Abstract
Adherent cells interact with extracellular matrix via cell-substrate contacts at focal adhesions. The dynamic assembly and disassembly of focal adhesions enables cell attachment, migration and growth. While the influence of mechanical forces on the formation and growth of focal adhesions has been widely observed, the force loading on specific proteins at focal adhesion complex is not clear. By co-expressing force sensitive α-actinin FRET probes and fluorescence labeled paxillin in MDCK cells, we have simultaneously observed the time-dependent changes in tension in α-actinin and the dynamics of focal adhesion during cell migration. We show that increase in tension in α-actinin at the focal adhesion coincides with elongation of the adhesion in its growth phase. The enlargement of focal adhesion is through a force sensitive recruitment of α-actinin and paxillin to the adhesion sites. Changes in α-actinin tension and correlated relocation of α-actinin in an active adhesion also guide the growth direction of the adhesion. The results support the model that cytoskeletal tension is coupled to focal adhesion via the linking protein, α-actinin at the adhesion complex. Lysophosphatidic Acid caused an immediate increase in α-actinin tension followed by drastic focal adhesion formation and elongation. Application of Rho-ROCK inhibitor, Y27632, resulted in reversible reduction in tension in α-actinin and disassociation of focal adhesion, suggesting the involvement of myosin-II mediated contractile force in the focal adhesion dynamics. These findings suggest that α-actinin not only serves as a physical linker between cytoskeleton and integrin, but also participates in force transmission at adhesion sites to facilitate adhesion’s growth.
Keywords: Mechanical force, cytoskeleton, α-actinin, FRET, focal adhesions
Introduction
Adherent cells interact with extracellular matrix (ECM) through focal adhesions (FAs), complex protein structures that link actin cytoskeleton to integrins. Through the cell-ECM interactions, cells exert forces onto the substrate that regulates ECM arrangement and adhesion structure; the resisting force of the substrate causes conformational changes of adhesion proteins that modify composition and morphology of adhesion assembly, and therefore, regulates cell migration and growth [1]. Many studies have shown that the integrin-cytoskeleton linkage is mechanosensitive and can both transmit and respond to mechanical forces between cytoskeleton and integrins [2–6]. However, how the forces are coupled to specific proteins in FA complex is unclear, because multiple cross-linking proteins participate in the mechanical linkage between actin cytoskeleton and integrin at the adhesion complex suggesting a complicated force map. In addition, changes of tension in linking proteins modulate their binding probability to other FA proteins and concurrently modify cytoskeleton structure, which further increases the complexity of force transmission at FAs.
The force-induced FA growth has been reported in various cell types [7–9]. Myosin-II mediated contractility is thought to be the major source of the internal force that influences the FA growth in resting cells [9–12]. Activation of Myosin-II driven contractility is via Rho target known as Rho-associated kinase (ROCK) [10, 11]. Tensional force from myosin II contraction can be transmitted to FAs through the actin cytoskeleton [3, 12]. Alternatively, external force can be applied to FAs by using pipette or ligand-coated beads bound to the cell surface, that causes the recruitment of FA proteins to adhesion sites [13, 14].
Several cross-linking proteins in FA complex, including vinculin and talin, have shown tension-dependent conformational changes that affect their binding strength and adhesion development [3, 12, 15]. Stretching talin using magnetic tweezers increased its binding probability with vinculin, resulting in force dependent vinculin recruitment to FAs [15]. Application of mechanical force using fibronectin coated pipette or microbeads induced the recruitment and rearrangement of vinculin [13, 16]. It has been further shown that adhesion growth requires both force transmission by vinculin and vinculin recruitment, and that these two parameters are separately regulated [3]. Alpha-actinin is another important cross-linking protein in FA complex due to its role in binding actin cytoskeleton to the adhesions and binding the actin filaments. The recruitment of α-actinin has been observed at the early phase of adhesion formation, and α-actinin dynamics regulates FA growth via its interaction with other adhesion proteins, such as Zyxin [12, 17–19]. In tracheal smooth muscle tissue, incorporation of α-actinin into integrin complex was found to be necessary for tension development [20]. In non-muscle cells, depletion of α-actinin inhibits cell’s sensation and adaptation to mechanical force from ECM, preventing adhesion maturation [21, 22]. These observations suggest that cytoskeletal linking proteins not only serve as mechanical linkers between actin filaments and integrin, but also participate in force transmission at adhesion sites to facilitate adhesion’s growth.
Here, we simultaneously measured time-dependent changes in tension in α-actinin and the dynamics of FAs by co-expressing force sensitive FRET probes and fluorescence-tagged paxillin, thereby, establishing a direct link between tension in α-actinin and FA dynamics in live cells. We show that an increase in tension in α-actinin at the FAs coincides with the elongation of FAs or change in direction of the adhesion movement. The observed cytoskeletal tension is myosin-II dependent.
Materials and methods
Actinin-sstFRET construction
sstFRET DNA plasmid was constructed as previously described [23, 24]. We genetically connected FRET acceptor Venus and donor Cerulean with a spectrin repeat domain as linker, named it sstFRET (spectrin repeat stretch sensitive FRET sensor). To create actinin-sstFRET chimeric constructs, we subcloned α-actinin into pEYFP-C1 vector (Clontech, Mountain View, CA, USA) by replacing the original YFP gene, then inserted sstFRET between first and second spectrin repeat domains in α-actinin, amino acid position 300. The linker length is chosen to be equal to the Forster distance of this particular FRET pair, at which there is 50% energy transfer. The Forster distance of CFP/YFP is 5nm. We used a linker with length close to 5nm so that any force induced distance variation between the FRET pair will lead to maximum FRET change, reflecting on the distance-FRET curve with steepest slope. The force probe has been carefully characterized previously, it has been shown that distribution of actinin-sstFRET is indistinguishable from actinin-GFP [24]. The specificity of actinin-sstFRET was characterized using a force free variant, actinin-C-sstFRET, showing that actinin-C-sstFRET is unable to sense the tension in α-actinin [24]. The construction of Paxillin-mApple has been described previously [25].
