Abstract
The optimal markers for human spermatogonial stem cells (SSCs) are not known. Among the genes recently linked to SSCs in mice and other animals are the basic helix-loop-helix transcription factor ID4 and the orphan G-protein coupled receptor GPR125. While ID4 and GPR125 are considered putative markers for SSCs, they have not been evaluated for co-expression in human tissue. Further, neither the size nor the character of the human spermatogonial populations that express ID4 and GPR125, respectively, are known. A major barrier to addressing these questions is the availability of healthy adult testis tissue from donors with no known reproductive health problems. To overcome this obstacle, we have employed healthy testicular tissue from a novel set of organ donors (n=16; aged 17-68 years) who were undergoing post-mortem clinical organ procurement. Using immunolabeling, we found that ID4 and GPR125 are expressed on partially overlapping spermatogonial populations and are more broadly expressed in the normal adult human testis. Additionally, we found that expression of ID4 remained stable during aging. These findings suggest that ID4 and GPR125 could be efficacious for identifying previously unrecognized human spermatogonial subpopulations in conjunction with other putative human stem cell markers, both in younger and older donors.
Introduction
Adult male germline stem cells, referred to as spermatogonial stem cells (SSCs), comprise a small population within the mammalian testis (de Rooij and Griswold, 2012; Dym, et al., 2009). While the actual size of the SSC population is a matter of debate, fertility is maintained into advanced age in most healthy males. The ability to maintain homeostatic organ function implies an exquisitely robust self-renewal system that can withstand environmental challenges. SSCs are typically considered to be isolated type A spermatogonia (SPG), referred to as Asingle or As; these can differentiate into committed progenitors to produce syncitia of paired cells (Apr) or larger chains of cells (de Rooij and Griswold, 2012). However, classical morphological features (e.g., nuclear morphology, or single cells vs. chains) have been challenging to correlate with newer molecular markers (von Kopylow, et al., 2012).
Compared to SSCs in animals, human SPG, including SSCs and committed progenitors, present unique challenges to study due to limited availability of normal tissue and a paucity of experimental assays. Chromatin structure was used historically to classify human SPG as Adark or Apale, but the functional significance of such differences has been controversial (Dym, et al., 2009; Ehmcke and Schlatt, 2006; Hermann, et al., 2010). Recently, comparisons between cellular morphology and expression of molecular markers have revealed surprising degree of diversity within human type A SPG (Lim, et al., 2011; von Kopylow, et al., 2012). Furthermore, only a small number of markers are available to delineate the cell populations encompassing human SPG or SSCs, respectively (Waheeb and Hofmann, 2011). Xenotransplantation has been developed to assess SSC activity in human testicular cells (Nagano, et al., 2002; Zohni, et al., 2012). However, it remains quite unclear what fraction of germ cells in the adult human testis are SSCs.
As a basis for developing effective functional assays, two essential preliminary components for studying human SSCs and committed progenitors include the ability to enrich the target cell population using immunoselection and to be able to confirm that the selected cells are phenotypically pure SPG or, more specifically, enriched for true SSCs. Toward this end, a plethora of markers, either with internal or cell surface expression, have been developed for SSCs in model systems, such as the mouse; surface markers include Thy1, Itga6, CD9, Gfra1, and others (Nagano and Yeh, 2013). In humans, proposed SSC markers include CD9, GPR125, SSEA-4, ITGA6 (He, et al., 2010; Izadyar, et al., 2011; Zohni, et al., 2012). Only CD9 and SSEA-4 have been evaluated by xenotransplantation (Izadyar, et al., 2011; Zohni, et al., 2012). GPR125 was discovered by recognition of an expression pattern in the adult mouse testis consistent with that of SPG, including SSCs (Seandel, et al., 2007). More recently GPR125 was employed for immunoselection of human testicular cells (He, et al., 2010). In the latter study, a very small fraction of SPG (~1-2 cells per tubular cross section) was positive for GPR125 expression. GPR125 expression is also detectable in long-term pre-pubertal testicular cell cultures that contain SSCs (Sadri-Ardekani, et al., 2011). It is not known whether adult GPR125+ testicular cells have functional stem cell activity in vivo.
Among intracellular markers, PLZF has been used effectively both in mice and in primates to identify undifferentiated SPG (He, et al., 2010; Hermann, et al., 2007; Hobbs, et al., 2010). UTF1 (undifferentiated embryonic cell transcription factor 1) is a pluripotency-associated gene that also clearly marks SPG in the adult human testis (Kristensen, et al., 2008; von Kopylow, et al., 2010). Id4 is a member of the basic helix-loop-helix transcription factor family (Benezra, et al., 1990; Yokota, 2001). Previously, ID4 expression was demonstrated in SPG in human testicular tumors (Sablitzky, et al., 1998). Recently, Id4 was shown not only to mark type Asingle SPG in mice but also to be required for SSC self-renewal in vitro and to prevent the Sertoli cell-only phenotype in vivo (Oatley, et al., 2011). These findings raise the questions of whether ID4 expression could mark SSCs in the normal adult human testis and to what extent different subpopulations of SPG share ID4 expression.
Typical sources of human testis tissue for adult SSC studies include men with infertility, cancer patients, and cadaveric organ donors, across a range of ages, depending on availability. Cadaveric organ donors represent a particularly attractive source of viable adult testis tissue, since other donor organs (e.g., heart, lungs, kidneys, and/or liver) are healthy enough for transplant into patients (He, et al., 2010). While most healthy men maintain fertility into advanced age, hypospermatogenesis is not uncommon in testicular biopsies (McLachlan, et al., 2007). Therefore, it is important to determine how much age-related variation exists in expression of candidate SPG markers. In this study, we sought to evaluate several recently identified candidate human SPG markers in normal adult testis tissue from cadaveric organ donors. We hypothesized that ID4 and GPR125, respectively, would mark very small populations of type A SPG in normal adults. We found large numbers of ID4+ SPG in adult human testis (n=16, age range = 17-67 years) in all seminiferous tubules from all donors. ID4 expression partially overlapped with UTF1, a well-documented human SPG marker (Kristensen, et al., 2008). ID4 expression also overlapped partially with GPR125, which itself was expressed on a larger number of cells per tubule than expected. No age-related changes in expression of ID4 or GPR125 in SPG were seen in this sample set.
