Abstract
Aging leads to a decline in strength and an associated loss of independence. The authors examined changes in muscle volume, maximum isometric joint moment, functional strength, and 1-repetition maximum (1RM) after resistance training (RT) in the upper extremity of older adults. They evaluated isometric joint moment and muscle volume as predictors of functional strength. Sixteen healthy older adults (average age 75 ± 4.3 yr) were randomized to a 6-wk upper extremity RT program or control group. The RT group increased 1RM significantly (p < .01 for all exercises). Compared with controls, randomization to RT led to greater functional pulling strength (p = .003), isometric shoulder-adduction moment (p = .041), elbow-flexor volume (p = .017), and shoulder-adductor volume (p = .009). Shoulder-muscle volumes and isometric moments were good predictors of functional strength. The authors conclude that shoulder strength is an important factor for performing functional reaching and pulling tasks and a key target for upper extremity RT interventions.
Keywords: biomechanics, isometric joint moment, reaching, aging
Aging leads to a decline in strength, which is in part due to sarcopenia or the loss of skeletal-muscle mass (Thompson, 2007; Vidt et al., 2012). This decrease in strength is associated with a loss of independence; approximately 14% of adults age 65 years and older require assistance with activities of daily living (ADLs), and 35% are limited in their ability to perform other functional tasks (Fuller-Thomson, Yu, Nuru-Jeter, Guralnik, & Minkler, 2009). Many of the tasks vital for independence, including dressing, grooming, and housework, require strength and coordination of the upper extremity (Lundgren-Lindquist & Sperling, 1983), and the ability to perform upper extremity functional tasks has been associated with better outcomes for the hospitalized elderly (Abizanda et al., 2007). While functional loss typically occurs first in the lower extremity, loss of function in the upper extremity follows and precedes loss of cognition and dexterity (Seidel et al., 2009). Therefore, strategies that preserve and improve upper extremity function are an important aspect of maintaining independence in the growing population of older adults.
Current evidence clearly shows that resistance training (RT) leads to increases in muscle volume (Fiatarone et al., 1990), isometric joint moment (Hunter et al., 2001), 1-repetition maximum (1RM; Fiatarone et al., 1990; Frontera, Hughes, Lutz, & Evans, 1991), and functional ability (Vincent et al., 2002) in the lower extremity of older adults. In addition, RT can induce hypertrophy (Brown, McCartney, & Sale, 1990; Fiatarone et al., 1990; Frontera, Meredith, O'Reilly, Knuttgen, & Evans, 1988) and lead to increases in 1RM (Brown et al., 1990) in the upper extremity of older adults. For example, Brown et al. found that a 12-week upper extremity RT intervention in an older adult population led to a 17.4% increase in the cross-sectional area of the elbow flexors and a 48% increase in biceps-curl 1RM. However, while some studies show that, in older adults, upper extremity RT leads to significant increases in maximum isometric moment (Hunter et al., 2001) and functional ability (Nelson et al., 2004), other studies have found no significant increases in either maximum isometric joint moment (Brown et al., 1990) or functional ability (Miszko et al., 2003) after RT. Therefore, it remains unsettled to what extent RT results in changes in strength as measured by maximum isometric joint moment and functional strength or ability. While research has shown that the most effective way to increase strength for a movement or task is to perform that task (training specificity; de Vreede et al., 2004; Ingebrigtsen, Holtermann, & Roeleveld, 2009), it may not always be feasible or practical to train this way due to time or cost constraints. In addition, it may not always be possible to quantitatively assess changes in functional strength in the clinic. Thus, it would be useful to determine if metrics such as muscle volume or isometric joint moment are robust predictors of functional strength.
To our knowledge, there are no studies that have assessed changes in multijoint strength and volume for the muscles actuating all the major upper limb joints after RT in a healthy older adult population. Comprehensive description of the relationships between an upper extremity RT program and several measures of strength, including functionally relevant strength measures, will inform future RT interventions designed to improve upper extremity function in older adults. In particular, a study goal is to identify important muscle targets for RT by elucidating the functional muscle groups most likely to experience hypertrophy after RT and those most associated with improvements in functional strength. Another goal is to clarify whether there is support for using simple measures of strength, such as isometric joint moment, to predict more clinically relevant functional strength outcomes. The aims of this study were to determine (a) the effects of randomization to a 6-week RT intervention on changes in 1RM, maximal isometric joint moment, functional strength, and muscle volume for six upper extremity functional groups representing all of the major muscles actuating the shoulder, elbow, and wrist joints and (b) if maximum isometric joint moment or functional-group muscle volume can be used to predict functional strength in a healthy older adult population.
Method
Study Sample
Sixteen healthy adults (8 male, 8 female) over the age of 65 were recruited for this study and included if they were not currently participating in aerobic or resistance training, were free of any medical condition that might be exacerbated by physical testing or strength training, had no contraindications to undergoing magnetic resonance imaging (MRI), had no history of neuromuscular disorder or injury that might affect the upper limb (including, but not limited to, stroke or Parkinson's disease), and were able to stand independently of assistive devices (such as walkers or canes). This study was approved by the Wake Forest University institutional review board, and all of the participants provided written informed consent. Participants were randomly assigned to either a control group or RT group, and the assessment staff were blinded to this assignment. After the blocked (sex) randomization, the groups each had 4 men and 4 women, with an average age of 75 ± 5.3 years for the control group and 74.8 ± 3.2 years for the RT group. The average height (1.71 ± 0.10 m) and body mass (78.5 ± 10.3 kg) of the RT group were not statistically different from the average height (1.69 ± 0.09 m) and body mass (74.3 ± 14.8 kg) of the control group.
RT
The RT group participated in 6 weeks of upper extremity RT under the supervision of master's-level exercise physiologists certified in emergency-management procedures. The RT intervention included 19 sessions of testing or training: one orientation session including 1RM testing, sixteen 1-hr RT sessions, and a 1RM test on all machines at 3 weeks and 6 weeks to monitor changes in strength. During the orientation session, participants were introduced to the equipment and instructed in proper technique, and a 1RM test was performed for each of the lifting exercises to determine the starting exercise intensity. The 1RM was determined by having the participants lift a given weight twice with good form and then adding weight incrementally until only one repetition could be completed with good form. Between efforts, 60–90 s of rest was provided. A recent study by Theou, Gareth, and Brown (2008) indicated that rest intervals of 60 s are sufficient to recover strength and that older women required less rest than younger women. For all participants, three to six efforts were required to determine the 1RM. We believe that this single training session was sufficient to reduce the effects of learning on performance based on research by Schroeder et al. (2007), who previously reported that there is not a significant change in maximal force production between two testing sessions. In addition, work by Phillips, Batterham, Valenzuela, and Burkett (2004) indicates that two or three test trials are sufficient to achieve repeatable results when testing 1RM.
Nine exercises—six using Nautilus weight machines and three using free weights—were completed at each training session (Table 1). The order of the exercises was designed to minimize muscle-group fatigue, determined for each participant at the first training session and maintained throughout the intervention. During the first three sessions, participants performed three sets of eight repetitions at 60% 1RM with 60–90 s of rest between sets. Beginning with the fourth session, the weight was incrementally increased so that it reached 70% 1RM by the end of the sixth session. Starting in the seventh session, two sets of eight repetitions were performed at 75% 1RM, and on the third set, the participants completed as many repetitions as possible while maintaining good form. If a participant performed 12 or more repetitions in the third set, the weight was increased at the next session.
Table 1. Exercises Performed During the Resistance-Training Intervention.
| Exercise | Type | # of repetitions, sets |
|---|---|---|
| Triceps press | Machine | 8 repetitions, 3 setsa |
| Preacher curl | Machine | 8 repetitions, 3 setsa |
| Chest press | Machine | 8 repetitions, 3 setsa |
| Overhead press | Machine | 8 repetitions, 3 setsa |
| Compound row | Machine | 8 repetitions, 3 setsa |
| Incline press | Machine | 8 repetitions, 3 setsa |
| Lateral raise | Free weights | 8 repetitions, 3 setsa |
| Wrist curl | Free weights | 10 repetitions, 1 set |
| Wrist extension | Free weights | 10 repetitions, 1 set |
After Session 6, two sets of eight repetitions, and then on the third set, as many repetitions as possible.
