Abstract
The pathology of age-related macular degeneration (AMD) is characterized by degeneration of photoreceptors and retinal pigment epithelial cells as well as by changes of choroidal capillaries in the macula. Although AMD is not a typical uveitis, there is a consistence and an imbalance of ocular para-inflammation. Ocular inflammation, particularly in the macula, plays a critical role in AMD pathogenesis. The inflammatory and immune-related elements involved in AMD include inflammatory and related cells as well as the secreted molecules and factors from these cells. Innate immune system elements such as macrophages and cytokines play an important role in AMD pathology and pathogenesis. This chapter reviews the observed deviation in macrophage plasticity and the elevated expression of interleukin-17 in AMD eyes while discussing potential contributions to AMD pathogenesis. Targeting of these specific inflammatory pathways and molecules at appropriate times should be explored and may become promising novel adjunct agents to AMD therapy.
Keywords: Age-related macular degeneration, Macrophage, IL-17, Inflammation, Eye
25.1 Introduction
In 1875, Hutchison and Tay described 10 cases of “symmetrical central chorioretinal disease occurring in senile persons” characterized by whitish spots (drusen) in the macula [1]. This was the first description of what was then called “senile macular degeneration” and what has since been renamed “age-related macular degeneration” (AMD) in the 1980s. The aging eye exists in a para-inflammatory state to keep normal physiological functions of photoreceptors and retinal pigment epithelium (RPE) cells and thus maintain retinal homeostasis [2]. Loss of retinal homeostasis is permissive for development of AMD in the macula resulting from photoreceptor and RPE pathology as well as subtle or mild chronic inflammation.
The etiology of AMD involves multiple factors such as aging, genetic predisposition, environmental elements including smoke and diet, oxidative stress, and inflammation [3]. Unlike uveitis, inflammation in the AMD eye is subtle or mild, never severe or overwhelming. This chapter describes recent pathological findings of macrophage and interleukin (IL)-17 involvements in AMD.
25.2 Macrophage
Macrophages, a predominant cell type associated with chronic inflammation, are the most prominent inflammatory cells observed in AMD tissue, outnumbering subretinal microglia (resident myeloid cells/macrophages) as well as lymphocytes in AMD eyes [3–5]. The findings have been well documented in and/or near the lesions of drusen, neovascular, and geographic atrophy AMD [6–8]. Macrophages secrete a wide range of cytokines, chemokines, complement factors, and growth factors, including vascular endothelial growth factor in response to pathogens and damaged tissues; most of them are implicated in AMD. Using immunohistochemistry, choroidal macrophages expressing inducible nitric oxide (iNO) were only found in the Bruch’s membrane of early AMD eyes with soft drusen or thick basal laminar deposits, in active disciform scars, and in eyes with subclinical choroidal neovascularization. Choroidal macrophages in the normal macula do not express iNO [9].
Each macrophage can secrete more than 100 different molecules for biologic activities including inflammation, immunity, phagocytosis, cell growth, and cell death [10]. The secretion of these products depends on the inciting stimulus, macrophage subtype, and location. Diversity and plasticity have long been recognized for cells of monocyte-macrophage lineage. In response to signals derived from microbes, damaged tissues, or activated lymphocytes, macrophages polarize into distinct functional phenotypes. Two main macrophage phenotypes are classified based on functional properties, surface markers, and cytokine profiles: the classically activated M1 macrophage and the alternatively activated M2 macrophage [11, 12]. In general, M1 macrophages are pro-inflammatory, microbicidal, and anti-tumoral; M2 macrophages are anti-inflammatory, tissue remodeling, pro-tumoral, immunoregulatory, and proliferative. Recently, some suggested that the M2 macrophages should be further divided into at least two groups: pro-angiogenic and anti-angiogenic [13]. However, there are still uncertainties regarding distinct expression patterns of surface markers that clearly define macrophage subtypes, particularly in the case of human macrophages. Furthermore, macrophages often retain their plasticity, so the phenotypes of a macrophage population can change over time [14]. M1 and M2 macrophages may undergo phenotype switching towards M2 prominence during the normal aging process [15, 16].
Distinct chemokine patterns are also associated with M1 and M2 macrophage activation and production. CXCL9, CXCL10, and CXCL11 represent M1 chemokines, and CCL17 and CCL22 represent M2 chemokines [17]. Using molecular pathology including microdissection (Fig. 25.1) and immunochemistry, a pathological imbalance of macrophage polarization was reported in AMD lesions; a relatively higher M1 infiltration in geographic atrophy AMD and an aberrantly higher M2 in neovascular AMD were reported [15]. The findings suggest that macrophage polarization and plasticity could contribute to AMD development and progression.
Fig. 25.1.