Cell culture and transfection
MDCK cells (ATCC) were cultured on fibronectin coated coverslips in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum and 1% penicillin and streptomycin. The details of coating method have been described previously [26]. At ~50–60% confluency, the cells were co-transfected with 0.4 μg plasmid of actinin-sstFRET and 0.2 μg plasmid of paxillin-mApple using transfection reagent Effectene (Qiagen, Valencia, CA), and were cultured for additional 24 hours. For long-duration imaging, the coverslip was mounted in an environmental control chamber (INUB-ZILCSD-F1-LU, Tokai Hit CO., Ltd, Japan) maintained at 37°C and 5% CO2.
Chemicals
Alexa Fluor 568 conjugated phalloidin was obtained from Invitrogen (Grand Island, NY). Rho-associated kinase (ROCK) inhibitor, Y27632, was purchased from CalBiocham (La Jolla, CA). Human fibronectin was obtained from BD Bioscience (Bedford, MA). Lysophosphatidic Acid (LPA) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Live cell imaging
Fluorescence images were acquired using an inverted microscope (Axiovert 200M, Zeiss) with a 63x oil immersion objective and a Hamamatsu EM-CCD camera (ImagEM C9100-13, Hamamatsu, Japan). FRET images were obtained using two filter sets, CFP (Ex=436/20, Em=480/40) and YFP (Ex=500/20, Em=535/30), and paxillin-mApple images were captured by RFP filter set (Ex=550/25, Em=605/70). Time-lapse images from three channels were recorded using Zeiss software (AxioVision, Zeiss).
Image analysis and FRET ratio calculation
We used software (CellProfiler) to identify and Image-J to analyze all growing FAs in the cells. Briefly, the RFP images were first filtered using spatial convolution method to eliminate the background and cytoplasmic fluorescence. The images were then processed using CellProfiler to identify FAs as primary objects in the image. In this process, typical diameter of FA was kept as 3–15 pixel units (0.75 to 3.75 μm) and the intensity threshold was determined referring to global intensity. Scattered fault objects or FA that showed no activity with time were deselected and the outline of the FA of interest was retained. The objects were then converted to a binary image to create a mask for the selected FAs. Since we are interested in the growing FAs, the last frame in the time sequence was processed first for FA identification. This image mask was then transferred to ImageJ to define the outline of the FA for measuring size and stress variation during FA growth. The size of a FA was calculated in two ways, the dimensions (surface area and length) or fluorescence intensity. Length of the focal adhesion was taken as the major axis of the ellipse fit of the selection outline. For a selection window containing multiple FAs, the fluorescence intensity was pixel averaged across the width and the averaged intensity was plotted along the window length.
The FRET efficiency was calculated as acceptor to donor ratio, where the donor signal was measured with donor excitation (CFP channel) and acceptor signal with acceptor excitation (YFP channel) following previously published methods [24]. The FRET signal is from the quenching of donor fluorescence that indicates the energy transfer and is inversely related to the tension in α-actinin. Since the molar ratio of CFP and YFP in the sensor is always 1:1, the ratio of these two channels faithfully reflects the FRET energy transfer, and is independent of concentration [24]. The YFP channel signal correlates only to the protein concentration (given the same excitation intensity). The images from CFP and YFP channels were processed using Image-J (NIH) as described previously [24, 27]. Briefly, after subtracting the background, the images were aligned and the ratio of acceptor to donor intensity was calculated and displayed in a 16-color map. Red color illustrates a higher ratio, indicating a lower tension; blue color illustrates a lower ratio, a higher tension. Because the signals of donor and acceptor were obtained independently with the specified filter sets, there is no need for bleed-through correction during image processing [28]. To compare changes in FRET between two different probes, actinin-sstFRET and actinin-C-sstFRET, time dependent FRET ratio was normalized with the ratio at time zero.
Results
Direct observation of tension in α-actinin using FRET probes
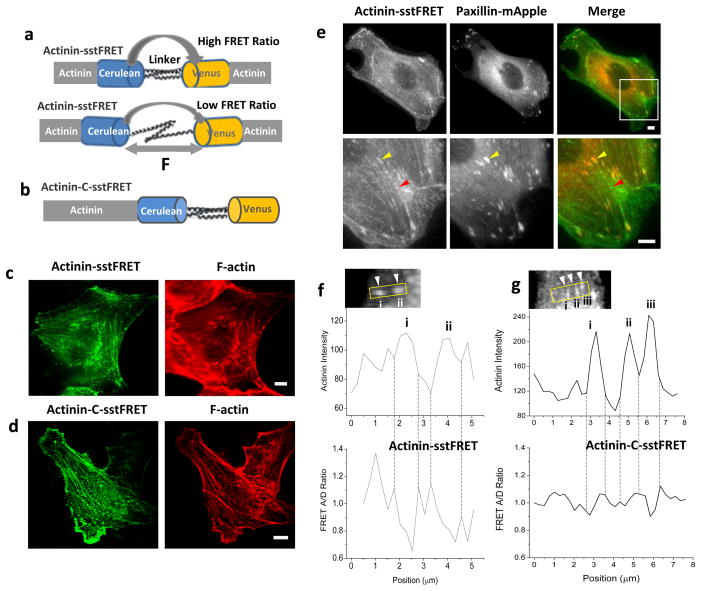
The change in tension across α-actinin was obtained in real time using actinin-sstFRET probes in MDCK cells. The sensor consists of the mutant GFP fluorophores - Cerulean (donor) and Venus (acceptor), linked by a spectrin repeat domain. The sensor cassette is inserted close to the middle of α-actinin (termed as actinin-sstFRET), as shown in Fig. 1a [23, 24, 28]. Resting sensor is set at higher FRET ratio; an increase in distance between fluorophores due to force causes a decrease in the FRET ratio (Fig. 1a). The force probes have been carefully characterized previously so that the chimeric constructs behave as endogenous proteins and the labeling does not interfere with physiological functions [24]. As control, a force insensitive variant of FRET probe was made by attaching the linked fluorophore pair to the C-terminal of α-actinin, termed as actinin-C-sstFRET (Fig. 1b). We have shown that actinin-C-sstFRET is insensitive to force changes in actinin [24]. By expressing FRET probes in MDCK cells and subsequently staining the actin with phalloidin, we have also shown that both actinin-sstFRET and actinin-C-sstFRET co-localize with F-actin in cells (Fig. 1c,d) [27].