Subject Testing
All participants were assessed in three pretesting sessions, each lasting ∼1 hr, during which muscle volume was assessed via MRI and isometric strength and functional strength testing were performed. The dominant arm (the right arm for all but 1 participant) was evaluated. After baseline assessments, participants randomized to the RT group participated in 6 weeks of RT, while participants randomized to the control group were asked to maintain their normal routines. Participants in the control group were contacted after 3 weeks to determine if there were any changes in health status. The follow-up evaluations were repeated after 6 weeks for both groups.
MRI
We acquired axial images of the dominant arm at baseline and completion of the study with a 1.5T MRI scanner (GE Healthcare, Milwaukee, WI) using a protocol described previously (Holzbaur, Murray, Gold, & Delp, 2007). A spoiled gradient sequence with 3-mm axial slices was used to image the upper limb from the shoulder to wrist. A single scan with the body coil was used to image muscles crossing the shoulder with echo time of 3 ms, a relaxation time of 11.6 ms, flip angle of 30°, matrix of 512 × 192, bandwidth of ±31.25 kHz, and field of view of 32 cm, resulting in a scan time of 16 min. In two successive scans, a flexed-array long-bone coil (Invivo, Orlando, FL) was used to obtain images with better spatial resolution for the smaller muscles of the arm and forearm, with the following scanning parameters: echo time = 5 ms, relaxation time = 23 ms, flip angle = 45°, matrix = 320 ×192, bandwidth = ± 15.63 kHz, and field of view = 16 cm, with each scan lasting 11 min. Total scan time was approximately 40 min.
To calculate muscle volumes, three-dimensional polygonal surfaces were created for each of the muscles using the identified muscle boundaries (Figure 1). The accuracy of estimating muscle volumes using MRI has been previously established by Tingart et al. (2003), and the accuracy of this imaging protocol was established by Holzbaur, Murray et al. (2007).
Figure 1.
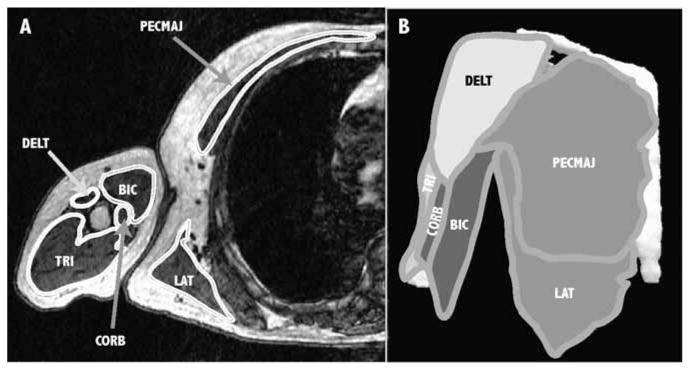
Three-dimensional (3D) muscle surfaces created from axial MR images. (A) Example axial slice showing the boundaries of the shoulder adductors (medium gray), shoulder abductors (white), elbow flexors (dark gray), and elbow extensors (light gray). In this slice, visible shoulder adductors include pectoralis major (PECMAJ), latissimus dorsi (LAT), and coracobrachialis (CORB); visible abductors include deltoid (DELT); visible elbow flexors include biceps (BIC); and the visible elbow extensor is triceps (TRI). (B) A 3D surface rendering of each muscle is created from the segmented images to determine muscle volume.
The axial images were manually segmented to identify muscle boundaries for 21 upper limb muscles (Table 2; 3D Doctor, Able Software Corp., Lexington, MA). The remaining wrist-flexor and -extensor muscles were segmented in two groups of muscles due to close association of the muscles. The muscles were categorized into functional groups based on their primary action (largest moment arm), and the volume of each functional group was determined as the sum of the individual muscle volumes for that group.
Table 2. Functional Muscle Groups.
| Joint | Muscle type | Muscles |
|---|---|---|
| Wrist | Extensor | Extensor carpi radialis longus and brevis |
| Extensor carpi ulnarisa | ||
| Extensor digitorum communisa | ||
| Extensor digiti minimia | ||
| Extensor indicis propioa | ||
| Extensor pollicis longusa | ||
| Extensor pollicis brevisa | ||
| Flexor | Flexor carpi radialis | |
| Flexor carpi ulnaris | ||
| Palmaris longusb | ||
| Flexor digitorum superficialisb | ||
| Flexor digitorum profundusb | ||
| Flexor pollicis longusb | ||
| Abductor pollicis longusb | ||
| Elbow | Extensor | Triceps |
| Anconeus | ||
| Supinator | ||
| Flexor | Biceps | |
| Brachioradialis | ||
| Brachialis | ||
| Pronator teres | ||
| Shoulder | Adductor | Latissimus dorsi |
| Pectoralis major | ||
| Teres major | ||
| Teres minor | ||
| Coracobrachialis | ||
| Abductor | Deltoid | |
| Infraspinatus | ||
| Subscapularis | ||
| Supraspinatus |
Note. Specified
wrist extensors
wrist flexors were segmented as a group due to close association of muscles anatomically.
Isometric Joint-Moment Assessment
Maximum isometric joint moments were evaluated at the wrist, elbow, and shoulder using a dynamometer (KIN-COM, Isokinetic International, Harrison, TN), following a previously described standard protocol (Holzbaur, Delp, Gold, & Murray, 2007). Participants were seated with their torso in a vertical posture and the hip flexed to 90°. The torso was restrained using straps to prevent changes in posture during the trials. Maximum isometric moment-generating capacity was measured at the wrist for flexion and extension with the forearm fully pronated and the elbow flexed to 90°. Maximum isometric joint moment at the elbow was assessed for both flexion and extension with the forearm fully supinated and the elbow flexed to 90°. At the shoulder, maximum isometric moment was assessed for abduction and adduction with the shoulder abducted to 60° and the elbow braced in full extension. Three trials of each of the six isometric-moment tests were obtained, and participants were provided with standardized verbal and visual feedback to encourage maximal voluntary activation. Participants were given 60 s rest between trials and 5 min of rest between functional-group evaluations. Each trial was analyzed using custom software (Matlab, The Mathworks Inc., Natick, MA, version R2008a) to identify the peak force sustained for at least 0.5 s. Forces were multiplied by the distance from the joint center of rotation to the location of the load cell (moment arm) to determine the moment generated about a joint. The maximum peak moment achieved over the three trials for each functional group was used for analysis.
Functional Strength Assessment
Functional strength was evaluated for two functional movements (a forward reach and a pull) as the maximum load with which a participant could complete the movement successfully (functional 1RM). While these tasks have not been described for maximum strength in this group previously, a similar reaching task was used to evaluate functional ability in a poststroke population (Wagner, Rhodes, & Patten, 2008). In addition, pulling strength has been used to evaluate functional capacity in individuals with chronic pain (Hart, 1988). The cited studies each reported that similar reaching and pulling tasks are reliable measures (intraclass correlation coefficient of .57–.99 for reaching and .95 for pulling) of functional ability in these populations. For the forward reach and pull, the wrist was braced. For the forward reach, participants started with the elbow flexed to 90°, reached forward while holding a dumbbell in their dominant hand to a line placed such that their elbow was flexed 10–20°, and returned to the starting posture (Figure 2[A]). A trial was considered successful if the dumbbell did not drag on the table during the reaching movement. Each participant started with a 1-lb (∼0.5-kg) dumbbell, and the weight was increased incrementally until the participant could not perform a successful reach. For the functional pulling task, a pulley resistance system was used. The participants held the handle in the dominant hand and pulled it toward the torso. They began with the elbow flexed 10–20°, pulled until the elbow was flexed to at least 90° for a successful trial, and returned to the starting posture (Figure 2[B]). Each participant started with 6 lb (∼2.7 kg; the minimal loading possible), and the weight was incrementally increased, as in the 1RM determination, until a maximal effort was found. One minute of rest was given between attempts for both the reach and the pull. Weight increments were based on perceived exertion of the participant to identify the maximal effort quickly (within ∼6 efforts) so as to limit the effects of fatigue on the maximal effort. The same sequence of resistance was used at the second testing period, and the participants were allowed to attempt a new maximal effort.