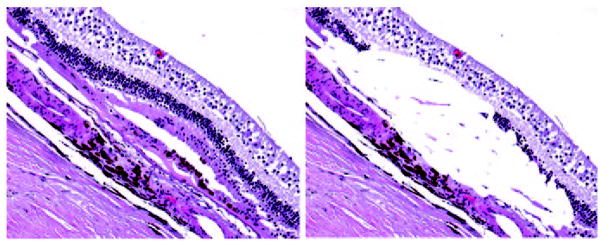
Macular lesion in an AMD eye before and after microdissection. The outer neuroretinal cells, hypertrophic RPE, and irregular Bruch’s membrane were microdissected and subjected for quantitative real-time polymerase chain reaction (RT-PCR). (left, before microdissection, right, after microdissection; hematoxylin and eosin, x100)
25.3 IL-17A
The IL-17 cytokine family includes six members named A–F. IL-17A is the main cytokine in the IL-17 family produced mostly by Th17 cells [18, 19]. However, other inflammatory cells such as neutrophils and even macrophages under specific conditions may produce IL-17A [20, 21]. IL-17A homodimers bind IL-17 receptor (R)C/IL-17RA heterodimers. The most notable role of IL-17 is its involvement in inducing and mediating pro-inflammatory responses. It controls extracellular pathogens and induces matrix destruction and neovascularization. Th17-type cytokines have been linked to neurodegenerative diseases such as multiple sclerosis and Alzheimer’s disease [22, 23].
In a recent report, serum levels of IL-17 were significantly higher in 23 AMD patients compared to 30 age-matched non-AMD individuals; serum levels of IL-22, a Th17 family cytokine, were also significantly higher in 25 AMD patients as compared to 29 control individuals [24]. This study also showed that the C5a ana-phylatoxin can promote Th17 cytokine expression from human CD4+ T cells. More recently, hypomethylation of the IL-17RC promoter was associated with AMD [25]. The epigenetic alteration leads to elevation of IL-17RC transcript and protein in peripheral blood as well as in macular cells of AMD patients. Since the IL-17RC subunit plays a key role in modulating the IL-17 response [26], the association of IL-17RC with AMD suggests that IL-17 could be an important player in AMD pathogenesis.
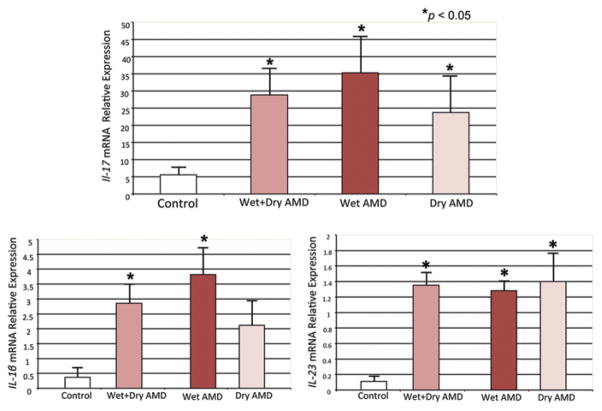
Our preliminary experiments have shown that IL-17A reduces cellular viability, alters cell metabolism, and induces apoptosis in ARPE-19 cells [27]. This in vitro study supports the harmful role of IL-17 on RPE cells, a critical cell in AMD. In addition, we detected significantly high expression of not only IL-17 but also IL-1β and IL-23 mRNA in AMD lesions (Fig. 25.2) [28]; these two cytokines promote Th17 cell differentiation and are secreted mainly by macrophages [18, 19]. Our data offer convincing support for an increase of IL-17 in AMD eyes and the role of IL-17 in neurodegenerative disease. Two recent independent studies have linked the inflammasome to AMD pathogenesis [29, 30]. Inflammasome is expressed in macrophages and activates the release of IL-1β and IL-18, which can subsequently drive an IL-17 response [31].
Fig. 25.2.
Transcript expression of IL-1β, IL-23, and IL-17 in macular cells of four normal and nine age-related macular degeneration (AMD, five geographic atrophy “dry” and four neovascular “wet” AMD) eyes. Significant elevations of these three cytokines are detected in maculae with AMD lesions compared to the normal controls
25.4 Conclusion
Immunopathology and molecular pathology of AMD lesions clearly prove that there is an important role for inflammation and innate immune cells such as macrophages in AMD. IL-17A and IL-17RC in AMD eyes and patients demonstrate IL-17 involvement in AMD pathogenesis. Targeting IL-17, IL-17RC, and cells producing IL-17 to deter retinal degeneration might be a potential treatment strategy for AMD. However, we should consider genetic background and clinical manifestation of each patient, duration of the therapy, and adverse effects of individual therapeutic agents.
Abbreviations
- AMD
Age-related macular degeneration
- RPE
Retinal pigment epithelium
- IL-17
Interleukin-17
- iNO
Inducible nitric oxide
- IL-17R
Interleukin-17 Receptor
- RT-PCR
Real time polymerase chain reaction
Contributor Information
Chi-Chao Chan, Email: chanc@nei.nih.gov.
Daniel Ardeljan, Email: ardeljand@mail.nih.gov.
