 10.1095/biolreprod.104.031070. [DOI] [PubMed] [Google Scholar]
- Schafer KS, Kegley SE. Persistent toxic chemicals in the US food supply. J. Epidemiol. Comm. Health. 2002;56:813–817. doi: 10.1136/jech.56.11.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M, Coksaygan T, Rakers F, Nathanielsz PW. Glucocorticoid exposure of sheep at 0.7 to 0.75 gestation augments late-gestation fetal stress responses. Am. J. Obstet. Gynecol. 2012;206:253. doi: 10.1016/j.ajog.2011.11.006. e16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma TP, Herkimer C, West C, Ye W, Birch R, Robinson J, Foster DL, Padmanabhan V. Fetal programming: prenatal androgen excess disrupts positive feedback actions of estradiol but has no effect on timing of onset of puberty in female sheep. Biol. Reprod. 2002;66:924–933. doi: 10.1095/biolreprod66.4.924. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Meyts ERajpert-De, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum. Reprod. 2001;6:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Shavlakadze T, Grounds M. Of bears, frogs, meat, mice and men: complexity of factors affecting skeletal muscle mass and fat. BioEssays. 2006;28:994–1009. doi: 10.1002/bies.20479. [DOI] [PubMed] [Google Scholar]
- Smith P, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment and depletion of follicular reserve. Biol. Reprod. 2009;80:726–736. doi: 10.1095/biolreprod.108.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. J. Endocrinol. 1998;158:327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- Staub C, Hardy VB, Chapin RE, Harris MW, Johnson L. The hidden effect of estrogenic/antiandrogenic methoxychlor on spermatogenesis. Toxicol. Appl. Pharmacol. 2002;180:129–135. doi: 10.1006/taap.2002.9369. [DOI] [PubMed] [Google Scholar]
- Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146:3185–3193. doi: 10.1210/en.2004-1444. [DOI] [PubMed] [Google Scholar]
- Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental Programming: Follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology. 2007a;148:3532–3540. doi: 10.1210/en.2007-0339. [DOI] [PubMed] [Google Scholar]
- Steckler TL, Roberts EK, Doop DD, Lee TM, Padmanabhan V. Developmental programming in sheep: Administration of testosterone during 60 to 90 days of pregnancy reduces breeding success and pregnancy outcome. Theriogenology. 2007b;67:459–467. doi: 10.1016/j.theriogenology.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Steckler TL, Herkimer C, Dumesic DA, Padmanabhan V. Developmental Programming: excess weight gain amplifies the effects of prenatal testosterone excess on reproductive cyclicity- implication to PCOS. Endocrinology. 2009;150:1456–1465. doi: 10.1210/en.2008-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc. Natl. Acad. Sci. USA. 2004;101:7129–7134. doi: 10.1073/pnas.0308058101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WY, Newbold R, Mardilovich K, Jefferson W, Cheng RY, Medvedovic M, Ho SM. Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology. 2008;149:5922–5923. doi: 10.1210/en.2008-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Methoxychlor reregistration eligibility decision (RED) EPA publication no. EPA 738-R-04-010; 2004. [Accessed April 8 2014]. www.epa.gov/oppsrrd1/REDs/methoxychlor_red.htm. [Google Scholar]
- Unsworth WP, Taylor JA, Robinson JE. Prenatal programming of reproductive neuroendocrine function: the effect of prenatal androgens on the development of estrogen positive feedback and ovarian cycles in the ewe. Biol. Reprod. 2005;72:619–927. doi: 10.1095/biolreprod.104.035691. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr. Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJR, Schoenfelder G. Urinary, Circulating and tissue biomonitoring studies indicate widespread exposure to Bisphenol A. Environ. Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Kraak GJ, Munkittrick KR, McMaster ME, Portt CB, Chang JP. Exposure to bleached kraft pulp mill effluent disrupts the pituitary-gonadal axis of white sucker at multiple sites; Toxicol. Appl. Pharmacol. 1992;115:224–233. doi: 10.1016/0041-008x(92)90327-o. [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Astapova OI, Aizenberg E, Lee JS, Padmanabhan V. Developmental programming: contribution of prenatal androgen and estrogen in organizing estradiol feedback systems and periovulatory hormonal dynamics in sheep. Biol. Reprod. 2009;80:718–725. doi: 10.1095/biolreprod.108.074781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Steckler TL, Abbott DH, Welch KB, MohanKumar PS, Phillips DJ, Refsal K, Padmanabhan V. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biol Reprod. 2011;84:87–96. doi: 10.1095/biolreprod.110.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Ye W, Padmanabhan V. Developmental programming: prenatal testosterone excess disrupts anti-Müllerian hormone expression in preantral and antral follicles. Fertil. Steril. 2012;97:748–756. doi: 10.1016/j.fertnstert.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C, Foster DL, Evans NP, Robinson J, Padmanabhan V. Intra follicular activin availability is altered in prenatally-androgenized lambs. Mol. Cell. Endocrinol. 2001;185:51–59. doi: 10.1016/s0303-7207(01)00632-3. [DOI] [PubMed] [Google Scholar]
- Wood RI, Foster DL. Sexual differentiation of reproductive neuroendocrine function in sheep. Rev. Reprod. 1998;3:130–140. doi: 10.1530/ror.0.0030130. [DOI] [PubMed] [Google Scholar]