Figure 2.
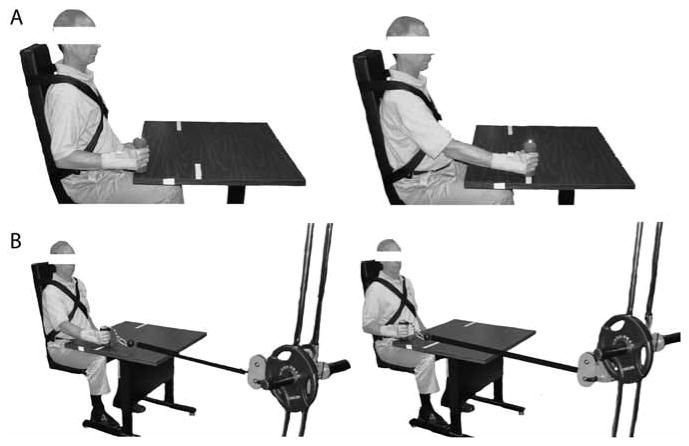
Upper limb posture at the start and finish (left) and midpoint (right) of the functional (A) reaching and (B) pulling tasks. For the forward reach, participants began with the elbow flexed to 90°, reached forward until the elbow was flexed to 10–20°, and returned to the starting posture. The pull began with the elbow flexed to 10–20°; each participant pulled until the elbow was flexed to at least 90° and then returned to the starting posture.
Statistical Analysis
To assess the effectiveness of the RT intervention to elicit change in 1RM strength, we used mixed models for repeated measures to estimate 1RM means at all time points, while adjusting for sex, and used a contrast to evaluate the time-specific 1RM means for a linear time trend. To determine the effects of randomization to the RT or control group on changes in functional-group muscle volume, we used multifactorial ANCOVA with change in functional-group muscle volume between baseline and follow-up as the dependent variable and terms for group, the baseline measure of the strength outcome, sex, and body mass as predictors. To determine the effects of randomization to the RT or control group on changes in functional strength and maximum isometric joint moment, we used multifactorial ANCOVA with changes in functional strength and maximum isometric joint moment between baseline and follow-up as the dependent variables and terms for group, the baseline measure of the strength outcome, and sex as predictors. All models were fit twice, as main effect models and again including a Group × Sex interaction. Adjustment for the baseline measurement is considered the appropriate approach for analysis of randomized comparisons (Fitzmaurice, 2001).
To evaluate muscle volume and maximum isometric joint moment as predictors of functional strength, we used multiple linear-regression analyses on baseline data collected before randomization. We ran a series of models to evaluate the volume of elbow flexors and shoulder adductors as predictors of functional pulling strength, adjusted for sex and body mass. Models 1a and 1b included either elbow flexors or shoulder adductors, without adjustment. Model 2 included both elbow flexors and shoulder adductors, without adjustment. Model 3 included both elbow flexors and shoulder adductors, with adjustment for sex. Model 4 included both elbow flexors and shoulder adductors, with adjustment for sex and body mass. These functional groups were chosen a priori because they are functionally relevant to the pulling task. For functional reaching, we evaluated the same four models using the volume of elbow extensors and shoulder abductors as predictors both alone and together, adjusted for sex and body mass. These functional groups were also expected a priori to be relevant to the performance of reaching.
We also evaluated maximum isometric joint moments as predictors of functional strength. Specifically, Models 1a and 1b included either elbow-flexion or shoulder-adduction isometric joint moments, without adjustment. Model 2 included both elbow-flexion and shoulder-adduction isometric joint moments, without adjustment. Model 3 included both elbow-flexion and shoulder-adduction isometric joint moments, with adjustment for sex. For functional reaching, we evaluated the same three models using maximum isometric joint moment of elbow extensors and shoulder abductors as predictors, both alone and together, adjusted for sex.
Because this was a preliminary study with a small sample size, all analyses were considered exploratory. Therefore, we report the nominal p values and make no corrections for Type I error.
Results
All participants completed the study, and none experienced any adverse events during testing or training. All participants in the RT group completed 16 RT sessions and 3 1RM testing sessions. Functional pulling test data for 3 participants in the control group could not be included in the analysis because participant strength exceeded the maximum capacity of the apparatus.
Increased 1RM Strength With RT
The RT program led to significant increases in 1RM strength for all exercises at all time points (p < .01 for each; Figure 3). These 1RM increases followed a linear trend for all exercises (p < .001 for each; Table 3). After 3 weeks of RT, the smallest average improvement in dynamic 1RM strength was seen for the triceps press (11% increase), and the largest improvement was for the overhead press (26% increase), as calculated based on the mean percentage change for all the individuals in the RT group. In the second 3 weeks of the RT intervention, we observed increases in dynamic 1RM strength ranging from 8% for the biceps curl to 19% for the incline press. The total increase in 1RM strength for the entire 6-week intervention ranged from 21% for the triceps press to 49% for the incline press.
Figure 3.
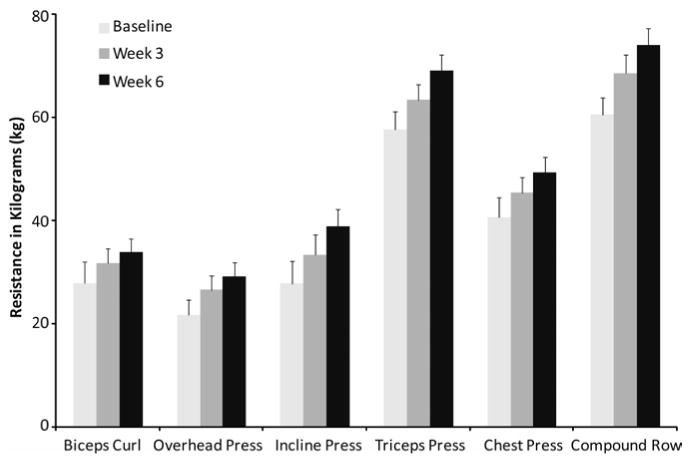
Changes in one-repetition maximum for each of the machine exercises for participants in the resistance-training group. Least-squares means and standard errors are shown for baseline, 3 weeks, and 6 weeks. Changes were linear and significant for all time points and all exercises.
Table 3. Least-Squares Means for One-Repetition Maximum Strength (kg) at Three Time Points for Resistance-Training Group.
| Task | Baseline | 3 weeks | 6 weeks | Test of linear trend, F(1, 6) | p |
|---|---|---|---|---|---|
| Compound row | 60.4 | 68.3 | 73.8 | 33.06 | .001 |
| Overhead press | 21.5 | 26.5 | 29.1 | 71.04 | <.001 |
| Incline press | 27.7 | 33.3 | 38.7 | 55.47 | <.001 |
| Triceps press | 57.5 | 63.2 | 68.9 | 67.05 | <.001 |
| Chest press | 40.5 | 45.3 | 49.2 | 37.90 | <.001 |
| Biceps curl | 27.8 | 31.6 | 33.7 | 39.08 | <.001 |
Functional-Group Muscle Volume
Our model analyses indicate that there was a significant main effect for group such that randomization to RT was associated with significantly greater increases in muscle volume for the elbow flexors and shoulder adductors with respect to the changes observed in the control group (Table 4). In addition, there was a significant Group × Sex interaction for the wrist extensors and elbow flexors (Table 4), indicating a different effect of the RT intervention in men versus women.