Materials and Methods
Tissue procurement
Testes were obtained from deceased organ donors through collaboration with the New York Organ Donor Network after specific consent was obtained from next of kin. All available donors suitable for clinical organ transplantation during the study period were considered eligible for the research study irrespective of past medical history, except those with known HIV or hepatitis C. All human tissue procurement procedures were conducted in accordance with Weill Cornell Medical College guidelines and National Institutes of Health regulations. The data in this study were obtained from 16 donors (17 to 67 years old). The causes of death included cerebrovascular accident, homicide, suicide, cardiac death, and trauma. Following resection of other viable organs for clinical transplantation, a bilateral orchiectomy was performed. Time from cross-clamp of the aorta to orchiectomy ranged from 70 to 135 minutes. Immediately following orchiectomy, tissue was transported to the laboratory packed in ice. Each human testis sample was dissected on top of an ice bath into ~0.5-1.5 cm3 tissue fragments and immediately allocated for different procedures. Fragments were snap frozen in liquid nitrogen for subsequent mRNA analysis. Additional fragments were fixed by immersion in •10-fold excess by volume of 4% paraformaldehyde (PFA) in PBS or in Bouin's fixative at 4° C for 8-16 hours. Samples were washed and later processed for paraffin embedding. Tissue integrity was subsequently confirmed by routine histopathological evaluation (see below).
Immunostaining
Bouin's fixed tissue was employed for immunohistochemistry (IHC) and immunofluorescence (IF) except as specifically noted otherwise. Five micron sections were warmed for 30 minutes at 60° C, de-waxed in xylene and rehydrated through a series of graded alcohols. After deparaffinization, antigen retrieval was performed by heat-induced epitope retrieval in an electric steamer using Tris-EDTA antigen retrieval buffer (pH 9) for 15-45 minutes. Hydrogen peroxide (3%) was used to block endogenous peroxidase activity. After blocking with either 10% normal donkey serum or tyramide signal amplification buffer (1% BSA, 0.2% powdered skim milk, 0.3% Triton X-100), sections were treated with Avidin/Biotin Blocking Kit (Vector Laboratories, SP-2001) to block all endogenous biotin, biotin receptors, and avidin binding sites. Sections were then incubated with primary antibodies at optimal dilutions (as listed in Table 1) overnight at 4° C. For IHC, detection of primary antibodies was performed with biotinylated donkey anti-rabbit IgG, biotinylated donkey anti-mouse IgG, or biotinylated donkey anti-goat (all from Jackson Immunoresearch) at a concentration of 1 μg/mL. Biotinylated secondary antibody was followed by streptavidin-horseradish peroxidase conjugate (Jackson Immunoresearch) and amino-ethyl carbazole (AEC; Life Technologies). Specificity of immunostaining was confirmed by staining with the immunoglobulin fraction of the species corresponding to the primary antibody. Color images of IHC staining were captured using an Olympus microscope and Spot camera and contrast enhanced uniformly for all images within each experiment using ImageJ (Schneider, et al., 2012). For KIT and WT1, 5 micron thick paraffin-embedded tissue sections were stained using the BOND III Autostainer and supplied reagents (Leica Microsystems, Buffalo Grove, IL, USA).
Table 1.
Primary antibodies used in this study
| Antigen | Company | Code | Species | Host | Clonality | Working Concentration |
|---|---|---|---|---|---|---|
| GPR125 | Novus Biologicals | NBP1-03561 | Human | Goat | Polyclonal | 0.4 μg/mL |
| ID4 | Calbioreagents | M106 | Human/Mouse | Rabbit | Monoclonal (82-12) | 5 μg/mL |
| UTF1 | Chemicon (Millipore) | MAB 4337 | Human/Mouse | Mouse | Monoclonal (5G10.2) | 5 μg/mL |
| Ki67 | Dako | M7240 | Human | Mouse | Monoclonal (MIB-1) | 0.1 μg /mL |
| KIT | Dako | A4502 | Human | Rabbit | Polyclonal | 26 μg/mL |
| WT1 | Dako | IS055 | Human | Mouse | Monoclonal (6F-H2) | Ready-to- use format |
| PLZF | Santa Cruz Biotechnology | SC-28319 | Human/Mouse/Rat | Mouse | Monoclonal (D-9) | 0.1 μg/mL |
For IF detection of ID4, Ki76, and UTF1, Alexa Fluor® 488-conjugated goat anti-rabbit IgG (Jackson Immunoresearch) and Cy3-conjugated donkey anti-mouse (Jackson Immunoresearch) or Alex Fluor® 488-conjugated anti-mouse secondary antibodies were used respectively. GPR125, and PLZF were detected using signal amplification with biotinyl tyramide (Jacobs, et al., 1998). Following overnight incubation with the primary antibody, sections were incubated for 90 minutes with biotinylated donkey anti-mouse IgG and biotinylated donkey anti-goat (Jackson Immunoresearch) respectively at a concentration of 1 μg/mL. Following incubation with the biotinylated secondary antibody, sections were incubated subsequently with streptavidin-horseradish peroxidase conjugate (Jackson Immunoresearch) for 30 minutes and biotinyl tyramide for 3-20 minutes. Incubation with biotinyl tyramide was followed by a 30-minute incubation with a streptavidin-conjugated fluorophore (as listed in Table S1). Counterstaining was performed with TO-PRO®-3 iodide (Life Technologies). Specificity of staining was again confirmed by staining with the corresponding normal immunoglobulin. Lack of cross-reactivity in co-staining protocols was confirmed by incubation with a single primary antibody followed by the detection system for the second antibody (data not shown). For example, the rabbit ID4 antibody was treated with the anti-goat and anti-mouse detection systems in order to ensure that there was no cross-reactivity. Fluorescent images were captured using Zeiss LSM 510 Meta and 710 confocal microscopes (Carl Zeiss). For each channel, signal was enhanced uniformly for all fields within each experiment using the LSM or ImageJ software. While occasional non-specific reactivity was seen interstitial areas or in the tubule lumen, species matched controls for primary and secondary antibodies yielded little signal (Supplemental Fig. S1).