Figure 1.
Construction and characterization of actinin-sstFRET sensors. (a) Construction of actinin-sstFRET sensor. The sensor consists of Cerulean as donor, Venus as acceptor, and a linker (spectrin repeat domain). The sensor is inserted close to the middle of actinin. Resting senor shows higher FRET ratio; under axial force ‘F’ the distance between donor and acceptor is extended leading to lower FRET ratio. (b) Construction of actinin-C-sstFRET, a probe inserted at C-terminal of α-actinin. (c),(d) Fluorescence images of actinin-sstFRET (c) and actinin-C-sstFRET (d) expressing cells (YFP channel, green) and the cells stained with phalloidin-Alexa Fluor 568 for F-actin (red), showing co-localization of FRET probes with F-actin. (e) Fluorescence images of a cell co-expressed with actinin-sstFRET and paxillin-mApple (upper panel), and zoom-in images (lower panel) showing elongated clusters of α-actinin (YFP channel, green) co-localized with paxillin (red) at FAs (yellow arrow heads) and dot-shaped α-actinin distributed along the stress fibers (red arrow heads). (f),(g) Actinin intensity (upper panels) and normalized FRET ratio (lower panels) along the selected windows measured in actinin-sstFRET (f) and actinin-c-sstFRET (g) respectively, showing that higher actinin stress (lower FRET ratio) is correlated with peak intensity in actinin-sstFRET (f). Scale bars represent 5 μm.
To visualize in situ tension in α-actinin and the formation and enlargement of FAs, we co-expressed actinin-sstFRET and paxillin-mApple in cells. Paxillin is present at the early stage of adhesion formation [18]. It has been shown that the paxillin is located in the bottom layer while α-actinin locates in the upper layer, the separation between the two is ~40 nm [29]. Therefore, we do not expect cross energy transfer between FRET sensor and paxillin-mApple and the stress measurement should not be affected by co-expression. This is supported by control experiments (see supplemental materials, S1). As shown in Fig. 1e, α-actinin demonstrates two different morphologies: (1) elongated or spike shaped clusters that are co-localized with paxillin in protrusions or at the ends of stress fibers in inner region (yellow arrow heads in Fig. 1e), and (2) small dots that are periodically distributed along the middle section of the stress fibers (red arrow heads in Fig. 1e). The dot-like α-actinin functions as cross-linker of actin filaments, this is consistent with previous findings [30, 31]. Alpha-actinin co-localizing with paxillin is frequently observed at the basal region of the cells, indicating its location in the adhesion complex. This work is focused on α-actinin that localizes with FAs.
Using actinin-sstFRET we measured α-actinin density and tension along a window that includes several FAs at the cell protrusion edge (insert in Fig. 1f). Higher tension (lower FRET ratio) associated with higher actinin concentration is observed at FAs compared to the surrounding areas (Fig. 1f). The localized high tension in α-actinin was observed in 82% of analyzed FAs. This distinction is largely reduced in mature FAs in the middle of the cell where FAs are shown as long spikes. In contrast, the FRET ratio measured using actinin-C-sstFRET did not show sharp contrast between FAs and their surrounding regions (Fig. 1g). These observations suggest that the FA-associated α-actinin at the cell protrusions is under tension in cultured cells.
FA growth is dynamically correlated to the increase in tension in α-actinin
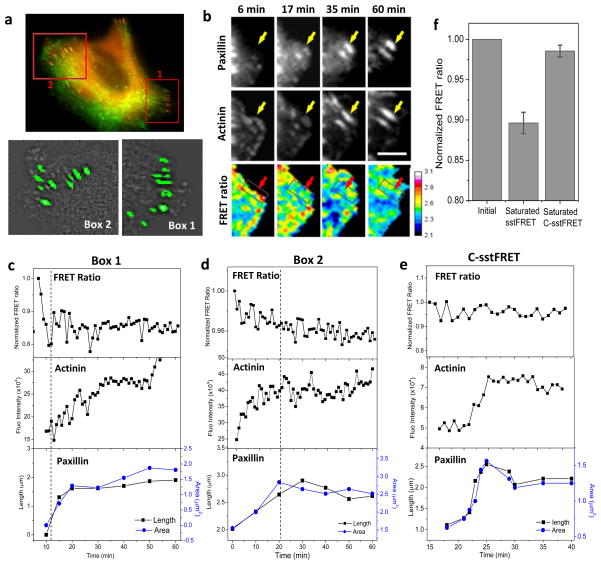
To determine whether the increase in α-actinin tension coincides with α-actinin recruitment and FA growth during cell migration, we followed tension in α-actinin using actinin-sstFRET and size of FAs using paxillin-mApple in MDCK cells. The growing FAs in two regions were identified and analyzed, the protrusion region where nascent FAs form (box-1 in Fig. 2a) and the inner region where FAs show inward growth (box-2 in Fig. 2a). Typical dynamic correlations of tension in α-actinin and FA growth at protrusion and inner regions are shown in Fig. 2b,c and Fig. 2d, respectively. An increase in actinin stress (decrease in FRET ratio) occurs during the enlargement of FAs in both regions. This FA growth involves the recruitment of both α-actinin and paxillin. At the protrusion edge in Fig. 2b,c, the tension increases dramatically within several minutes preceding the apparent growth of the focal complex (Fig. 2c), suggesting that α-actinin tension might provide the driving force for the FA growth. Moreover, the growth phase takes ~10 min in the protrusion region, much shorter than that in the inner region of the cell. In the inner region, tension increases in α-actinin to a less extent and the change in tension coincides with the FA elongation (Fig. 2d). The time dependence of α-actinin tension, correlated actinin accumulation and FA elongation for a selected FA (in box-2 in Fig. 2a) is shown in Fig. 2d (also see Supplemental Materials, S2). The tension reaches saturation at the end of FA growth that lasts approximately 10–20 min (Fig. 2d). In this region, the clusters of α-actinin and paxillin are present before the start of observation and they have shown a continuous elongation towards the cell body (Fig. 2d, Supplemental Materials, S2). During 60 min of observation, both α-actinin and paxillin clusters show inward elongation up to 135–160% of their original length. The effect has been observed in repeated experiments. As controls, cells were transfected with actinin-C-sstFRET. Figure 2e shows the changes in FRET ratio and corresponding changes in FA configurations from an actinin-C-sstFRET expressing cell. Actinin-C-sstFRET expressing cells did not show such correlation. Note that the time dependent FRET ratio is normalized with the initial value so that the two probes can be directly compared. The statistical analysis was performed for growing FAs in protrusion region. The FRET ratio at the end of the growth (typically takes ~15 min) normalized with the initial ratio is shown in Fig. 2f. A consistent increase in tension is shown in actinin-sstFRET (n=32 FAs from 20 cells) during this period, whereas random variation is shown in actinin-C-sstFRET (n=11 FAs from 6 cells).