Table 4. Change in Muscle Volume, Functional Strength, and Maximum Isometric Joint Moment in the Resistance-Training (RT) and Control (Con) Groups.
| Model Containing Only Main Effect of Group | Model Containing Main Effects and Interaction of Group and Sex | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Least-Squares Mean of Change | Mens Least-Squares Mean of Change | Women's Least-Squares Mean of Change | ||||||||||
|
|
|
|
||||||||||
| Con | RT | Fa | P | η2b | Con | RT | Con | RT | Fc | Pd | η2b | |
| Muscle volume (cm3) | ||||||||||||
| wrist flexs | 4.73 | 18.57 | 1.84 | .203 | .08 | 20.87 | 49.39 | −11.34 | −12.32 | 2.4 | .156 | .09 |
| wrist exts | −10.67 | 2.10 | 3.59 | .085 | .16 | −1.95 | 23.25 | −18.82 | −19.62 | 5.4 | .042* | .18 |
| elbow flexs | −10.03 | 16.47 | 7.80 | .017* | .40 | 1.25 | 45.92 | −21.71 | −12.58 | 5.4 | .043* | .19 |
| elbow exts | −4.25 | 14.14 | 4.20 | .065 | .25 | 12.64 | 28.08 | −21.09 | 0.15 | 0.1 | .757 | .01 |
| shoulder abds | −10.78 | 6.05 | 1.310 | .277 | .09 | −4.93 | 16.54 | −16.39 | −4.68 | 0.1 | .744 | .01 |
| shoulder adds | −18.74 | 28.17 | 10.10 | .009* | .46 | −11.08 | 58.34 | −28.70 | 0.30 | 2.0 | .191 | .08 |
| Functional strength (N) | ||||||||||||
| pulling | 7.55 | 30.14 | 15.50 | .003* | .52 | 21.56 | 34.77 | −0.76 | 23.10 | 0.4 | .569 | .01 |
| reaching | 1.68 | 2.20 | 0.22 | .644 | .01 | 3.23 | 3.63 | 0.12 | 0.77 | 0 | .910 | 0 |
| Maximum isometric joint moments (Nm) | ||||||||||||
| wrist flexn | 2.84 | 7.02 | 2.43 | .145 | .12 | 7.35 | 12.63 | −1.67 | 1.43 | 0.2 | .700 | .01 |
| wrist extn | −1.34 | 0.41 | 2.58 | .134 | .07 | 1.18 | 3.07 | −3.86 | −2.25 | 0 | .907 | 0 |
| elbow flexn | 2.48 | 9.37 | 0.82 | .382 | .04 | 9.03 | 22.47 | −4.71 | −3.09 | 0.6 | .471 | .03 |
| elbow extn | 5.67 | 1.86 | 0.11 | .743 | .01 | 22.42 | −5.55 | −8.12 | 6.30 | 4.9 | .049* | .24 |
| shoulder abdn | −5.20 | −7.73 | 0.20 | .664 | .01 | −1.03 | −11.87 | −9.10 | −3.86 | 2.0 | .183 | .10 |
| shoulder addn | 2.14 | 24.59 | 5.23 | .041* | .25 | 9.88 | 42.72 | −5.46 | 6.34 | 0.9 | .369 | .04 |
Note. flexs = flexors; exts = extensors; abds = abductors; adds = adductors; flexn = flexion; extn = extension; abdn = abduction; addn = adduction.
Because models involving volume adjust for body mass, all tests for models including volume are performed as F(1, 11). Models for functional strength and maximum isometric joint moment do not adjust for body mass and are performed as F(1, 12).
η2 defined as SS (group)/SS (total).
A11 tests involving volume are performed as F(1, 10) and functional strength and maximum isometric joint moment as F(1, 11).
For Group × Sex.
Significant at an α level of .05.
Maximum Isometric Joint Moments and Functional Strength
There was a significant main effect for group such that randomization to RT was associated with a significantly greater increase in maximum isometric shoulder-adduction moment (p = .041) and functional pulling strength (p = .003, Table 4). There were no significant differences between the RT and control group for either the change in functional reaching strength or the change in maximum isometric joint moment for the other functional muscle groups. In addition, there was a significant Group × Sex interaction for maximum isometric elbow-extension moment (Table 4).
Predictors of Functional Pulling Strength
Separately, without adjustment for sex, shoulder-adductor volume (R2 = .70) and elbow-flexor volume (R2 = .55) explained a significant portion of the variation in functional pulling strength at baseline (Models 1a and 1b, Table 5). However, when both shoulder-adductor and elbow-flexor volumes were included in the model together, elbow-flexor volume was no longer a significant predictor of functional pulling strength, whereas shoulder-adductor volume remained a significant predictor (Model 2, Table 5). When sex and body mass were included in this model, they did not add significantly to the percentage variability explained (Models 3 and 4, Table 5). The total variability in functional pulling strength explained by including both shoulder-adductor and elbow-flexor volume was 72% (Model 2, Table 5), indicating that most of the variability in functional pulling strength can be explained by shoulder-adductor volume alone.
Table 5. Shoulder and Elbow Functional Group Volumes as Predictors of Reaching and Pulling Strength, Before Randomization.
| Pulling | Reaching | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Elbow flexors | Shoulder add | Sex | Body mass | Elbow extensors | Shoulder abd | Sex | Body mass | ||
| Models la, lb | |||||||||
| R2 | .55 | .70 | .26 | .40 | |||||
| beta | 0.24 | 0.13 | 0.03 | 0.03 | |||||
| ta | 3.68 | 5.08 | 2.22 | 3.07 | |||||
| P | .0036 | .0004 | .043 | .0083 | |||||
| Model 2 | F(2, 10) = 12.77, p = .002, R2 = .72 | F(2, 13) = 5.04, p = .024, R2 = .44 | |||||||
| beta | −0.13 | 0.19 | −0.03 | 0.05 | |||||
| tb | −0.79 | 2.43 | −0.89 | 2.02 | |||||
| P | .45 | .035 | .39 | .065 | |||||
| Model 3 | F(3, 9) = 8.81, p = .005, R2 = .75 | F(3, 12) = 3.15, p = .065, R2 = .44 | |||||||
| beta | −0.24 | 0.16 | 36.79 | −0.03 | 0.05 | 1.65 | |||
| tc | −1.21 | 1.99 | 0.98 | −0.90 | 1.60 | 0.28 | |||
| P | .26 | .078 | .35 | .38 | .13 | .78 | |||
| Model 4 | F(4, 8) = 5.97, p = .016, R2 = .75 | F(4, 11) = 2.17, p = .140, R2 = .44 | |||||||
| beta | −0.26 | 0.15 | 43.23 | 0.11 | −0.03 | 0.05 | 1.72 | 0.003 | |
| td | −1.19 | 1.55 | 0.98 | 0.32 | −0.86 | 1.32 | 0.27 | 0.38 | |
| P | .27 | .16 | .35 | .76 | .41 | .21 | .79 | .97 | |
Note. add = adductors; abd = abductors.
t(11) for pulling and t(14) for reaching.
t((10) for pulling and t(13) for reaching.
t(9) for pulling and t(12) for reaching.
t(8) for pulling and t(11) for reaching.
Separately, without adjustment for sex, maximum isometric shoulder-adduction moment (R2 = .73) and maximum isometric elbow-flexion moment (R2 = .42) explained a significant portion of the variation in functional pulling strength at baseline (Models 1a and 1b, Table 6). However, when both shoulder-adduction and elbow-flexion moment were included in the model together, elbow-flexion moment was no longer a significant predictor of functional pulling strength, whereas maximum isometric shoulder adduction remained a significant predictor (Model 2, Table 6). The inclusion of sex in this model did not add significantly to the percentage variability explained (Model 3, Table 6). The total variability in functional pulling strength explained by including both maximum isometric shoulder-adduction and elbow-flexion moments was 73% (Model 2, Table 6), indicating that elbow-flexion moment does not add significantly to the variability explained by shoulder-adduction moment alone.