Quantification of spermatogonial markers and statistical analysis
Length of outer border of the tubular cross section was measured by tracing the tubule perimeter free hand in ImageJ and using the software to measure the perimeter length in relative units (Supplemental Fig. S2). Positively marked cells were determined in comparison with normal rabbit IgG controls. Captured images were contrast-enhanced uniformly for each experiment and thresholded in ImageJ prior to counting which was done in a blinded fashion by two independent observers. Those cells around the basement membrane of the seminiferous tubule, which displayed a strong stain, were counted as positive. Positively stained cells in the interstitium or located adluminal to the basement membrane within the tubule were not counted. For serial sections, only cells that could be unequivocally identified (based on counterstain and location) in both sections were counted. The average number of cells per unit length was calculated from four random fields per patient. To avoid introducing bias due to known azoospermia, the single patient with complete maturation arrest was omitted from the statistical analysis of ID4. Cell number data were analyzed using the Mann-Whitney test for non-parametric data (GraphPad Prism 6).
Results
Pathological evaluation of cadaveric human testis samples
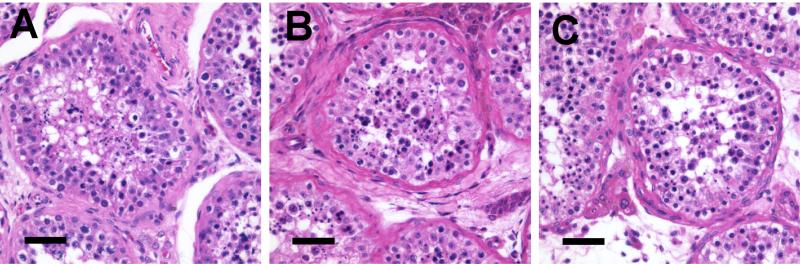
Testis samples were procured from cadaveric organ donors (n=16), ages 17 to 67, after specific consent was obtained from the next of kin (Table 2). All potential donors were included except as noted above (see Methods). Testis histology was evaluated using H&E staining of Bouin's or paraformaldehyde-fixed, paraffin-embedded tissue samples. With a single exception, all donors exhibited spermatogenesis (Fig. 1). One donor had germ cells arrested at the SPG stage. Five other donors (all age •42) had occasional tubular atrophy had occasional tubular atrophy or hypospermatogenesis to varying degrees but were otherwise unremarkable (Table 2).
Table 2.
Demographic and histological summary of donors.
| Donor # | Age (yrs) | Cause of death* | Pathological Findings** |
|---|---|---|---|
| 1 | 17 | CVA | None |
| 2 | 21 | CVA | None |
| 3 | 22 | Suicide | None |
| 4 | 25 | MVA | None |
| 5 | 42 | CVA | Tubular atrophy (5-10% of tubules) |
| 6 | 43 | CVA | None |
| 7 | 49 | Cardiac arrest | None |
| 8 | 49 | Cardiac arrest | Marked hypospermatogenesis; tubular atrophy (10% of tubules) |
| 9 | 51 | CVA | None |
| 10 | 52 | Homicide | None |
| 11 | 54 | Cardiac arrest | Complete maturation arrest (azoospermia), predominantly at the primary spermatocyte level; tubular atrophy (5% of tubules); moderate to marked diffuse basement membrane thickening |
| 12 | 57 | CVA | None |
| 13 | 62 | CVA | Mild hypospermatogenesis; mild diffuse basement membrane thickening |
| 14 | 63 | CVA | None |
| 15 | 67 | CVA | Tubular atrophy (5-10% of tubules); mild diffuse basement membrane thickening |
| 16 | 67 | MI | Moderate hypospermatogenesis; tubular atrophy (10-20% of tubules); moderate diffuse basement membrane thickening |
CVA, cerebrovascular accident; MVA, motor vehicle accident; MI, myocardial infarction.
Spermatogonia were observed in all samples. Samples with decreased spermatogenesis or other findings are specifically noted.
Figure 1.
Histology of adult cadaveric organ donor testes of varying ages. Hematoxylin and eosin staining of Bouin's fixed tissue: (A) 22 year old, (B) 43 year old, and (C) 62 year old, demonstrating excellent tissue preservation as well as active spermatogenesis with complete maturation.
ID4 is expressed in a subpopulation of spermatogonia in normal human testis
Detection of ID4 by either IF or IHC demonstrated consistent strong anti-ID4 reactivity localized either to the nucleus alone or more commonly to both the cytoplasm and nucleus of a subpopulation of SPG found immediately adjacent to the basement membrane of the seminiferous tubule (Fig. 2). More differentiated germ cell types were negative. Sertoli cells, identifiable based on expression of WT1, stained weakly or negative for ID4 (Mundlos, et al., 1993)(Supplemental Fig. S7). IgG controls for the primary antibody yielded no signal (data not shown). Of note, specificity of the anti-ID4 clone 82-12, which lacks cross-reactivity to human ID1, ID2, and ID3, was confirmed by a similar expression pattern found in normal human testis in the Human Protein Atlas, using a different primary antibody (Sharma, et al., 2012; Uhlen, et al., 2010; Wen, et al., 2012).