Figure 2.
Direct observation of tension in α-actinin during FA growth. (a) Upper panel: Merged fluorescence images of actinin-sstFRET (YFP channel, green) and paxillin-mApple (red) of a MDCK cell. Lower panels: Identified FAs in two regions marked in upper penal. (b) Fluorescence images of paxillin-mApple, actinin-sstFRET (YFP channel) and the FRET ratio at various times for a zoomed in region in box-1 in (a). (c) Time course of normalized FRET ratio (upper panel), fluorescence intensity of α-actinin (middle panel), and elongation (left axis) and covered area (right axis) of paxillin cluster (lower panel) for a FA (indicated by arrows in b). (d) Same analysis performed for a selected FA from Box-2 in (a). Notice that the force in actinin dramatically increased before the apparent growth of FA in (c) (left of dash line), whereas the force gradually increased during the FA growth in (d) (left of dash line). (e) Same measurements for actinin-C-sstFRET, showing no correlation between force and FA growth. Scale bar represents 5 μm. (f) Statistics of FRET ratio at the end (saturation) of FA growth normalized with their initial ratios (called as saturated FRET) in actinin-sstFRET (P < 0.0001, Paired sample t-test, n=32) and actinin-C-sstFRET probes (n=11).
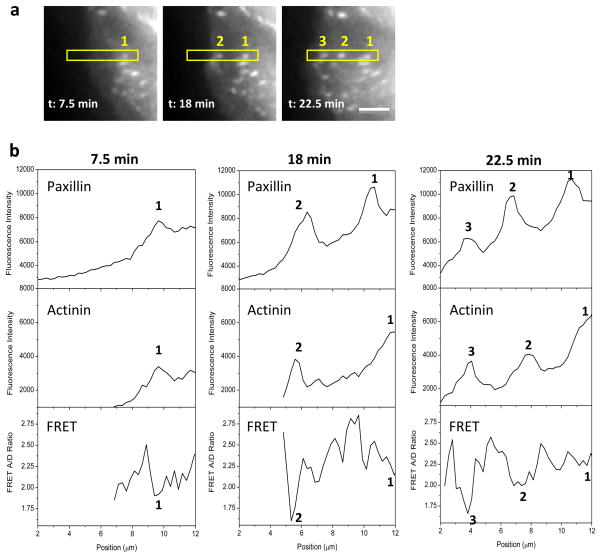
Detailed analysis of subsequent formation of FAs at the protrusion region shows an oscillatory distribution in tension in α-actinin along the protrusion direction and the tension peaks are co-localized with FAs. In Fig. 3a, FAs form subsequently in the order of 1 to 3 as highlighted in the box as the cell protruded from right to left. Fluorescence intensity of paxillin and α-actinin, as well as tension in α-actinin (FRET ratio) were measured along the selected window in Fig. 3a at various times and are shown in Fig. 3b. The largest tension was consistently observed in the newly formed FA (lowest FRET ratio for the new FA-3, Fig. 3b), while tension in α-actinin in the established FAs gradually reduced as the new FA appeared ahead along the protrusion direction (reduction of peak FRET ratio from peak 3 to 1 at 22.5 min, Fig. 3b). This result indicates that in the protrusion area the highest tension in α-actinin is shown at the early stage of FA assembly. This observation is consistent with Fig. 2c.
Figure 3.
Time dependent distribution of α-actinin tension and FA proteins (paxillin and α-actinin) at protrusion edge. (a) Fluorescence images of paxillin-mApple in protrusion region of a MDCK cell, showing three newly formed FAs in time sequence, 1 to 3. (b) Fluorescence intensity of paxillin and α-actinin as well as FRET ratio along the selected window in (a) at various times, showing that an increase in tension in α-actinin coincides with the formation of new FAs. Scale bar represents 5 μm.
The cell migration involves FA maturation at the leading edge and FA disassembly at the trailing edge of the cell. We also followed the tension in α-actinin and FA dynamics at the trailing edge of the cell. We observed a small reduction in α-actinin tension at early stage of FA disassembly in some regions and random tension variation in other regions (data not shown). One possible reason is that the large portion of FA associated α-actinin submerges with F-actin binding α-actinin during retraction, making it difficult to define the force in FA region. This result is consistent with previous reports [32–34].