Table 6. Maximum Isometric Elbow and Shoulder Joint Moment as Predictors of Functional Pulling and Reaching Strength, Before Randomization.
| Pulling | Reaching | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Elbow flexion | Shoulder adduction | Sex | Elbow extension | Shoulder abduction | Sex | |
| Models 1a, 1b | ||||||
| R2 | .42 | .73 | .46 | .49 | ||
| beta | 0.98 | 1.49 | 0.23 | 0.38 | ||
| ta | 2.84 | 5.50 | 0.46 | 0.49 | ||
| p | .016 | .0002 | .0041 | .0024 | ||
| Model 2 | F(2, 10) = 13.75, p = .001, R2 = .73 | F(2, 13) = 9.33, p = .003, R2 = .59 | ||||
| beta | 0.026 | 1.47 | 0.13 | 0.25 | ||
| tb | 0.07 | 3.41 | 1.74 | 2.05 | ||
| p | .95 | .0067 | .11 | .061 | ||
| Model 3 | F(3, 9) = 11.52, p = .002, R2 = .79 | F(3, 12) = 5.78, p = .011, R2 = .59 | ||||
| beta | 0.04 | 0.99 | 25.59 | 0.13 | 0.24 | 0.61 |
| tc | 0.12 | 1.99 | 1.62 | 1.47 | 1.71 | 0.21 |
| p | .91 | .078 | .14 | .17 | .11 | .83 |
t(11) for pulling and t(14) for reaching.
t(10) for pulling and t(13) for reaching.
t(9) for pulling and t(12) for reaching.
Predictors of Functional Reaching Strength
In the analyses of functional reaching strength, elbow-extensor volume (R2 = .26) and shoulder-abductor volume (R2 = .40) explained a significant portion of the variation in this variable when considered separately (Models 1a and 1b, Table 5). However, neither shoulder-abductor volume nor elbow-extensor volume remained a significant predictor of functional reaching strength when both volume measures (p = .39 for elbow extensors, p = .065 for shoulder abductors) were included in the model, not controlling for sex (Model 2, Table 5). The total variability in functional reaching strength explained by including both shoulder-abductor and elbow-extensor volume was 44% (Model 2, Table 5), only a 4% increase over shoulder abduction alone. Adjusting for sex and body mass did not add significantly to the percentage variability explained (Models 3 and 4, Table 5).
In the analyses of functional reaching strength, maximum isometric elbow-extension moment (R2 = .46) and maximum isometric shoulder-abduction moment (R2 = .49) explained a significant portion of the variation in this variable when considered separately (Models 1a and 1b, Table 6). Neither shoulder-abduction moment nor elbow-extension moment remained a significant predictor of functional reaching strength when both maximum isometric joint moments (p = .11 for elbow extension, p = .06 for shoulder abduction) were included in the model, not controlling for sex (Model 2, Table 6). The total variability in functional reaching strength explained by including both maximum isometric shoulder-abduction and elbow-extension moments was 59% (Model 2, Table 6), indicating that elbow-extension moment explained an additional 10% of variability beyond shoulder abduction alone, although this additional explained variability was not statistically significant in this small sample. Adjusting for sex did not add significantly to the percentage variability explained (Model 3, Table 6).
Discussion
In the RT group, increases in upper extremity strength measured using dynamic 1RM strength were achieved after 6 weeks of RT. The muscle volumes for all functional groups tended to increase more in the RT group than in the control group. However, only the changes observed in elbow-flexor and shoulder-adductor muscle volumes were significantly greater for the RT group than the changes observed for the control group. The RT group also had significantly greater increases in functional pulling strength and maximum isometric shoulder-adduction moment compared with the control group. In addition, we found that in this group of healthy older adults, the volume and maximum isometric joint moments of muscles actuating the shoulder accounted for more of the variability in functional reaching and pulling strength than the volume and maximum isometric joint moments of muscles actuating the elbow. These results clearly demonstrate the effectiveness of the RT protocol for increasing muscle strength, as measured by increases in 1RM, and suggest that the shoulder joint plays an important role in functional strength.
The RT group increased dynamic 1RM strength by 21–49% over the course of the intervention, comparable to increases seen in previous studies in which interventions lasting from 12 to 24 weeks with older adult subjects led to increases in upper extremity 1RM ranging from 16% to 48% (Brown et al., 1990; Coke, Staffileno, Braun, & Gulanick, 2008; Vincent et al., 2002). Strength increases of this magnitude have been associated with increases in functional ability in an impaired older adult population (Venturelli, Lanza, Muti, & Schena, 2010). Venturelli et al. reported that after a 6-month upper body RT intervention, older adults increased biceps-curl 1RM by an average of 26%, and, in the same cohort of older adults, functional ability, as measured by the Barthel ADL Index, increased by an average of 78%. However, the Barthel Index, which scores functional ability from zero to 100 based on the capacity to perform 10 ADLs, includes lower extremity function, as well as upper extremity function.
While increases in dynamic 1RM strength for the RT exercises can be used to demonstrate the effectiveness of an RT program, a criticism of this approach is that it is simply “training for the test.” Thus, other strength measures such as maximal isometric joint moment have been used to assess more general increases in strength after an RT intervention (Brown et al., 1990; Hunter et al., 2001; Vincent et al., 2002). Only maximum isometric shoulder-adduction moment had a significantly greater change for the RT than for the control group. Similar lack of improvement in isometric joint moment has been previously reported (Brown et al., 1990) and may be due to the level of activation during training (Ingebrigtsen et al., 2009). Tests of isometric moment-generating capacity and functional ability may place different demands on the neuromuscular system compared with the 1RM exercises due to the range of motion involved, level of muscle activation required, posture of the joints, and lack of a velocity component for the isometric joint-moment testing (Ingebrigtsen et al., 2009). A previous study reported significant changes in maximum isometric joint moment after training with a high load and explosive intention (higher initial muscle activation) but no changes in maximum isometric joint moment after training with a high load and a slow contraction velocity (lower initial muscle activation; Ingebrigtsen et al., 2009). The latter training paradigm was used in this RT intervention because of its efficacy in increasing dynamic 1RM strength.
This phenomenon of activation-level-dependent gains is similar to that of task-specific training, which has been reported to be the most effective method of increasing strength for a movement of interest, in accordance with the principle of specificity of training (de Vreede et al., 2004). Several groups have provided evidence that RT more effectively increases functional ability, in both impaired and healthy individuals, when it is combined with practicing functional movements (Manini et al., 2007; Patten, Dozono, Schmidt, Jue, & Lum, 2006; Thielman, Dean, & Gentile, 2004). The RT program included a training exercise that was very similar to the functional pulling test (the compound row) but no exercise that was similar to the functional reaching task. We observed a significant difference in the change in functional pulling strength between the RT group and the control group but no significant difference between the groups for the changes in functional reaching strength.