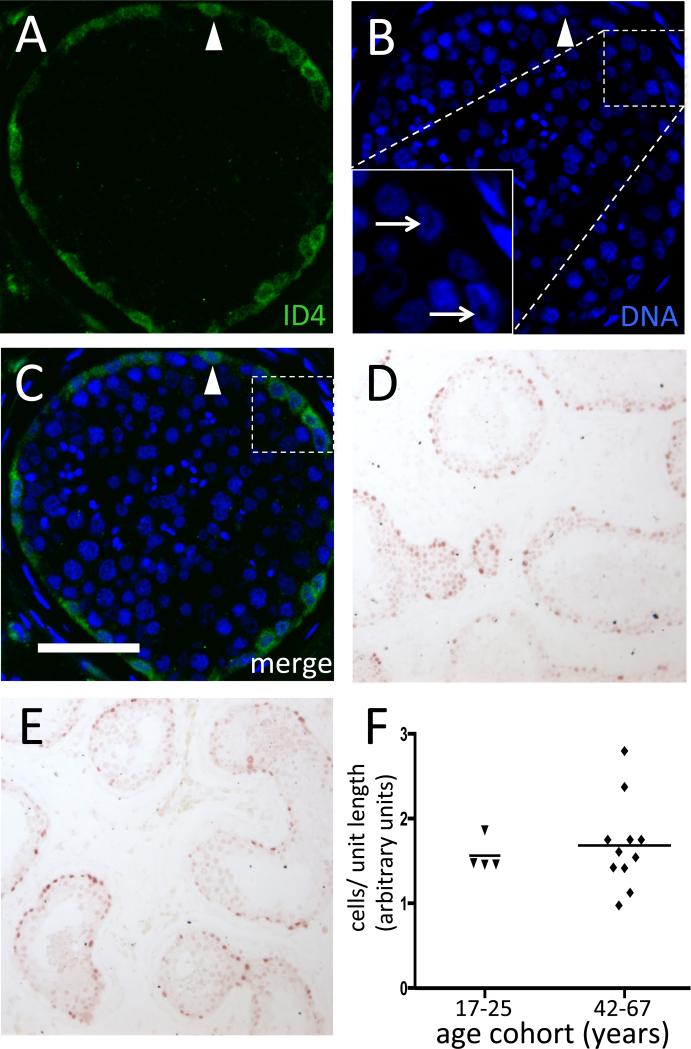
Figure 2.
ID4 expression in normal testis from early through later adulthood. (A-C) IF demonstrating rabbit monoclonal anti-ID4 signal (green; panel A), DNA counterstain alone (blue; panel B), or merged image (C). Arrows denote ID4+ cells with nuclear rarefaction zones, seen as dark areas within the nucleus in the inset. Arrowhead denotes a cell without a nuclear rarefaction zone. Bar is 50 μm. (D-E) Representative ID4+ IHC (reddish-brown) on PFA-fixed tissue in a 21 year old (D) and 49 year old (E). (F) Quantification of ID4+ cells in PFA-fixed samples grouped by age (Mann-Whitney U-test, p >0.05)
Germ cell chromatin structure, specifically the presence or absence of a nuclear rarefaction zone, has been used previously to classify human SPG and can distinguish the Adark and the Apale SPG subtypes (Clermont, 1966; von Kopylow, et al., 2012). Accordingly, DNA counterstaining confirmed that the ID4-positive cells included cells with a clearly recognizable nuclear rarefaction zone and also those without (Fig. 2). Based on the location of the population intensely expressing ID4, which was restricted to the cells lining the basement membrane, in conjunction with the nuclear morphology, it is likely that these cells include type A SPG.
In order to determine whether the number of ID4+ cells changes with age within the limits of the available samples, we quantified the number of ID4+ cells in each donor and arbitrarily subdivided the available specimens into age brackets comprising younger (17-25 years; n=4) vs. older (42-67 years; n=12) donors. Since human seminiferous tubule cross sections are of irregular shape, we determined the number of ID4+ cells per unit length of tubule and found no significant difference based on age brackets (Mann-Whitney U-test, p > 0.05), with an average of ~17 ID4+ cells per transverse (i.e., circular) tubule cross-section in the entire set (Fig. 2). Thus, normal aging appeared to have no effect on the frequency of SPG that exhibit strong ID4 expression.
Overlap in expression of ID4 and markers of spermatogonia
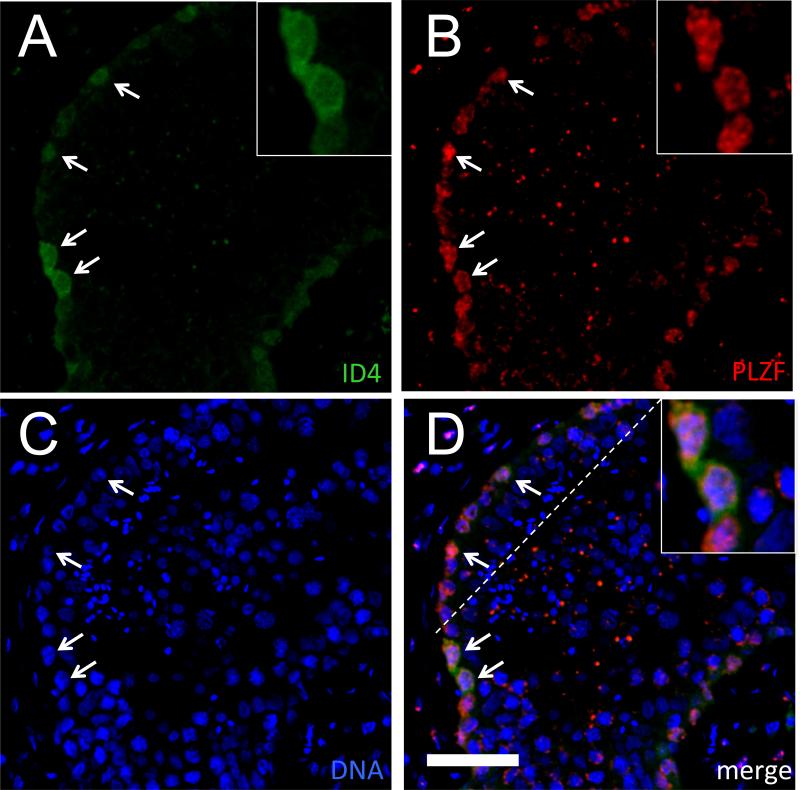
To further characterize the ID4-expressing SPG, immunostaining was performed for ID4 and additional markers of spermatogonia. As a surrogate marker for differentiating spermatogonia, KIT was co-localized at the cellular level with ID4 using IHC on serial thin sections (Supplemental Fig. S7). The KIT+ fraction was a minor population of the ID4+ germ cell pool (13.3±4.1% [mean±S.D.], Table S2). In contrast, PLZF was employed as a marker of undifferentiated spermatogonia (Buaas, et al., 2004; Hermann, et al., 2007). Consistent with the expected pattern based on prior studies (von Kopylow, et al., 2012), PLZF expression was restricted to the nuclei of SPG adjacent to the basement membrane of the seminiferous tubules (Fig. 3). Co-labeling with anti-ID4 and anti-PLZF revealed that the vast majority of ID4-positive cells co-expressed PLZF (96.1±1.8% [mean±S.D.]; Table S2). Negative controls (normal, species-matched IgG) showed little or no signal along the basement membrane (Supplemental Fig. S1).