Change in FA growth direction is facilitated by force-dependent α-actinin translocation
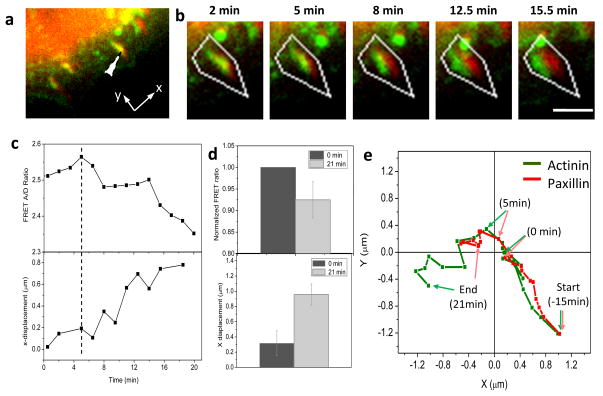
Interestingly, the change in direction of FA expansion is also facilitated by force-induced translocation of α-actinin clusters. We followed a transitioning FA and found that an increase in α-actinin tension was associated with diversion of α-actinin cluster from the original growth direction, leading to a change in FA orientation and subsequent growth in a different direction (Fig. 4). The selected FA (indicated by arrow in Fig. 4a) undergoes inward growth (towards positive y axis) for ~15 min (images not shown), and then expands perpendicular to the original growth direction (Fig. 4b). The history of the movement of α-actinin and paxillin shown in Fig. 4b is plotted in Fig. 4e. In Fig. 4, the time zero was chosen right before the separation of the two proteins, so we can focus the analysis on the separation. Note that the change of growth direction is initiated with a displacement of α-actinin from FA location at time zero followed by paxillin relocalization (Fig. 4b). A clear separation between the two proteins was seen at 5 min (Fig. 4b,e). This displacement results in a reorientation of the FA growth from radial (along y-axis) to peripheral direction (along x-axis). Figure 4c shows that α-actinin displacement coincides with the increase of tension in α-actinin, suggesting that the change of FA growth direction might be directed by the force coupled α-actinin bundle. Similar growth was observed in repeated experiments, the normalized FRET ratio and separation is shown in Fig. 4d (n=5) from three cells. There is no substantial recruitment of α-actinin to the adhesion cluster during this reorientation of growth. It has been proposed that FA growth involves myosin generated high tension on cell protrusion, inward expansion by myosin II pulling, initiation of new adhesion, and separation of lamellipodium actin from new adhesion site [9]. Our results further suggest that the FA growth direction can be changed if the direction of pulling force is altered. This could occur via the association of α-actinin with peripheral actin bundles.
Figure 4.
Correlation of tension in α-actinin with redirection of FA growth. (a) Merged fluorescence images of actinin-sstFRET (YFP channel, green) and paxillin-mApple (red) at the active edge of a MDCK cell. (b) Time sequence of merged images of α-actinin (green) and paxillin (red) of a selected FA indicated in (a). The FA is initially orientated in radial direction (along y-axis). The α-actinin cluster changes moving direction towards left (along x-axis) at 5 min and paxillin follows later. (c) Time course of FRET ratio (upper panel) and x-displacement between the centers of the two protein clusters, actinin and paxillin, (lower panel), showing that the change in growth direction is accompanied with increase of tension in α-actinin. (d) Statistics of changes in FRET ratio (P < 0.17, Paired sample t-test) and x-displacement between α-actinin and paxillin (P < 0.008, Paired sample t-test) in 21 min. Error bars represent s.e.m. from five FAs from three cells. (e) Trace of α-actinin (green) and paxillin (red) locations of a single FA (x, y directions are shown in (a)). The locations of two proteins at time zero and 5 min are indicated by arrows to show the initial separation. Scale bar represents 1 μm.
Lysophosphatidic Acid (LPA)-induced tension in α-actinin promotes FA growth
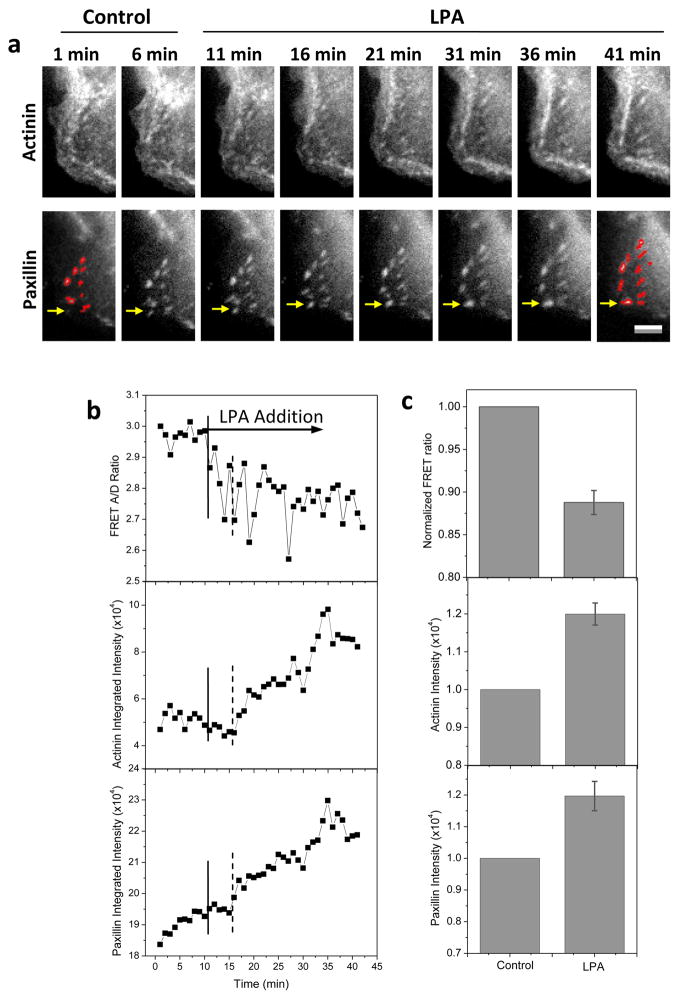
To examine the role of myosin-II mediated cytoskeletal tension in early phase of FA growth we modulated the cell tension using LPA that promotes cell contraction via activation of Rho-ROCK [35–37]. LPA (30 μM) was added at 10 min and a decrease in FRET ratio occurred immediately, reaching a minimum level at 20 min (~10 min post drug application) (Fig. 5). This increase in tension in α-actinin leads to a drastic growth of FAs at protrusion edges. However, the FA growth started at ~16 min (indicated by dashed lines in Fig. 5b), showing a ~5 min delay from the FRET change. The process is reversible and drug washout terminates the FA growth. We have tested eight cell cultures and the results are consistent. The statistics of the tension in α-actinin at FAs and α-actinin and paxillin intensities before and 30 min after LPA application has been obtained from 18 FAs in eight cells and is shown in Fig. 5c. This data supports our argument that tension in α-actinin precedes the FA growth at protrusions. It also provides the evidence that FA growth is driven by myosin II mediated contractility, in agreement with previous model [9].