The lack of change in muscle volume may in part explain the lack of improvement in functional reaching strength and maximum isometric joint moment at the elbow and wrist. In contrast, the significant increase in shoulder-adductor volume is consistent with the significant increase in maximum isometric shoulder-adduction moment and functional pulling strength in the RT group. The increases in muscle volume and corresponding increases in 1RM that we observed are similar to those reported by Popadic Gacesa et al. (2009), who observed a 5% increase in elbow-extensor volume and 15% increase in maximum isometric elbow-extension moment after a 6-week RT intervention. In the RT training group, we observed a 4% increase in elbow-flexor and -extensor volume and a 3% increase in shoulder-adductor volume, which corresponded to increases in 1RM by 27% for the biceps curl, 21% for the triceps press, and 26% for the compound row. The disparity between increases in the functional-group muscle volumes and their associated 1RMs suggests that much of the improvement seen in 1RM was due to factors other than muscle hypertrophy, such as the training-specific improvements discussed herein. The increases in strength that occur before hypertrophy are often attributed to neuromuscular adaptation or changes in coordination associated with task-specific training (Aagaard, Suetta, Caserotti, Magnusson, & Kjaer, 2010; Abe, DeHoyos, Pollock, & Garzarella, 2000; Patten et al., 2006). A recent review by Narici and de Boer (2011) also suggests a disparity among muscle groups in their response to RT based on findings by Tesch and colleagues (Alkner & Tesch, 2004; Tesch, Trieschmann, & Ekberg, 2004) and Trappe et al. (2004), which indicate that the soleus muscle has a decreased response to exercise in terms of protein synthesis and hypertrophy in comparison with the quadriceps muscle. Some reports suggest that it takes 4–8 weeks to induce significant increases in the muscle thickness of some upper extremity functional groups in a healthy adult population (Abe et al., 2000); however, to the best of our knowledge, no study has characterized the time course for changes in upper extremity muscle volume or thickness in an older population. Our results are consistent with the study in younger adults, with significant increases in the muscle volume of some functional groups (shoulder adductors and elbow flexors) occurring after 6 weeks of RT but other functional groups showing nonsignificant increases after this time period.
Randomization to RT was associated with significantly greater increases in shoulder-adductor muscle volumes with respect to the control group, and this functional group of muscles is involved in producing the maximum isometric shoulder-adduction moment and functional pulling strength. Isometric shoulder moment and functional pulling strength are also the only two measures of strength where the RT group showed significant improvement compared with the control group. It remains to be determined if a longer RT intervention would lead to greater gains in hypertrophy, resulting in greater gains in functional strength, and transfer to measures of strength not practiced during the intervention. We also observed a significant interaction between sex and RT intervention for wrist-extensor and elbow-flexor volume and for elbow-extension maximum isometric joint moment. This is an area that deserves further study with larger, more diverse, populations, since we had only 8 men and 8 women in this cohort.
We found that both maximum isometric moment and muscle volume at the shoulder explained more of the variability in functional strength than either maximum isometric moment or muscle volume at the elbow. This suggests that the volume and strength of functional groups actuating the shoulder may be more important than the elbow musculature in the performance of functional reaching and pulling tasks. Our data are consistent with previous electromyographic and kinematic analyses of reaching that suggest that the shoulder is responsible for movement generation and the acceleration and deceleration of the shoulder and elbow, while the elbow stabilizes endpoint trajectory (Buchanan, Kelso, & de Guzman, 1997; Dounskaia, 2005; Galloway & Koshland, 2002; Levin, Forner-Cordero, Li, Ouamer, & Swinnen, 2008).
To develop an optimal upper extremity RT protocol for increasing functional strength and ability for a variety of tasks, it is important to identify both the type of exercises to include (activation level, task-specific), and the key muscle groups to target. Our preliminary results indicate that maximum isometric shoulder moments explained more of the variation in functional reaching and pulling strength than maximum isometric elbow moments. This suggests that shoulder strength may be an important factor for performing reaching and pulling tasks. Therefore, future RT programs aimed at increasing functional ability for these tasks may choose to focus on muscles that cross the shoulder joint. For example, in the current study the forward reaching task took place in the sagittal plane, while the RT exercises targeting shoulder-abductor muscles were performed in the frontal plane. It is possible that augmenting the RT program to include additional movements in the sagittal plane that target muscles contributing to shoulder-flexion strength would lead to greater improvements in functional reaching strength. Similarly, we did not include exercises for the rotator-cuff muscles, which may have limited the improvements we observed in the reaching task because these muscles function to stabilize the glenohumeral joint.
This study had several limitations. We did not attempt to control diet, medication, or supplements, which may be important factors to consider in future studies. In addition, it is well documented that the aging process includes the infiltration of fat and connective tissue into muscle fibers (Ryall, Schertzer, & Lynch, 2008). The presence of this noncontractile tissue within the volume of a muscle does not contribute to the moment-generating capabilities. We did not include a scan designed to highlight intramuscular fat in addition to the spoiled gradient sequences used, and therefore we could not quantify the fat content of the muscles. Previous studies evaluating the percentage of intramuscular fat in the calf muscles of older adults reported between 2.3% and 10.7% depending on the muscle evaluated and genetic makeup of the sample (Miljkovic et al., 2009; Schwenzer et al., 2009). Thus, intramuscular fat could be substantially increasing the muscle volumes we measured. Because our study sample was older adults, the amount of time spent in the MRI was an important consideration, and adding a fat-suppression sequence would have dramatically increased the acquisition time. The inclusion of a fat scan may lead to more accurate measurements of muscle gain after RT and therefore more statistically significant gains in muscle volume in the event that RT group participants lose fat in addition to gaining muscle.
We chose to assess functional strength instead of functional ability. Many groups use clinical tests such as the Barthel ADL Index to measure functional ability. These tests are often aimed at impaired populations, use measurements that capture the ability to perform a task or the time it takes to complete a functional task, and may include both upper and lower extremity function. These tests can have a ceiling effect when used to evaluate a healthy population, where no or only very small improvements in test score are possible. By using functional strength as an outcome, we are able to assess improvement regardless of ability. We chose not to normalize the improvements in 1RM strength by body mass. Studies in the lower extremity often include this type of normalization because lower extremity strength and body weight are related measures. However, body weight is not strongly related to strength in the upper extremity (Beliaeff, Bouchard, Hautier, Brochu, & Dionne, 2008; Holzbaur, Murray et al., 2007).
The RT protocol was designed to minimize muscle-group fatigue and decrease the risk of injury due to the high loads seen at the shoulder when performing ADLs (Westerhoff et al., 2009) and the high incidence of rotator-cuff tears in this population (Kim et al., 2009). There are other approaches that are used to determine the order of exercises performed, such as moving from smaller to larger muscle groups or single-joint to multijoint exercises, that may have led to different outcomes. Similarly, there are other approaches to increasing the resistance used that are more aggressive (use higher percentages of 1RM or more repetitions) and may lead to greater increases in 1RM over the course of the intervention. We chose to be more conservative in our approach to increasing resistance to minimize the risk of injury. However, our RT protocol still allowed the participants to increase the resistance more rapidly if appropriate. To determine the participants who needed to increase the resistance of an exercise after 2 weeks of the intervention (6 sessions), individuals performed as many repetitions as possible in the third set. If participants exceeded 12 repetitions, the resistance for that exercise was increased at the next session to ensure that they were progressively overloading the muscles. Clearly, the 1RM increased as individuals gained strength between 1RM testing sessions, leading to an increase in the number of repetitions possible in the third set.
We relied on self-reported assessment of injuries. There may be a large number of asymptomatic rotator-cuff tears in an older adult population (Kim et al., 2009). Maximal isometric shoulder-abduction moment and functional reaching strength highly depend on the muscles in the rotator cuff. Unknown injury to these muscles, if present, could have contributed to the decreases in maximal isometric shoulder-abduction moment over the course of the study in both groups. While the current study focused on a small sample of healthy, unimpaired older adults, future efforts will explore the role of RT in improving strength in those with impairments that frequently affect this population, including rotator-cuff disease and impairment due to stroke.
In addition to decreases in maximal isometric shoulder-abduction moment over the course of the study in both groups, we observed decreases in muscle volume for most functional groups in the control group (average 2% decrease). These participants reported that they were not engaged in any exercise program during the course of the study. A recent study showed that 2 weeks of immobilization led to a 5% decline in quadriceps muscle volume in a cohort of older adults (Suetta et al., 2009). If the participants were engaged in limited upper limb activity or were avoiding use of the shoulder due to asymptomatic rotator-cuff injuries, this amount of muscle-volume loss may not be unusual. These data suggest that the time course of changes in muscle volume in older adults not engaged in exercise deserves further study in a larger group of participants.
Finally, this study was a preliminary study performed in a small group of healthy older adults. Due to the small sample size, all statistical analyses should be considered exploratory in nature. We believe that due to the relatively small p values for many of the analyses, particularly the increases in 1RM strength, many of the relationships will remain true for a larger population. In the future, we aim to perform work to further establish the importance of the shoulder in performing these and other functional tasks.