Figure 3.
Co-localization of anti-ID4 and anti-PLZF signals in the human seminiferous epithelium. Representative IF using rabbit monoclonal anti-ID4 (green; A) or mouse monoclonal anti-PLZF (red; B), DNA counterstain (blue; C), or merged image (D) from a 21 year old. Arrows denote ID4+/PLZF+ cells. Bar is 50 μm. Insets show selected ID4+/PLZF+ cells with both cytoplasmic and nuclear staining.
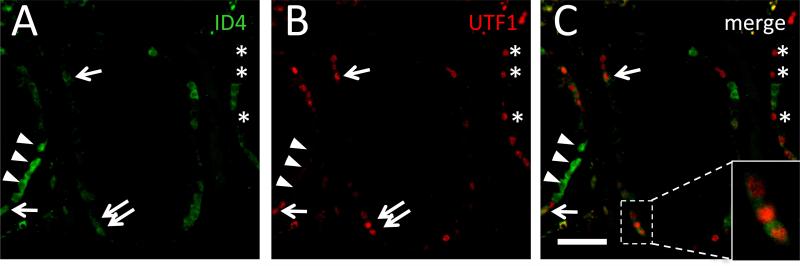
To confirm that ID4 is expressed relatively broadly across subsets of SPG, we selected UTF1 as a well-defined SPG marker. UTF1 expression was consistent with previously documented antibody specificities and was localized to the nuclei of SPG immediately adjacent to the basement membrane of the seminiferous tubules (Fig. 4; Supplemental Fig. S3) (Kristensen, et al., 2008). There was no discernible age-related change in the number of UTF1-positive cells (Supplemental Fig. S3 and data not shown). The co-expression pattern of ID4 and UTF1 revealed both UTF1-positive and UTF1-negative cells among the ID4+ SPG (Fig. 4; Supplemental Fig. S4; Table S2). The ID4+/UTF1+ SPG constituted approximately 37.1±15.1% (mean±S.D.) of ID4-positive cells. Therefore, ID4 and UTF1 mark distinct but partially overlapping subpopulations of SPG. In conjunction with the results for PLZF, these data indicate that the ID4+ population encompasses a broad spectrum of human SPG, including the undifferentiated subset.
Figure 4.
Co-localization of anti-ID4 and anti-UTF1 signals in the human seminiferous epithelium. Representative IF using rabbit monoclonal anti-ID4 (green; A) or mouse monoclonal anti-UTF1 (red; B), or merged image (C) from a 21 year old. Cell populations labeled: ID4+/UTF1+ (arrows), ID4+/UTF1− (arrowheads), and ID4−/UTF1+ (asterisks). Bar is 50 μm.
ID4-expressing cells tend to be rarely dividing
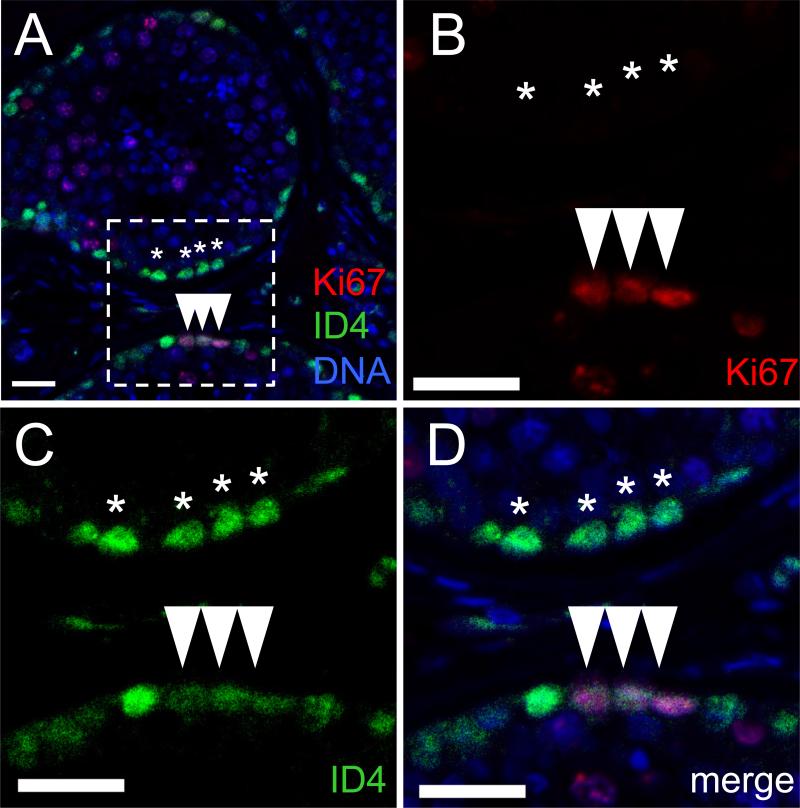
To determine whether the ID4+ SPG population is part of the mitotically-active cell fraction in the seminiferous tubule, Ki67 staining was employed. Co-labeling for ID4 and Ki67 revealed expression in generally non-overlapping germ cell populations, with only 0.6-2.8% of ID4-expressing cells co-stained for Ki67 (Fig. 5; Table S2). While the aforementioned ID4+ population was located in the monolayer along the basement membrane of the seminiferous epithelium, the Ki67+ population extended toward the adluminal zone of the tubule. This population comprised an irregular distribution of cell clusters, consistent with the expected appearance of type B SPG. These data indicate that the majority of the ID4+ population is rarely dividing.