References
- 1.Hutchison J, Tay W. Symmetrical central chorioretinal disease occurring in senile persons. R London Ophthal Hosp Rep. 1875;8:231–244. [Google Scholar]
- 2.Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28(5):348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Coleman HR, Chan CC, Ferris FL, III, Chew EY. Age-related macular degeneration. The Lancet. 2008;372(9652):1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration: the involvement of immunocompetent cells. Graefes Arch Clin Exp Ophthalmol. 1985;223(2):69–76. doi: 10.1007/BF02150948. [DOI] [PubMed] [Google Scholar]
- 5.Dastgheib K, Bressler SB, Green WR. Clinicopathologic correlation of laser lesion expansion after treatment of choroidal neovascularization. Retina. 1993;13(4):345–352. doi: 10.1097/00006982-199313040-00013. [DOI] [PubMed] [Google Scholar]
- 6.Grossniklaus HE, Miskala PH, Green WR, Bressler SB, Hawkins BS, Toth C, et al. Histopathologic and ultrastructural features of surgically excised subfoveal choroidal neovascular lesions: submacular surgery trials report no. 7. Arch Ophthalmol. 2005;123(7):914–921. doi: 10.1001/archopht.123.7.914. [DOI] [PubMed] [Google Scholar]
- 7.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28(1):1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Killingsworth MC, Sarks JP, Sarks SH. Macrophages related to Bruch’s membrane in age-related macular degeneration. Eye (Lond) 1990;4(Pt 4):613–621. doi: 10.1038/eye.1990.86. [DOI] [PubMed] [Google Scholar]
- 9.Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol. 2010;94(7):918–925. doi: 10.1136/bjo.2009.165563. [DOI] [PubMed] [Google Scholar]
- 10.Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 12.Biswas SK, Chittezhath M, Shalova IN, Lim JY. Macrophage polarization and plasticity in health and disease. Immunol Res. 2012;53(1–3):11–24. doi: 10.1007/s12026-012-8291-9. [DOI] [PubMed] [Google Scholar]
- 13.Dick AD. Road to fulfilment: taming the immune response to restore vision. Ophthalmic Res. 2012;48(1):43–49. doi: 10.1159/000335982. [DOI] [PubMed] [Google Scholar]
- 14.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao X, Shen D, Patel MM, Tuo J, Johnson TM, Olsen TW, et al. Macrophage polarization in the maculae of age-related macular degeneration: a pilot study. Pathol Int. 2011;61(9):528–535. doi: 10.1111/j.1440-1827.2011.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahbub S, Deburghgraeve CR, Kovacs EJ. Advanced age impairs macrophage polarization. J Interferon Cytokine Res. 2012;32(1):18–26. doi: 10.1089/jir.2011.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 19.Gaffen SL. Recent advances in the IL-17 cytokine family. Curr Opin Immunol. 2011;23(5):613–619. doi: 10.1016/j.coi.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song C, Luo L, Lei Z, Li B, Liang Z, Liu G, et al. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181(9):6117–6124. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- 21.Vazquez N, Rekka S, Gliozzi M, Feng CG, Amarnath S, Orenstein JM, et al. Modulation of innate host factors by Mycobacterium avium complex in human macrophages includes interleukin 17. J Infect Dis. 2012;206(8):1206–1217. doi: 10.1093/infdis/jis492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gold R, Luhder F. Interleukin-17-extended features of a key player in multiple sclerosis. Am J Pathol. 2008;172(1):8–10. doi: 10.2353/ajpath.2008.070862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu WT, Chen-Plotkin A, Grossman M, Arnold SE, Clark CM, Shaw LM, et al. Novel CSF biomarkers for frontotemporal lobar degenerations. Neurology. 2010;75(23):2079–2086. doi: 10.1212/WNL.0b013e318200d78d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu B, Wei L, Meyerle C, Tuo J, Sen HN, Li Z, et al. Complement Component C5a Promotes Expression of IL-22 and IL-17 from Human T cells and its Implication in Age-related Macular Degeneration. J Transl Med. 2011;9(1):111. doi: 10.1186/1479-5876-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei L, Liu B, Tuo J, Shen D, Chen P, Li Z, et al. Hypomethylation of the IL17RC Promoter Associates with Age-Related Macular Degeneration. Cell Rep. 2012;2(5):1151–1158. doi: 10.1016/j.celrep.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho AW, Gaffen SL. IL-17RC: a partner in IL-17 signaling and beyond. Semin Immunopathol. 2010;32(1):33–42. doi: 10.1007/s00281-009-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ardeljan D, Wang Y, Shen D, Tuo J, Chan CC. Treatment with recombinant interleukin-17A reduces ARPE-19 cell viability. ARVO Abstr. #1227. 2012 May 6; 2012. [Google Scholar]
- 28.Chan CC, Shen D, Cao X, Wang VM, Wang Y, Tuo J. Expression of IL-17 in Eyes of Age-related Macular Degeneration. ARVO Abstr. #1228. 2011 2011. [Google Scholar]
- 29.Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149(4):847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doyle SL, Campbell M, Ozaki E, Salomon RG, Mori A, Kenna PF, et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012;18(5):791–798. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mills KH, Dungan LS, Jones SA, Harris J. The role of inflammasome-derived IL-1 in driving IL-17 responses. J Leukoc Biol. 2012;93(4):489–497. doi: 10.1189/jlb.1012543. [DOI] [PubMed] [Google Scholar]