In conclusion, 6 weeks of RT produced significant gains in 1RM strength, functional pulling strength, maximum isometric shoulder-adduction moment, elbow-flexor volume, and shoulder-adductor volume in a cohort of healthy older adults. A longer RT intervention may clarify whether significant gains in hypertrophy for other functional groups would lead to more general gains in strength than those possible due to neuromuscular adaptation. Our results indicate that shoulder strength, as measured here by shoulder-muscle volume and maximum isometric joint moment, is a potential limiting factor for reaching and pulling ability. We believe that these data provide a basis for development of targeted RT interventions to more effectively increase functional ability in older adults.
Acknowledgments
We acknowledge Eric C. Haakonssen for participant training. In addition, we acknowledge funding support from the Wake Forest University Cross Campus Collaborative Research Fund and Science Research Fund. The work of Michael Miller, Anthony Marsh, and Katherine Saul was partially supported by the WFU Claude Pepper Older Americans Independence Center, National Institutes for Aging P30 AG021332. This work was also supported in part by the Center for Biomolecular Imaging of Wake Forest School of Medicine
Contributor Information
Melissa Daly, Athletic Dept., Williams College, Williamstown, MA.
Meghan E. Vidt, Virginia Tech–Wake Forest School of Biomedical Engineering and Sciences, Winston-Salem, NC
Joel D. Eggebeen, Dept. of Health and Exercise Science, Wake Forest University, Winston-Salem, NC
W. Greg Simpson, Dept. of Health and Exercise Science, Wake Forest University, Winston-Salem, NC.
Michael E. Miller, Dept. of Health and Exercise Science, Wake Forest University, Winston-Salem, NC
Anthony P. Marsh, Dept. of Health and Exercise Science, Wake Forest University, Winston-Salem, NC
Katherine R. Saul, Virginia Tech–Wake Forest School of Biomedical Engineering and Sciences, Winston-Salem, NC
References
- Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: Strength training as a countermeasure. Scandinavian Journal of Medicine & Science in Sports. 2010;20(1):49–64. doi: 10.1111/j.1600-0838.2009.01084.x. PubMed. [DOI] [PubMed] [Google Scholar]
- Abe T, DeHoyos DV, Pollock ML, Garzarella L. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. European Journal of Applied Physiology. 2000;81(3):174–180. doi: 10.1007/s004210050027. PubMed. [DOI] [PubMed] [Google Scholar]
- Abizanda P, Navarro JL, Romero L, Leon M, Sanchez-Jurado PM, Dominguez L. Upper extremity function, an independent predictor of adverse events in hospitalized elderly. Gerontology. 2007;53(5):267–273. doi: 10.1159/000102541. PubMed. [DOI] [PubMed] [Google Scholar]
- Alkner BA, Tesch PA. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. European Journal of Applied Physiology. 2004;93(3):294–305. doi: 10.1007/s00421-004-1172-8. PubMed. [DOI] [PubMed] [Google Scholar]
- Beliaeff S, Bouchard DR, Hautier C, Brochu M, Dionne IJ. Association between muscle mass and isometric muscle strength in well-functioning older men and women. Journal of Aging and Physical Activity. 2008;16(4):484–493. doi: 10.1123/japa.16.4.484. PubMed. [DOI] [PubMed] [Google Scholar]
- Brown AB, McCartney N, Sale DG. Positive adaptations to weight-lifting training in the elderly. Journal of Applied Physiology. 1990;69(5):1725–1733. doi: 10.1152/jappl.1990.69.5.1725. PubMed. [DOI] [PubMed] [Google Scholar]
- Buchanan JJ, Kelso JA, de Guzman GC. Self-organization of trajectory formation. I. Experimental evidence. Biological Cybernetics. 1997;76(4):257–273. doi: 10.1007/s004220050338. PubMed. [DOI] [PubMed] [Google Scholar]
- Coke LA, Staffileno BA, Braun LT, Gulanick M. Upper-body progressive resistance training improves strength and household physical activity performance in women attending cardiac rehabilitation. Journal of Cardiopulmonary Rehabilitation and Prevention. 2008;28(4):238–245. doi: 10.1097/01.HCR.0000327180.29122.83. quiz 246–237. [DOI] [PubMed] [Google Scholar]
- de Vreede PL, Samson MM, van Meeteren NL, van der Bom JG, Duursma SA, Verhaar HJ. Functional tasks exercise versus resistance exercise to improve daily function in older women: A feasibility study. Archives of Physical Medicine and Rehabilitation. 2004;85(12):1952–1961. doi: 10.1016/j.apmr.2004.05.006. PubMed. [DOI] [PubMed] [Google Scholar]
- Dounskaia N. The internal model and the leading joint hypothesis: Implications for control of multi-joint movements. Experimental Brain Research. 2005;166(1):1–16. doi: 10.1007/s00221-005-2339-1. PubMed. [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. Journal of the American Medical Association. 1990;263(22):3029–3034. doi: 10.1001/jama.1990.03440220053029. PubMed. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice G. Clustered data. Nutrition (Burbank, Los Angeles County, Calif) 2001;17(6):487–488. doi: 10.1016/S0899-9007(01)00539-1. PubMed. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. Journal of Applied Physiology. 1991;71(2):644–650. doi: 10.1152/jappl.1991.71.2.644. PubMed. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: Skeletal muscle hypertrophy and improved function. Journal of Applied Physiology. 1988;64(3):1038–1044. doi: 10.1152/jappl.1988.64.3.1038. PubMed. [DOI] [PubMed] [Google Scholar]
- Fuller-Thomson E, Yu B, Nuru-Jeter A, Guralnik JM, Minkler M. Basic ADL disability and functional limitation rates among older AMERICANS from 2000-2005: The end of the decline? The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2009;64(12):1333–1336. doi: 10.1093/gerona/glp130. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway JC, Koshland GF. General coordination of shoulder, elbow and wrist dynamics during multijoint arm movements. Experimental Brain Research. 2002;142(2):163–180. doi: 10.1007/s002210100882. PubMed. [DOI] [PubMed] [Google Scholar]
- Hart D. Test–retest reliability of the static push–pull tests for functional-capacity evaluations. Physical Therapy. 1988;68(5):824. [Google Scholar]
- Holzbaur KR, Delp SL, Gold GE, Murray WM. Moment-generating capacity of upper limb muscles in healthy adults. Journal of Biomechanics. 2007;40(11):2442–2449. doi: 10.1016/j.jbiomech.2006.11.013. PubMed. [DOI] [PubMed] [Google Scholar]
- Holzbaur KR, Murray WM, Gold GE, Delp SL. Upper limb muscle volumes in adult subjects. Journal of Biomechanics. 2007;40(4):742–749. doi: 10.1016/j.jbiomech.2006.11.011. PubMed. [DOI] [PubMed] [Google Scholar]
- Hunter GR, Wetzstein CJ, McLafferty CL, Jr, Zuckerman PA, Landers KA, Bamman MM. High-resistance versus variable-resistance training in older adults. Medicine and Science in Sports and Exercise. 2001;33(10):1759–1764. doi: 10.1097/00005768-200110000-00022. PubMed. [DOI] [PubMed] [Google Scholar]
- Ingebrigtsen J, Holtermann A, Roeleveld K. Effects of load and contraction velocity during three-week biceps curls training on isometric and isokinetic performance. Journal of Strength and Conditioning Research. 2009;23(6):1670–1676. doi: 10.1519/JSC.0b013e3181b3f37b. PubMed. [DOI] [PubMed] [Google Scholar]