Figure 5.
Effect of LPA on FA formation and tension in α-actinin. (a) Live cell images of actinin-sstFRET (YFP channel) and paxillin-mApple (RFP channel) before (control) and after application of 30 μM LPA, showing that LPA promotes FA growth (initial and post drug FAs are highlighted by mask outlines). (b) Time course of FRET ratio and α-actinin and paxillin intensities of an individual FA indicated by arrow in (a, lower panels). LPA was added at 10 min indicated by the solid line. The dashed lines indicate delayed FA growth. (d) Statistics of FRET ratio (P < 0.0001, Paired sample t-test), intensity of α-actinin (P < 0.002, Paired sample t-test) and paxillin (P < 0.001, Paired sample t-test) in single FAs after LPA treatment normalized with the corresponding control values. Error bars represent s.e.m. from 18 FAs of eight cells. Scale bar represents 5 μm.
Blockage of Rho-ROCK reversibly disassociates FAs
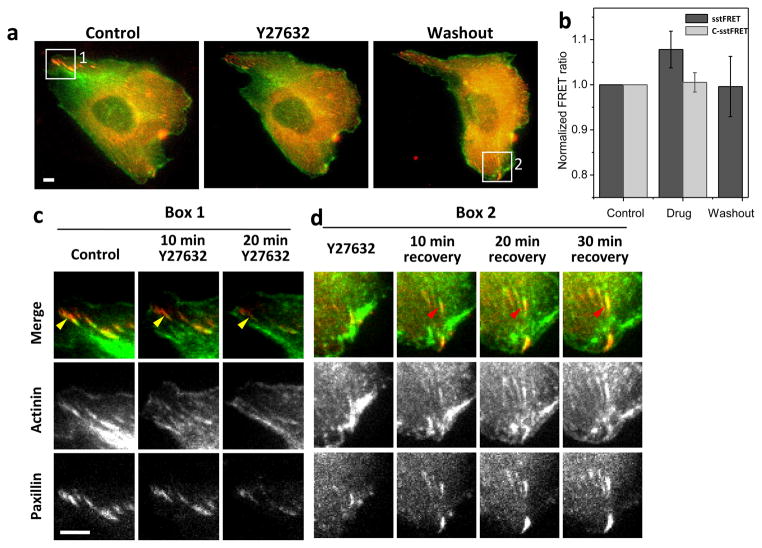
We further tested the effect of inhibition of myosin-II on cytoskeletal tension and FA disassembly and reassembly using the Rho-associated kinase (ROCK) inhibitor Y27632. Addition of 20 μM Y27632 causes disassembly of the FAs and associated actin bundles, and both FAs and actin bundles are reassembled and reorganized after washout of the inhibitor (Fig. 6a). An example of FA disassembly is indicated by yellow arrows in Fig. 6c, and recovery is indicated by red arrows in Fig. 6d. We simultaneously measured the changes in tension in α-actinin using sstFRET during the disassembly and reassembly, and found that the drug reduced the average tension of the cell to a minimum level (highest FRET ratio). Following the washout, the cell returns to a higher tension state (lower FRET ratio); this is consistent with the idea that α-actinin cross-links actin and restores the cell homeostasis after the washout. In actinin-C-sstFRET expressing cells, application of drug alters FA assembly, although the FRET ratio remains unchanged. Fig. 6b shows the statistics of FRET ratio from actinin-sstFRET (n=4) and actinin-C-sstFRET (n=5). Similar level of tension is reached in all cells in the presence of inhibitor, although a large variation was shown in initial tension. This may be related to the different pre-stressed states of individual cells, which we have shown in our previous studies [27]. Interestingly, α-actinin clusters diminish faster than paxillin during the application of drug, and reassemble faster in the recovery (Fig. 6c,d), supporting the idea that attached F-actin bundles are required for FA assembly. This behavior is consistent over repeated experiments.
Figure 6.
Effect of Rho-ROCK inhibitor on FA formation and cytoskeletal tension. (a) Merged live cell images of actinin-sstFRET (YFP channel, green) and paxillin-mApple (red) before (control), during the application, and washout of 20 μM Y27632. (b) Statistics of FRET ratio averaged over whole cell before, during and after wash out of the drug for actinin-sstFRET (P < 0.13, Paired sample t-test, n=4) and actinin-C-sstFRET (n=5). Error bars represent s.e.m. (c) Images from box-1 at various times showing disassembly of FAs due to the application of inhibitor. (d) Images from box-2 showing FA reassembly on washout. Scale bars are 5 μm.