Figure 5.
The majority of ID4-positive in the human seminiferous epithelium are not mitotically-active. Representative IF showing a small minority of anti-ID4 reactive cells (green) that co-expressed Ki67 (red). DNA counterstain is (blue). (A) Merged image at low magnification. (B-D) Higher magnification views of the field denoted by dashed line in (A). (B) Ki67 (red). (C) ID4 (green). (D) Merged image. (Arrowheads denote ID4+/Ki67+ cells; Asterisks denote selected ID4+/Ki67− cells.) Bar is 25 μm.
The ID4-positive spermatogonial population overlaps substantially with GPR125-expressing germ cells
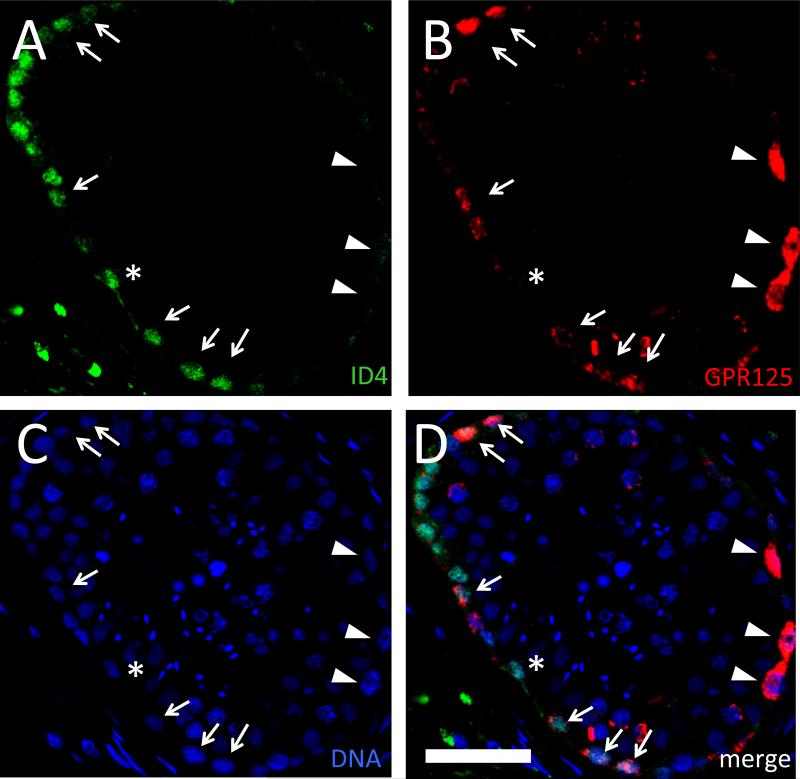
GPR125 marks undifferentiated SPG in mice and has been employed for selection of putative SSCs in humans (He, et al., 2010; Seandel, et al., 2007). Using an amplified detection system for enhanced sensitivity, we found that GPR125 is expressed in a comparatively large subpopulation of germ cells residing along the basement membrane of the seminiferous tubule but not in Sertoli cells, consistent with the reported GPR125 expression pattern in the adult mouse testis (Fig. 6 and Supplemental Fig. S5). Of note, GPR125 signal intensity varied widely both between patients and within patients, possibly due to slight differences in tissue fixation of labile, low abundance antigens. GPR125 was only detectable in Bouin's-fixed tissue but not in paraformaldehyde-fixed tissue. In each field that could be evaluated, •5 cells expressed GPR125. Co-staining for ID4 and GPR125 revealed substantial but not complete overlap between the ID4+ and GPR125+ SPG populations, respectively, along the basement membrane of both younger and older donors, although variations in signal intensity precluded reliable counting of GPR125+ cells (Fig. 6; Supplemental Fig. S6).
Figure 6.
Partial overlap of anti-ID4 and anti-GPR125 signal in the human seminiferous epithelium. Representative IF using rabbit monoclonal anti-ID4 (green; A) or goat polyclonal anti-GPR125 (red; B), DNA counterstain (blue; C), or merged image (D) from a 49 year old. Cell populations labeled: ID4+/GPR125+ (arrows), ID4-/low/GPR125+ (arrowheads), and ID4+/GPR125− (asterisk). Bar is 50 μm.
Discussion
The ideal stem cell marker is both highly sensitive and specific, and such features are critical not only for uncovering the molecular mechanisms of stem cell control but also for implementing clinical stem cell-based protocols safely (e.g., for exclusion of contaminants). To date, a relatively small array of candidate stem cell markers have been evaluated in normal adult human testis tissue, whereas a larger array of studies employed tissue from patients with azoospermia or other disorders. Herein, we have confirmed that expression of candidate SSC markers ID4 and GPR125 is maintained in SPG within the normal adult human testis into the seventh decade of life but that ID4+ and GPR125+ cells represent a larger fraction of SPG than expected based on prior work.
Expression of basic helix-loop-helix transcription factors of the ID family has been found in conjunction with a variety of stem types (Yokota, 2001). Based on firm genetic data linking ID4 expression to the SSC phenotype in mice, we sought to evaluate ID4 in normal adult human testis (Oatley, et al., 2011). Previously, it was shown that type A SPG contained within human testicular seminomatous tumors express ID4 in the cytoplasm, but normal, non-malignant tissue has not been studied (Sablitzky, et al., 1998). We found both nuclear and cytoplasmic expression of ID4 in the normal testis, irrespective of donor age. The biological significance of the nuclear-to-cytoplasmic ratio of ID4 in SPG is unknown but could represent the effect of a dynamic signal rather than a marker for cellular differentiation status. Our data suggest that the distribution of ID4 positive cells in humans may be generally less restricted than in rodents (Oatley, et al., 2011).