- Kim HM, Teefey SA, Zelig A, Galatz LM, Keener JD, Yamaguchi K. Shoulder strength in asymptomatic individuals with intact compared with torn rotator cuffs. Journal of Bone and Joint Surgery American Volume. 2009;91(2):289–296. doi: 10.2106/JBJS.H.00219. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin O, Forner-Cordero A, Li Y, Ouamer M, Swinnen SP. Evidence for adaptive shoulder-elbow control in cyclical movements with different amplitudes, frequencies, and orientations. Journal of Motor Behavior. 2008;40(6):499–515. doi: 10.3200/JMBR.40.6.499-515. PubMed. [DOI] [PubMed] [Google Scholar]
- Lundgren-Lindquist B, Sperling L. Functional studies in 79-year-olds. II. Upper extremity function. Scandinavian Journal of Rehabilitation Medicine. 1983;15(3):117–123. PubMed. [PubMed] [Google Scholar]
- Manini T, Marko M, VanArnam T, Cook S, Fernhall B, Burke J, Ploutz-Snyder L. Efficacy of resistance and task-specific exercise in older adults who modify tasks of everyday life. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2007;62(6):616–623. doi: 10.1093/gerona/62.6.616. PubMed. [DOI] [PubMed] [Google Scholar]
- Miljkovic I, Cauley JA, Petit MA, Ensrud KE, Strotmeyer E, Sheu Y, Zmuda JM. Greater adipose tissue infiltration in skeletal muscle among older men of African ancestry. The Journal of Clinical Endocrinology and Metabolism. 2009;94(8):2735–2742. doi: 10.1210/jc.2008-2541. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miszko TA, Cress ME, Slade JM, Covey CJ, Agrawal SK, Doerr CE. Effect of strength and power training on physical function in community-dwelling older adults. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2003;58(2):171–175. doi: 10.1093/gerona/58.2.M171. PubMed. [DOI] [PubMed] [Google Scholar]
- Narici MV, de Boer MD. Disuse of the musculo-skeletal system in space and on earth. European Journal of Applied Physiology. 2011;111(3):403–420. doi: 10.1007/s00421-010-1556-x. PubMed. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Layne JE, Bernstein MJ, Nuernberger A, Castaneda C, Kaliton D, Fiatarone MA. The effects of multidimensional home-based exercise on functional performance in elderly people. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2004;59(2):154–160. doi: 10.1093/gerona/59.2.M154. PubMed. [DOI] [PubMed] [Google Scholar]
- Patten C, Dozono J, Schmidt S, Jue M, Lum P. Combined functional task practice and dynamic high intensity resistance training promotes recovery of upper-extremity motor function in post-stroke hemiparesis: A case study. Journal of Neurologic Physical Therapy; JNPT. 2006;30(3):99–115. doi: 10.1097/01.npt.0000281945.55816.e1. PubMed. [DOI] [PubMed] [Google Scholar]
- Phillips WT, Batterham AM, Valenzuela JE, Burkett LN. Reliability of maximal strength testing in older adults. Archives of Physical Medicine and Rehabilitation. 2004;85(2):329–334. doi: 10.1016/j.apmr.2003.05.010. PubMed. [DOI] [PubMed] [Google Scholar]
- Popadic Gacesa JZ, Kozic DB, Dragnic NR, Jakovljevic DG, Brodie DA, Grujic NG. Changes of functional status and volume of triceps brachii measured by magnetic resonance imaging after maximal resistance training. Journal of Magnetic Resonance Imaging. 2009;29(3):671–676. doi: 10.1002/jmri.21690. PubMed. [DOI] [PubMed] [Google Scholar]
- Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9(4):213–228. doi: 10.1007/s10522-008-9131-0. PubMed. [DOI] [PubMed] [Google Scholar]
- Schroeder ET, Wang Y, Castaneda-Sceppa C, Cloutier G, Vallejo AF, Kawakubo M, Sattler FR. Reliability of maximal voluntary muscle strength and power testing in older men. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2007;62(5):543–549. doi: 10.1093/gerona/62.5.543. PubMed. [DOI] [PubMed] [Google Scholar]
- Schwenzer NF, Martirosian P, Machann J, Schraml C, Steidle G, Claussen CD, Schick F. Aging effects on human calf muscle properties assessed by MRI at 3 Tesla. Journal of Magnetic Resonance Imaging. 2009;29(6):1346–1354. doi: 10.1002/jmri.21789. PubMed. [DOI] [PubMed] [Google Scholar]
- Seidel D, Crilly N, Matthews FE, Jagger C, Clarkson PJ, Brayne C. Patterns of functional loss among older people: A prospective analysis. Human Factors: The Journal of the Human Factors and Ergonomics Society. 2009;51:669–680. doi: 10.1177/0018720809353597. [DOI] [PubMed] [Google Scholar]
- Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. Journal of Applied Physiology. 2009;107(4):1172–1180. doi: 10.1152/japplphysiol.00290.2009. PubMed. [DOI] [PubMed] [Google Scholar]
- Tesch PA, Trieschmann JT, Ekberg A. Hypertrophy of chronically unloaded muscle subjected to resistance exercise. Journal of Applied Physiology. 2004;96(4):1451–1458. doi: 10.1152/japplphysiol.01051.2003. PubMed. [DOI] [PubMed] [Google Scholar]
- Theou O, Gareth JR, Brown LE. Effect of rest interval on strength recovery in young and old women. Journal of Strength and Conditioning Research. 2008;22(6):1876–1881. doi: 10.1519/JSC.0b013e3181821928. PubMed. [DOI] [PubMed] [Google Scholar]
- Thielman GT, Dean CM, Gentile AM. Rehabilitation of reaching after stroke: Task-related training versus progressive resistive exercise. Archives of Physical Medicine and Rehabilitation. 2004;85(10):1613–1618. doi: 10.1016/j.apmr.2004.01.028. PubMed. [DOI] [PubMed] [Google Scholar]
- Thompson DD. Aging and sarcopenia. Journal of Musculoskeletal & Neuronal Interactions. 2007;7(4):344–345. PubMed. [PubMed] [Google Scholar]
- Tingart MJ, Apreleva M, Lehtinen JT, Capell B, Palmer WE, Warner JJ. Magnetic resonance imaging in quantitative analysis of rotator cuff muscle volume. Clinical Orthopaedics and Related Research. 2003;415:104–110. doi: 10.1097/01.blo.0000092969.12414.e1. PubMed. [DOI] [PubMed] [Google Scholar]
- Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. The Journal of Physiology. 2004;557(Pt 2):501–513. doi: 10.1113/jphysiol.2004.062166. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturelli M, Lanza M, Muti E, Schena F. Positive effects of physical training in activity of daily living–dependent older adults. Experimental Aging Research. 2010;36(2):190–205. doi: 10.1080/03610731003613771. PubMed. [DOI] [PubMed] [Google Scholar]
- Vidt ME, Daly M, Miller ME, Davis CC, Marsh AP, Saul KR. Characterizing upper limb muscle volume and strength in older adults: A comparison with young adults. Journal of Biomechanics. 2012;45(2):334–341. doi: 10.1016/j.jbiomech.2011.10.007. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent KR, Braith RW, Feldman RA, Magyari PM, Cutler RB, Persin SA, Lowenthal DT. Resistance exercise and physical performance in adults aged 60 to 83. Journal of the American Geriatrics Society. 2002;50(6):1100–1107. doi: 10.1046/j.1532-5415.2002.50267.x. PubMed. [DOI] [PubMed] [Google Scholar]
- Wagner JM, Rhodes JA, Patten C. Reproducibility and minimal detectable change of three-dimensional kinematic analysis of reaching tasks in people with hemiparesis after stroke. Physical Therapy. 2008;88(5):652–663. doi: 10.2522/ptj.20070255. PubMed. [DOI] [PubMed] [Google Scholar]
- Westerhoff P, Graichen F, Bender A, Halder A, Beier A, Rohlmann A, Bergmann G. In vivo measurement of shoulder joint loads during activities of daily living. Journal of Biomechanics. 2009;42(12):1840–1849. doi: 10.1016/j.jbiomech.2009.05.035. PubMed. [DOI] [PubMed] [Google Scholar]