Discussion
Adherent cells migrate via formation and enlargement of focal adhesions, a process involving increase in cytoskeleton tension, polymerization of actin, and activation of associated plaque proteins. A growing body of evidence shows that FA formation and growth is a force-dependent process mediated by the cross-linking proteins at adhesion complex. Through their connection to cytoskeleton, the specific linking proteins could transmit mechanical force onto integrin or other adhesion proteins that modulate the adhesion assembly [13, 38–40], enabling reorganization of focal adhesions [41]. This paper presents a direct observation of tension in α-actinin, an actin cross-linking protein, at adhesion complex. By simultaneously measuring the changes in tension in α-actinin and FA configuration, we have established a direct link between α-actinin tension and FA dynamics and show that an increase in tension in α-actinin precedes the initial growth or translocation of FAs in cell protrusion region.
The activity of α-actinin is crucial for maturation of FAs at the leading edge of migrating cells due to their important role in binding actin cytoskeleton to the adhesions and binding the actin filaments. Our observations show that α-actinin participates in the force transmission process during FA growth, and this is accomplished by both changes in α-actinin tension and α-actinin recruitment (Fig. 2). An increase in tension precedes assembly of FAs in the protrusion region (Fig. 2b,c); similar changes in tension are also shown during the continuing growth of mature FAs (Fig. 2d). The time-dependent recruitment of α-actinin and the initial adhesion elongation suggests that the recruitment of α-actinin to newly formed adhesions is also a crucial step for initial adhesion development (Fig. 2b–d). Since the increase in tension in α-actinin at FAs precedes adhesion growth at protrusion edge and the increase in tension coincides with adhesion growth in the relative interior region (compared Fig. 2c and 2d), we suggest that higher α-actinin tension is required for the recruitment of adhesion proteins (α-actinin and paxillin) to newly forming adhesions, while sustained tension is required for further expansion (Fig. 3). A recent study has shown that α-actinin is responsible for transmitting force between specific integrin and actin; depletion of α-actinin impairs the force-dependent stress fiber formation and subsequent FA maturation [22]. Our results are consistent with this report. Interestingly, in this study it has been shown that traction force is enhanced in initial adhesions in α-actinin knock down cells, suggesting that α-actinin may compete with another cross-linking protein, talin, for binding in the FAs during the formation and maturation [22]. Since our FRET probes measure the forces in α-actinin directly, the observed increase in actinin tension since its participation and correlated α-actinin recruitment provides direct evidence that α-actinin becomes major player in force transmission during FA growth.
The mechanical force has been found necessary for assembly of focal adhesions during cell migration [17, 41]. Although an earlier report has shown that large contractile force can be exerted onto substrate in the absence of the cross-linked stress fibers, however, without stress fibers it is unable to stimulate the adhesion growth [41]. Thus, the local force without linkage of stress fibers to FAs is not sufficient; an effective force coupling to force sensitive adhesion proteins is required for the growth of FAs.
Interestingly, we have observed that the redirection of the adhesion expansion is initiated by the separation of α-actinin from the FA site and this process is associated with an increase in tension in α-actinin (Fig. 4). Although paxillin has been observed as the first component of adhesion proteins that appears at adhesion sites [18], α-actinin appears to be leading the expansion of a mature FA to a new direction (Fig. 4). This result is consistent with the force driven FA dynamics model, where FA formation and growth is accomplished by myosin driven inward adhesion expansion, initiation of new adhesion, and separation of lamellipodium actin from new adhesion site [9]. Our results suggest that the force to the newly formed FA could be applied by α-actinin from stress fibers of different orientation, such as the peripheral actin bundles. Since we have only observed tension increase but not recruitment of α-actinin during the relocation of FA, we think the force transmission and the protein recruitment might be regulated separately, as in the case of vinculin [3].
In resting cells, myosin-II mediated contraction force causes conformational changes of adhesion proteins, driving recruitment of adhesion proteins and adhesion growth [12, 42, 43]. Our study shows that induction of myosin II mediated force by LPA stimulates FA growth at cell protrusion (Fig. 5). In contrast, inhibition of myosin activated force by Rho-ROCK inhibitor Y27632 results in a reversible disassembly of mature FAs and associated actin bundles (Fig. 6). These results not only support our observation that the increase in tension in α-actinin precedes FA growth, but also support the involvement of myosin II activated contraction in the process.
In conclusion, we have directly observed the changes in tension in α-actinin and force-dependent focal adhesion assembly, disassembly, and relocation in MDCK cells. The results show that α-actinin participates in the formation and growth of FAs by transmitting mechanical force and being recruited to adhesions. These data offer the insights on force transduction mechanisms in the adhesion complex that regulate cell adhesion and migration.
Supplementary Material
Highlights.
Directly measured the changes in tension in α-actinin using stress sensitive FRET probes.
Focal adhesion growth is dynamically correlated to the increase in α-actinin tension and its recruitment.
Change in FA growth direction is facilitated by force-dependent α-actinin translocation.
Focal adhesion growth is mediated by Rho-ROCK pathway.