In contrast to the sparse distribution of ID4+ type Asingle SPG in the murine testis (Oatley, et al., 2011), we found large numbers of strongly-positive cells in the human testis by immunostaining using a highly specific monoclonal antibody for ID4. In conjunction with the prior findings of Sablitzky et al. (1998), the expression pattern that we observed, consistent with the pattern observed with another unrelated antibody, is likely to represent authentic ID4 protein (Sablitzky, et al., 1998; Uhlen, et al., 2010). One interpretation of the unexpectedly large pool of ID4 positive SPG in humans is that expression is not restricted to stem cells but this would need to be validated using functional assays.
GPR125 is an orphan G-protein coupled receptor that marks a large pool of murine undifferentiated SPG including SSCs (Seandel, et al., 2007). The implementation of GPR125 for cell sorting, prospective validation of putative human SSCs, or immunostaining has been hampered by the lack of quality antibodies and inconsistent results (von Kopylow, et al., 2012). Our data are consistent with a similar pattern of GPR125 expression in the human testis as was seen in mice, with GPR125+ cells in a more broad distribution than would be expected for SSCs alone. Whereas He et al. (2010) found the immunoreactivity of GPR125 to be limited to 1-2 cells per seminiferous tubule cross-section (He, et al., 2010), we confirmed the existence of GPR125+ cells in a similar distribution but at a higher frequency (•5 cells) in transverse sections through the tubules using a different antibody. This difference could be due to the highly sensitive immunodetection system that we employed.
While the function of GPR125 remains enigmatic, anti-GPR125 antibodies have been employed for immunoselection of human SSCs (He, et al., 2010). Various studies have successfully detected GPR125 at the mRNA level, but mixed results have been obtained in analysis of GPR125 protein (von Kopylow, et al., 2012). In fact, we tested multiple commercial human anti-GPR125 antibodies that yielded non-specific staining in the human testis (data not shown) before finding effective ones. We speculate that low levels of GPR125 antigen density and/or stability necessitate a highly amplified detection system when employing available commercial anti-GPR125 antibodies (e.g., from Novus Biologicals), given that GPR125 antigen(s) were generally only detectable in Bouin's fixed tissue but not after paraformaldehyde fixation.
The common expression of ID4 and UTF1, PLZF, or GPR125 in some but not all SPG suggests that substantial heterogeneity exists within the undifferentiated SPG. However, given the limitations of archived samples, it is difficult to ascertain whether expression of such markers is stable or varies in a dynamic fashion, related to the status of the cell and surrounding microenvironment. For example, GPR125 expression in mice varied substantially with the stage of the spermatogenic cycle of the seminiferous tubules but it is presently unclear whether or not the same is true in humans (Seandel, et al., 2007). Interestingly, ID4+ cells did not tend to include the Ki67+ fraction, presumed to be proliferating cells. However, it is unknown what fraction of authentic human SSCs are likely to be mitotically actively vs. quiescent.
Whereas most human gametes yield healthy offspring, subtle yet pathogenic alterations can occur in a cell autonomous or non-cell autonomous manner with aging of men (Paul and Robaire, 2013). We did not detect substantial age-related changes in the populations of SPG labeled by the candidate markers in this study, although the sample size is small. This result is generally consistent with conclusions obtained using morphological criteria to identify Adark and Apale SPG which do not decrease substantially until after about the sixth decade (Nistal, et al., 1987). Furthermore, potential age-related declines in spermatogenic function are unlikely to be constrained by the size of the ID4+ population. Finally, the absence of substantial age-related changes in the Type A SPG populations indicates that cadaveric organ donor tissue even from older adults is useful for further studies of undifferentiated SPG and possibly SSCs.
Supplementary Material
Acknowledgements
We thank the families of all the organ donors for their participation. We also thank the staff of the New York Organ Donor Network, Eugene K. Cha, M.D., Christopher Barbieri, M.D., Ph.D., and Wayland Hsiao, M.D. for their gracious help in tissue procurement.
Funding
Supported in part by Research Grant No. 5-FY11-571 from the March of Dimes Foundation and National Institutes of Health grant 1DP2HD080352-01 (M.S.). M.S. was a New York Stem Cell Foundation-Druckenmiller Fellow. Work was also supported in part by the Translational Research Program of the WCMC Department of Pathology and Laboratory Medicine.
Footnotes
Disclosures
A patent application has been submitted by Weill Cornell Medical College for the use of GPR125 as a stem cell marker with Drs. Seandel and Rafii as co-inventors.