Acknowledgments
This work was supported by National Institutes of Health grant DK77302 (S.Z.H.) and National Science Foundation Grant CMMI-0825707 (S.Z.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science (New York, NY. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 2.Brown CM, Hebert B, Kolin DL, Zareno J, Whitmore L, Horwitz AR, Wiseman PW. Probing the integrin-actin linkage using high-resolution protein velocity mapping. Journal of cell science. 2006;119:5204–5214. doi: 10.1242/jcs.03321. [DOI] [PubMed] [Google Scholar]
- 3.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, Schwartz MA. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Wichert G, Haimovich B, Feng GS, Sheetz MP. Force-dependent integrin-cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. The EMBO journal. 2003;22:5023–5035. doi: 10.1093/emboj/cdg492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morimatsu M, Mekhdjian AH, Adhikari AS, Dunn AR. Molecular tension sensors report forces generated by single integrin molecules in living cells. Nano Lett. 2013;13:3985–3989. doi: 10.1021/nl4005145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stricker J, Falzone T, Gardel ML. Mechanics of the F-actin cytoskeleton. Journal of biomechanics. 43:9–14. doi: 10.1016/j.jbiomech.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nature cell biology. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 9.Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, Beaver W, Dobereiner HG, Freund Y, Borisy G, Sheetz MP. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128:561–575. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science (New York, NY. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 11.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 12.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. The Journal of cell biology. 2010;188:877–890. doi: 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. The Journal of cell biology. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. The Journal of cell biology. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science (New York, NY. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galbraith CG, Sheetz MP. Forces on adhesive contacts affect cell function. Curr Opin Cell Biol. 1998;10:566–571. doi: 10.1016/s0955-0674(98)80030-6. [DOI] [PubMed] [Google Scholar]
- 17.Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nature cell biology. 2008;10:1039–1050. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential Dynamics of α5 Integrin, Paxillin, and α-Actinin during Formation and Disassembly of Adhesions in Migrating Cells. The Journal of cell biology. 2001;153:1427–1440. doi: 10.1083/jcb.153.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinhard M, Zumbrunn J, Jaquemar D, Kuhn M, Walter U, Trueb B. An α-Actinin Binding Site of Zyxin Is Essential for Subcellular Zyxin Localization and α-Actinin Recruitment. Journal of Biological Chemistry. 1999;274:13410–13418. doi: 10.1074/jbc.274.19.13410. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Gunst SJ. Dynamic association between alpha-actinin and beta-integrin regulates contraction of canine tracheal smooth muscle. The Journal of physiology. 2006;572:659–676. doi: 10.1113/jphysiol.2006.106518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sen S, Dong M, Kumar S. Isoform-specific contributions of alpha-actinin to glioma cell mechanobiology. PloS one. 2009;4:e8427. doi: 10.1371/journal.pone.0008427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roca-Cusachs P, del Rio A, Puklin-Faucher E, Gauthier NC, Biais N, Sheetz MP. Integrin-dependent force transmission to the extracellular matrix by alpha-actinin triggers adhesion maturation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E1361–1370. doi: 10.1073/pnas.1220723110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng F, Suchyna TM, Sachs F. A fluorescence energy transfer-based mechanical stress sensor for specific proteins in situ. The FEBS journal. 2008;275:3072–3087. doi: 10.1111/j.1742-4658.2008.06461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng F, Sachs F. Visualizing dynamic cytoplasmic forces with a compliance-matched FRET sensor. Journal of cell science. 2011;124:261–269. doi: 10.1242/jcs.071928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaner NC, Lin MZ, McKeown MR, Steinbach PA, Hazelwood KL, Davidson MW, Tsien RY. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat Methods. 2008;5:545–551. doi: 10.1038/nmeth.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye N, Bathany C, Hua SZ. Assay for molecular transport across gap junction channels in one-dimensional cell arrays. Lab on a chip. 2011;11:1096–1101. doi: 10.1039/c0lc00350f. [DOI] [PubMed] [Google Scholar]
- 27.Rahimzadeh J, Meng F, Sachs F, Wang J, Verma D, Hua SZ. Real-time observation of flow-induced cytoskeletal stress in living cells. Am J Physiol Cell Physiol. 2011;301:C646–652. doi: 10.1152/ajpcell.00099.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng F, Sachs F. Orientation-based FRET sensor for real-time imaging of cellular forces. Journal of cell science. 2012;125:743–750. doi: 10.1242/jcs.093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–584. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallenius T, Luukko K, Makela TP. CLP-36 PDZ-LIM protein associates with nonmuscle alpha-actinin-1 and alpha-actinin-4. The Journal of biological chemistry. 2000;275:11100–11105. doi: 10.1074/jbc.275.15.11100. [DOI] [PubMed] [Google Scholar]
- 31.Otey CA, Pavalko FM, Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. The Journal of cell biology. 1990;111:721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annual review of cell and developmental biology. 2010;26:315–333. doi: 10.1146/annurev.cellbio.011209.122036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo JC, Han X, Hsiao CT, Yates JR, 3rd, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nature cell biology. 2011;13:383–393. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiller HB, Friedel CC, Boulegue C, Fassler R. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 2011;12:259–266. doi: 10.1038/embor.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JH, Adelstein RS. LPA(1) -induced migration requires nonmuscle myosin II light chain phosphorylation in breast cancer cells. Journal of cellular physiology. 2011;226:2881–2893. doi: 10.1002/jcp.22631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manning TJ, Jr, Rosenfeld SS, Sontheimer H. Lysophosphatidic acid stimulates actomyosin contraction in astrocytes. Journal of neuroscience research. 1998;53:343–352. doi: 10.1002/(SICI)1097-4547(19980801)53:3<343::AID-JNR8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 37.Khurana S, Tomar A, George SP, Wang Y, Siddiqui MR, Guo H, Tigyi G, Mathew S. Autotaxin and lysophosphatidic acid stimulate intestinal cell motility by redistribution of the actin modifying protein villin to the developing lamellipodia. Experimental cell research. 2008;314:530–542. doi: 10.1016/j.yexcr.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibers. The Journal of cell biology. 2006;172:259–268. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirata H, Tatsumi H, Sokabe M. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. Journal of cell science. 2008;121:2795–2804. doi: 10.1242/jcs.030320. [DOI] [PubMed] [Google Scholar]
- 40.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 41.Oakes PW, Beckham Y, Stricker J, Gardel ML. Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. The Journal of cell biology. 2012;196:363–374. doi: 10.1083/jcb.201107042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. The Journal of cell biology. 2007;176:573–580. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lo CM, Buxton DB, Chua GC, Dembo M, Adelstein RS, Wang YL. Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol Biol Cell. 2004;15:982–989. doi: 10.1091/mbc.E03-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.