References
- Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Renewal of spermatogonia in man. Am J Anat. 1966;118:509–524. doi: 10.1002/aja.1001180211. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, Griswold MD. Questions about spermatogonia posed and answered since 2000. J Androl. 2012;33:1085–1095. doi: 10.2164/jandrol.112.016832. [DOI] [PubMed] [Google Scholar]
- Dym M, Kokkinaki M, He Z. Spermatogonial stem cells: mouse and human comparisons. Birth defects research Part C, Embryo today : reviews. 2009;87:27–34. doi: 10.1002/bdrc.20141. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Schlatt S. A revised model for spermatogonial expansion in man: lessons from non-human primates. Reproduction. 2006;132:673–680. doi: 10.1530/rep.1.01081. [DOI] [PubMed] [Google Scholar]
- He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biology Of Reproduction. 2010;82:363–372. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Hansel MC, Orwig KE. Spermatogonial stem cells in higher primates: are there differences from those in rodents? Reproduction. 2010;139:479–493. doi: 10.1530/REP-09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Lin CC, Sheng Y, Tomko J, Rodriguez M, et al. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25:2330–2338. doi: 10.1634/stemcells.2007-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs RM, Seandel M, Falciatori I, Rafii S, Pandolfi PP. Plzf regulates germline progenitor selfrenewal by opposing mTORC1. Cell. 2010;142:468–479. doi: 10.1016/j.cell.2010.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadyar F, Wong J, Maki C, Pacchiarotti J, Ramos T, Howerton K, et al. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Hum Reprod. 2011;26:1296–1306. doi: 10.1093/humrep/der026. [DOI] [PubMed] [Google Scholar]
- Jacobs W, Dhaene K, Van Marck E. Tyramine-amplified immunohistochemical testing using “homemade” biotinylated tyramine is highly sensitive and cost-effective. Arch Pathol Lab Med. 1998;122:642–643. [PubMed] [Google Scholar]
- Kristensen DM, Nielsen JE, Skakkebaek NE, Graem N, Jacobsen GK, Rajpert-De Meyts E, et al. Presumed pluripotency markers UTF-1 and REX-1 are expressed in human adult testes and germ cell neoplasms. Hum Reprod. 2008;23:775–782. doi: 10.1093/humrep/den010. [DOI] [PubMed] [Google Scholar]
- Lim J, Goriely A, Turner GD, Ewen KA, Jacobsen GK, Graem N, et al. OCT2, SSX and SAGE1 reveal the phenotypic heterogeneity of spermatocytic seminoma reflecting distinct subpopulations of spermatogonia. J Pathol. 2011;224:473–483. doi: 10.1002/path.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan RI, Rajpert-De Meyts E, Hoei-Hansen CE, de Kretser DM, Skakkebaek NE. Histological evaluation of the human testis--approaches to optimizing the clinical value of the assessment: mini review. Hum Reprod. 2007;22:2–16. doi: 10.1093/humrep/del279. [DOI] [PubMed] [Google Scholar]
- Mundlos S, Pelletier J, Darveau A, Bachmann M, Winterpacht A, Zabel B. Nuclear localization of the protein encoded by the Wilms' tumor gene WT1 in embryonic and adult tissues. Development. 1993;119:1329–1341. doi: 10.1242/dev.119.4.1329. [DOI] [PubMed] [Google Scholar]
- Nagano M, Patrizio P, Brinster RL. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil Steril. 2002;78:1225–1233. doi: 10.1016/s0015-0282(02)04345-5. [DOI] [PubMed] [Google Scholar]
- Nagano MC, Yeh JR. The identity and fate decision control of spermatogonial stem cells: where is the point of no return? Curr Top Dev Biol. 2013;102:61–95. doi: 10.1016/B978-0-12-416024-8.00003-9. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistal M, Codesal J, Paniagua R, Santamaria L. Decrease in the number of human Ap and Ad spermatogonia and in the Ap/ Ad ratio with advancing age. New data on the spermatogonial stem cell. J Androl. 1987;8:64–68. doi: 10.1002/j.1939-4640.1987.tb00950.x. [DOI] [PubMed] [Google Scholar]
- Oatley MJ, Kaucher AV, Racicot KE, Oatley JM. Inhibitor of DNA binding 4 is expressed selectively by single spermatogonia in the male germline and regulates the self-renewal of spermatogonial stem cells in mice. Biology Of Reproduction. 2011;85:347–356. doi: 10.1095/biolreprod.111.091330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C, Robaire B. Ageing of the male germ line. Nat Rev Urol. 2013;10:227–234. doi: 10.1038/nrurol.2013.18. [DOI] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Hikim APS, Clegg ED. Histological and Histopathological evaluation of the testis. Cache River Press; St. Louis: 1990. [Google Scholar]
- Sablitzky F, Moore A, Bromley M, Deed RW, Newton JS, Norton JD. Stage- and subcellular-specific expression of Id proteins in male germ and Sertoli cells implicates distinctive regulatory roles for Id proteins during meiosis, spermatogenesis, and Sertoli cell function. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 1998;9:1015–1024. [PubMed] [Google Scholar]
- Sadri-Ardekani H, Akhondi MA, van der Veen F, Repping S, van Pelt AM. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA : the journal of the American Medical Association. 2011;305:2416–2418. doi: 10.1001/jama.2011.791. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–350. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Chinaranagari S, Patel D, Carey J, Chaudhary J. Epigenetic inactivation of inhibitor of differentiation 4 (Id4) correlates with prostate cancer. Cancer Med. 2012;1:176–186. doi: 10.1002/cam4.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, et al. Towards a knowledge-based Human Protein Atlas. Nature biotechnology. 2010;28:1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- von Kopylow K, Kirchhoff C, Jezek D, Schulze W, Feig C, Primig M, et al. Screening for biomarkers of spermatogonia within the human testis: a whole genome approach. Hum Reprod. 2010;25:1104–1112. doi: 10.1093/humrep/deq053. [DOI] [PubMed] [Google Scholar]
- von Kopylow K, Staege H, Spiess AN, Schulze W, Will H, Primig M, et al. Differential marker protein expression specifies rarefaction zone-containing human Adark spermatogonia. Reproduction. 2012;143:45–57. doi: 10.1530/REP-11-0290. [DOI] [PubMed] [Google Scholar]
- Waheeb R, Hofmann MC. Human spermatogonial stem cells: a possible origin for spermatocytic seminoma. Int J Androl. 2011;34:e296–305. doi: 10.1111/j.1365-2605.2011.01199.x. discussion e305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen YH, Ho A, Patil S, Akram M, Catalano J, Eaton A, et al. Id4 protein is highly expressed in triple-negative breast carcinomas: possible implications for BRCA1 downregulation. Breast Cancer Res Treat. 2012;135:93–102. doi: 10.1007/s10549-012-2070-0. [DOI] [PubMed] [Google Scholar]
- Yokota Y. Id and development. Oncogene. 2001;20:8290–8298. doi: 10.1038/sj.onc.1205090. [DOI] [PubMed] [Google Scholar]
- Zohni K, Zhang X, Tan SL, Chan P, Nagano M. CD9 is expressed on human male germ cells that have a long-term repopulation potential after transplantation into mouse testes. Biology Of Reproduction. 2012;87:27. doi: 10.1095/biolreprod.112.098913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.